The Utility of Uterine Artery Doppler Velocimetry in Prediction of Preeclampsia in a Low-Risk Population (original) (raw)
. Author manuscript; available in PMC: 2013 Oct 1.
Abstract
Objective
The underlying pathophysiology of preeclampsia is thought to be abnormal trophoblast invasion of the spiral arteries, leading to maldevelopment of uteroplacental perfusion. We estimated whether uterine artery Doppler measurements made in the early second trimester would predict the subsequent development of preeclampsia.
Methods
Uterine artery Doppler measurements prior to 21 weeks of gestation (median 16.6 weeks) were correlated with subsequent development of preeclampsia in a cohort of 2,188 low-risk nulliparous women in a randomized control trial of antioxidant supplementation for prevention of preeclampsia. Preeclampsia developed in 165 (7.5%) women.
Results
Development of preeclampsia overall was associated with increased resistance index (RI), pulsatility index (PI), a PI or RI multiples of the median (MoM) at or above the 75th %ile, but not the presence of a notch or a bilateral notch prior to 21 weeks. The sensitivity was 43% (95% CI 35–51) and specificity 67% (95% CI 65–69) for prediction of preeclampsia overall. The presence of a notch or bilateral notch, RI and PI MoM were significantly associated with early onset (before 34 weeks of gestation) vs late onset or no preeclampsia (OR = 6.9 (95% CI 2.3–20.9), sensitivity 78% (95% CI 52–94), specificity 66% (95% CI 64–68)). The presence of a notch or RI MoM at or above the 75%ile increased the odds of developing severe vs mild or no preeclampsia (OR=2.2 (95% CI 1.4–3.7), sensitivity 53% (95% CI 40–65), specificity 66% (95% CI 64–68)).
Conclusion
Our data show poor sensitivity of second-trimester Doppler ultrasound measurements for prediction of preeclampsia overall in a well-characterized, low-risk, nulliparous population. The technique has utility in identifying poor trophoblast invasion of spiral arteries of a magnitude that severely compromises uteroplacental blood flow and gives early-onset disease.
Introduction
Preeclampsia is a pregnancy-specific syndrome characterized by the onset of hypertension and proteinuria after the 20th week of gestation. It is the leading cause of fetal growth restriction, indicated premature delivery, and is responsible for over 50,000 maternal deaths annually worldwide (1–2). In the USA the syndrome affects up to 7% of the 4.25 million pregnancies resulting in live births each year. A screening test that could identify, early in pregnancy, those women who would later develop preeclampsia would allow increased surveillance of those at risk, and reduce surveillance for those unlikely to develop the syndrome (3–4).
Our current state of knowledge describes preeclampsia as a two stage phenomenon resulting in the systemic preeclampsia syndrome in women “sensitive” to the insult. In this paradigm the initial insult is thought to be abnormal placentation leading to maldevelopment of uteroplacental perfusion that then leads to the increased inflammatory response and endothelial dysfunction of the syndrome. In several large prospective studies, Doppler measurements of the uteroplacental vasculature performed in the second trimester or in the late first trimester reportedly could identify women who subsequently develop preeclampsia or intra-uterine growth restriction (IUGR) (5). It was most sensitive identifying women requiring delivery before 32 weeks gestation (6).
This study was performed by the Maternal-Fetal Medicine Units (MFMU) Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) as part of the Combined Antioxidant and Preeclampsia Prediction Studies (CAPPS) in which the overall objective was to identify potential biochemical and biophysical markers for prediction of preeclampsia. In this analysis we address the hypothesis that uterine artery doppler measurements made in the early second trimester would predict the subsequent development of preeclampsia.
Methods
Study design
The NICHD MFMU Network conducted this study as a planned observational cohort of a larger randomized controlled trial to estimate whether antioxidant supplementation (1000mg vitamin C and 400IU of vitamin E) prevented preeclampsia in nulliparous women at low risk for developing the syndrome. The full details of the trial have been reported previously (7). Women were eligible to participate in this cohort if they were enrolled in the randomized clinical trial between 9 weeks, 0 days and 12 weeks, 6 days. Exclusion criteria were: a prior pregnancy lasting beyond 19 weeks 6 days, an elevated blood pressure (systolic pressure ≥ 135 mmHg or diastolic blood pressure ≥ 85 mmHg), proteinuria (24 hour urine collection of ≥ 300 mg protein or a dipstick value more than trace), use of anti-hypertensive medication, pre-gestational diabetes, regular use or use within 7 days of platelet active drugs or non-steroidal anti-inflammatory agents, known fetal abnormalities or demise at the time of enrolment, or a history of medical complications. These blood pressures were chosen to ensure that individuals with blood pressures of ≥140 mmHg systolic or ≥90 mmHg diastolic later in gestation, truly had pregnancy-associated hypertension. Written informed consent was obtained from every patient prior to enrolment and the study was approved by the institutional review board at each clinical site and the data coordinating center.
Clinical information including demographics, medical, obstetrical, social and sexual history was obtained at the time of enrollment by patient interview and chart review.
Uterine artery Doppler
All women in the cohort were scheduled to have an initial uterine artery Doppler at 16 weeks gestation performed specifically for the research protocol. If a notch was present at the time of the initial ultrasound measurement, a second ultrasound was scheduled for 24 weeks gestation to determine if a notch was still present. The earliest the Doppler was to be performed was 14 weeks, 0 days and the latest was 26 weeks, 6 days. To focus on early prediction and ensure there was no overlap in gestational age for the initial and repeat Dopplers, this analysis only included initial Dopplers performed prior to 21 weeks’ gestation. Prior to performing any uterine artery Doppler examination, sonographers completed a certification examination that included written questions and expert review of images to ensure standard procedures were employed across the clinical centers. The Doppler examinations were performed as follows: All examinations were performed transabdominally. The transducer was placed in the lower lateral quadrant, angled medially and color Doppler used to identify the common iliac bifurcation into the internal and external iliacs. The internal iliac was followed medially until close to the lateral edge of the uterus until the main branch entering the uterus was identified and insonated as it entered the uterus and 1 cm distal to its apparent crossing of the external iliac artery. Gate, gain and scale were optimized to obtain the waveform. If the sonographer was unable to obtain an adequate image transabdominally a transvaginal approach was used. Three waveforms from the right and left uterine arteries were recorded. A diastolic notch, defined as the presence of a clear upswing in the waveform at the beginning of diastole, on any waveform, was considered positive for the presence of a notch. The resistance index (RI) and pulsatility index (PI) for each waveform were calculated using the software packages on the ultrasound machine or, if not available, manually using the formulas;
RI=(systolic-diastolic)/systolic
PI=(systolic-diastolic)/[(systolic+diastolic)/2]
The mean RI and PI were calculated by taking the average of the waveforms from both the left and right uterine arteries. No data related to Doppler findings was revealed to the treating clinician.
Primary outcome
The primary outcome was the development of preeclampsia. Secondary outcomes included the severity and gestational age of preeclampsia onset. The diagnosis of hypertension was based on blood-pressure measurements obtained during or after the 20th week of pregnancy, excluding intra-operative blood pressures and intra-partum systolic pressures. Mild pregnancy-associated hypertension was defined as a systolic pressure between 140 and 159 mmHg or a diastolic pressure between 90 mmHg and 109 mmHg on two occasions 2 to 240 hours apart. Severe pregnancy-associated hypertension was defined as a systolic pressure of 160 mmHg or more or a diastolic pressure of 110 mmHg or more on two occasions 2 to 240 hours apart, or a single blood-pressure measurement that was severely elevated and that led to treatment with an antihypertensive medication. Mild preeclampsia was defined as mild pregnancy-associated hypertension with documentation of proteinuria within 72 hours before or after an elevated blood-pressure measurement. Proteinuria was defined as total protein excretion of 300 mg or more in a 24-hour urine sample or 2+ or higher on dipstick testing, or a protein-to-creatinine ratio of 0.35 or more if a 24-hour urine sample was not available. Severe preeclampsia was defined as preeclampsia with either severe pregnancy-associated hypertension or protein excretion of 5 g or more in a 24-hour urine sample or as mild pregnancy-associated hypertension with oliguria (<500 ml in a 24-hour urine sample), pulmonary edema (confirmed by radiography), or thrombocytopenia (platelet count of <100,000 per cubic millimeter). Preeclampsia included mild and severe preeclampsia, HELLP syndrome and eclampsia. For this analysis, severe preeclampsia, HELLP syndrome and eclampsia were combined as severe preeclampsia. The time of onset of preeclampsia (early onset defined as <34 weeks or late onset defined as ≥34 weeks gestation) was determined as the time at which individuals first met the criteria for diagnosis of preeclampsia given above.
To determine the diagnosis of preeclampsia, de-identified medical charts of all women with pregnancy-associated hypertension were reviewed centrally by at least three reviewers.
Statistical analysis
Categorical variables were compared using the chi-square test and continuous variables using the Wilcoxon rank sum test for comparison of 2 groups and the Kruskal-Wallis for comparison of 3 groups. Tests for trend were performed using the Cochran-Armitage trend test for categorical variables and the Jonckheere-Terpstra test for continuous variables. Multiples of the median (MoM) were computed for pulsatility index and resistance index by taking the observed measurement and dividing by the expected median The expected median was derived from multiple regression of gestational age at Doppler, maternal weight in kilograms and racial group in the women that did not have an elevated blood pressure or proteinuria. All variables that were significant with a p-value less than 0.1 were included in the final calculation. The equation for the expected median RI is as follows: exp{−0.06651 − 0.00333*gestational age of Doppler − 0.0008466*maternal weight + 0.02524*(1 if African American, 0 otherwise) + 0.05276*(1 if Hispanic, 0 otherwise)}. The equation for the expected median PI is follows: exp{1.05827 − 0.00766* gestational age of Doppler − 0.00112* maternal weight + 0.05973*(1 if African American, 0 otherwise)+ 0.10852*(1 if Hispanic, 0 otherwise)}. The 75th percentile cutoff was defined using the women that did not have an elevated blood pressure or proteinuria. The ability of the model to accurately discriminate between women with and without preeclampsia was determined by receiver operating characteristics (ROC) curves and by calculating sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios. ROC curves were compared using the nonparametric approach of DeLong et al (8).A nominal p value less than 0.05 was considered to indicate statistical significance and no adjustments were made for multiple comparisons. Analyses were performed using SAS software (Cary, NC).
Results
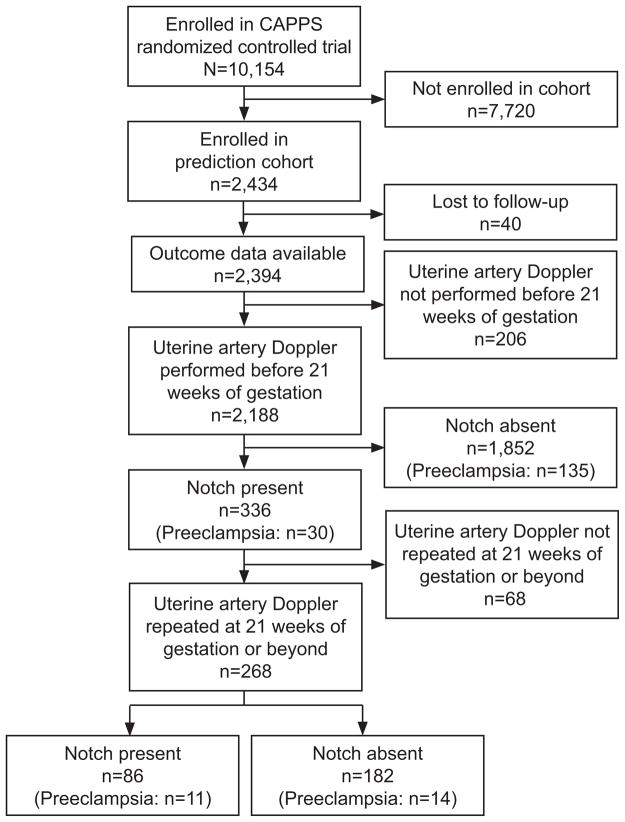
A total of 2,434 women were enrolled in the cohort and outcome data was available on 2,394 (Figure 1). Of these, 2,188 women (91%) had an initial Doppler < 21 weeks’ gestation (median=16.6, range=13.6–20.9). Population characteristics are presented in Table 1. The incidence of notching of the waveform, the resistance index or the pulsatility index were not different between patients assigned to either the antioxidant or placebo groups and data were therefore combined for the analysis.
Figure 1.
Flow of participants in Combined Antioxidant and Preeclampsia Prediction Studies (CAPPS).
Table 1.
Population Characteristics
| Characteristic | Preeclampsia (n=165) | No Preeclampsia (n=2,023) | P |
|---|---|---|---|
| Gestational age at enrollment | 11.6 [10.6–12.3] | 11.6 [10.7–12.3] | 0.78 |
| Maternal age | 22 [19–25] | 23 [20–27] | 0.02 |
| Race | <0.001 | ||
| African American | 59 (35.8) | 454 (22.4) | |
| Hispanic | 49 (29.7) | 496 (24.5) | |
| Caucasian or other | 57 (34.5) | 1,073 (53.0) | |
| Previous pregnancy (before 20weeks) | 34 (20.6) | 454 (22.4) | 0.59 |
| Family history of preeclampsia | 24 (14.5) | 266 (13.1) | 0.61 |
| Smoked during pregnancy | 27 (16.4) | 334 (16.5) | 0.96 |
| BMI at enrollment | 27.1 [23.0–31.6] | 24.6 [21.8–28.7] | <0.001 |
| Blood pressure at enrollment | |||
| Systolic | 112 [106–118] | 110 [102–118] | 0.007 |
| Diastolic | 66 [60–70] | 66 [60–70] | 0.78 |
| Treatment group | 0.31 | ||
| Vitamins C & E | 89 (53.9) | 1,008 (49.8) | |
| Placebo | 76 (46.1) | 1,015 (50.2) |
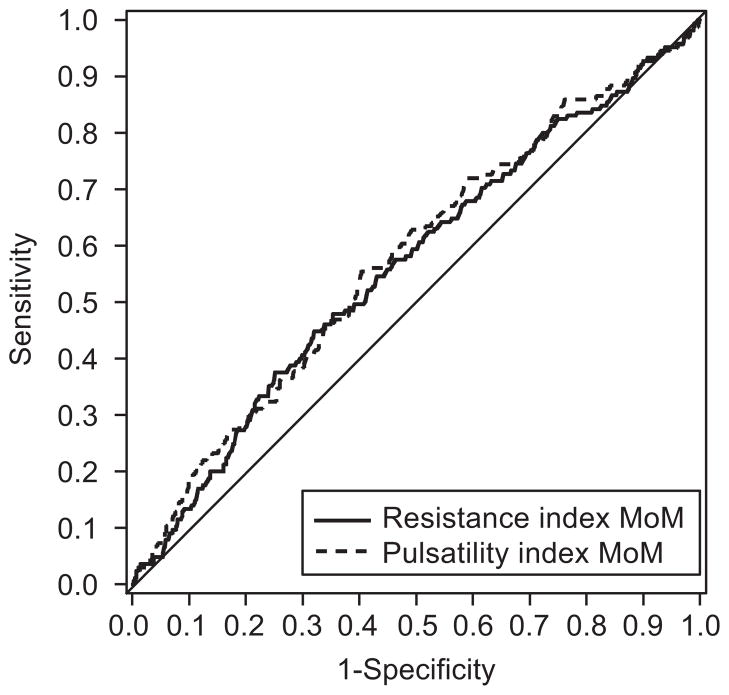
Of the 2,188 women who had an initial uterine artery Doppler examination performed at < 21 weeks’ gestation, 165 women subsequently developed preeclampsia. The presence of a notch or a bilateral notch was not significantly associated with the development of preeclampsia (Table 2). However, RI and PI were significantly higher in women who developed preeclampsia (Table 2). The presence of a RI or PI MoM at or above the 75%ile (values of 1.10 and 1.20 respectively) also was associated with a significantly increased incidence of preeclampsia (Table 2). However, the diagnostic utility was not great for any of these measurements; notch or RI MoM at or above the 75th %ile had a sensitivity of 43% (95% CI 35–51), specificity of 67% (95% CI 65–69), positive predictive value of 10% (95% CI 8–12), negative predictive value of 93%, (95% CI 92–95) positive likelihood ratio 1.29 (95% CI 1.07–1.55) and negative likelihood ratio 0.86 (95% CI 0.75–0.98). ROC curves were also constructed for RI and PI MoM and the development of preeclampsia (Figure 2). The area under the curve was significantly higher for PI compared with RI (0.58 vs 0.56, p=0.04).
Table 2.
Relationship of Preeclampsia and Uterine Artery Notch, Resistance Index and Pulsatility Index
| Preeclampsia (n=165) | No Preeclampsia (n=2,023) | P | |
|---|---|---|---|
| Notch | 30 (18.2) | 306 (15.2) | 0.31 |
| Bilateral notch | 14 (8.5) | 116 (5.7) | 0.15 |
| RI | 0.63 [0.57–0.70] | 0.61 [0.55–0.68] | 0.009 |
| RI MoM | 1.04 [0.94–1.15] | 1.01 [0.91–1.11] | 0.006 |
| PI | 1.20 [1.00–1.53] | 1.12 [0.92–1.37] | 0.001 |
| PI MoM | 1.09 [0.88–1.33] | 1.00 [0.83–1.22] | 0.001 |
| RI MoM 75th %ile or higher | 62 (37.6) | 533 (26.3) | 0.002 |
| PI MoM 75th %ile or higher | 60 (36.6) | 530 (26.2) | 0.004 |
Figure 2.
Receiver operating characteristics (ROC) curve for the development of preeclampsia. ROC curves were constructed for resistance index and pulsatility index multiple of the median (MoM) data. The area under the curve was significantly higher for pulsatility index compared with resistance index RI (0.58 compared with 0.56, _P_=.04).
Data were additionally analyzed to estimate if Doppler characteristics were related to the time of onset of preeclampsia or to the severity of preeclampsia. Of the 165 women that developed preeclampsia, 18 had early onset (<34 weeks) and 66 had severe preeclampsia. The presence of a notch or bilateral notch, and RI and PI MoM were significantly associated with time of onset of preeclampsia (Table 3). Women with early onset preeclampsia were more likely to have the presence of a notch (44.4% vs 15.2%, p=0.003) and bilateral notch (22.2% vs 5.8%, p=0.019) compared with women with late onset preeclampsia or no preeclampsia. Similarly, both RI and PI MoM were significantly associated with early onset preeclampsia compared with women that had late onset or no preeclampsia (p=0.002, p=0.002 respectively). The presence of a notch or an RI MoM at or above the 75th percentile significantly increase a women’s odds of developing early onset preeclampsia compared with women that had late onset or no preeclampsia (OR=6.9, 95% CI 2.3–20.9) and had a sensitivity of 78% (95% CI 52–94), specificity of 66% (95% CI 64–68), positive predictive value of 1.9% (95% CI 1.0–3.1), negative predictive value of 99.7% (95% CI 99.3–99.9), positive likelihood ratio of 2.30 (95% CI 1.79–2.97) and negative likelihood ratio of 0.34 (95% CI 0.14–0.80). When severity of preeclampsia was examined, there was a significant trend for PI MoM but not notch, bilateral notch or RI MoM (Table 4). Women with severe preeclampsia were more likely to have the presence of a notch (24.8% vs 15.1%, p=0.018) but not a bilateral notch (12.3% vs 5.8%, p=0.05) compared with women that had mild or no preeclampsia. Both RI and PI MoM were significantly associated with development of severe preeclampsia compared with women that had mild or no preeclampsia (p<0.001 for both). The presence of a notch or an RI MoM at or above the 75th percentile significantly increase a women’s odds of developing severe preeclampsia compared with women that had mild or no preeclampsia (OR=2.2, 95% CI 1.4–3.7) and had a sensitivity of 53% (95% CI 40–65), specificity of 66% (95% CI 64–68), positive predictive value of 5% (95% CI 3–6), negative predictive value of 98% (95% CI 97–99), positive likelihood ratio of 1.58 (95% CI 1.25–2.00) and negative likelihood ratio of 0.71 (95% CI 0.55–0.91).
Table 3.
Relationship of Time of Onset of Preeclampsia and Uterine Artery Notch, Resistance Index and Pulsatility Index
| Early-Onset Preeclampsia (n=18) | Late-Onset Preeclampsia (n=147) | No Preeclampsia (n=2,023) | P | |
|---|---|---|---|---|
| Notch | 8 (44.4) | 22 (15.0) | 306 (15.2) | 0.003 |
| Bilateral notch | 4 (22.2) | 10 (6.8) | 116 (5.7) | 0.01 |
| RI | 0.71 [0.61–0.78] | 0.63 [0.57–0.69] | 0.61 [0.55–0.68] | 0.003 |
| RI MoM | 1.19 [0.99–1.36] | 1.04 [0.94–1.13] | 1.01 [0.91–1.11] | 0.002 |
| PI | 1.69 [1.14–1.89] | 1.19 [1.00–1.42] | 1.12 [0.93–1.37] | <0.001 |
| PI MoM | 1.57 [1.03–1.81] | 1.08 [0.87–1.28] | 1.00 [0.83–1.22] | <0.001 |
| RI MoM 75th %ile or higher | 12 (66.7) | 50 (34.0) | 533 (26.3) | <0.001 |
| PI MoM 75th %ile or higher | 11 (64.7) | 49 (33.3) | 530 (26.2) | <0.001 |
| Notch and RI MoM 75th %ile or higher | 6 (33.3) | 15 (10.2) | 165 (8.2) | <0.001 |
| Notch and PI MoM 75th %ile or higher | 6 (33.3) | 15 (10.2) | 178 (8.8) | 0.001 |
| Notch or RI MoM 75th %ile or higher | 14 (77.8) | 57 (38.8) | 674 (33.4) | <0.001 |
| Notch or PI MoM 75th %ile or higher | 13 (76.5) | 56 (38.1) | 658 (32.7) | <0.001 |
Table 4.
Relationship of Severity of Preeclampsia and Uterine Artery Notch, Resistance Index and Pulsatility Index
| Severe Preeclampsia (n=66) | Mild Preeclampsia (n=99) | No Preeclampsia (n=2,023) | P | |
|---|---|---|---|---|
| Notch | 17 (25.8) | 13 (13.1) | 306 (15.2) | 0.09 |
| Bilateral notch | 8 (12.3) | 6 (6.1) | 116 (5.7) | 0.05 |
| RI | 0.65 [0.60–0.72] | 0.62 [0.56–0.68] | 0.61 [0.55–0.68] | 0.75 |
| RI MoM | 1.09 [0.97–1.18] | 1.02 [0.93–1.13] | 1.01 [0.91–1.11] | 0.17 |
| PI | 1.25 [1.04–1.61] | 1.16 [0.98–1.40] | 1.12 [0.93–1.37] | 0.013 |
| PI MoM | 1.15 [0.97–1.41] | 1.07 [0.84–1.25] | 1.00 [0.83–1.22] | 0.001 |
| RI MoM 75th %ile or higher | 31 (47.0) | 31 (31.3) | 533 (26.3) | <0.001 |
| PI MoM 75th %ile or higher | 30 (45.5) | 30 (30.6) | 530 (26.2) | <0.001 |
| Notch and RI MoM 75th %ile or higher | 13 (19.7) | 8 (8.1) | 165 (8.2) | 0.005 |
| Notch & PI MoM 75th %ile or higher | 13 (19.7) | 8 (8.1) | 178 (8.8) | 0.014 |
| Notch or RI MoM 75th %ile or higher | 35 (53.0) | 36 (36.4) | 674 (33.4) | 0.002 |
| Notch or PI MoM 75th %ile or higher | 34 (51.5) | 35 (35.7) | 658 (32.7) | 0.003 |
Of the 336 women that had a notch on their initial Doppler examination, 268 (80%) had a repeat Doppler performed at ≥ 21 weeks’ gestation (median=24.1, range=22.0–27.9) (Figure 1). Of these 268 women, only 25 developed preeclampsia. Among the 268, the persistence of a notch occurred in 86 women (32%) however this was not associated with subsequent development of preeclampsia; a notch was present in 44% of women with preeclampsia and 31% of women without preeclampsia, p=0.19. The presence of a bilateral notch also was not associated the development of preeclampsia in these women. MoMs of the PI and RI were not calculated since there was not an adequate group of women in which to derive the expected median. PI but not RI was associated with the subsequent development of preeclampsia (median 1.10 in women with preeclampsia, 0.98 in women without preeclampsia, p=0.02).
Discussion
We estimated whether uterine artery Doppler measurements made prior to 21 weeks gestation would predict the subsequent development of preeclampsia. Whereas the presence of a notch or bilateral notch in the waveform was not associated with preeclampsia we found a significant relationship of RI and PI MoM to preeclampsia at this time. However selection of a notch or RI or PI MoM at or above the 75th %ile as a positive predictor did not yield clinically useful sensitivity (43%) and specificity (67%) for predicting preeclampsia.
Previous studies reported that Doppler ultrasound performed at 11–14 weeks gestation (9) (10) has the best utility for prediction of delivery before 32 weeks gestation and if performed in the second trimester will identify those women who develop preeclampsia or IUGR (5) but particularly severe preeclampsia (11) or preeclampsia requiring delivery before 32 weeks gestation (6). In this low risk population, the presence of a notch or RI MoM at or above the 75%ile gave an odds ratio for developing early onset preeclampsia of 6.9 with a sensitivity of 78% and specificity of 66%. The presence of a notch or an RI MoM at or above the 75%ile had an odds ratio of 2.2 with a sensitivity of 53% specificity 66% for development of severe preeclampsia. The high negative predictive value for early onset and severe preeclampsia suggests Doppler ultrasound is useful as a rule out test. However the cost of screening large numbers of women to identify the small number who will subsequently develop early onset preeclampsia, for which we have no current treatment, brings the benefit into question. Overall our data show poor sensitivity of second trimester Doppler ultrasound measurements for prediction of preeclampsia overall in a well characterized low risk nulliparous population.
Doppler ultrasound demonstrates that uteroplacental impedance (12) and pulsatility index (13) are reduced with advancing gestational age in line with increasing uteroplacental blood flow. Inclusion of an early diastolic notch, present in 55% of patients at 11–14 weeks (9), to define abnormal flow velocity waveform improved the sensitivity for predicting preeclampsia, but while decreasing with gestational age (15–16) it may persist in 24% of patients at 24–26 weeks gestation (14) giving a remaining high false positive rate. Inclusion of a second screening ultrasound at 24 weeks to increase specificity (15) could identify women who subsequently develop preeclampsia or IUGR (5) particularly those requiring delivery before 32 weeks gestation (6). We found that 15% of women had a notch at <21 weeks gestation and with repeat Doppler at ≥21 weeks, approximately one third still displayed the notch suggesting uteroplacental impedance was still high. However, only 13% of these women subsequently developed preeclampsia. A recent meta-analysis and systematic review highlighted the heterogeneity of patient characteristics, timing of studies and clinical definitions used in previous Doppler ultrasound studies (16) and the consequent disparity in findings. This meta-analysis claimed that of second trimester parameters measured, increased pulsatility index with notching had the highest positive likelihood ratio, being 7.5, for development of preeclampsia in low risk patients (16). This contrasts with the positive likelihood ratio of 1.29 we found for RI or a notch for development of preeclampsia. The difference in findings from those previously reported may be due to the particular focus on a well defined low risk nulliparous population with no obvious pre-existing risk factors, the definitions of outcomes used and the heterogeneity of ethnicity in this population.
Preeclampsia is modeled as abnormal trophoblast invasion and development of the uteroplacental circulation leading to subsequent inflammation with maternal endothelial dysfunction (17). There is increasing evidence that there may be different phenotypes of preeclampsia (18) and indeed that early or late onset and mild or severe preeclampsia may have differing underlying pathophysiologies (19). Our data suggest that in this low risk population, abnormal development of the uterine vasculature has a stronger involvement in the development of early onset and severe preeclampsia than in late onset or mild preeclampsia.
Also women who are preeclamptic during pregnancy have an increased risk of developing cardiovascular disease later in life (3–4), suggesting they may have subclinical vascular disease prior to pregnancy, which the vascular stress test of pregnancy exposes as preeclampsia. Hence abnormal trophoblast invasion may not be the primary etiologic factor in all cases of preeclampsia and as shown here, Doppler interrogation of uteroplacental blood flow may not identify all women at risk of preeclampsia. Thus the technique has poor predictive power when applied to the overall population of low risk women. The need to perform large population-based studies evaluating multiple markers has been recently stressed (20) and may aid in identifying subgroups of at risk women. We have recently shown that despite evaluation of multiple first trimester clinical and biochemical parameters in this same low risk population, we were unable to identify an algorithm that could predict subsequent preeclampsia, only achieving a sensitivity of 46% (95% CI 38–54) for 80% specificity (21). Addition of the pulsatility index from this Doppler data to the best biomarkers identified (ADAM12, PlGF and PAPP-A) only yielded a sensitivity of 43% (95% CI 35–51) reinforcing the limited utility of Doppler measurements in predicting preeclampsia.
Acknowledgments
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD34208, HD27869, HD40485, HD40560, HD40544, HD34116, HD40512, HD21410, HD40545, HD40500, HD27915, HD34136, HD27860, HD53118, HD53097, HD27917, and HD36801]; the National Heart, Lung, and Blood Institute; and the National Center for Research Resources [M01 RR00080, UL1 RR024153, UL1 RR024989] and its contents do not necessarily represent the official view of NICHD, NHLBI, NCRR or NIH.
The authors thank Sabine Bousleiman, R.N.C., M.S.N. and Margaret Cotroneo, R.N., for participating in protocol development and coordination between clinical research centers; Elizabeth Thom, PhD, for protocol and data management and statistical analysis; and Jay D. Iams, M.D., Alan M. Peaceman, M.D., and Gail D. Pearson, M.D., Sc.D, for protocol development and oversight.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.World Health Organization. Estimates of maternal mortality: a new approach by WHO and UNICED. Geneva: World Health Organization; 1996. [Google Scholar]
- 2.Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455–63. [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007 Nov 10;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008 Nov;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 5.Papageorghiou AT, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2004 Jun;18(3):383–96. doi: 10.1016/j.bpobgyn.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol. 2001 Nov;18(5):441–9. doi: 10.1046/j.0960-7692.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010 Apr 8;362(14):1282–91. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep;44(3):837–45. [PubMed] [Google Scholar]
- 9.Martin AM, Bindra R, Curcio P, Cicero S, Nicolaides KH. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11–14 weeks of gestation. Ultrasound Obstet Gynecol. 2001 Dec;18(6):583–6. doi: 10.1046/j.0960-7692.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- 10.Parra M, Rodrigo R, Barja P, Bosco C, Fernandez V, Munoz H, et al. Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am J Obstet Gynecol. 2005 Oct;193(4):1486–91. doi: 10.1016/j.ajog.2005.02.109. [DOI] [PubMed] [Google Scholar]
- 11.Papageorghiou AT. Predicting and preventing pre-eclampsia-where to next? Ultrasound Obstet Gynecol. 2008 Apr;31(4):367–70. doi: 10.1002/uog.5320. [DOI] [PubMed] [Google Scholar]
- 12.Campbell S, Diaz-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Willson K, et al. New doppler technique for assessing uteroplacental blood flow. Lancet. 1983 Mar 26;1(8326 Pt 1):675–7. doi: 10.1016/s0140-6736(83)91970-0. [DOI] [PubMed] [Google Scholar]
- 13.Plasencia W, Maiz N, Poon L, Yu C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks and 21 + 0 to 24 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2008 Aug;32(2):138–46. doi: 10.1002/uog.5402. [DOI] [PubMed] [Google Scholar]
- 14.Fleischer A, Schulman H, Farmakides G, Bracero L, Grunfeld L, Rochelson B, et al. Uterine artery Doppler velocimetry in pregnant women with hypertension. Am J Obstet Gynecol. 1986 Apr;154(4):806–13. doi: 10.1016/0002-9378(86)90462-x. [DOI] [PubMed] [Google Scholar]
- 15.Bower S, Bewley S, Campbell S. Improved prediction of preeclampsia by two-stage screening of uterine arteries using the early diastolic notch and color Doppler imaging. Obstet Gynecol. 1993 Jul;82(1):78–83. [PubMed] [Google Scholar]
- 16.Cnossen JS, Morris RK, ter Riet G, Mol BW, van der Post JA, Coomarasamy A, et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ. 2008 Mar 11;178(6):701–11. doi: 10.1503/cmaj.070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009 Mar;30( Suppl A):S32–7. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myatt L, Carpenter L. Prediction of Preeclampsia. In: FL, MB, editors. Preeclampsia Etiology and Clinincal Practice. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 19.Roberts JM, Catov JM. Preeclampsia more than 1 disease: or is it? Hypertension. 2008 Apr;51(4):989–90. doi: 10.1161/HYPERTENSIONAHA.107.100248. [DOI] [PubMed] [Google Scholar]
- 20.Giguere Y, Charland M, Bujold E, Bernard N, Grenier S, Rousseau F, et al. Combining biochemical and ultrasonographic markers in predicting preeclampsia: a systematic review. Clin Chem. 2010 Mar;56(3):361–75. doi: 10.1373/clinchem.2009.134080. [DOI] [PubMed] [Google Scholar]
- 21.Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, et al. First-Trimester Prediction of Preeclampsia in Nulliparous Women at Low Risk. Obstet Gynecol. 2012 Jun;119(6):1234–42. doi: 10.1097/AOG.0b013e3182571669. [DOI] [PMC free article] [PubMed] [Google Scholar]