Quantitative monitoring of activity-dependent bulk endocytosis of synaptic vesicle membrane by fluorescent dextran imaging (original) (raw)
. Author manuscript; available in PMC: 2012 Sep 26.
Abstract
Activity-dependent bulk endocytosis (ADBE) is the dominant synaptic vesicle (SV) retrieval mode in central nerve terminals during periods of intense neuronal activity. Despite this fact there are very few real time assays that report the activity of this critical SV retrieval mode. In this paper we report a simple and quantitative assay of ADBE using uptake of large flourescent dextrans as fluid phase markers. We show that almost all dextran uptake occurs in nerve terminals, using co-localisation with the fluorescent probe FM1-43. We also demonstrate that accumulated dextran cannot be unloaded by neuronal stimulation, indicating its specific loading into bulk endosomes and not SVs. Quantification of dextran uptake was achieved by using thresholding analysis to count the number of loaded nerve terminals, since monitoring the average fluorescence intensity of these nerve terminals did not accurately report the extent of ADBE. Using this analysis we showed that dextran uptake occurs very soon after stimulation and that it does not persist when stimulation terminates. Thus we have devised a simple and quantitative method to monitor ADBE in living neurones, which will be ideal for real time screening of small molecule inhibitors of this key SV retrieval mode.
Keywords: Dextran, Endocytosis, Fluid phase, Synaptic vesicle, Fluorescence, FM1-43, Nerve terminal
1. Introduction
Neurotransmitter release is dependent on the fusion of small synaptic vesicles (SVs) with the neuronal plasma membrane. The maintenance of neurotransmitter release is dependent on the subsequent retrieval and recycling of fused SVs. There are at least three modes by which a SV can be internalised. Both clathrin-dependent endocytosis and kiss-and-run modes of retrieval internalise single SVs ([Edeling et al., 2006] and [Harata et al., 2006]) and are the dominant modes of SV retrieval during low intensity stimulation ([Granseth et al., 2006], [Zhang et al., 2009] and [Zhu et al., 2009]). However, during high intensity stimulation another SV endocytosis mode is triggered to increase the retrieval capacity within the nerve terminal, called activity-dependent bulk endocytosis (ADBE) (Cousin, 2009). ADBE is an activity-dependent fluid phase uptake mode that generates endosome-like structures direct from the plasma membrane. SVs can then bud from these endosomes to rejoin the recycling pool of SVs (Richards et al., 2000). Due to its large capacity, ADBE is the dominant SV retrieval mode in central nerve terminals during high intensity stimulation.
Fluorescence-based approaches have been predominantly employed to visualise SV recycling in neuronal culture, mainly due to the fact that it is difficult to directly measure either SV fusion or retrieval in a typical small central nerve terminal. The great majority of these methods utilise either the uptake of small fluorescent molecules (such as FM1-43, [Cochilla et al., 1999] and [Cousin and Robinson, 1999]) or the fusion of SV proteins to fluorescent proteins that report the pH of their immediate environment (Ryan, 2001). Unfortunately these methods do not differentiate between different SV retrieval modes such as clathrin-dependent endocytosis and ADBE. Therefore it is impossible to determine the contribution of either mode to SV retrieval during intense stimulation.
Because of the limitations in existing fluorescence approaches, we decided to establish a selective assay of ADBE, using dextran, a large inert fluid phase marker. Fluorescent-tagged dextrans are too large to be internalised within a single SV ([Berthiaume et al., 1995], [Araki et al., 1996], [Holt et al., 2003] and [Teng et al., 2007]). This means that any observed internalised fluorescence should be due to ADBE, since all other SV retrieval modes occur at the level of a single SV. We now report the development of a reliable, quantifiable and accurate method to monitor ADBE in a typical central nerve terminal in culture. The extent of ADBE was monitored by quantifying the number of nerve terminals loaded with dextran, rather than the fluorescence intensity of the nerve terminals themselves. This simple and efficient assay will allow the molecular mechanism of ADBE to be specifically monitored using both pharmacological and molecular technologies.
2.1. Materials
FM1-43, tetramethyrhodamine–dextran, penicillin/streptomycin, phosphate buffered salts, foetal calf serum and Minimal Essential Medium were obtained from Invitrogen (Paisley, UK). All other reagents were from Sigma (Poole, UK).
2.2. Primary culture of cerebellar granule neurones
Primary cultures of cerebellar granule neurones were prepared from the cerebella of 7-day old Sprague–Dawley rat pups as previously described (Tan et al., 2003). All experiments were performed on neuronal cultures between 8 and 10 days in vitro. In all experiments granule neurone cultures were removed from culture medium and repolarised in incubation medium (170 mM NaCl, 3.5 mM KCl, 0.4 mM KH2PO4, 20 mM TES (N-tris[hydroxy-methyl]-methyl-2-aminoethane-sulphonic acid), 5 mM NaHCO3, 5 mM glucose, 1.2 mM Na2SO4, 1.2 mM MgCl2, 1.3 mM CaCl2, pH 7.4) for 10 min before starting experiments.
2.3. Dextran internalisation protocol
Cultures were mounted in a Warner (Hamden, CT, USA) imaging chamber (RC-21BRFS) and SV turnover was stimulated by application of 50 mM KCl (50 mM NaCl removed to maintain osmolarity) for defined time periods. Tetramethyrhodamine–dextran (either 10 kDa or 40 kDa, both 50 μM) was present either during the stimulus followed by immediate washing, or absent during stimulation and then present for 2 min after stimulation. Cultures were imaged immediately after these experimental protocols had finished. Dextran uptake was visualised by exciting the cultures at 550 nm and gathering fluorescence emission at greater than 575 nm using a Nikon Diaphot epifluorescence microscope (Yokyo, Japan) and 20× air objective. In all experiments the gain and exposure settings were fixed. Fluorescent images were captured using a Hamamatsu (Hamamatsu City, Japan) Orca-ER CCD digital camera and processed using offline imaging software (Simple PCI, Compix Imaging Systems, USA). In all experiments 10 fields of view were captured for each coverslip of neurones and at least 3 coverslips were investigated for each experimental condition (30 fields of view in total).
2.4. Analysis of dextran uptake
Dextran uptake was analysed using two different approaches. Firstly the average fluorescence intensity of individual dextran puncta was calculated. This was achieved by selecting a defined region of interest and manually assigning cloned copies of the region to all labelled puncta in a field of view. For each field of view the average fluorescent intensity per region of interest was measured, then by combining all fields across coverslips for each condition the final average dextran fluorescence per puncta was determined. Second, the number of nerve terminals containing dextran puncta was determined. This was achieved by counting the number of fluorescent puncta in a defined field of view (130 μm × 130 μm). Thresholding analysis was performed using the Simple PCI software to discount regions that were too large to represent individual nerve terminals (diameter greater than 2 μm) and regions so small (less then 300 nm) that they represented background noise. This lower limit was selected since it is the resolution limit for fluorescence light microscopy (1.22λ/2 × N.A.). The number of dextran puncta in all 10 fields of view was averaged, and then these values were averaged between coverslips from the same experimental condition. To ensure the density of nerve terminals was consistent between experimental conditions, experiments were performed on the same set of cultures.
2.5. Co-localisation of dextran uptake with nerve terminals
Cultures were stimulated with elevated KCl (50 mM) for 2 min in the presence of either 10 kDa or 40 kDa tetramethyrhodamine–dextran (50 μM). Dextran was washed away immediately after stimulation and loaded dextran was imaged using excitation at 550 nm (emission of >575 nm). After a 10-min rest period cultures were loaded with FM1-43 (10 μM) that was co-applied during a 2-min KCl stimulation. Dye was washed away immediately after stimulation. Dye loading was visualised by excitation at 480 nm, while monitoring emission at greater than 510 nm. Co-localisation of dextran and FM1-43 puncta was achieved by overlaying captured images from both the dextran and FM1-43 loaded fields. The extent of co-localisation was determined by counting the number of dextran labelled puncta in a defined region, and then calculating the number of dextran puncta which corresponded to a FM1-43 labelled synapse and vice versa. These values were calculated and averaged across 3 fields of view from independent coverslips.
2.6. Unloading of dextran puncta from nerve terminals
Cultures were stimulated with elevated KCl (50 mM) for 2 min in the presence of either 10 kDa or 40 kDa tetramethyrhodamine–dextran (50 μM). Dextran was washed away immediately after stimulation and loaded dextran was imaged using excitation at 550 nm (emission of >575 nm). After a 30-min rest period cultures were stimulated for 2 min with elevated KCl (50 mM). A second image was then captured using the same imaging settings. The number of dextran puncta released by stimulation was calculated by counting the number of dextran labelled puncta in a defined region before and after stimulation. This value was averaged with others from identical experiments from 3 independent coverslips.
3. Results
3.1. Fluorescent dextrans are internalised in typical central nerve terminals in culture
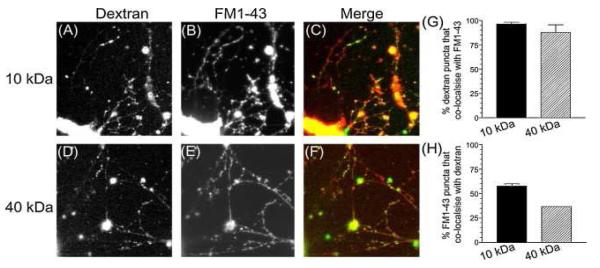
To confirm the specificity of dextran uptake as a marker of ADBE, we first determined whether uptake occurred at synaptic sites. Two different dextran sizes (10 kDa or 40 kDa tetramethyrhodamine–dextran) were chosen to determine the size of particle that could be accumulated by fluid phase bulk uptake. Cultures were stimulated with 50 mM KCl to evoke ADBE in the presence of either the 40 kDa or 10 kDa dextran. After washing, a punctate loading was observed for both dextrans (Fig. 1A and D). To determine whether these puncta corresponded to synaptic sites, the same cultures were stimulated in the presence of the small fluorescent dye FM1-43 to load all recycling membrane compartments. A characteristic punctate pattern of FM1-43 loading was observed after this protocol (Fig. 1B and E). The subcellular distribution of both accumulated dextran and FM1-43 was then compared by overlaying the images (Fig. 1C and F). There was a striking co-localisation of dextran puncta with FM1-43 for both 10 kDa and 40 kDa dextrans (Fig. 1G) illustrating that almost all dextran uptake occurred in nerve terminals. When the reciprocal analysis was performed, only a subset of nerve terminals were found to accumulate dextran (approximately 60% for 10 kDa dextran and 35% with 40 kDa dextran, Fig. 1H). Thus both 40 kDa and 10 kDa dextrans are only internalised by central nerve terminals during strong stimulation, however this uptake occurs in a distinct subpopulation of nerve terminals.
Fig. 1.
Large fluorescent dextrans are accumulated in nerve terminals. Granule neurone cultures were stimulated with elevated KCl (50 mM) for 2 min in the presence of 50 μM of either 10 kDa or 40 kDa tetramethyrhodamine–dextran. Dextran was washed away immediately after stimulation. After 10 min cultures were loaded with 10 μM FM1-43 using a 2-min stimulation with 50 mM KCl. Dye was washed away immediately after stimulation. Images show cultures loaded with either 10 kDa (A) or 40 kDa (D) dextran and then loaded with FM1-43 (B, for 10 kDa dextran or E for 40 kDa dextran). Merged images (C and F) show the overlay between dextran (green) and FM1-43 (red). Quantification of either the extent of dextran co-localisation with FM1-43 (G) or FM1-43 co-localisation with dextran (H) is displayed (±S.E.M., n = 3).
3.2. Fluorescent dextrans are not released after their internalisation
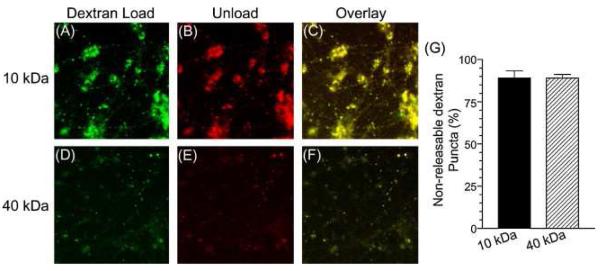
Having shown that both 10 kDa and 40 kDa dextrans could be internalised into nerve terminals, we next assessed whether they could also be released in a stimulation-dependent manner. A large component of stimulation-dependent unloading of either dextran would suggest a non-specific uptake into single SVs, since these will readily fuse with the plasma membrane on stimulation. Cultures were loaded with either 10 kDa or 40 kDa dextran using KCl stimulation and after a 30-min rest period were subjected to a KCl stimulus to attempt to release the marker. Images were acquired both before and after KCl application and then overlaid to determine the number of dextran puncta that disappeared during stimulation (Fig. 2). When this analysis was performed very few puncta disappeared, suggesting little release of either 10 kDa or 40 kDa dextran had occurred (Fig. 2G). This confirms that both dextrans were not internalised into SVs, since they would have been released by this stimulation protocol.
Fig. 2.
Large fluorescent dextrans are not released by nerve terminal stimulation. Granule neurone cultures were loaded with 50 μM of either 10 kDa (A–C) or 40 kDa tetramethyrhodamine–dextran (D–F) using elevated KCl (50 mM) for 2 min. Dextran was washed away immediately after stimulation. After 30 min cultures were stimulated with 50 mM KCl for 2 min. Images were captured either before (A and D) or after (B and E) the second KCl stimulation. Merged images (C and F) show an overlaid image of the field of view before (green) and after (red) stimulation. (G) Quantification of the number of dextran puncta that did not disappear with stimulation (±S.E.M., n = 3).
3.3. Fluorescent dextran uptake is rapid and does not occur after stimulation
Having confirmed that fluorescent dextrans can be internalised but not released during intense nerve terminal stimulation, we next determined the speed of their uptake. ADBE has traditionally been thought to be a slow process that persists for minutes after termination of stimulation ([Koenig and Ikeda, 1989], [Takei et al., 1996], [Gad et al., 1998] and [Richards et al., 2000]). However this view has been recently challenged, with a number of complementary studies demonstrating a rapid activation and inactivation that closely follows the depolarising stimulus ([Teng et al., 2007], [Wu and Wu, 2007] and [Clayton et al., 2008]).
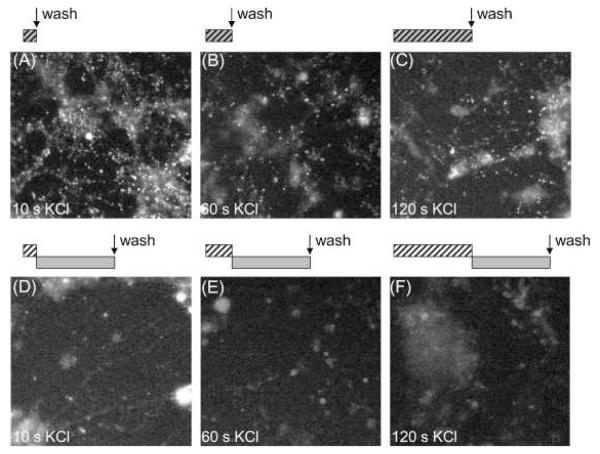
To determine the kinetics of dextran internalisation, we incubated cultures with the 40 kDa dextran and then stimulated for increasing periods of time with 50 mM KCl (Fig. 3A-C). A robust uptake of dextran was observed within 10 s of stimulation, confirming that ADBE is rapidly triggered in central nerve terminals (Fig. 3A). A comparable loading was also observed for all longer stimulation periods, suggesting that the majority of dextran uptake occurred within the first 10 s (Fig. 3B and C). To determine whether ADBE also persisted after termination of stimulation, a similar protocol was performed, however in this instance the 40 kDa dextran was applied for 2 min immediately after varying periods of KCl stimulation. A dramatic decrease in the extent of dextran loading was now observed at all time points (Fig. 3D-F), indicating that ADBE terminated co-incident with cessation of stimulation. Thus ADBE is triggered immediately by intense stimulation and does not persist when this stimulation ceases.
Fig. 3.
Large fluorescent dextrans are only accumulated during, not after, strong stimulation. Granule neurone cultures were loaded by co-applying 50 μM of 40 kDa tetramethyrhodamine–dextran with elevated KCl (50 mM) for increasing periods of time (A, 10 s; B, 60 s; C, 2 min) followed by immediate washing. Alternatively cultures were stimulated with elevated KCl (50 mM) for increasing periods of time (D, 10 s; E, 60 s; F, 2 min) followed by application of 50 μM dextran in the absence of stimulation for 2 min. The schematic located above the images illustrates the loading protocol – where hatched bars indicate KCl stimulation, and grey bars indicate dextran addition. Arrow indicates where dextran was washed away. Images displayed are representative fields of view for these stimulation conditions.
3.4. Quantification of activity-dependent dextran uptake
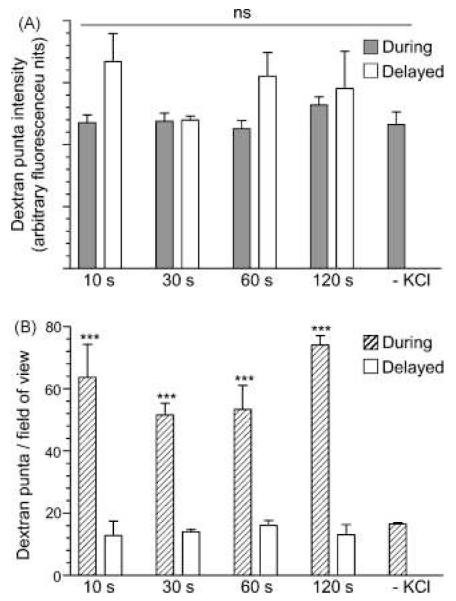
The differences in the extent of 40 kDa dextran uptake occurring either during or after stimulation are striking, however for this assay to be used as a readout of the extent of ADBE these differences have to be easily quantified. Quantification of the extent of loading of small extracellular probes such as FM1-43 is usually performed by determining the fluorescence intensity of visible puncta ([Cochilla et al., 1999] and [Cousin and Robinson, 1999]). This provides a reliable estimate of the number of SVs that have retrieved during the loading period, since the uptake of these molecules is quantal (Ryan et al., 1997). We performed a similar analysis to determine whether differences in the intensity of fluorescent dextran puncta were a reliable reporter of the extent of ADBE. To our surprise we observed no difference in the average fluorescence intensity of dextran puncta for any experimental condition (Fig. 4A). To highlight the fact that average fluorescence intensity does not accurately report the extent of ADBE, the few dextran puncta that were observed in the complete absence of stimulation had the same average intensity as those that were formed during stimulation (Fig. 4A). Therefore average intensity of individual dextran puncta do not accurately report the extent of ADBE in central nerve terminals. these stimulation conditions.
Fig. 4.
The number of dextran puncta, but not their fluorescence intensity reports the extent of ADBE. Granule neurone cultures were loaded with 50 μM of 40 kDa tetramethyrhodamine–dextran using the protocols described in Fig. 3. Cultures were also incubated with dextran for 2 min in the absence of stimulation (–KCl). (A) Average fluorescence intensity of puncta that were labelled with 40 kDa dextran either during stimulation (grey bars) or after stimulation (open bars). (B) Number of fluorescent puncta that occurred either during stimulation (hatched bars) or after stimulation (open bars). All experiments n = 3 ± S.E.M. One-way ANOVA, ***<0.001 between during and delayed for each KCl stimulation period; all other conditions > 0.05.
Since there is no correlation between the fluorescence intensity of dextran puncta and the extent of ADBE, we quantified dextran uptake using a different approach. Rather than measuring their fluorescence intensity, we instead determined the number of dextran puncta occurring within a defined region. We achieved this by using standard thresholding analysis software to discount any dextran puncta above 2 μm (which is too large for a single nerve terminal) and below 300 nm (which is likely to be noise, since it is at the resolution limit of fluorescence microscopy). This method of analysis now revealed striking differences between the number of dextran puncta in cultures that were exposed to dextran during stimulation, in comparison to after stimulation (Fig. 4B). For cultures incubated with the 40 kDa dextran during stimulation, a maximum number of puncta was observed within 10 s, confirming that the majority of ADBE occurred very soon after initiation of stimulation (Fig. 4B). Very little dextran uptake was observed in the absence of stimulation, indicating the great majority of the recorded puncta during stimulation came from internalised dextran. The lack of dextran uptake without stimulation also indicates that it cannot drive its own internalisation. In addition, the analysis highlighted that no dextran puncta were observed when the marker was applied after termination of stimulation, since the number of puncta was equivalent to those in cultures that had received no stimulation (Fig. 4B). Thus the extent of ADBE can be accurately quantified by calculating the number of nerve terminals that take up dextran but not by the average amount of dextran accumulated.
4. Discussion
We have developed a simple, quantitative assay of ADBE in primary neuronal culture using the uptake of large fluorescent dextrans. The assay accurately reports the extent of this SV retrieval mode by quantifying the number of nerve terminals undergoing ADBE, not the amount of ADBE within individual nerve terminals.
4.1. Dextran uptake selectively labels ADBE
The major hypothesis underlying the assay is that large inert dextrans will be excluded from retrieving SVs due to their size, but will be accumulated by ADBE as a fluid phase marker since this mode retrieves larger volumes of extracellular fluid. Evidence presented in this paper suggests that this is the case. Firstly, only a subpopulation of nerve terminals accumulates dextran in comparison to FM1-43. If dextran were labelling single SVs, then a much higher co-localisation between FM1-43 and dextran-containing nerve terminals would have been observed. Secondly, the great majority of accumulated dextran cannot be released by a subsequent stimulus. This suggests that dextran is not inside single SVs that are competent for fusion, in agreement with previous studies (Holt et al., 2003).
Theoretically both dextrans could fit inside a single SV. Assuming the dextrans are globular proteins, the approximate diameter of the 40 kDa dextran is 9 nm and the 10 kDa dextran is 5 nm (Lynch et al., 2008). Considering the lumen of a SV is approximately 24 nm in diameter (Zhang et al., 2009), at least one molecule of dextran could theoretically be accumulated during stimulation. However this is unlikely, since the loading concentration of dextran employed (50 μM) should be low enough not to bias non-specific fluid phase uptake into single SVs (volume accumulated by single SV − 7 × 10−211). This is exacerbated by the fact that dextrans are simple fluid phase markers and thus are not enriched on membranes for internalisation like FM1-43 ([Cochilla et al., 1999] and [Cousin and Robinson, 1999]), or are not actively clustered on membranes as cargo for clathrin-dependent endocytosis (Edeling et al., 2006). The same arguments also hold true for dextran accumulation into SVs that are budding from endosomes. Since dextran is not actively accumulated by ADBE, it will not be enriched within endosomes and therefore it is unlikely that it will be a internalised inside a budding SV.
The smaller 10 kDa dextran labels almost 60% of active nerve terminals, whereas the larger 40 kDa dextran only labels approximately 35%. At first glance this seems to suggest that the smaller dextran may partially label SVs, however its inability to be subsequently released strongly argues against this. In agreement with a selective labelling of ADBE by this smaller dextran, its loading follows an identical time course and pattern in response to trains of action potentials of differing intensity (data not shown) to that seen with 40 kDa dextran (Clayton et al., 2008). The disproportionality in loading between the dextrans is most likely explained by the fact that the 10 kDa dextran is accessible to the lumen of a formative bulk endosome for a longer period of time than the large dextran. For example, during both the formation and closure of the bulk invagination a stage should be reached where the smaller dextran will have access, but the 40 kDa will not. Since little is known about the morphology of nascent bulk endosomes, differential accessibility of dextran molecules could provide important real time information on both the dilation and closure of these structures.
4.2. Quantification of dextran uptake
Large fluorescent dextrans have been employed to monitor fluid phase uptake routes in non-neuronal cells for many years. However, only a few groups have attempted to monitor ADBE in neurones using the same approach ([Holt et al., 2003] and [Teng et al., 2007]). Dextran uptake was used to visualise fluid phase uptake in lizard neuromuscular junctions (Teng et al., 2007), however no quantification was attempted. ADBE was also monitored using dextran uptake in large bipolar nerve terminals (Holt et al., 2003). However neither of these studies examined the uptake of dextran in a typical small central nerve terminal.
The key finding during characterisation of the assay was that standard approaches previously used to quantify differences in fluorescence intensity of loaded puncta do not accurately report the obvious visual discrepancies in bulk dextran uptake. This is probably due to a combination of factors. Firstly, extensive morphological studies have shown that only a few bulk endosomes are formed in a small nerve terminal during strong stimulation (typically between 3 and 8 per nerve terminal; [Evans and Cousin, 2007], [Clayton et al., 2008] and [Clayton et al., 2009]). Secondly it is unlikely that many dextran molecules are accumulated into bulk endosomes even considering their larger size. Considering these two factors it is easy to see why the dynamic range of the assay is not large enough to accurately monitor differences in fluorescence intensity of individual puncta. Fluorescent intensity measurements have been used to estimate dextran uptake in bipolar nerve terminals (Holt et al., 2003), however this system had a far higher proportion of endosomes per nerve terminals (lower approximation 29 per nerve terminal) possibly providing a narrow but useable dynamic range.
Since monitoring the fluorescence intensity of individual nerve terminals did not provide an accurate quantification of ADBE, we instead monitored the number of nerve terminals that accumulated dextran within a defined region. Once thresholding analysis was performed to discount puncta that were too large to be nerve terminals and small enough to be discounted as noise, we obtained an accurate estimation of the extent of ADBE throughout the neuronal culture. This method of quantification is robust and has recently been employed to monitor both the physiology and molecular mechanism of ADBE. For example, this approach was used to monitor the activity-dependent triggering of ADBE by different trains of action potentials (Clayton et al., 2008). In addition it has been used to quantitatively measure inhibition of ADBE by a range of pharmacological antagonists (Clayton et al., 2009). Importantly the results obtained from these dextran uptake assays were independently corroborated by morphological analysis at the level of the single nerve terminal using the fluid phase marker horse radish peroxidase ([Clayton et al., 2008] and [Clayton et al., 2009]).
A potential drawback of the assay is that it does not differentiate between internalised and non-internalised dextran. We are confident that the majority of the signal we observe is due to internalised probe, since very dextran puncta are observed in the absence of stimulation. To overcome this problem we are currently attempting to conjugate dextran to the pH-sensitive dye CypHer5E which only fluoresces in acidic environments (Adie et al., 2002). This should increase the dynamic range of the assay further by only reporting signal from dextran accumulated inside acidic endosomes.
Since this dextran uptake assay has an extremely simple protocol and post-hoc analysis, it should easily translated into a high throughput assay system. This would allow the screening of small molecule inhibitors with potential effects on ADBE. Alternatively the assay can also be translated into a high-resolution technique, allowing investigations of ADBE at the level of the single neurone. This has recently been achieved using shRNA silencing of syndapin expression in primary neuronal culture (Clayton et al., 2009). In this assay the number of dextran puncta in individual transfected neurones were calculated to estimate the inhibition of ADBE during syndapin knockdown. Thus this simple assay has a number of potential applications to investigate both the physiology and molecular mechanism of ADBE, a key retrieval mode for the nerve terminal during intense neuronal activity.
Acknowledgements
This work was supported by grants from the Wellcome Trust (Ref: 070569 & 084277) and Epilepsy Research UK (0503).
Abbreviations
SV
synaptic vesicle
ADBE
activity-dependent bulk endocytosis
References
- Adie EJ, Kalinka S, Smith L, Francis MJ, Marenghi A, Cooper ME, Briggs M, Michael NP, Milligan G, Game S. A pH-sensitive fluor, CypHer 5, used to monitor agonist-induced G protein-coupled receptor internalization in live cells. BioTechniques. 2002;33:1152–1157. doi: 10.2144/02335dd10. [DOI] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume EP, Medina C, Swanson JA. Molecular size-fractionation during endocytosis in macrophages. J Cell Biol. 1995;129:989–998. doi: 10.1083/jcb.129.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Anggono V, Smillie KJ, Chau N, Robinson PJ, Cousin MA. The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. J Neurosci. 2009;29:7706–7717. doi: 10.1523/JNEUROSCI.1976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Evans GJ, Cousin MA. Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J Neurosci. 2008;28:6627–6632. doi: 10.1523/JNEUROSCI.1445-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Cousin MA. Activity-dependent bulk synaptic vesicle endocytosis—a fast, high capacity membrane retrieval mechanism. Mol Neurobiol. 2009;39:185–189. doi: 10.1007/s12035-009-8062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Mechanisms of synaptic vesicle recycling illuminated by fluorescent dyes. J Neurochem. 1999;73:2227–2239. doi: 10.1046/j.1471-4159.1999.0732227.x. [DOI] [PubMed] [Google Scholar]
- Edeling MA, Smith C, Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat Rev Mol Cell Biol. 2006;7:32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- Evans GJ, Cousin MA. Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5. J Neurosci. 2007;27:401–411. doi: 10.1523/JNEUROSCI.3809-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Harata NC, Aravanis AM, Tsien RW. Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. J Neurochem. 2006;97:1546–1570. doi: 10.1111/j.1471-4159.2006.03987.x. [DOI] [PubMed] [Google Scholar]
- Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci. 2003;23:1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KL, Gerona RR, Kielar DM, Martens S, McMahon HT, Martin TF. Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Mol Biol Cell. 2008;19:5093–5103. doi: 10.1091/mbc.E08-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Ryan TA. Presynaptic imaging techniques. Curr Opin Neurobiol. 2001;11:544–549. doi: 10.1016/s0959-4388(00)00247-6. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Smith SJ. Optical detection of a quantal presynaptic membrane turnover. Nature. 1997;388:478–482. doi: 10.1038/41335. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Teng H, Lin MY, Wilkinson RS. Macroendocytosis and endosome processing in snake motor boutons. J Physiol. 2007;582:243–262. doi: 10.1113/jphysiol.2007.130989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Wu LG. Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse. Proc Natl Acad Sci USA. 2007;104:10234–10239. doi: 10.1073/pnas.0611512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Heinemann SF. Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron. 2009;61:397–411. doi: 10.1016/j.neuron.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]