Reprogramming axonal behavior by axon-specific viral transduction (original) (raw)
. Author manuscript; available in PMC: 2013 Mar 1.
Published in final edited form as: Gene Ther. 2012 Jan 26;19(9):947–955. doi: 10.1038/gt.2011.217
Abstract
The treatment of axonal disorders, such as diseases associated with axonal injury and degeneration, is limited by the inability to directly target therapeutic protein expression to injured axons. Current gene therapy approaches rely on infection and transcription of viral genes in the cell body. Here we describe an approach to target gene expression selectively to axons. Using a genetically engineered mouse containing epitope-labeled ribosomes, we find that neurons in adult animals contain ribosomes in distal axons. To use axonal ribosomes to alter local protein expression, we utilized a Sindbis virus containing an RNA genome that has been modified so that it can be directly used as a template for translation. Selective application of this virus to axons leads to local translation of heterologous proteins. Furthermore, we demonstrate that selective axonal protein expression can be used to modify axonal signaling in cultured neurons, enabling axons to grow over inhibitory substrates typically encountered following axonal injury. We also show that this viral approach also can be used to achieve heterologous expression in axons of living animals, indicating that this approach can be used to alter the axonal proteome in vivo. Together, these data identify a novel strategy to manipulate protein expression in axons, and provides a novel approach for using gene therapies for disorders of axonal function.
Keywords: viral vector gene transfer, Sindbis, axon regeneration
INTRODUCTION
Axon injury is a feature of traumatic nerve injury as well as numerous peripheral neuropathies. In these conditions, axon regeneration, elongation, and reinnervation of target tissue is needed to restore neuronal connectivity and function. However, axon regeneration is typically slow in the peripheral nervous system (PNS), and is highly inefficient in the central nervous system (CNS) due to inhibitors of axonal growth at injury sites1, 2. A major therapeutic goal is to develop approaches to reactivate the developmental axonal growth and guidance programs in injured axons in order to reestablish functional neuronal circuitry2.
Although gene therapy can be used to manipulate gene expression in a variety of cell types, it cannot readily be used to express growth-promoting genes specifically in injured axons3. Many gene therapy viruses require transcription steps in the cell body or nucleus4. Additionally, gene therapy directed towards neurons may lead to ubiquitous expression of transgenes throughout the cell, instead of selective expression in axons. Proteins that influence axonal growth may have undesirable effects on neuronal morphology if they are not axonally restricted, which is a feature of several signaling proteins that have roles in axonal elongation during development5.
A mechanism to selectively control the spatial expression of proteins in neurons is local translation. Local translation is particularly important during embryonic development, when elongating axons contain readily detectable ribosomes and mRNA6. Many mRNAs in embryonic axons encode for proteins that influence axonal growth and guidance6, 7. After the establishment of neuronal circuitry, ribosomes and mRNA are no longer readily detectable in axons8. For example, electron microscopy (EM) studies of axons from mature, myelinated neurons have not detected the classical rosette structures that reflect actively translating polyribosomes in the distal axon9-12. Alternative approaches for imaging ribosomes, such as RNA-binding dyes and electron spectroscopic detection of phosphorus, which is abundant in ribosomes, support the presence of ribosomes in the axoplasm of myelinated neurons in adults13, but the potential nonspecificity of these techniques has prevented definitive conclusions regarding axonal localization of ribosomes6.
Here we describe the design of a virus that utilizes axonal ribosomes and local translation to reprogram axonal protein expression specifically in axons and promote axonal growth. Using a transgenic mouse that expresses eGFP-tagged ribosomes in cortical neurons, we show that ribosomes are present in adult CNS myelinated axons and are selectively enriched at the nodes of Ranvier. We describe the construction of a modified Sindbis virus that contains an RNA genome that can be used as a template for translation by axonal ribosomes, and show that this virus can be used to induce heterologous gene expression specifically in axons. We use this virus to induce axonal expression of soluble adenylyl cyclase (sAC), an enzyme that generates the axon-growth enhancing second messenger cAMP. Selective axonal expression of sAC increases axonal growth rates and overcomes the axon-growth inhibitory effects of components of the glial scar. We also show that application of this virus to axons in rat spinal cord leads to heterologous gene expression in axons in vivo, indicating that this modified Sindbis virus can be used to target and manipulate protein expression in axons in adult animals.
RESULTS
Ribosomes are localized to nodes of Ranvier in corticospinal tract axons
Although EM studies have not provided convincing evidence of axonal ribosomes in the CNS of adult animals14, there are several reasons why axonal ribosomes may have been overlooked. For example, if axonal ribosomes do not aggregate into electron dense rosette structures or if they are obscured by other electron dense structures, they will not be readily detected9.
Immunohistochemical techniques to detect ribosomes have been complicated by nonspecificity of the antibodies15. Experiments to demonstrate the specificity of antibody staining would require demonstrating the loss of immunoreactivity in knockout animals, which is not possible for genes that encode proteins required for cell viability, such as ribosome subunits.
To determine if ribosomes are present in axons of mature neurons, we used a genetic strategy to overcome the technical issues associated with labeling and identifying ribosomes in axons. We used mice expressing eGFP fused to the large ribosomal subunit protein, L10a. The eGFP-L10a fusion is stable, and is not associated with significant cleavage of the eGFP from the L10a16. The expression of this transgene is under the control of the Glt25d2 promoter, which drives expression in layer 5b cortical neurons17. These neurons include neurons of the motor cortex that project axons into the spinal cord and constitute the corticospinal tract18. Since the corticospinal tract is the only portion of the spinal cord that derives from cortical neurons, any eGFP labeling in the spinal cord must derive from axons of cortical motor neurons, and not from any adjacent supporting cells. Additionally, since wild-type mice do not express eGFP, anti-eGFP immunostaining in these mice can be used to establish the specificity of labeling in axons.
We first confirmed that eGFP-L10a-labeled ribosomes are localized to rough endoplasmic reticulum and dendrites in a manner consistent with previous reports19. Immunoelectron microscopy using anti-eGFP antibodies revealed characteristic ribosome labeling in rough endoplasmic reticulum and dendrites in the cortex of adult Glt25d2::eGFP-L10a mice (Figure 1a). These localizations are essentially identical to previously reported ribosome localizations based on their characteristic electron-dense structure19. No specific signal was detected in the cell body or dendrites of neurons in wild-type mice (Figure 1b).
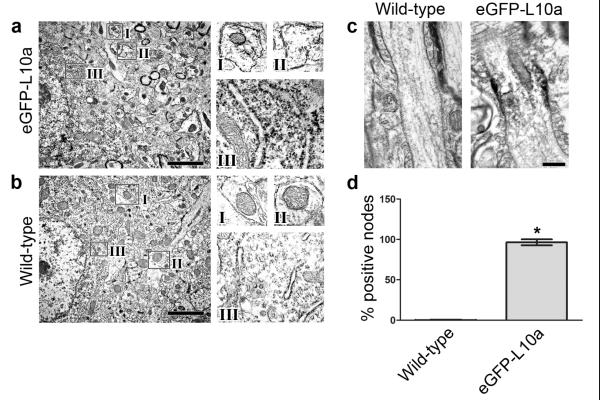
Figure 1. Immunoelectron microscopy shows eGFP-labeled ribosomes localized to nodes of Ranvier in the corticospinal tract.
(a) eGFP-L10a exhibits characteristic ribosomal localization in Glt25d2::eGFP-L10a mice. Immunoelectron microscopy was performed on cortical sections of 8-10 week old transgenic mice using anti-GFP antibodies and peroxidase labeling. Anti-eGFP immunoreactivity is detected in dendrite cross sections (boxes I and II, false-colored yellow) and rough endoplasmic reticulum (box III, false-colored blue) in the cortex of Glt25d2::eGFP-L10a mice. Enlarged images of regions I, II, and III are shown on the right. The localization of the eGFP-labeled ribosomes is consistent with previous descriptions of endogenous ribosomes19. Scale bar, 1 μm.
(b) Anti-eGFP immunoreactivity is not present in the dendrites (boxes I and II, false-colored yellow) or rough endoplasmic reticulum (box III, false-colored blue) of wild-type animals. Enlarged images of regions I, II, and III are shown on the right. Scale bar, 1 μm.
(c) eGFP-labeled ribosomes are localized to nodes of Ranvier in the corticospinal tract. Corticospinal tracts from 8 – 10 week old Glt25d2::eGFP-L10a or wild-type mice were examined with anti-GFP immunoelectron microscopy and visualized with peroxidase. Specific eGFP signal in Glt25d2::eGFP-L10a mice is localized beneath the membrane in nodes of Ranvier, which are recognized by characteristic loops of myelin (false-colored green). Scale bar, 500 nm.
(d) Quantification of percent of positive nodes in the corticospinal tract of Glt25d2::eGFP-L10a mice and wild-type controls. Nodes were considered positive if the average signal intensity in a 67 nm2 area below the nodal membrane was at least two standard deviations higher than the average signal intensity in wild-type controls. The number of positive nodes was significantly higher in Glt25d2::eGFP-L10a mice compared to wild-type controls (*P < 0.0001, unpaired two-tailed Student’s _t_-test, n = 30 nodes measured from each animal. N =3 per group). Data are expressed as mean ± s.e.m.
We next examined whether ribosomes are present in axons of adult animals. We sectioned thoracic level (T) 7-8 spinal cords and examined eGFP expression in the corticospinal tract of wild-type and Glt25d2::eGFP-L10a mice by anti-eGFP immunoelectron microscopy. In these mice, a striking enrichment of eGFP labeling was detected at nodes of Ranvier (Figure 1c). Labeling primarily appeared as a cluster of immunoreactive signals just under the membrane at the node. Anti-eGFP labeling was detected in approximately 100% of nodes of Ranvier in the corticospinal tract of Glt25d2::eGFP-L10a mice, while labeling was not observed in any nodes in wild-type mice (Figure 1d). This nodal localization of immunoreactivity was also confirmed using anti-GFP antibodies with an alternate method of visualization (Supplementary Figure 1). Together, these data indicate that ribosomes are present in the corticospinal tract in adult animals and are selectively localized to nodes of Ranvier.
We next examined whether this labeling was altered after axonal injury. Adult mice were subjected to a dorsal hemisection injury at the level of T8 and then analyzed approximately 500 μm rostral to the injury site at 24 h and 7 d after injury. Anti-eGFP labeling remained detectable at the nodes, with no obvious change in signal localization, suggesting that injury of corticospinal tracts does not lead to increased translocation of ribosomes in the injured axon within this time period (Supplementary Figure 2). Because of their low frequency and small size, regenerating growth cones are technically challenging to locate in tissue. Nevertheless, we located a regenerating growth cone from an injured myelinated axon in the corticospinal tract of a Glt25d2::eGFP-L10a mouse 7 d after injury. This growth cone also exhibited eGFP-positive labeling, indicating that ribosomes are present within regenerating growth cones (Supplementary Figure 3).
Design of a Sindbis genome that expresses transgenes in axons
Because axons of adult animals contain ribosomes, we considered the possibility that these ribosomes could be used to translate heterologous RNA that is selectively introduced into axons, thereby influencing local protein expression to promote axonal regeneration. We considered the possibility of using an RNA virus to selectively introduce RNA into axons. Alphaviruses, such as Sindbis and Semliki forest virus, consist of a single-stranded, plus-strand RNA genome with a 5′ cap and a 3′ poly(A) tail20. The Sindbis virus genome encodes a coat protein which renders the virus highly neuron specific, although the coat protein can be altered to affect viral tropism21, 22. Upon entry into the cell, virally encoded RNA-dependent RNA polymerase amplifies the genomic RNA, and generates transcripts that are under the control of a subgenomic promoter within the genomic RNA. Because Sindbis is not retrogradely trafficked from axons20, and because axons may not contain sufficient ribonucleotide triphosphates substrates used by the nucleus-localized cellular RNA polymerases, axonal infection of Sindbis is unlikely to lead to productive infection.
Several studies have utilized internal ribosome entry site (IRES) sequences to drive transgene expression in Semliki forest and Sindbis viruses as an alternative to the subgenomic promoter23, 24. We considered that the IRES sequence could permit the Sindbis genome to be used as a direct template for translation by axonal ribosomes (Figure 2a). We used a Sindbis virus containing the 586-nt human encephalomyocarditis virus IRES sequence based on its ability to promote internal initiation of RNA translation in a broad range of cell types, including neurons25. The IRES sequence was inserted into the Sindbis virus expression plasmid, SINrep(nsP2S726), that contains a point mutation in the nonstructural protein nsP2, which minimizes neurotoxicity21, 26.
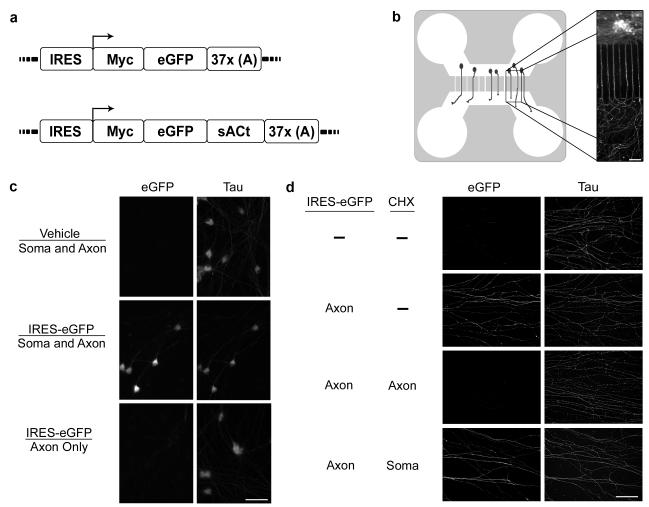
Figure 2. The Sindbis-IRES virus enables axon-specific protein expression.
(a) Schematic representation of Sindbis-IRES virus expression cassette. The Sindbis-IRES-eGFP virus is a plus-strand, single-stranded RNA genome which contains an IRES for direct translation by axonal ribosomes. The open reading frame of the top schematic expresses a Myc epitope fused to the eGFP sequence. The 3′ untranslated region includes a string of 37 adenosines. The poly(A) tract is expected to confer stability to the RNA genome. Below is a schematic of a similar construct that expresses a sACt fusion protein.
(b) Diagram of microfluidic chamber. The culture chamber consists of a PDMS mold suitable for cell culture. The chamber is comprised of a cell body and an axonal compartment connected by microgrooves that are 10μm wide and 450 μm long. When neurons (drawn in green in schematic on left) are cultured in the cell-body compartment, axons grow through the microgrooves into the fluidically isolated axonal compartment. Shown on the right is an immunofluorescence image of a microfluidic chamber. Rat DRG neurons with axons extending through the microgrooves are visualized by anti-GAP43 immunohistochemistry (green). This cell culture platform enables experimental treatments to be selectively applied to the axonal compartment, without introduction into the cell body compartment. Scale bar, 100 μm.
(c) Application of Sindbis-IRES-eGFP to the axonal compartment does not result in detectable eGFP in cell bodies. E15 rat DRG neurons were cultured in microfluidic chambers and either Sindbis-IRES-eGFP or vehicle as indicated (above the line) was added to the indicated compartments (below the line). eGFP expression in cell bodies was detected by anti-eGFP immunofluorescence (green), and Tau expression (red) was used to label neuronal cell bodies and processes after 8 h. eGFP was readily detected in the cell bodies of neurons infected with Sindbis-IRES-eGFP in both the axonal and cell body compartments. No eGFP was detected in the cell bodies of neurons infected with Sindbis-IRES-eGFP in the axonal compartment alone. Taken together, these data indicate that infection of axons by Sindbis-IRES virus does not lead to retrograde transport and Sindbis expression in the cell body. Scale bar, 50 μm.
(d) Axon-specific infection with Sindbis-IRES-eGFP leads to local translation of eGFP. Sindbis-IRES-eGFP was applied to the axonal compartment as indicated, and axonal expression of eGFP (green) was detected by immunofluorescence. Axons were counterstained with Tau (red). Application of Sindbis-IRES-eGFP to the axonal compartment induced the appearance of eGFP in axons. This effect was blocked by selective application of cycloheximide to the axonal compartment at the time of infection. Axonal eGFP was not blocked by selective application of cycloheximide to the cell body compartment, confirming that the expression of eGFP in axons does not involve translocation of the virus to the cell body and somatic translation, but is a consequence of axonal mRNA translation. Scale bar, 100 μm.
To determine if the Sindbis-IRES RNA genome can serve as a template for translation within axons, embryonic day 15 (E15) rat dorsal root ganglia (DRG) neurons were plated in compartmentalized microfluidic culture chambers. These chambers allow the fluidic isolation of axons from cell bodies by means of a volume differential of media between the two compartments. Microfluidic chambers provide an experimental system in which Sindbis virus can be selectively applied to either cell bodies or axons without diffusion to the other compartment27 (Figure 2b). We applied Sindbis-IRES virus driving the expression of eGFP (Sindbis-IRES-eGFP) either to both the axonal and cell body compartments or to only the axonal compartment. As expected, application of Sindbis-IRES-eGFP to both compartments resulted in robust eGFP expression throughout the neuron and axon (Figure 2c). However, application of Sindbis-IRES-eGFP selectively to the axonal compartment resulted in eGFP expression in essentially all axons with no detectable eGFP in cell bodies (Figure 2d). The absence of eGFP expression in the cell bodies and proximal axons located within the cell body compartment indicates that the axonally localized eGFP was synthesized within axons, without the need for viral replication and eGFP transcription or translation in the cell body.
To further confirm that the eGFP detected in axons was synthesized locally, we preincubated the axonal compartment with the protein synthesis inhibitor cycloheximide for 30 min before axonal infection with Sindbis-IRES-eGFP. With cycloheximide in the axonal compartment, eGFP levels in axons were abolished, demonstrating that intra-axonal synthesis is necessary for protein expression elicited by axonal infection with Sindbis-IRES virus particles (Figure 2d). Addition of cycloheximide to the cell body compartment 30 min before axonal infection did not alter eGFP expression in axons, demonstrating that axonal synthesis is sufficient for protein expression (Figure 2d). The eGFP expression level observed in distal axons after application of the Sindbis-IRES particles specifically to the axonal compartment was ~20% as intense as when the Sindbis-IRES virus was applied to both compartments reflects the increase in the efficiency of eGFP expression resulting from the amplification of the Sindbis viral genome in the cell body20, 28. Although eGFP could diffuse from axons to cell bodies, we did not detect significant increases in eGFP in the cell body after axon-specific expression of eGFP, possibly due to dilution of the protein as it diffused into the cell body.
Expression and functional activity of transgenes in axons
We next sought to identify proteins that could be used to promote axonal regeneration when selectively expressed in axons. cAMP and cAMP-stimulating molecules have been shown to increase axonal growth both in vitro and when administered systemically to animals that have been subjected to models of spinal cord injury29, 30. However, cAMP-stimulating molecules affect many organ systems and systemic administration may have numerous nonspecific effects31. Selectively increasing cAMP in axons by genetic manipulation might provide an approach to bypass problems of systemic administration of cAMP. Unlike canonical transmembrane adenylyl cyclases, sAC is a cytosolic cAMP-generating enzyme. Since it is not inserted into membranes, it may be readily expressed in axons, which may have limited capacity to insert locally synthesized proteins into membranes32. We focused on a truncated form of sAC, sACt, which is considerably shorter than the longer isoforms expressed in neurons and has high constitutive basal activity33.
We next asked if axonal expression of sACt mimics the effects of pharmacologic manipulation of cAMP in axons. One mechanism by which cAMP stimulates axonal growth across inhibitory substrates is by inducing transcription and translation of growth promoting genes in the cell body29,34. Indeed, pharmacological increases in axonal cAMP induce the phosphorylated form of cAMP response element-binding protein (pCREB) in the nucleus, which stimulates expression of genes required for axonal growth35. To determine if axonal sAC expression exhibits a similar effect, we infected axons with Sindbis-IRES virus driving the expression of eGFP-sACt (Sindbis-IRES-eGFP-sACt). Postnatal rat DRG neurons were cultured in microfluidic chambers, and axons were observed to cross into the axonal compartment by day in vitro two (DIV 2). Selective application of Sindbis-IRES-eGFP-sACt to axons on DIV 2 led to the expression of eGFP-sACt in axons by DIV 3, with little to no expression detected in the cell body (Figure 3a). To determine if axonally expressed sACt mimicked the effects of pharmacologic manipulation of cAMP, we monitored levels of pCREB after selective application of Sindbis-IRES-eGFP-sACt. Nuclear pCREB levels were identical in neurons whose axons were treated with vehicle or infected with the control virus, Sindbis-IRES-eGFP. However, infection of axons with Sindbis-IRES-eGFP-sACt increased the levels of pCREB to a level similar to that induced by axonal application of 10 μM 8-Br-cAMP, a cell-permeable, non-hydrolysable cAMP analog (Figure 3b). The effect of axonal application of Sindbis-IRES-eGFP-sACt was blocked by pretreatment with cycloheximide in the axonal compartment, confirming that the effect of Sindbis-IRES-eGFP-sACt was mediated by axonal synthesis of eGFP-sACt (Figure 3c). The ability of axonal eGFP-sACt expression to induce an increase in nuclear pCREB levels indicates that transgene expression encoded by the Sindbis-IRES virus is sufficient to induce retrograde signaling to the nucleus.
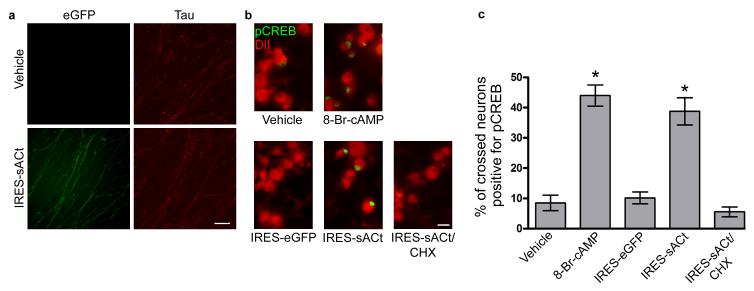
Figure 3. Axon-specific expression of soluble adenylyl cyclase alters signaling in the cell body.
(a) Local translation of eGFP-sACt in axons upon axonal application of Sindbis-IRES-eGFP-sACt. Vehicle or Sindbis-IRES-eGFP-sACt was applied to the axonal compartment of microfluidic chambers containing P6 rat DRG neurons. eGFP-sACt was visualized by anti-eGFP immunofluorescence (green) in axons counterstained with Tau immunofluorescence (red). Axonal application of Sindbis-IRES-eGFP-sACt induced appearance of eGFP-sACt, confirming that postnatal DRG axons can translate proteins. Scale bar, 50 μm.
(b) Axonal expression of sACt increases pCREB in neuronal nuclei. P6 DRG neurons were cultured in microfluidic chambers. Sindbis-IRES virus and experimental treatments were added to the axonal compartment as indicated. pCREB levels in neuronal nuclei were detected by anti-pCREB immunofluorescence (green). The cell bodies of neurons with distal axons extended into the axonal compartment were identified by retrograde DiI labeling (red). Few pCREB positive neurons were detected after vehicle or Sindbis-IRES-eGFP infection of axons. Application of 8-Br-cAMP (10 μM) to axons for 1 h increased the number of pCREB-positive neurons. Axonal application of Sindbis-IRES-eGFP-sACt for 8 h resulted in a similar increase in pCREB positive neurons. This effect was blocked by administration of cycloheximide (1 μM) to the axon at the time of infection, confirming that axonal translation is required for the induction of pCREB by axonally applied Sindbis-IRES-sACt. Scale bar, 50 μm.
(c) Quantification of the increase in pCREB-positive nuclei in (b). Treatment of axons with either 10 μM 8-Br-cAMP or IRES-eGFP-sACt resulted in a statistically significant increase in the number of pCREB positive neurons relative to vehicle (PBS) treatment (*P < 0.0001, n = 176 neurons measured across three experiments, and *P < 0.0001, n = 93 neurons measured across three experiments, respectively). The number of pCREB-positive nuclei were not significantly increased following treatment with either IRES-eGFP (P = 0.62, n = 166 neurons measured across three experiments) or IRES-eGFP-sACt with CHX (P = 0.34, n = 179 nuclei measured across three experiments) as compared with vehicle control. Student’s _t_-test (unpaired, two-tailed) was applied. Data are expressed as mean ± s.e.m.
Axonal expression of eGFP-sACt promotes axonal growth on inhibitory substrates
Axon regeneration after injury is limited due to inhibitory substances found in myelin and the glial scar36. A component of the glial scar, chondroitin sulfate proteoglycan (CSPG)37, acts on receptors on distal axons to inhibit axonal growth38, 39. In most studies, the inhibitory effects of CSPG on axon growth are detected by plating neurons directly on a substratum containing CSPG. However, this approach results in CSPG exposure to both the cell body and axons, which differs from the selective axonal exposure to CSPG that occurs after injury. To study pathways affecting CSPG signaling in axons, we developed a microfluidic culture system to model the selective exposure of elongating axons to CSPG. In this system, CSPG is present only in the axonal compartment, so that only the distal axons are exposed to CSPG, similar to the selective exposure of CSPG to axon terminals in nerve injuries. We next addressed whether axons that encounter CSPG exhibit reduced elongation rates. To test this, we used postnatal DRG neurons, since these neurons exhibit reduced axonal growth in response to CSPG40. Axons growing into the axonal compartments containing CSPG in the substratum exhibited an approximately 50% reduction in axon growth rate compared to axons growing into axonal chambers containing laminin alone (Figure 4a). The reduced growth rate indicates that selective exposure of axons to CSPG induces an inhibitory effect on axonal growth rates.
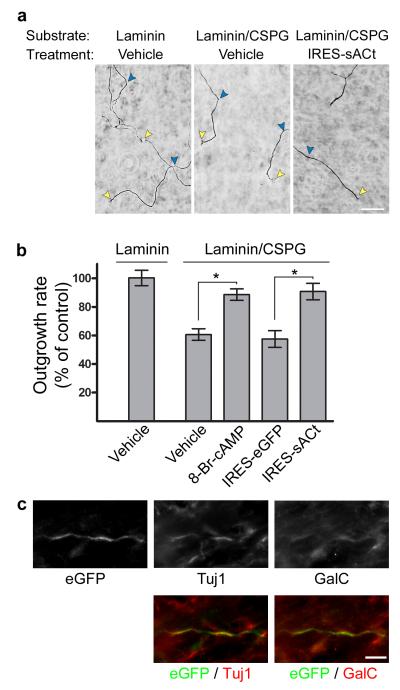
Figure 4. Axon-specific expression of soluble adenylyl cyclase alters axon behavior.
(a) Axonal application of Sindbis-IRES-eGFP-sACt results in increased axonal growth on CSPG. P6 DRG neurons were cultured in microfluidic chambers in which the axonal compartment was coated with either laminin, or laminin containing CSPG as indicated. The axonal compartment was incubated with either vehicle or Sindbis-IRES-eGFP-sACt as indicated. Axonal growth was measured by imaging axon terminals over 3 h by phase contrast microscopy. Shown are phase contrast images at t = 3 h. Axon termini are marked with yellow arrowheads. The position of axon termini at t = 0 h are indicated with blue arrowheads. Axon growth was slowed in microfluidic chambers containing CSPG in the axonal compartment. However, axonal growth rates of axons expressing Sindbis-IRES-eGFP-sACt extending across CSPG were comparable to those of the laminin control. Scale bar, 50 μm.
(b) Quantification of axonal outgrowth rate in (a). Axon growth was normalized to the rate of vehicle-treated axons grown on laminin. Axons grow significantly slower across laminin containing CSPG than laminin alone (*P < 0.0001, n = 34 axons measured across a minimum of three experiments). Treatment of axons with 10 μM 8-Br-cAMP significantly increased outgrowth rates across CSPG as compared to vehicle control (*P < 0.0001, n = 41 axons measured across a minimum of three experiments). Axonal expression of eGFP-sACt significantly increased outgrowth rates across CSPG compared to axonal expression of eGFP, which itself was not significantly different than vehicle treatment on CSPG (*P = 0.0002, n = 29 axons measured across three experiments and P = 0.657, n = 28 axons measured across three experiments, respectively). Comparisons were made using the unpaired, two-tailed Student’s _t_-test. Data are expressed as mean ± s.e.m.
(c) In vivo expression of eGFP-sACt in axons in the corticospinal tract. Sindbis-IRES-eGFP-sACt was stereotaxically injected into the corticospinal tract of adult C57bl/6 mice and allowed to express for 4 d. eGFP-sACt levels in axons were detected by anti-eGFP immunofluorescence (green) and colocalized with Tuj1 staining (red). eGFP immunofluorescence (green) does not strongly colocalize with a marker of myelinating glial cells, GalC (red). Taken together, these data indicate that eGFP-sACt is expressed in myelinated axons in the corticospinal tract. Scale bar, 5 μm.
We next sought to use the Sindbis-IRES system to overcome the growth inhibition of CSPG. Axon growth inhibitory signaling from the components of the glial scar, such as CSPG, has been found to be attenuated by cAMP-regulated signaling pathways29. Thus, we next examined the growth properties of axons expressing eGFP-sACt across CSPG containing substratum. Compared to axons expressing eGFP, axons expressing eGFP-sACt exhibited growth rates comparable to axons treated with 10 μM 8-Br-cAMP, and to control axons grown on CSPG-free substratum (Figure 4b). These data indicate that axon-specific expression of eGFP-sACt allows axons to overcome the inhibitory effects of CSPG. Together, these data indicate that axonal eGFP-sACt expression can activate CREB signaling and increase axonal growth on CSPG, suggesting that the Sindbis-IRES approach can be used to identify candidate proteins that might be suitable for promoting axon regeneration.
Targeting eGFP-sACt to spinal cord axons
We next asked whether Sindbis-IRES virus can be used to target axons in the corticospinal tract. Cortical injections of the Sindbis virus from which the Sindbis-IRES virus was derived results in marked neuronal specific infection, and transgene expression for up to four weeks21, suggesting that Sindbis-IRES virus may be useful for manipulating protein expression in axons in vivo. To test the ability of the Sindbis-IRES virus to selectively infect axons in vivo, we stereotaxically injected Sindbis-IRES-eGFP-sACt into the spinal cord of adult mice. After four days, animals were sacrificed and the corticospinal tract was immunostained with anti-eGFP antibodies. Immunolabeling revealed eGFP-positive axons colocalized with Tuj1, an axonal marker. These axons were surrounded by, but not colocalized with, galactosylceramidase (GalC) labeling, a marker for myelinating oligodendrocytes characteristic of corticospinal tract axons (Figure 4c, Supplementary Figure 4). These data indicate that the Sindbis-IRES virus can be used to infect axons in vivo.
DISCUSSION
Here we describe a viral strategy for targeting protein expression to the axonal compartment, rather than targeting protein expression throughout the neuron. This strategy relies on selective axonal application of an RNA virus that contains a genome designed to be a template for axonal ribosomes. Previous studies have described the mechanism of local translation in postnatal tissue, and this is the first study to utilize local translation to modify the protein content, and thus behavior, of axons both in vitro and in vivo. Selective expression of sACt in axons markedly affects axonal behavior, resulting in retrograde signaling and axonal growth over inhibitory substrates, in a manner similar to pharmacologic application of cell-permeable, non-hydrolysable cAMP analogs. We also describe a platform for testing whether candidate proteins can influence axonal behavior when selectively expressed in axons. Thus these experiments provide a strategy for identifying proteins, such as sACt, which may be suitable for future in vivo studies of axon regeneration. Axon regeneration is a common therapeutic goal in the treatment a variety of disease states such as spinal cord injury and peripheral neuropathy. Our studies demonstrate a novel form of axon-specific gene therapy that can be used to influence axonal protein expression and promote axonal growth in these and other therapeutic contexts.
An important feature of the Sindbis-IRES system is the rapidity in which protein expression is induced. Direct translation of proteins from the IRES incorporated in the Sindbis genome can permit rapid changes in protein expression. Indeed, we see Sindbis-IRES-mediated protein expression in axons of cultured neurons at 8 h, the earliest time point tested. This is considerably faster than lentiviral and adenoviral–mediated transgene expression, which require viral genome amplification, and may also require time for protein transport into axons4. Thus, the Sindbis-IRES system may be particularly well-suited for applications requiring rapid manipulation of protein expression after injury.
Selective expression of proteins in axons relies on the existence of axonal ribosomes. However, ribosome localization to axons and local translation is largely thought to be a phenomenon limited to growing embryonic axons8. Demonstration of ribosome localization in axons after the embryonic period has been limited to sensory axons of the peripheral nervous system13. However, these studies found that the electron dense structures consistent with ribosome clusters were in the portion of the axons near the cell body. For example, ribosomes were only found adjacent to the first node of Ranvier9, which may reflect diffusion of ribosomes from the cell body. Additionally, these studies did not localize ribosomes within the node, but in the adjacent paranode area of the first node of Ranvier9. The previous inability to detect ribosomes in more distal portions of the axon may have been due to the difficulty in detecting unclustered ribosomes without a specific antibody. Alternatively, the difference between our study and previous studies may reflect fundamental differences between axons in the peripheral and central nervous system. Our data provide the first electron microscopic demonstration of ribosomes in distal axons of an adult animal. Additionally, our finding of Sindbis-IRES-mediated eGFP-sACt expression in axons in the corticospinal tract of adult animals indicates that these ribosomes are functional and can translate heterologous RNA.
The localization of ribosomes to nodes of Ranvier may be particularly important to allow for local translation. Nodes are sites of nutrient transport41 which may be important for providing amino acid precursors for local translation. Additionally, mitochondria can be recruited to nodes of Ranvier in electrically active axons42. Since mitochondria provide amino acid precursors and ATP required for translation, ribosome localization at nodes of Ranvier may be required for efficient local translation. Alternatively, a major function of local translation is to synthesize proteins required for mitochondrial function43. The colocalization of ribosomes and mitochondria may be important for mitochondrial function in axons. Our finding that axons of adult animals contain ribosomes suggests that axons have a greater capacity for protein translation than was previously appreciated, and may play previously unappreciated roles in axonal maintenance or signaling. Identification of mRNAs that associate with axonal ribosomes may provide insights into the function of intra-axonal translation in adult animals.
Notably, our data demonstrate that ribosomes are present in axons, even in the absence of injury. Axonal injury has been linked to the transfer of ribosomes from neighboring myelinating Schwann cells to axons in the peripheral nervous system44. The ribosomes we detect in axons are unlikely to have been transferred from neighboring cells since these cells do not express the eGFP-L10a construct, which is controlled by the cortical neuron-specific Glt25d2 promoter. However, ribosomes transferred from neighboring cells into axons would also contribute to the local translation capacity of axons, which may also be utilized by Sindbis-IRES constructs.
Our data also show an approach for identifying proteins that can function in axons to promote axon regeneration after injury. Using a novel microfluidic system to model spinal cord injury, we identified sACt as a protein that can induce increased pCREB levels and promote axonal growth across inhibitory substrates. Sindbis-IRES viruses utilized in the context of the microfluidic system can be used to test other axonally expressed proteins for their growth promoting effects. Although we used a neurotropic Sindbis coat protein to target expression in axons, Sindbis infection could potentially be limited to specific neuronal types by modifying Sindbis coat proteins. We envision that the approach described here will provide an approach for gene therapy directed towards axons in axonal injury and peripheral neuropathy, enabling axons to be reprogrammed to promote axon growth and regeneration.
METHODS
Immunoelectron microscopy
Three 8 – 10 week old C57bl/6 or Glt25d2::eGFP-L10a mice were compared in the uninjured group. Three Glt25d2::eGFP-L10a mice were subjected to dorsal hemisection and cared for post-operatively for 24 h or 7d. Mice were deeply anesthetized with sodium pentobarbital (150 mg/kg, i.p.) and perfused through the ascending aorta with 5 ml of heparin-saline, followed by 30 ml of 3.75% acrolein (Polysciences) and 2% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.4). The spinal cord was removed and post-fixed in the fixative for 30 min. Transverse sections, 40 μm thick, were cut through the cervical and thoracic spinal cords with a Leica vibratome. Sections were processed for EM immunocytochemistry as described previously45. Briefly, sections were incubated in 1% sodium borohydride in PB, rinsed, freeze-thawed, and incubated in 0.5% bovine serum albumin (BSA) in 0.1M Tris-Saline (TS, pH 7.6). Sections were incubated in a chicken anti-GFP mAb (Abcam) diluted 1:7,500 in 0.1% BSA for 48 h at 4°C. Signal was visualized by incubating with species anti-chicken IgG conjugated to biotin followed by incubation in avidtin-biotin complex then H2O2 and diaminobenzidine. To confirm peroxidase labeling, a subset of sections were incubated a goat anti-chicken secondary antibody conjugated with 1 nm gold particles (Electron Microscopy Sciences). The conjugated gold particles were intensified with IntenSE silver solution (Amersham) for 5 min. Sections for EM were embedded in Embed 812 (Electron Microscopy Sciences), and ultrathin sections (70-72nm) were prepared from the region of interest on a Leica UCT ultratome. Sections were counterstained with uranyl acetate and Reynold’s lead citrate. All nodes within an approximately 1,650 μm2 area of corticospinal tract for each animal were imaged with an Advanced Microscopy Techniques camera on a Philips CM10 electron microscope, and captured images were analyzed using NIS-Elements software (Nikon). Nodes were deemed positive if a 67 nm2 area below the nodal membrane was at least two standard deviations more intensely labeled the wild-type average.
Cell culture and infection
All reagents were obtained from Invitrogen, unless otherwise indicated. Coverslips (Assistant brand, Carolina Biological Supply) were treated with a hand-held corona discharger (BD20-AC, Electro-Technic Products) and precoated with 0.01% poly-L-lysine (Trevigen) overnight in a 37°C incubator. The coverslips were rinsed with water, dried, and the microfluidic chambers were attached. The cell body side of the chamber was coated with 0.3 mg/ml laminin (Trevigen) and the axonal side was coated either with 0.3 mg/ml laminin alone or 0.3 mg/ml laminin mixed with 750 ng/ml CSPG (Millipore) overnight at 37°C.
E15 DRG neurons were obtained as described previously46. Postnatal DRG neurons were harvested from neonates on postnatal day 4-6 and dissociated as described47. In brief, thoracic DRG ganglia were dissected from the spinal cord and treated twice at 37°C with 1 ml of 0.1% collagenase (Wellington) for twenty minutes followed by 1 ml TrypLE to detach satellite cells. Ganglia were allowed to settle by gravity, and the TrypLE was removed. Ganglia were mechanically dissociated with a flame-polished pasture pipette in 1 ml growth medium. Myelin debris were removed by passing the cell suspension through a 100 μm cell strainer (BD Biosciences), and the suspended cells were layered onto a 32%/54% Percoll gradient and centrifuged for 8 min at 0.4xg to remove glial cells48. The enriched neuron population was collected from the interface and loosely pelleted in HBSS. Viable neurons were counted using Trypan Blue, and approximately 50,000 neurons were plated on the cell body side of each microfluidic chamber.
On DIV 2, approximately 200,000 particles of Sindbis-IRES-eGFP-sACt or Sindbis-IRES-eGFP were selectively applied to axons and incubated for 8 to 12 h.
Virus cloning
The IRES-containing viral plasmid SINrep(nsP2S726) was derived from the Sindbis virus expression system vector, pSinRep5, and contains a point mutation in the non-structural protein 2 (P726S) reducing its neurotoxicity21. The IRES element from the human encephalomyocarditis virus was amplified by PCR from pIRES-hyg (Clontech) and inserted into pSinRep5 via XbaI and MluI restriction sites.
Myc-eGFP and myc-eGFP-sACt were assembled using the eGFP coding sequence from pEGFP (Clontech). A plasmid containing the sequence for rat sACt was a generous gift of Dr. Levin and Dr. Buck (Weill-Cornell Medical College). The myc tag was incorporated into the PCR primer. myc-eGFP-sACt constructs and myc-eGFP constructs were then inserted into pSinRep-IRES at the MluI and SphI restriction sites.
Virus expression
The Sindbis plasmid and virus particles were generated according to the instructions provided by Invitrogen, which previously sold the Sindbis viral system. The Sindbis plasmids and the helper plasmid TE12 were a generous gift of S. Schlesinger and A. Jeromin49. Briefly, both the Sindbis-IRES plasmid and helper plasmid were electroporated into BHK-21 cells and cultured for 48 h. Virus particle-containing cell supernatant was purified over a three-step sucrose gradient and concentrated on Centricon YM-100 centrifugal filter devices (Millipore). Particles were resuspended in PBS, and PBS was used as the vehicle in control experiments. After freezing aliquots at −80°C, the titer of viral stocks was determined by infecting a monolayer of BHK-21 cells with serial stock dilutions.
Immunocytochemistry
Neurons were fixed with 4% paraformaldehyde in PBS for 15 min at 25°C, permeabilized in PBS/0.1% Triton X-100 for 10 min, and blocked with TSA blocking reagent for 30 min. Immunofluorescence was performed using the TSA fluorescence system (PerkinElmer), using anti-eGFP 1:2,000 (Abcam) and anti-Tau 1:500 (Millipore). Secondary antibodies were anti-chicken HRP 1:1,000 (Santa Cruz) and anti-mouse Alexa Fluor 568 (Invitrogen). Images were captured in NIS-Elements with a Nikon TE2000 microscope with a 40x objective and a CoolSNAP HQ2 camera. Only linear adjustments were made to the original images.
pCREB assay
Postnatal neurons were grown for 72 h in microfluidic chambers with a microgroove length of 450 μm. 100μl of medium was removed from the axonal compartment to ensure fluidic isolation. Axons were preincubated for 30 min with either 1 μM cycloheximide or vehicle before approximately 750,000 infectious Sindbis virus particles or vehicle was added to the axonal compartment. Cells were incubated for 8 h. 2 h before fixation, crossed neurons were labeled with Vybrant DiI (Invitrogen). 1 h before fixation either 10 μM 8-Br-cAMP (Sigma Aldrich) or vehicle were added to the axonal compartment. Neurons were fixed with 4% paraformaldehyde in PBS for 15 min at 25°C, permeabilized in PBS/0.1% Triton X-100 for 10 min, and blocked with 10% normal goat serum for 30 min. pCREB was visualized with anti-pCREB (Ser133) clone 87G3 at 1:500 (Cell Signaling) and anti-Rabbit Alexa Fluor 647 (Invitrogen) antibodies. The background level of pCREB was set according to the average nuclear intensity of the vehicle control slide, and nuclei more than two standard deviations above the average in DiI positive cells were considered positive.
Outgrowth assay
Neurons were grown in microfluidic chambers with microgroove length of 450 μm. After 48 h, 100 μl of fresh medium was added to the cell body compartment to ensure microfluidic isolation from the axonal compartment, and approximately 750,000 infectious Sindbis virus particles or vehicle were added to the axonal compartment. Axons were incubated for an additional 24 - 30 h. 30 min before imaging, 10 μM of 8-Br-cAMP (Sigma Aldrich) or vehicle was added to the axonal compartment. Phase contrast images of the axonal compartment were captured at time point zero, and again 3 h later, on a Nikon TE2000 microscope with a 20x objective and a CoolSNAP HQ2 camera. Differences in axon length were measured using NIS-Elements software (Nikon) by an investigator blinded to treatment condition. Experimental groups were uncoded only after imaging and data analysis.
Intraparenchymal injection of virus
All procedures and animal care were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines. Male, wild-type, c57bl/6 mice between 8 and 10 weeks old were anesthetized with isoflourane and placed in a stereotaxic unit (model 960; David Kopf Instruments). Vertebrae T7 and T8 were removed by laminectomy. One injection of 1 μl of virus (~15,000 particles per μl) was administered with a Hamilton syringe mounted on a microinjector (model 5000; David Kopf Instruments) at the level of T7 and a depth of 0.5 mm below the dura. Virus was injected over the span of three minutes, and the needle was held in place for an additional five minutes to increase viral spread. After removal of the needle, the spinal cord was completely transected with a scalpel at the level of T8.
Immunofluorescence of spinal cords
Mice were deeply anesthetized with sodium pentobarbital (150 mg/ kg i.p.) and transcardially perfused with 5 ml heparin-saline followed by 20 ml of 4% paraformaldehyde in PBS. The spinal cord was placed in 4% paraformaldehyde for 1 h before being cryoprotected in 30% sucrose for 72 h. The spinal cord was cut into four 5 mm sections frozen together in a mold with Optimal Cutting Temperature (OCT) compound. The cords were cut longitudinally on a cryostat in 14 μm thick sections and collected on Superfrost Plus slides. Sections were blocked with 10% BSA/0.25% Tween 20 for 30 min before incubating with primary antibody in a humidified chamber overnight at 4°C. Immunofluorescence was performed using anti-GFP 1:1,000 (Abcam), anti-galactosylceramidase 1:500 (Millipore), and anti-Tuj1 1:500 (Covance). Secondary antibodies were anti-rabbit Alexa Fluor 488, anti-chicken Alexa Fluor 568, and anti-mouse Alexa Fluor 647. Images were captured in NIS-Elements with a Nikon TE2000 microscope with a 40x objective and a CoolSNAP HQ2 camera. Only linear adjustments were made to the original images.
Statistical analysis
As the data is normal and without significant variances, statistical analysis was performed using two-tailed, unpaired Student’s t-tests and one-way analysis of variance (ANOVA) with Bonferroni post-tests. GraphPad Prism 5 (GraphPad Software, CA) was used for statistical analysis. All alpha levels were set to be 5%.
Supplementary Material
Supplementary Figure 1
Supplemental Figure 1 Localization of eGFP-positive signals to nodes of Ranvier using an alternative visualization strategy.
We sought to confirm the axonal localization of eGFP-L10a using an alternate visualization method. In these experiments, we used an immunogold protocol to label eGFP-L10a.
(a) Anti-eGFP signal is not present in the nodes of Ranvier in wild-type animals. Corticospinal tracts of Glt25d2::eGFP-L10a mice and wild type controls were incubated with anti-eGFP mAb and visualized with a secondary antibody conjugated to 1 nm gold particles. Gold signal was intensified by incubating in a silver solution for 5 min. Nodes are recognized by characteristic myelin loops (green). Scale bar, 500 nm.
(b) eGFP signal colocalizes to nodes of Ranvier in Glt25d2::eGFP-L10a mice visualized with gold-conjugated secondary antibody. Gold particles (orange arrows) localize beneath the membrane of nodes of Ranvier in Glt25d2::eGFP-L10a mice. Due to the modest penetration of gold particles through tissue, signal intensity with gold visualization is lower than that of immunoperoxidase. eGFP signal, visualized with either immunogold in this panel, or immunoperoxidase (shown in Fig 1), specifically localizes below the membrane at the node of Ranvier, which are recognized by characteristic loops of myelin (green). The detection of eGFP-L10a by two different labeling methodologies helps to rule out the possibility that the nodal labeling is an artifact of the labeling protocol. Scale bar, 500 nm.
Supplementary Figure 2
Supplemental Figure 2 Localization of eGFP signals in nodes of Ranvier after axonal injury.
(a) Anti-eGFP signal at nodes of Ranvier at different time points after injury. In these experiments, Glt25d2::eGFP-L10a mice were subjected to a dorsal hemisection injury, and the corticospinal tract 500 μm rostral to the site of transection was harvested for immunoelectron microscopy at the indicated time points. At all time points, eGFP signal consistently localizes to nodes of Ranvier. Although there are subtle differences in labeling in the images shown above, there was no consistent increase in the intensity of eGFP-positive labeling at nodes within 500 μm of the injury site at either 24 h or 7 d. The image in the 7 d post-injury section shows a mitochondrion (orange arrow) within the node, which is occasionally observed in either the injured or uninjured sections, and consistent with previous reports that mitochondria can be observed in nodes42. Myelin labeling is shown in green. The node is the area that is not surrounded by myelin. Scale bar, 500 nm.
(b) Quantification of percent positive nodes in the corticospinal tract of injured and uninjured Glt25d2::eGFP-L10a mice. Incidence of positive nodes in injured Glt25d2::eGFP-L10a mice (*P < 0.640, n = 2 animals in each group) as compared with uninjured control. Positive signal was defined as the presence of a 67 nm2 area below the nodal membrane with average signal intensity at least two standard deviations higher than the average signal intensity in wild-type controls. Comparisons were made with a one-way analysis of variance. Data are expressed as mean ± s.e.m.
Supplementary Figure 3
Supplemental Figure 3 eGFP-L10a in growth cone of a regenerating adult axon.
We sought to determine if ribosomes are found in growth cones after injury. As described in the text, eGFP-L10a-expressing mice were subjected to dorsal hemisection. Spinal cords sections within 500 μm of the injury site were harvested 4 days after injury and subjected to immunoelectron microscopy. Due to their low abundance, growth cones are technically challenging to locate. The growth cone that was identified was emanating from a myelinated axon, consistent with corticospinal tract axons. The growth cone was identified based on its similarity in morphology and composition to previous electromicroscopic descriptions, such as that by Tennyson50. This regenerating growth cone is characteristically enriched in mitochondria and vesicular bodies, and it contains eGFP labeling (orange arrows). Myelin is colored green. Scale bar, 2 μm.
Supplementary Figure 4
Supplemental Figure 4 Additional examples of eGFP-sACt expression in axons in the corticospinal tract.
In Figure 4C, we showed an example of an axon in the CST which expresses eGFP-sACt. In these experiments, the Sindbis-IRES-eGFP or Sindbis-IRES-eGFP-sACt virus (1 μl) was stereotaxically injected into spinal cords at T7. In each of the animals (n = 3), myelinated axons in the CST were found to be positive for eGFP labeling. Shown are additional examples of Tuj1 positive axons (red) within the CST expressing eGFP immunoreactivity (green) after injection of the Sindbis-IRES virus. Scale bar, 5 μm.
ACKNOWLEDGEMENTS
We thank S. Schlesinger and A. Jeromin for Sindbis plasmids, Joe Harris (U.C. Irvine), for preparing masters for casting microfluidic chambers, M. S. Cohen, A. Deglincerti, L. Levin, and J. Buck (Weill Cornell Medical College) for useful comments and suggestions. This work was supported by the Christopher Reeve Paralysis Foundation, the New York State Spinal Cord Injury Board, NIH grants NINDS NS56306 (S.R.J.), DA08259 (TAM), ML096571 (TAM), ML098351 (TAM), the Korea Research Foundation funded by the Ministry of Education, Science and Technology (NLJ), and training grant T32DA007274 (B.A.W).
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- 1.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4(9):703–13. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 2.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–52. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 3.Franz S, Weidner N, Blesch A. Gene therapy approaches to enhancing plasticity and regeneration after spinal cord injury. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4(5):353–64. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 5.Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10(5):332–43. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piper M, Holt C. RNA translation in axons. Annu Rev Cell Dev Biol. 2004;20:505–23. doi: 10.1146/annurev.cellbio.20.010403.111746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hengst U, Deglincerti A, Kim HJ, Jeon NL, Jaffrey SR. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat Cell Biol. 2009;11(8):1024–30. doi: 10.1038/ncb1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengst U, Jaffrey SR. Function and translational regulation of mRNA in developing axons. Semin Cell Dev Biol. 2007;18(2):209–15. doi: 10.1016/j.semcdb.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelena J. Ribosomes in myelinated axons of dorsal root ganglia. Z Zellforsch Mikrosk Anat. 1972;124(2):217–29. doi: 10.1007/BF00335680. [DOI] [PubMed] [Google Scholar]
- 10.Conradi S, Ronnevi LO. Ultrastructure and synaptology of the initial axon segment of cat spinal motoneurons during early postnatal development. J Neurocytol. 1977;6(2):195–210. doi: 10.1007/BF01261505. [DOI] [PubMed] [Google Scholar]
- 11.Li YC, Cheng CX, Li YN, Shimada O, Atsumi S. Beyond the initial axon segment of the spinal motor axon: fasciculated microtubules and polyribosomal clusters. J Anat. 2005;206(6):535–42. doi: 10.1111/j.1469-7580.2005.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pannese E, Ledda M. Ribosomes in myelinated axons of the rabbit spinal ganglion neurons. J Submicrosc Cytol Pathol. 1991;23(1):33–8. [PubMed] [Google Scholar]
- 13.Koenig E, Martin R, Titmus M, Sotelo-Silveira JR. Cryptic peripheral ribosomal domains distributed intermittently along mammalian myelinated axons. J Neurosci. 2000;20(22):8390–400. doi: 10.1523/JNEUROSCI.20-22-08390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palay SL, Sotelo C, Peters A, Orkand PM. The axon hillock and the initial segment. J Cell Biol. 1968;38(1):193–201. doi: 10.1083/jcb.38.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kun A, Otero L, Sotelo-Silveira JR, Sotelo JR. Ribosomal distributions in axons of mammalian myelinated fibers. J Neurosci Res. 2007;85(10):2087–98. doi: 10.1002/jnr.21340. [DOI] [PubMed] [Google Scholar]
- 16.Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–48. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135(4):749–62. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez LF, Kalil K. Critical stages for growth in the development of cortical neurons. J Comp Neurol. 1985;237(4):506–18. doi: 10.1002/cne.902370407. [DOI] [PubMed] [Google Scholar]
- 19.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2(3):284–91. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeromin A, Yuan LL, Frick A, Pfaffinger P, Johnston D. A modified Sindbis vector for prolonged gene expression in neurons. J Neurophysiol. 2003;90(4):2741–5. doi: 10.1152/jn.00464.2003. [DOI] [PubMed] [Google Scholar]
- 22.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8(8):573–87. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrengruber MU, Lundstrom K, Schweitzer C, Heuss C, Schlesinger S, Gahwiler BH. Recombinant Semliki Forest virus and Sindbis virus efficiently infect neurons in hippocampal slice cultures. Proc Natl Acad Sci U S A. 1999;96(12):7041–6. doi: 10.1073/pnas.96.12.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, et al. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436(7053):1020–4. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Salas E. Internal ribosome entry site biology and its use in expression vectors. Curr Opin Biotechnol. 1999;10(5):458–64. doi: 10.1016/s0958-1669(99)00010-5. [DOI] [PubMed] [Google Scholar]
- 26.Dryga SA, Dryga OA, Schlesinger S. Identification of mutations in a Sindbis virus variant able to establish persistent infection in BHK cells: the importance of a mutation in the nsP2 gene. Virology. 1997;228(1):74–83. doi: 10.1006/viro.1996.8364. [DOI] [PubMed] [Google Scholar]
- 27.Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2(8):599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong C, Levis R, Shen P, Schlesinger S, Rice CM, Huang HV. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243(4895):1188–91. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- 29.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7(8):628–43. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 30.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10(6):610–6. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 31.Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006;5(8):660–70. doi: 10.1038/nrd2058. [DOI] [PubMed] [Google Scholar]
- 32.Spencer GE, Syed NI, van Kesteren E, Lukowiak K, Geraerts WP, van Minnen J. Synthesis and functional integration of a neurotransmitter receptor in isolated invertebrate axons. J Neurobiol. 2000;44:72–81. doi: 10.1002/1097-4695(200007)44:1<72::aid-neu7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289(5479):625–8. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44(4):609–21. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21(13):4731–9. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 37.McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11(11):3398–411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, et al. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22(3):405–16. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 39.Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7(3):261–8. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- 40.Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci. 2004;24(29):6531–9. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6(9):683–90. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 42.Ohno N, Kidd GJ, Mahad D, Kiryu-Seo S, Avishai A, Komuro H, et al. Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J Neurosci. 2011;31(20):7249–58. doi: 10.1523/JNEUROSCI.0095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hillefors M, Gioio AE, Mameza MG, Kaplan BB. Axon viability and mitochondrial function are dependent on local protein synthesis in sympathetic neurons. Cell Mol Neurobiol. 2007;27(6):701–16. doi: 10.1007/s10571-007-9148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Court FA, Hendriks WT, MacGillavry HD, Alvarez J, van Minnen J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J Neurosci. 2008;28(43):11024–9. doi: 10.1523/JNEUROSCI.2429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milner TA, Thompson LI, Wang G, Kievits JA, Martin E, Zhou P, et al. Distribution of estrogen receptor beta containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res. 2010;1351:74–96. doi: 10.1016/j.brainres.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banker G, Goslin K. Culturing Nerve Cells. 2nd edn MIT Press; Cambridge, MA: 1998. [Google Scholar]
- 47.Delree P, Leprince P, Schoenen J, Moonen G. Purification and culture of adult rat dorsal root ganglia neurons. J Neurosci Res. 1989;23(2):198–206. doi: 10.1002/jnr.490230210. [DOI] [PubMed] [Google Scholar]
- 48.Goldenberg SS, De Boni U. Pure population of viable neurons from rabbit dorsal root ganglia, using gradients of Percoll. J Neurobiol. 1983;14(3):195–206. doi: 10.1002/neu.480140304. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Dittgen T, Nimmerjahn A, Waters J, Pawlak V, Helmchen F, et al. Sindbis vector SINrep(nsP2S726): a tool for rapid heterologous expression with attenuated cytotoxicity in neurons. J Neurosci Methods. 2004;133(1-2):81–90. doi: 10.1016/j.jneumeth.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 50.Tennyson VM. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J Cell Biol. 1970;44(1):62–79. doi: 10.1083/jcb.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplemental Figure 1 Localization of eGFP-positive signals to nodes of Ranvier using an alternative visualization strategy.
We sought to confirm the axonal localization of eGFP-L10a using an alternate visualization method. In these experiments, we used an immunogold protocol to label eGFP-L10a.
(a) Anti-eGFP signal is not present in the nodes of Ranvier in wild-type animals. Corticospinal tracts of Glt25d2::eGFP-L10a mice and wild type controls were incubated with anti-eGFP mAb and visualized with a secondary antibody conjugated to 1 nm gold particles. Gold signal was intensified by incubating in a silver solution for 5 min. Nodes are recognized by characteristic myelin loops (green). Scale bar, 500 nm.
(b) eGFP signal colocalizes to nodes of Ranvier in Glt25d2::eGFP-L10a mice visualized with gold-conjugated secondary antibody. Gold particles (orange arrows) localize beneath the membrane of nodes of Ranvier in Glt25d2::eGFP-L10a mice. Due to the modest penetration of gold particles through tissue, signal intensity with gold visualization is lower than that of immunoperoxidase. eGFP signal, visualized with either immunogold in this panel, or immunoperoxidase (shown in Fig 1), specifically localizes below the membrane at the node of Ranvier, which are recognized by characteristic loops of myelin (green). The detection of eGFP-L10a by two different labeling methodologies helps to rule out the possibility that the nodal labeling is an artifact of the labeling protocol. Scale bar, 500 nm.
Supplementary Figure 2
Supplemental Figure 2 Localization of eGFP signals in nodes of Ranvier after axonal injury.
(a) Anti-eGFP signal at nodes of Ranvier at different time points after injury. In these experiments, Glt25d2::eGFP-L10a mice were subjected to a dorsal hemisection injury, and the corticospinal tract 500 μm rostral to the site of transection was harvested for immunoelectron microscopy at the indicated time points. At all time points, eGFP signal consistently localizes to nodes of Ranvier. Although there are subtle differences in labeling in the images shown above, there was no consistent increase in the intensity of eGFP-positive labeling at nodes within 500 μm of the injury site at either 24 h or 7 d. The image in the 7 d post-injury section shows a mitochondrion (orange arrow) within the node, which is occasionally observed in either the injured or uninjured sections, and consistent with previous reports that mitochondria can be observed in nodes42. Myelin labeling is shown in green. The node is the area that is not surrounded by myelin. Scale bar, 500 nm.
(b) Quantification of percent positive nodes in the corticospinal tract of injured and uninjured Glt25d2::eGFP-L10a mice. Incidence of positive nodes in injured Glt25d2::eGFP-L10a mice (*P < 0.640, n = 2 animals in each group) as compared with uninjured control. Positive signal was defined as the presence of a 67 nm2 area below the nodal membrane with average signal intensity at least two standard deviations higher than the average signal intensity in wild-type controls. Comparisons were made with a one-way analysis of variance. Data are expressed as mean ± s.e.m.
Supplementary Figure 3
Supplemental Figure 3 eGFP-L10a in growth cone of a regenerating adult axon.
We sought to determine if ribosomes are found in growth cones after injury. As described in the text, eGFP-L10a-expressing mice were subjected to dorsal hemisection. Spinal cords sections within 500 μm of the injury site were harvested 4 days after injury and subjected to immunoelectron microscopy. Due to their low abundance, growth cones are technically challenging to locate. The growth cone that was identified was emanating from a myelinated axon, consistent with corticospinal tract axons. The growth cone was identified based on its similarity in morphology and composition to previous electromicroscopic descriptions, such as that by Tennyson50. This regenerating growth cone is characteristically enriched in mitochondria and vesicular bodies, and it contains eGFP labeling (orange arrows). Myelin is colored green. Scale bar, 2 μm.
Supplementary Figure 4
Supplemental Figure 4 Additional examples of eGFP-sACt expression in axons in the corticospinal tract.
In Figure 4C, we showed an example of an axon in the CST which expresses eGFP-sACt. In these experiments, the Sindbis-IRES-eGFP or Sindbis-IRES-eGFP-sACt virus (1 μl) was stereotaxically injected into spinal cords at T7. In each of the animals (n = 3), myelinated axons in the CST were found to be positive for eGFP labeling. Shown are additional examples of Tuj1 positive axons (red) within the CST expressing eGFP immunoreactivity (green) after injection of the Sindbis-IRES virus. Scale bar, 5 μm.