Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7 (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 28.
Published in final edited form as: Sci Signal. 2012 Feb 7;5(210):ra11. doi: 10.1126/scisignal.2002585
Abstract
The transition element zinc, which has recently been identified as an intracellular second messenger, has been implicated in various signaling pathways, including those leading to cell proliferation. Zinc channels of the ZIP protein family (Solute Carrier Family 39A, SLC39A) transiently increase the cytosolic free zinc (Zn2+) concentration in response to extracellular signals. Here, we show that phosphorylation of evolutionarily conserved residues in zinc transporter ZIP7 is associated with the gated release of Zn2+ from intracellular stores, leading to activation of tyrosine kinases and the phosphorylation of AKT and extracellular signal-regulated kinases 1 and 2 (ERK1/2). Through pharmacological manipulation, proximity assay, and mutagenesis, we identified CK2 as the kinase responsible for ZIP7 activation. Together, the present results show that eukaryotic transition element channels can be activated post-translationally by phosphorylation eliciting a cell signaling cascade. Our study links the regulated release of zinc from intracellular stores to phosphorylation of kinases involved in proliferative responses and cell migration, suggesting a functional role for ZIP7 and zinc signals for these events which are characteristic of cancerous cells. Furthermore, the interaction of ZIP7 with CK2, a kinase that is antiapoptotoc and promotes cell division, highlights the potential for ZIP7 as a target for anti-cancer drug development.
Introduction
The essential role of zinc 1 is demonstrated by the deleterious effects of zinc deficiency 2 and by the link between zinc regulatory dysfunction and the pathophysiology of various disease states, including neurodegeneration 3, inflammation 4, diabetes 5, cancer 6, and others 7. Recent data reinforces zinc’s essential role, confirming its widespread involvement in development, immunity, reproduction, endocrinology, and neurotransmission 3, 4, 8-10. Zinc acts as a cofactor for an estimated 3,000 human proteins 11, representing 10% of the genome, and has a well-established role in regulation of gene expression through metal-responsive transcription-factor-1 (MTF1) 12, 13. However, the true extent of zinc’s participation in cellular processes is only now emerging and suggests a complexity and importance comparable to that attributed to calcium. This is emphasized by zinc’s role as a second messenger 14 involved in a regulating various pathways 3, 8, 9, often by inhibiting protein tyrosine phosphatases (PTPs) 15. Transient increases in the cytosolic free zinc ion (Zn2+) can occur through the release of zinc from zinc-binding proteins driven by changes in cellular redox potential 16, and by flux of Zn2+ into the cytosol 6, 14, 17 through zinc channels.
The concentration of cytosolic Zn2+ must be tightly controlled to prevent cell death due to zinc insufficiency or toxicity 1. This requirement, together with the fact that zinc cannot traverse biological membranes unaided, is addressed through two families of proteins dedicated to mediating zinc movement across membranes. These are the ZnT (Official name: Solute Carrier Family 30A, SLC30A) family of zinc transporters that remove zinc from the cytosol into organelles or out of the cell and the ZIP (Official name: Solute carrier Family 39A, SLC39A) family of zinc channels that increase cytosolic zinc by mediating influx across the plasma membrane or from organelles. There are a total of 10 ZnT and 14 ZIP gene loci in humans allowing for fine-tuning of zinc distribution among tissues and intracellular compartments 18, 19. A study of the prokaryotic homologue, ZIPB, which translocates zinc in a non-saturable fashion, indicates that the ZIP proteins function as Zn2+ ion channels 20. However, the mechanisms whereby zinc transport is regulated have been unclear.
Zinc channel ZIP7, which resides in the membrane of the endoplasmic reticulum (ER), the Golgi, or both 19, 21-23, has orthologs in many species, including Drosophila (CATSUP) and Arabidopsis (IAR1) 19, 24, that are also found in the ER 25. At least in some cells, human ZIP7 21 is essential for zinc release from the ER, a process that results in activation of downstream signaling pathways 6 that promote cell proliferation 26. Activation of these pathways may occur through phosphatase inhibition 15, thereby preventing de-activation of phosphorylated tyrosine kinases. The apparent role of ZIP7 as a gatekeeper of cytosolic zinc release from the ER 9, highlights the involvement of zinc in rapid signaling, in addition to, and contrasting with, its role in mediating transcriptional responses 13, 27, 28. Studies identifying ZIP7 as essential for zinc release from the ER employed Tamoxifen-resistant MCF-7 breast cancer cells (TamR), which natively exhibit increased intracellular zinc 6 and ZIP7, but not other ZIP transporters 29, compared to wild-type MCF-7 cells, and thus provide an excellent model for investigating the role of ZIP7 in cellular zinc homeostasis. TamR cells are more aggressive than wild-type MCF-7 cells; they show increased proliferation and increased invasiveness 30, both of which are driven by hyperactivation of the EGFR (epidermal growth factor receptor) and IGF1-R (insulin-like growth factor 1 receptor) signaling pathways and thereby of downstream effectors such as AKT and MAPK (mitogen-activated protein kinase) 31, 32. However, the ubiquitous expression of ZIP7 among organisms and cell types 21 suggests that it mediates a fundamental role of Zn2+ flux from intracellular stores.
We hypothesised that exposure of cells to exogenous stimuli, such as application of extracellular zinc, results in ZIP7-dependant release of zinc from the ER without a prior rise in cytosolic Zn2+ concentration. Here, we investigated the molecular mechanism underlying gated zinc release by ZIP7 and showed that extracellular stimulation activates ZIP7 through phosphorylation at specific residues by the protein kinase CK2 (formerly casein kinase 2). Protein kinase CK2 is a ubiquitously expressed tetrameric threonine/serine kinase composed of two catalytic α subunits and two regulatory β subunits 33. Although CK2 has numerous cellular targets and has not been previously thought to be regulated, it has been particularly implicated in cell survival and proliferation, participating in such processes as apoptosis and mitosis 34. CK2 shuttles between the cytosol and nuclei of cells in order to support apoptosis or mitosis, respectively. ZIP7 phosphorylation by CK2 results in ZIP7-mediated zinc release from the ER and the subsequent activation of multiple downstream pathways that enhance cell proliferation and migration.
Results
In silico analyses identify ZIP7 as a putative target for phosphorylation by CK2
Three independent genome-wide phosphorylation screens have revealed phosphorylation of two adjacent serine residues on ZIP7 35-37, Ser275 and Ser276, suggesting involvement of these residues in channel activity. We have discovered that both Ser275 and Ser276 are predicted 38 to be phosphorylated by CK2 fitting the consensus sequence [S/TXXE, where S is Ser, T is Thr, E is Glu and X is any amino acid]. The presence of additional acidic residues surrounding this sequence (Table 1) enhance the likelihood of phosphorylation by CK2. Ser275 and Ser276 are located cytosolically and juxtamembrane to ZIP7 transmembrane domain IV, the region implicated in zinc transport 19. CK2 promotes cell growth, proliferation, and progression through the cell cycle 39 and, because ZIP7 also increases cell proliferation 6, we investigated whether CK2 was involved in ZIP7 activation.
Table 1. Conservation of residues equivalent to Ser275 and Ser276 in human ZIP7.
| Species | Common name | No. | Alignment |
|---|---|---|---|
| Homo sapiens | human | 270 | TKEKQSSEEEEKE |
| Pan troglodytes | chimpanzee | 364 | TKEKQSSEEEEKE |
| Bosctaurus | cow | 270 | TKEKQSSEEEEKE |
| Pongo abelii | orangutan | 270 | TKEKQSSEEEEKE |
| Ovis aries | sheep | 270 | SKEKQSSEEEEKE |
| Mus musculus | mouse | 278 | SKEKPSTEEE–KE |
| Canis familiaris | dog | 270 | SKEKQSSEEEEKE |
| Sus scrofa | pig | 270 | SKEKQSSEEEEKE |
| Ailuropoda melanoleuca | panda | 273 | SKEKQSSEEEEKE |
| Rattus norvegicus | rat | 270 | SKGKPSSEDE–KE |
| Danio rerio | zebrafish | 257 | PKSKDSDEEDDKK |
| Deosophila melanogaster | fruit fly | 257 | PAKKKSSDKEDSG |
| Oncorhynchus mykiss | trout | 265 | PKAKESDGEKKKK |
| Oriza sativa | rice | 334 | PNKALSSEDSSVS |
| Zea mays | maize | 265 | LNYQKSDTDGKDI |
| Anopheles gambiae | mosquito | 313 | EAPTKSKKEEAKA |
| Xenopus laevis | frog | 276 | IVDDATEKEEEKD |
Exogenous zinc stimulates the association of CK2 and ZIP7, leading to Zn2+ release into the cytoplasm and tyrosine kinase activation
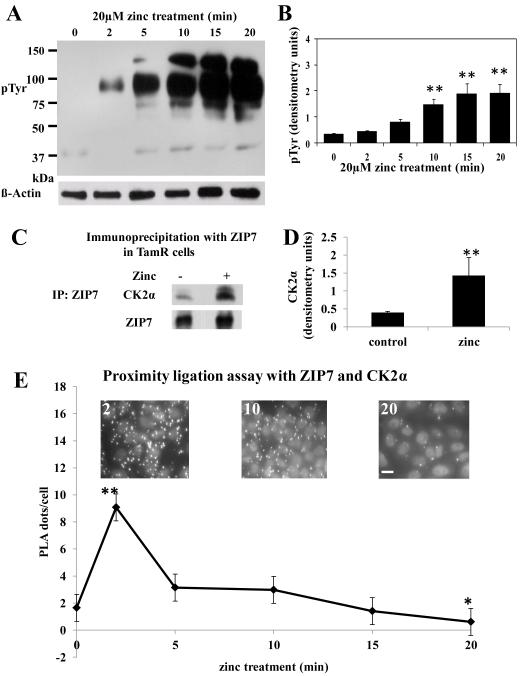
Zinc promotes activation of receptor tyrosine kinases, and thereby of downstream pathways that promote cell proliferation 6. We have previously established an experimental method 6 whereby application of exogenous zinc leads to ZIP7 mediated Zn2+ release resulting in tyrosine kinase activation. We dissected the time course of these events to investigate the underlying process., We then probed cell lysateswith a phospho-tyrosine antibody, observed that zinc treatment led to activation of unspecified tyrosine kinases, with significant (P ≤ 0.001) tyrosine phosphorylation apparent after 10 minutes and a maximal effect after 15 min (Fig. 1A-B), suggesting that molecules involved in ZIP7 activation had associated with it earlier than 10 min.
Figure 1. Zinc release activates tyrosine kinases downstream of CK2 binding ZIP7.
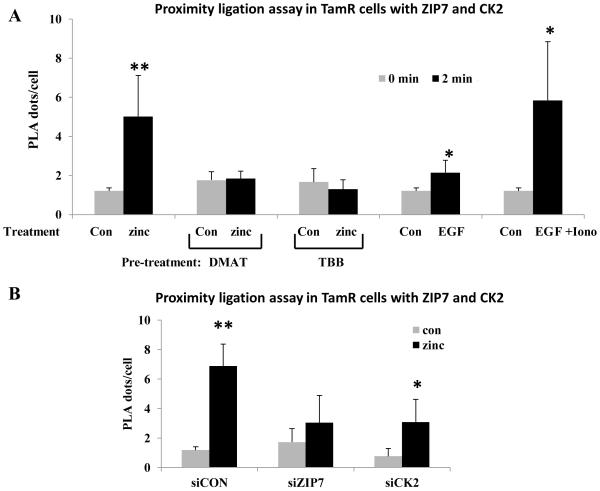
(A-B) TamR cells were treated with 20 μM zinc plus sodium pyrithione and lysates probed by Western analysis with a phosphotyrosine (pTyr) antibody (A). pTyr, quantified by densitometry on blots from three independent experiments was normalized to β-actin and displayed as mean ± SD with significant (P ≤ 0.001) changes over time 0 indicated by ** (B). (C-D) TamR cells, treated with zinc for 5 minutes, were immunoprecipitated with antibody directed against ZIP7. Western Blot was probed with antibodies directed against CK2α (C) or ZIP7 (as loading control); mean values ± SD calculated from N=3 experiments and normalized to ZIP7 (D) showed a significant change (P ≤ 0.001 indicated by **) in the association of CK2α and ZIP7. (E) Proximity ligation assay was performed with anti-ZIP7 and anti-CK2α antibodies in TamR cells treated with zinc. Fluorescent dots in inserts demonstrate ZIP7 and CK2α in close proximity (<40nm). Complete time course and antibody-free controls shown in fig. S2. Pooled results as 25 stacks taken 0.3μm apart from at least 6 representative fields of view in 3 experiments are expressed as mean dots/cell ± SD. Significant changes compared to time 0 are indicated by * (P ≤ 0.05) and ** (P ≤ 0.001). Scale bar=10μm.
To determine whether CK2 associated with ZIP7, we immunoprecipitated zinc-treated cells with ZIP7 antibody 6 and probed the precipitates for CK2α (Fig. 1C-D); this revealed a zinc-dependent association of CK2 with ZIP7, which preceded tyrosine kinase activation (Fig. 1A-B). We next deployed a proximity ligation assay (Duolink), in which a fluorescent dot appears wherever two molecules are within 40 nm, indicative of their physical interaction, using ZIP7 and CK2α antibodies (Fig. 1E, see also fig. S1 for pictorial data giving detailed time course). Quantification of these interactions (Fig. 1E) revealed a significant (P ≤ 0.001) increase in association of ZIP7 and CK2α at 2 min, with their association returning to pre-stimulation levels after 10 min and showing a significant (P ≤ 0.05) decrease in their association compared to the starting value by 20 min.
CK2 inhibition decreases ZIP7-dependent zinc signals
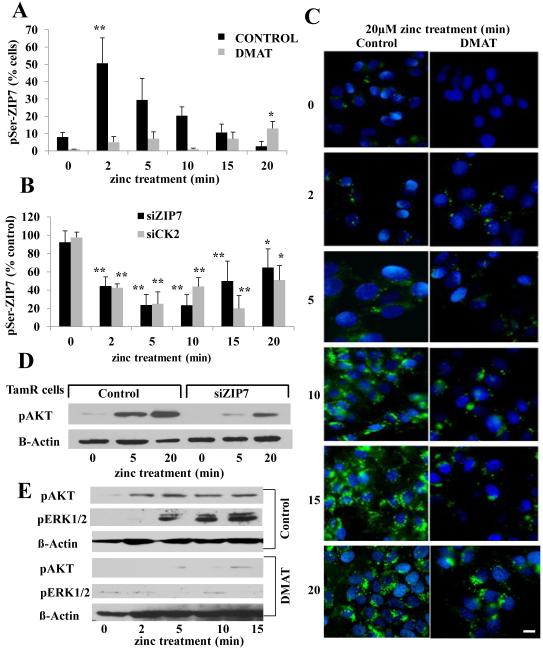
We next investigated the effect of inhibiting CK2 activity pharmacologically, using the CK2 inhibitor dimehtylamino-4,5,6,7-tetrabromo-1H-benzamidazole (DMAT) (Fig. 2A), or decreasing its abundance with CK2-specific siRNAs, on association of phospho-serine (pSer) with ZIP7 in TamR cells after zinc treatment (Fig. 2B). Using FACS (fluorescence activated cell sorting) analysis to assess increases in pSer while also probing for ZIP7, we were able to limit the investigation to the increased serine phosphorylation observed in those cells that were positive for ZIP7. These experiments established a maximal and significant (P ≤ 0.001) increase in pSer in the ZIP7-positive cell population at 2 min post zinc treatment (Fig. 2A), a time that coincided with the maximal association of ZIP7 and CK2 (Fig. 1E). This increase in pSer was suppressed by DMAT (Fig. 2A). The presence of siRNA to ZIP7 or CK2 significantly (P ≤ 0.001) decreased ZIP7-associated serine phosphorylation (Fig. 2B), reinforcing the hypothesis of a link between CK2 and phosphorylation of ZIP7. Consistent with previous observations, 5-20 min of zinc treatment elicited an increase in green fluorescence in TamR cells grown on coverslips and loaded with the zinc-specific dye FluoZin-3 (Fig. 2C), indicative of Zn2+ release into the cytosol 6. Pre-treatment with 1μM DMAT (Fig. 2C) substantially attenuated the increase in fluorescence, emphasizing the involvement of CK2. AKT and ERK1/2 (extracellular signal-regulated kinases 1 and 2), which are activated downstream of zinc release 6, were phosphorylated following the external zinc stimulus; prior transfection with ZIP7 siRNA significantly reduced the increase in AKT phosphorylation (P ≤ 0.05, Fig 2D and fig. S2), and inhibition of CK2 by DMAT eliminated the significant zinc effect on pAKT and pERK1/2 (P ≤ 0.05 and P ≤ 0.001, respectively Fig. 2E and fig S2).
Figure 2. CK2 inhibition decreases ZIP7-dependent zinc transport.
(A) FACS analysis of TamR cells treated with zinc plus sodium pyrithione and probed with antibodies directed against pSer and ZIP7. The increased percentage of cells with activated pSer abundance was recorded as maximum at 2 minutes after zinc treatment (** P ≤ 0.001) as a function of ZIP7 and was abolished by DMAT pretreatment. (B) Parallel FACS analysis demonstrated that CK2 or ZIP7 siRNA attenuated the increase in pSer as a function of ZIP7 by over 50%. Results displayed in panel B represent cells with increased pSer expressed as a percentage of control cells with the mean ± SD calculated from n=3 experiments. (C) TamR cells (control), loaded with Fluozin-3 showed increased green fluorescence after 10 minutes of zinc treatment; pre-treatment with CK2 inhibitor DMAT largely prevented this increase. Nuclei were counterstained blue with DAPI. Scale bar=10μm. (D-E) ZIP7 siRNA significantly decreased pAKT at 5 minutes (P ≤ 0.05, fig. S2) (D) and the CK2 inhibitor DMAT reduced the significant increase in pAKT at 5, 10 and 15 mins (P ≤ 0.05, Fig. S2) and pERK1/2 at 5 min (P ≤ 0.001, Fig. S2) and 10 and 15 mins (P ≤ 0.05, Fig. S2) to undetectable levels (E); representative of N=3 experiments. See fig. S2 for quantification of blots D-E.
Mutation of ZIP7 (S275A:S276A) prevents its association with CK2 and cytosolic zinc signals
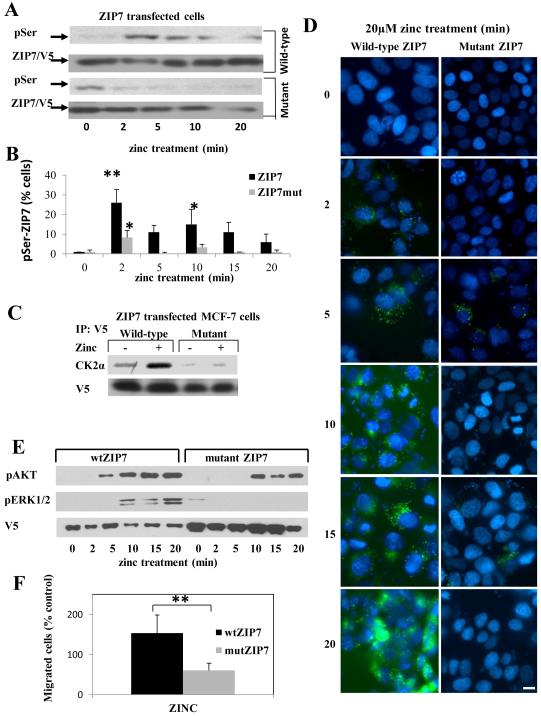
We generated a mutant form of ZIP7 (in which Ser275 and Ser276 were substituted with Ala, S275A, S276A, (fig. S3) containing a C-terminal V5-tag that does not compromise channel function 21; this mutant ZIP7 was expressed robustly in MCF-7 cells (fig. S3B). Western blot (Fig. 3A and fig. S4A for densitometry quantification) or FACS analysis (Fig. 3B) revealed that zinc treatment of MCF-7 cells expressing recombinant wild-type ZIP7 produced a significantly increased pSer profile (P ≤ 0.05 and P ≤ 0.001, respectively, fig S4) similar to that observed in TamR cells, whereas cells expressing mutant ZIP7 did not. Although zinc stimulation failed to induce serine phosphorylation of mutant ZIP7, we observed, by Western Blot, increased serine phosphorylation of mutant ZIP7, compared to that of wild-type ZIP7, at time zero but this was not apparent on FACS analysis (Fig. 3B). Zinc stimulated an increase in pSer in cells transfected with mutant ZIP7 that was smaller than that in cells transfected with wild-type ZIP7, but still significant (P ≤ 0.05), which we attributed to phosphorylation of endogenous ZIP7 (Fig. 3B). Immunoprecipitation with antibodies directed against the C-terminal V5-tag confirmed a significant association (P ≤ 0.05, fig S4) of wild-type but not mutant ZIP7 with CK2 after zinc stimulation (Fig. 3C and fig. S4B for quantification of blot). Furthermore, cells transfected with the mutant ZIP7 did not generate the Zn2+ signal observed in those transfected with the wild-type ZIP7 (Fig. 3D). pERK1/2 was below the detectable limits of our assay following zinc treatment of cells expressing mutant ZIP7, and pAKT, although detectable, possibly due to endogenous ZIP7, was significantly decreased (P ≤ 0.05, fig. S4) compared to that in cells expressing wild-type ZIP7 (Fig. 3E and fig. S4C-D for quantification of blots). To determine whether interfering with ZIP7 signaling caused any phenotypic changes, we investigated the migratory behaviour of transfected cells on fibronectin-coated membranes, a measure of their metastatic potential 30. We found significantly increased (P<0.001) migration of cells transfected with wild-type ZIP7 increased compared to untransfected controls, whereas that of cells transfected with mutant ZIP7 was decreased (Fig. 3F). Together, these results identify a functional mutation of ZIP7 that interferes with CK2 phosphorylation of ZIP7, prevents ZIP7-mediated zinc signals, decreases downstream signalling events, and interferes with ZIP7-mediated cell migration.
Figure 3. Mutation of ZIP7 (S275A:S276A) prevents its association with CK2 and zinc release.
(A) MCF-7 cells transfected with wild type or mutant ZIP7 were treated with zinc and analysed for pSer by Western blot. There was significant activation of pSer in cells expressing wild-type ZIP7 at 2 and 5 mins (P ≤ 0.001 and P ≤ 0.05, respectively, see Fig. S4) whereas this was absent in cells expressing ZIP7 mutant. Representative of n=3 blots. (B) Parallel FACS experiments determined the extent of activated pSer in ZIP7 positive cells in N=3 experiments and expressed as mean ± SD. Cells expressing wild-type ZIP7 showed increased activation of pSer throughout compared to time 0 (indicated by * (P ≤ 0.05) and ** (P ≤ 0.001), especially at 2 minutes, in contrast to cells expressing mutant ZIP7 which showed a small 10% increase only at the 2 minute time point. (C) Recombinant ZIP7 proteins immunoprecipitated with V5 antibody after 5 minutes of zinc treatment and probed for CK2α. Cells transfected with wild-type ZIP7 showed a significant increase in CK2α when treated with zinc (P ≤ 0.05, see Fig S4). Representative of N=3 experiments.(D) Cells expressing mutant ZIP7 did not show increased green Fluozin-3 fluorescence after zinc treatment, indicative of no cytosolic Zn2+ release. Nuclei were counterstained blue with DAPI. Representative of N =3 experiments Scale bar=10μm. (E) Cells expressing mutant ZIP7, in contrast to wild-type ZIP7, did not show a significant ERK1/2 phosphorylation in response to zinc treatment (P ≤ 0.05, Fig. S4) and showed a significantly decreased phosphorylation of AKT (see Fig.S4). Representative of N=3 experiments (F) Mutant ZIP7 transfected cells showed significantly decreased migratory potential over 48 hours in the presence of zinc compared to cells transfected with wild-type ZIP7 (P<0.001 using an independent t-test, indicated by **) N=3 experiments. See Fig. S4 for quantification of Western blots in this figure.
Zinc release from stores can be stimulated without exogenous zinc treatment
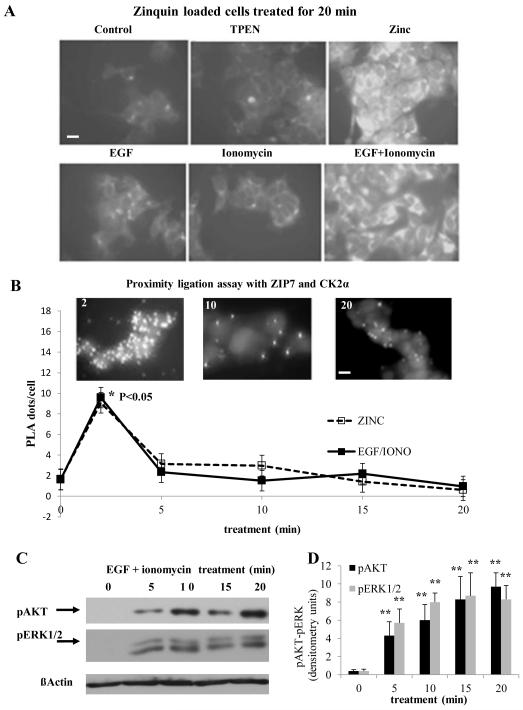
We tested the ability of ZIP7 to elicit a Zn2+ signal in the absence of external zinc using epidermal growth factor (EGF) or the calcium ionophore ionomycin, both of which trigger cytosolic Zn2+ waves originating from the ER 14, either separately or together. Cytosolic [Zn2+] was assessed at 20 minutes using Zinquin (Fig. 4A). Although we observed only a minor increase in [Zn2+] in cells treated with EGF or ionomycin individually, the combination induced a cytosolic [Zn2+] increase comparable to that induced by treatment with zinc plus zinc ionophore (Fig. 4A). Given that no external zinc was added during this experiment, this zinc signal was likely a result of zinc release from an intracellular store. The proximity ligation assay indicated that treatment with the combination of EGF and ionomycin also produced CK2 association with ZIP7 (Fig. 4B and fig. S5A). The time-course of ZIP7-CK2 interactions was identical to that observed with exogenous zinc (Fig. 4B) and, as with exogenous zinc, was coupled to the phosphorylation of AKT and ERK1/2 (Fig. 4C and D) 6.
Figure 4. Zinc release from stores can be stimulated without exogenous zinc treatment.
(A) TamR cells were loaded with zinquin, exposed to different stimuli, and imaged 20 minutes later to assess intracellular zinc. Fluorescence with EGF+ionomycin was comparable to that with zinc plus sodium pyrithione, indicating that EGF+ionomycin stimulated zinc release into the cytosol in the absence of extracellular zinc. Representative of N= 3 experiments. Scale bar=10μm. (B) Proximity ligation assay performed in TamR cells with anti-ZIP7 and anti-CK2α antibodies demonstrates that EGF + ionomycin generate a transient association between ZIP7 and CK2, with a maximal increase compared to time 0 (* P ≤ 0.05) at 2 minutes. Overlaid with effects of zinc treatment to aid comparison. Fluorescent dots in inserts demonstrate ZIP7 and CK2α in close proximity (<40nm). Complete time course and validation of CK2 antibody shown in Fig. S5. Pooled results from over 3 representative fields from each of N=3 experiments are expressed as mean ± SD dots/cell. Scale bar=10μm. (C-D) Western blot analysis of pAKT and pERK1/2 confirmed that treatment with EGF and ionomycin showed phosphorylation consistent with cytosolic Zn2+ release. Densitometry of n=3 blots normalized to β-actin demonstrate a significant increase compared to time 0 (** P ≤ 0.001), results are given as mean ± SD.
CK2 is essential for ZIP7 activation
Using the Duolink proximity ligation assay in TamR cells, we found that EGF alone increased the ZIP7-CK2 interaction slightly at 2 minutes after treatment, and that the combination of EGF+ionomycin induced a response equivalent to that produced by treatment with exogenous zinc plus zinc ionophore (Fig. 5A). Pre-treatment with the CK2 inhibitors DMAT or TBB (4,5,6,7-tetrabromobenzotriazole) prevented the increase in ZIP7-CK2 association after zinc stimulation (Fig. 5A) whereas the siRNAs attenuated the increase but didn’t actually prevent it (Fig. 5B), presumably due to the incomplete knockdown and reinforcing our observation here that the ZIP7-CK2 association could be induced by external stimuli and inhibited by blocking CK2’s catalytic activity.
Figure 5. CK2 is essential for ZIP7 activation.
(A-B) Duolink proximity assay performed with rabbit anti-ZIP7 and mouse anti-CK2 antibodies to assess the association of these molecules within TamR cells under various conditions. Pooled results from at least 3 representative fields of view from each of N=3 experiments are expressed as mean ± SD dots/cell. Significant changes compared to control cells with no treatment are indicated by * (P ≤ 0.05) and ** (P ≤ 0.001). (A) TamR cells stimulated with: 20μM zinc with 10μM zinc ionophore (sodium pyrithione), EGF (10ng/ml), or EGF (10ng/ml) and ionomycin (500nM). generated a significant increase in the association of ZIP7 with CK2α. The zinc-induced association was abolished by pre-treatment with the CK2 inhibitors DMAT or TBB. (B) Transfection of TamR cells for 3 days with siRNA directed toward either ZIP7 or CK2 suppressed the zinc stimulated increase in association observed between these molecules.
Exogenous treatment does not alter ZIP7 intracellular location but does change quaternary structure
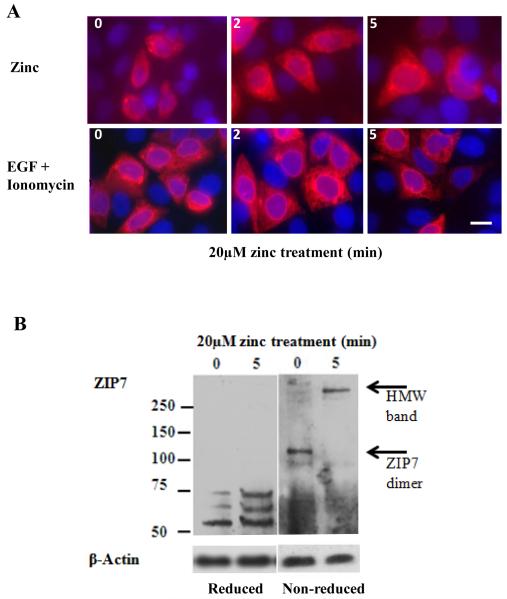
Fluorescent microscopy of TamR cells transfected with wild-type ZIP7 confirmed its localization to the ER (Fig. 6A), as observed in other cell lines 6, 21, and showed that it did not re-locate in response to treatment with either zinc or EGF+ionomycin. Furthermore, we used antibodies directed against the V5-tag engineered into the recombinant proteins to confirm localization of both wild-type and mutant recombinant ZIP7 in MCF-7 cells to the ER (fig. S3B), a location mirrored by our ZIP7 antibody (fig. S3C). We compared Western blots probed with a ZIP7 antibody under reducing or non-reducing conditions to investigate the ability of zinc to change the conformation of ZIP7 (Fig. 6B). In reducing conditions, ZIP7 was identified in a band close to the predicted molecular mass of 50kDa 19, 21, as well as in two bands of higher mass, suggestive of post-translational modifications. However, in non-reducing conditions, we observed a ZIP7 band at approximately 120kDa, suggestive of a possible dimer (Fig. 6B) that changed to a larger high-molecular weight band with 5min of zinc treatment, a time course consistent with the onset of ZIP7-mediated zinc transport, suggestive of the formation of a protein multimer incorporating ZIP7.
Figure 6. Intracellular location and quaternary structure of ZIP7 during zinc treatment.
(A) Imaging of recombinant wild-type ZIP7 in TamR cells after treatment with zinc or EGF + ionomycin in permeabilized cells probed with V5 antibody which was conjugated to Alexa-Fluor 594 (red). The nuclei were counterstained blue with DAPI. Representative of N= 3 experiments. Scale bar=10μm. (B) TamR cells treated with zinc were probed for ZIP7 under reducing or non-reducing conditions. Under non-reducing conditions the ZIP7 band approximating 50kDa, increased to100kDa, a size consistent with its dimerization. Under reducing conditions, zinc treatment increased the two bands between 50-75kDa that were replaced under non-reducing conditions with a high molecular mass band greater than 250kDa. The lower bands indicative of cellular processing were evident in both reducing and non-reducing conditions. Representative of N= 3 blots.
Discussion
The Ca2+ ion has been recognized as a second messenger since the early 1970’s 40. Compelling evidence now exists that the Zn2+ ion also fulfils the necessary criteria to be classified as a second messenger 41. Although cytosolic Zn2+ signals may arise from changes in abundance of zinc transporters6 there is at least one instance where an extracellular stimulus gives rise to a cytosolic Zn2+ wave, originating from the ER, within minutes 14. Furthermore, activation of proliferative tyrosine kinase pathways in breast cancer cells (MCF-7 and TamR) depends on the release into the cytosol of Zn2+ from the ER, mediated by the zinc channel ZIP7 6, 9. Here, we have demonstrated that treatment of TamR or MCF-7 cells with external zinc plus zinc ionophore, or with the combination of EGF and the calcium ionophore ionomycin, elicits a physical association between the protein kinase CK2 and the zinc channel ZIP7 that peaks within two minutes. These results strongly suggest that CK2 then phosphorylates ZIP7 at Ser275 and Ser276, and this is followed by an increase in cytosolic free [Zn2+], phosphorylation of ERK1/2 and AKT, and cell migration. Therefore, we propose a model whereby phosphorylation of ZIP7 by CK2 mediates the gated release of Zn2+ from ER stores into the cytosol (Fig. 6), producing a transient intracellular pool of Zn2+ that subsequently activates cellular signaling pathways. No cytosolic ‘free’ zinc was apparent in these cells until after zinc release from the ER (Figs. 2 and 3), as was also the case with EGF and ionomycin stimulation (Fig. 4). Thus we do not believe that the added exogenous zinc and ionophore cause a rise in cytosolic zinc before it is released from the ER by ZIP7. This is consistent with the belief that there is nofree zinc in cells but that it is mopped up by a ‘muffler’ (9).
There are two alternative explanations for our data: phosphorylation of ZIP7 could lead to its association with another ion channel, which then releases zinc from the ER; or could promote its relocation to the plasma membrane, enabling the influx of extracellular zinc. We consider both of these scenarios unlikely because ZIP7 was not observed in the plasma membrane and because treatment with EGF and ionomycin generated a Zn2+ signal, which was dependent on CK2 phosphorylation of ZIP7, in the absence of externally added zinc. Thus, although we cannot entirely reject either of these alternative explanations, we contend that the gated release of Zn2+ from the ER through ZIP7 following ZIP7 phosphorylation is more probable.
The zinc importer, SLC39A4 (Zip4) is functional in the apical membrane of several transporting epithelia 42, and are internalised and degraded in response to zinc excess. Transporters for copper and iron are regulated by trafficking in response to cellular metal levels. These include the divalent metal transporter-1 (DMT1) which relocates to lysosomes in high iron conditions 43, the copper importer, CTR1 which shuttles between the plasma membrane and internal vesicles depending on cellular copper status 44 and two P-type copper ATPases, ATP7A and ATP7B, which translocate to post-Golgi vesicles or plasma membrane 45 during high copper and traffic back to trans-Golgi network during copper depletion, a process that can involve modification by phosphorylation or glutathionylation 45. Thus, post-translational regulation of transition metal transporters involve cellular location changes and there is clear precedent for involvement of phosphorylation as a mechanism for transition metal trafficking, but the present study is the first to demonstrate activation of a eukaryotic transition metal channel resulting in a rapid influx of the solute. The requirement for such a mechanism may be explained by the action of the Zn2+ ion as a second messenger and in this respect, cellular roles of zinc much resemble those of Ca2+, which is also released into the cytosol by gated mechanisms 46.
ZIP7 abundance is increased in tumors being one of the top 10% of genes overexpressed in many poor prognostic cancer states 9 and shows a statistically significant positive correlation of mRNA expression with known indicators of poor outcome in breast cancer, such as the proliferation marker Ki67 29. This, together with current thinking that CK2 is essential for the neoplastic phenotype and can also act as an oncogene when overexpressed 47, suggests that these two molecules may have a common role in cell survival. The fact that CK2 inhibitors decrease the viability of various cancer cells, including prostate cancer cells and tamoxifen-resistant MCF-7 breast cancer cells 48 adds further weight to this view,, supporting the possibility that CK2 and ZIP7 may function together to mediate Zn2+ signals.
Targeting ZIP7-mediated release of zinc in breast cancers leads to loss of growth, invasion and signaling 6. The identification here of a CK2-mediated phosphorylation switch for activating ZIP7 to release zinc opens the door to the use of CK2 inhibitors, which are well tolerated by cancer patients 49, to treat breast cancer Furthermore, because intracellular zinc signals inhibit protein tyrosine phosphatases 15, targeting zinc release could prevent activation of multiple tyrosine kinases and their associated signalling pathways, thereby benefiting cancer patients 6, 50.
CK2, which is a key regulator of many proteins and pathways 51,34, has been considered to be constitutively active 51. Here, we observed that CK2 transiently associated with and serine-phosphorylated ZIP7 following the appropriate extracellular stimuli, indicating that CK2 must be regulated through changes in its activity, its physical location, or both. In any event, the involvement of CK2 in promoting zinc flux from the ER is intriguing, given that each regulatory β-subunit coordinates a zinc atom in a C4 zinc finger and these motifs are essential for assembly of the holoprotein 52. Thus, it is tempting to speculate that zinc itself regulates CK2 activity.
There are 14 human ZIP (SLC39) zinc channels and 10 ZnT (SLC30) zinc transporters that may be similarly activated by cell signaling events. Additionally, several Ca2+ channels are highly permeable to Zn2+ and it is possible that other ion channels can generate Zn2+ signals 41. Zinc signals can also occur in response to changes in the cellular redox state; the zinc-binding protein, metallothionein (MT), which releases Zn2+ under oxidative conditions, is at the center of this redox switch 53. . As with calcium, there is now substantial evidence for a role for cytosolic Zn2+ signals in regulating cell functions from the start of life 8 through to cell death 54, 55.
A zinc signal stimulates exit from meiosis in mouse oocytes 56, zinc influx through Zip6 controls gastrulation in zebrafish 8, and activation of mast cells produces cytosolic zinc release from ER 14. The ZIP7 residues that comprise the CK2 phosphorylation site are strongly conserved between species (Table 1), even in plants, suggesting that their interaction is fundamental to eukaryotic life. Further investigation of the evidence for phosphorylation of other zinc transporters from mass spectrometry analysis 9 is required to determine the full extent of the role of phosphorylation in regulation of zinc signals. Our identification of phosphorylation as responsible for activating regulated zinc release indicates that their interaction may provide a mechanism by which cells can evoke zinc signals. Further investigation of the evidence for phosphorylation of other zinc channels and transporters from mass spectrometry analysis 9is required to determine the full extent of the role of phosphorylation in regulation of zinc signals.
Materials and Methods
Cell preparation and treatments
The development of Tamoxifen-resistant (TamR) cells from MCF-7 breast cancer cells has been described 6. CK2 inhibitor dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT at 1μM) or 4,5,6,7-tetrabromobenzotriazole (TBB at 25μM) were added for 1 hour prior to stimulation with zinc. Zinc stimulation was with 20μM zinc in the presence of 10μM zinc ionophore (sodium pyrithione) in phenol red-free RPMI + 4-OH Tamoxifen without serum. Zinc-free stimulation was with EGF (10ng/ml) or 500nM ionomycin (or both) in phenol red-free RPMI + 4-OH Tamoxifen without serum, with no zinc added 57.
For immunoprecipitations, 1μg of antibody was incubated with 500μg protein and 20μl of EZview™ Red Protein A Affinity Gel (Sigma) beads overnight. Cells were lysed for 1 hour in ice-cold lysis buffer [5.5mm EDTA, 0.6% Nonidet P40, 1/10 mammalian protease inhibitor cocktail (Sigma) in Krebs-Ringer HEPES buffer (KRH)] and separated by 10% SDS-PAGE as described previously 21. Primary antibodies for Western Blot were as follows: p-ERK1/2T202/Y204 (1/1000) and pAKTS473 (1/1000), New England Biolabs Ltd., UK, β-actin (1/10,000) and cell-permeable zinc chelator N,N,N_,N_-tetrakis-(2-pyridylmethyl) ethylenediamine (TPEN), Sigma-Aldrich (Poole, UK) and pTyr, pSer, and CK2α antibodies from Santa Cruz Biotechnology, Inc., Germany. The ZIP7 polyclonal antibody (1/100) has been described previously 6. The mouse V5 antibody (1/1000) was from Invitrogen.
Preparation of cells for fluorescence microscopy has been described 6. Briefly, cells were seeded on coverslips and harvested when approximately 70% confluent. After appropriate treatment cells were fixed in 3.7% paraformaldehyde and permeabilised as required using 0.4% saponin in phosphate buffered saline with 1% albumin. All images, except PLA images, were processed with one level of deconvolution using Openlab software (Improvision). For imaging zinc, cells were loaded with the cell-permeable zinc specific dye Fluozin-3 (5μM, Invitrogen) or Zinquin (25μM, Cambridge Bioscience, Cambridge, UK) for 30 mins at 37°C prior to zinc treatment as described 6.
Transfections
The recombinant construct for ZIP7 with the C-terminal V5 tag in vector pcDNA3.1/V5-His-TOPO 21 was mutated (S275A and S276A) using the Stratagene Quickchange kit and its sequence confirmed by sequencing (fig. S4). MCF-7 cells were transfected for 18 hours with wild-type or mutant ZIP7 constructs using lipofectamine 2000 (Invitrogen) as previously described 6. TamR cells were transfected for 3 days with siRNA pools to ZIP7 or CK2 (Dharmacon) using siRNAMax reagent (Invitrogen) as described 6.
Proximity Ligation Assay
Cells on 8-well chamber slides (Lab-Tek, Fisher) were fixed in 3.7% formaldehyde in PBS for 15 minutes followed by a Proximity Ligation Assay (PLA) using the Duolink red kit (Cambridge Bioscience) according to manufacturer’s instructions. Wells were removed from the chamber slides before blocking (DUOLink kit) and incubation with ZIP7 (1/200) or CK2α antibodies (1/200, Santa Cruz) for 1 hour, followed by rabbit PLA plus and mouse PLA minus probes (containing oligonucleotides) at 37°C for 1 hour, ligase for 30 minutes at 37°C to hybridize PLA probes and generate a closed circle before DNA amplification (rolling circle amplification), and conjugation to Alexa Fluor 594 for 100 minutes at 37°C. Coverslips, mounted on slides with Vectashield with DAPI, were viewed on a Leica RPE automatic microscope using a×63 oil immersion lens acquired using a multiple band pass filter set appropriate for 4,6-diamidino-2-phenylindole (DAPI) and Texas Red and presented as maximal projections of 25 stacks taken 0.3μm apart. Numbers of dots per cell were determined using image tool software from Olink and presented as average values ±SD.
FACS analysis
Cells for FACS analysis were dislodged using 3mM EDTA in PBS and resuspended in Phenol red-free RPMI medium before treatment with zinc plus ionophore at 37°C and incubation with antibodies directed against ZIP7 (1/50) or pSer (1/50) antibodies for 1 hour on ice followed by secondary antibodies to Alexa-Fluor 488 and phycoerythrin PE for 30 minutes on ice. Results were expressed as a two parameter dot plot displaying FITC detection channel (FL1) on x-axis and PE detection channel (FL2) on y-axis. Values were adjusted for compensation of overlapping fluorescence using manufacturer’s instructions.
Migration Assay
Cells expressing wild-type or mutant ZIP7 were seeded onto fibronectin-coated (100ug/ml) microporous membranes (8mm pore size, Costar Ltd) at 50,000 cells/membrane ± 20μM zinc without ionophore and cultured for a period of 48hrs. The migratory cells, those which passed across the porous membrane from the inside of the insert to the outside of the membrane, were fixed, stained and counted. Cell migration was quantified as the mean number of cells observed in each of 6 random fields of view per sample, in duplicate. Differences between groups were statistically compared using the paired t-test.
Statistical analysis
Statistical analysis was performed on all data using ANOVA with post hoc Dunnett and Tamhane tests with the exception of the migration assay. Significance was assumed with P ≤ 0.05. Error bars shown represent standard deviations calculated from a minimum of n=3 different experiments.
Supplementary Material
Supplementary Material
Summary.
The present research unveils a post-translational modification that mediates gated zinc release from the endoplasmic reticulum
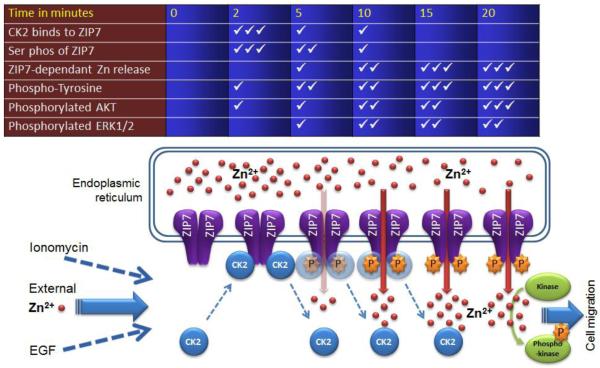
Figure 7. Schematic showing the temporal relationship of CK2 association with ZIP7 and zinc release.
Schematic illustrating the time course of the effect of zinc treatment on phosphorylation of ZIP7 by CK2 and the subsequent zinc release from the endoplasmic reticulum. The ticks represent relative abundance where 3 ticks represents maximum.
Acknowledgements
We thank Michaela Manisova for help generating the ZIP7 mutant, Samantha Huntley, Anna-Lena Stegmann and Jarmila Kralova for Western Blot assistance and Lynne Farrow for performing the statistical analysis. KMT was supported by a Wellcome Trust University Research Award [Grant number 091991/Z/10/Z]. and CH and PK were supported by King’s College, London and Cardiff University, respectively. Author contributions: KMT, PK and CH designed the experiments, interpreted the data and wrote the manuscript. KMT performed experiments and SH performed migration assays. All authors contributed to discussion and interpretation of the data. The authors declare that they have no competing interests.
References and Notes
- 1.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiological reviews. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Prasad AS. Impact of the discovery of human zinc deficiency on health. Journal of the American College of Nutrition. 2009;28:257–265. doi: 10.1080/07315724.2009.10719780. [DOI] [PubMed] [Google Scholar]
- 3.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nature reviews. Neuroscience. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 4.Haase H, Rink L. The immune system and the impact of zinc during aging. Immunity & ageing: I & A. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 6.Taylor KM, et al. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer Cells. Endocrinology. 2008;149:4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 7.Rink L. Zinc in human health. IOS Press; Amsterdam: 2011. [Google Scholar]
- 8.Yamashita S, et al. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;429:298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- 9.Hogstrand C, Kille P, Nicholson RI, Taylor KM. Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends in molecular medicine. 2009;15:101–111. doi: 10.1016/j.molmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Fukada T, Kambe T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics. 2011;3:662–674. doi: 10.1039/c1mt00011j. [DOI] [PubMed] [Google Scholar]
- 11.Passerini A, Andreini C, Menchetti S, Rosato A, Frasconi P. Predicting zinc binding at the proteome level. BMC Bioinformatics. 2007;8:39. doi: 10.1186/1471-2105-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laity JH, Andrews GK. Understanding the mechanisms of zinc-sensing by metal-response element binding transcription factor-1 (MTF-1) Arch Biochem Biophys. 2007;463:201–210. doi: 10.1016/j.abb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Hogstrand C, Zheng D, Feeney G, Cunningham P, Kille P. Zinc-controlled gene expression by metal-regulatory transcription factor 1 (MTF1) in a model vertebrate, the zebrafish. Biochemical Society transactions. 2008;36:1252–1257. doi: 10.1042/BST0361252. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki S, et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haase H, Maret W. Protein tyrosine phosphatases as targets of the combined insulinomimetic effects of zinc and oxidants. Biometals. 2005;18:333–338. doi: 10.1007/s10534-005-3707-9. [DOI] [PubMed] [Google Scholar]
- 16.Maret W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Experimental gerontology. 2008;43:363–369. doi: 10.1016/j.exger.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Colvin RA, Holmes WR, Fontaine CP, Maret W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics: integrated biometal science. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 18.Sekler I, Sensi SL, Hershfinkel M, Silverman WF. Mechanism and regulation of cellular zinc transport. Molecular medicine. 2007;13:337–343. doi: 10.2119/2007-00037.Sekler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta. 2003;1611:16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin W, Chai J, Love J, Fu D. Selective electrodiffusion of zinc ions in a Zrt-, Irt-like protein, ZIPB. J. Biol. Chem. 2010;285:39013–39020. doi: 10.1074/jbc.M110.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochemical Journal. 2004;377:131–139. doi: 10.1042/BJ20031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Kirschke CP, Zhang Y, Yu YY. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J Biol Chem. 2005;280:15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki A, Endo T. Ermelin, an endoplasmic reticulum transmembrane protein, contains the novel HELP domain conserved in eukaryotes. Gene. 2002;284:31–40. doi: 10.1016/s0378-1119(01)00885-x. [DOI] [PubMed] [Google Scholar]
- 24.Stathakis DG, et al. The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics. 1999;153:361–382. doi: 10.1093/genetics/153.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasswell J, Rogg LE, Nelson DC, Rongey C, Bartel B. Cloning and characterization of IAR1, a gene required for auxin conjugate sensitivity in Arabidopsis. Plant Cell. 2000;12:2395–2408. doi: 10.1105/tpc.12.12.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor KM. A distinct role in breast cancer for two LIV-1 family zinc transporters. Biochemical Society transactions. 2008;36:1247–1251. doi: 10.1042/BST0361247. [DOI] [PubMed] [Google Scholar]
- 27.Jackson KA, Valentine RA, Coneyworth LJ, Mathers JC, Ford D. Mechanisms of mammalian zinc-regulated gene expression. Biochemical Society transactions. 2008;36:1262–1266. doi: 10.1042/BST0361262. [DOI] [PubMed] [Google Scholar]
- 28.Andrews GK. Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochemical Society transactions. 2008;36:1242–1246. doi: 10.1042/BST0361242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor KM, et al. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Molecular medicine. 2007;13:396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiscox S, et al. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib (‘Iressa’, ZD1839) Clin Exp Metastasis. 2004;21:201–212. doi: 10.1023/b:clin.0000037697.76011.1d. [DOI] [PubMed] [Google Scholar]
- 31.Knowlden JM, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 32.Knowlden JM, Hutcheson IR, Barrow D, Gee JM, Nicholson RI. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: a supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–4618. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 33.Niefind K, Raaf J, Issinger OG. Protein kinase CK2 in health and disease: Protein kinase CK2: from structures to insights. Cell Mol. Life Sci. 2009;66:1800–1816. doi: 10.1007/s00018-009-9149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St-Denis NA, Litchfield DW. Protein kinase CK2 in health and disease: From birth to death: the role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell Mol. Life Sci. 2009;66:1817–1829. doi: 10.1007/s00018-009-9150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oppermann FS, et al. Large-scale proteomics analysis of the human kinome. Molecular & cellular proteomics: MCP. 2009;8:1751–1764. doi: 10.1074/mcp.M800588-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahedi RP, et al. Phosphoproteome of resting human platelets. J. Proteome Res. 2008;7:526–534. doi: 10.1021/pr0704130. [DOI] [PubMed] [Google Scholar]
- 37.Kim JE, Tannenbaum SR, White FM. Global phosphoproteome of HT-29 human colon adenocarcinoma cells. J. Proteome Res. 2005;4:1339–1346. doi: 10.1021/pr050048h. [DOI] [PubMed] [Google Scholar]
- 38.Puntervoll P, et al. ELM server: A new resource for investigating short functional sites in modular eukaryotic proteins. Nucleic Acids Res. 2003;31:3625–3630. doi: 10.1093/nar/gkg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinna LA. The raison d’etre of constitutively active protein kinases: the lesson of CK2. Accounts of chemical research. 2003;36:378–384. doi: 10.1021/ar020164f. [DOI] [PubMed] [Google Scholar]
- 40.Nagata N. Rasmussen, H. Parathyroid hormone, 3′5′ AMP, Ca++, and renal gluconeogenesis. Proc. Natl. Acad. Sci. U. S. A. 1970;65:368–374. doi: 10.1073/pnas.65.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011;16:1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biological chemistry. 2007;388:1301–1312. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson DM, Yamaji S, Tennant J, Srai SK, Sharp PA. Regulation of divalent metal transporter expression in human intestinal epithelial cells following exposure to non-haem iron. FEBS Lett. 2005;579:1923–1929. doi: 10.1016/j.febslet.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 44.Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J.Biol. Chem. 2003;278:9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- 45.van den Berghe PV, Klomp LW. Posttranslational regulation of copper transporters. J. Biol. Inorg. Chem. 2010;15:37–46. doi: 10.1007/s00775-009-0592-7. [DOI] [PubMed] [Google Scholar]
- 46.Hanson CJ BM, Roderick HL. Cell signalling: IP3 receptors channel calcium into cell death. Curr Biol. 2004;14:R933–935. doi: 10.1016/j.cub.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochimica et biophysica acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Yde CW, et al. Induction of cell death in antiestrogen resistant human breast cancer cells by the protein kinase CK2 inhibitor DMAT. Cancer letters. 2007;256:229–237. doi: 10.1016/j.canlet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Solares AM, et al. Safety and preliminary efficacy data of a novel casein kinase 2 (CK2) peptide inhibitor administered intralesionally at four dose levels in patients with cervical malignancies. BMC cancer. 2009;9:146. doi: 10.1186/1471-2407-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor KM, Gee JMW, Kille P. In: Zinc and Cancer, in Zinc in human health. Rink L, editor. IOS press; Amsterdam: 2011. [Google Scholar]
- 51.Pinna LA, Allende JE. Protein kinase CK2 in health and disease: Protein kinase CK2: an ugly duckling in the kinome pond. Cell Mol. Life Sci. 2009;66:1795–1799. doi: 10.1007/s00018-009-9148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chantalat L, et al. Crystal structure of the human protein kinase CK2 regulatory subunit reveals its zinc finger-mediated dimerization. Embo J. 1999;18:2930–2940. doi: 10.1093/emboj/18.11.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maret W. Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals. 2011;24:411–418. doi: 10.1007/s10534-010-9406-1. [DOI] [PubMed] [Google Scholar]
- 54.Formigari A, Irato P, Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:443–459. doi: 10.1016/j.cbpc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Truong-Tran AQ, Ho LH, Chai F, Zalewski PD. Cellular zinc fluxes and the regulation of apoptosis/gene-directed cell death. The Journal of nutrition. 2000;130:1459S–1466S. doi: 10.1093/jn/130.5.1459S. [DOI] [PubMed] [Google Scholar]
- 56.Kim AM, Vogt S, O’Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nature chemical biology. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore GE, Gerner RE, Franklin HA. Culture of normal human leukocytes. Jama. 1967;199:519–524. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material