Adoptive Cell Therapy for the Treatment of Patients with Metastatic Melanoma (original) (raw)
. Author manuscript; available in PMC: 2012 Sep 27.
Published in final edited form as: Curr Opin Immunol. 2009 Mar 21;21(2):233–240. doi: 10.1016/j.coi.2009.03.002
Abstract
Adoptive cell therapy (ACT) is the best available treatment for patients with metastatic melanoma. In a recent series of three consecutive clinical trials using increasing lymphodepletion prior to infusion of autologous tumor infiltrating lymphocytes (TIL), objective response rates between 49% and 72% were seen. Persistence of infused cells in the circulation at one month was highly correlated with anti-tumor response as was the mean telomere length of the cells infused and the number of CD8+ CD27+ cells infused. Responses occur at all sites and appear to be durable with many patients in ongoing response beyond three years. In the most recent trial of 25 patients receiving maximum lymphodepletion, seven of the 25 patients (28%) achieved a complete response. Of the 12 patients in the three trials who achieved a complete response all but one are ongong between 18 and 75 months. We recently demonstrated that ACT using autologous lymphocytes genetically modified to express anti-tumor T cell receptors can mediate tumor regression and this approach is now being applied to patients with common epithelial cancers.
Introduction
Melanoma appears to be unique among human cancers because of its ability to induce significant numbers of lymphocytes with anti-tumor activity during the natural course of tumor growth1. Thus, tumor infiltrating lymphocytes (TIL) or peripheral lymphocytes repeatedly stimulated in vitro with autologous melanoma cells often demonstrate in vitro recognition of melanoma cells based on assays of lysis or cytokine secretion. The ability to isolate and characterize anti-tumor lymphocytes from patients with melanoma has thus enabled the identification and characterization of multiple melanoma associated antigens that can be the target of immunotherapy2,3. The development of techniques to grow large numbers of anti-tumor lymphocytes has enabled the development of cell transfer therapies that are by far, the most effective treatment for patients with metastatic melanoma4–6.
Effective cancer immunotherapy is dependent on the presence of large numbers of anti-tumor lymphocytes with appropriate homing and effector functions that enable them to seek out and destroy cancer cells in vivo. Adoptive cell therapy (ACT) refers to an immunotherapy approach in which anti-tumor lymphocytes are identified and grown ex vivo and then infused into the cancer patient, often along with vaccines or growth factors that can augment the in vivo impact of the transferred cells6. Since ACT involves the administration of ex vivo cultured lymphocytes, very large numbers of anti-tumor lymphocytes can be generated and infused. The ability to test the activity of cells prior to infusion enables the identification of highly selected cells with high avidity for tumor antigens. Naturally occurring anti-tumor lymphocytes are often anergized or tolerized in vivo and thus the ability to culture these cells ex vivo away from suppressive influences that exist in vivo enables the administration of highly activated cells that exhibit multiple anti-tumor effector functions. Perhaps the most important factor responsible for the effectiveness of ACT is the ability to manipulate the host prior to cell transfer to provide an improved environment for the transferred cells. A variety of immunosuppressive influences can exist in the cancer patient including the presence of lymphocytes or myeloid cells with immunosuppressive activity. These suppressive factors may play a role in limiting the anti-tumor effectiveness of non-specific immune stimulants such as interleukin-2 (IL-2) and cancer vaccines are ineffective probably due in part to the presence of these suppressive influences7. This ability to remove lymphoid and myeloid suppressor cells prior to immune stimulation is unique to ACT as a form of immunotherapy.
It should be emphasized that although melanoma is unique in its ability to naturally result in the generation of anti-tumor T cells in vivo, there does not appear to be any difference in the susceptibility of different cancer types to the anti-tumor activity of lymphocytes. Virtually all tumors are equally susceptible to lysis and are able to stimulate cytokine release from anti-tumor lymphocytes when tumor antigen is encountered. Thus principles that are being learned concerning the effectiveness of ACT in patients with melanoma can be of value in applying ACT therapy to patients with other cancer types using lymphocytes genetically engineered to manifest anti-tumor function6,8.
Early clinical studies of ACT for melanoma
A critical step in the development of effective ACT for the treatment of patients with melanoma was the demonstration in 1987 that lymphocytes infiltrating into metastatic melanoma deposits could be grown in interleukin-2 (IL-2) and exhibit major histocompatability complex (MHC) restricted recognition of both fresh and cultured melanoma cells1. Utilizing current techniques reactivity against melanoma can be detected in TIL from approximately 80% of melanoma patients9,10. The first use of TIL to treat patients with metastatic melanoma was reported in 1988 and increased numbers of patients were reported in 199411,12. Eighty-six patients were treated with autologous TIL plus high dose IL-2. Twenty-eight patients received cells without a preparative regimen and 58 patients received a preinfusion dose of 25mg/kg cyclophosphamide. IL-2 was given at 720,000IU/kg every eight hours to tolerance13. Over 80% of patients received >1011 cells. Objective response rates in patients with metastatic melanoma treated with cells alone or with this low dose cyclophosphamide were 31% and 35% respectively. Objective responses were seen in 34% of patients who had not received prior IL-2 and in 32% of patients who were refractory to prior treatment with IL-2. In these initial trials single cell suspensions were generated from tumors using enzymatic digestion and grown in IL-2 until sufficient cell numbers were generated. Cells were administered irrespective of their anti-tumor activity as measured by in vitro assays although analysis of cell activity demonstrated a significant correlation between clinical response and the in vitro lysis of autologous tumor by the administered TIL14 (p=0.0008). Objective clinical responses were also associated with cells that were cultured for shorter periods of time and that had shorter doubling times (p=0.0001 and 0.03 respectively) 15. A subgroup of patients received cells that were labeled with indium-111 and traffic of the administered cells to tumor deposits also correlated with clinical response (p=0.022) 16–18. Although objective anti-tumor responses were seen in these early trials many of the responses were short-lived. Of the 29 patients who experienced an objective clinical response only five were complete and the median duration of all partial responders was only four months. Analysis of the survival of the transferred cells in vivo using cells genetically labeled with neomycin phosphotransferase demonstrated a lack of persistence of the infused cells19. Fewer than 0.1% of the cells in the circulation at one week following infusion were the transferred cells. These early studies, however, demonstrated the potential value of ACT and modifications of this approach have led to dramatic improvement in its effectiveness4–6.
ACT following lymphodepletion in patients with melanoma
Early animal models predicted that the effectiveness of cell transfer therapies could be improved by administering either total body irradiation or lymphodepleting chemotherapy prior to the cell transfer. The limited persistence of the transferred cells in our early human trials thus led us to explore the use of lymphodepletion in patients with metastatic melanoma prior to receiving ACT with TIL. A series of clinical trials have been performed in a total of 93 patients with metastatic melanoma exploring the use of increasing levels of lymphodepletion4–6,20. In the conduct of these recent trials a change was made in the procedures used to generate TIL for cell transfer. In the earlier trials entire excised tumors were subjected to enzymatic digestion to form a single cell suspension which was then cultured in 6,000IU/ml IL-2. Lymphocytes infiltrating into the tumor stroma grew and after 2–3 weeks, cultures were generally cleared of tumor cells and lymphocytes were continued in culture until the target number of cells was obtained. In the earlier trials these entire populations of TIL were administered without further selection. In the current trial a modified procedure was utilized10. Excised tumors were minced into tiny fragments and individual fragments placed in the wells of a 24 well culture plate, or alternatively limited numbers of cells from a single cell suspension were put in individual wells of a 24 well plate. All wells then were individually grown and separately tested for their ability to recognize either the autologous tumor or tumors sharing MHC antigens. Individual cultures showing appropriate reactivity were then further expanded, generally to a total of 1010 to 1011 cells before they were infused into patients. Although more labor intensive, this latter technique had the advantage of identifying cultures with anti-tumor activity but potentially had the disadvantage of limiting the heterogeneity or polyclonality of the cells administered21,22.
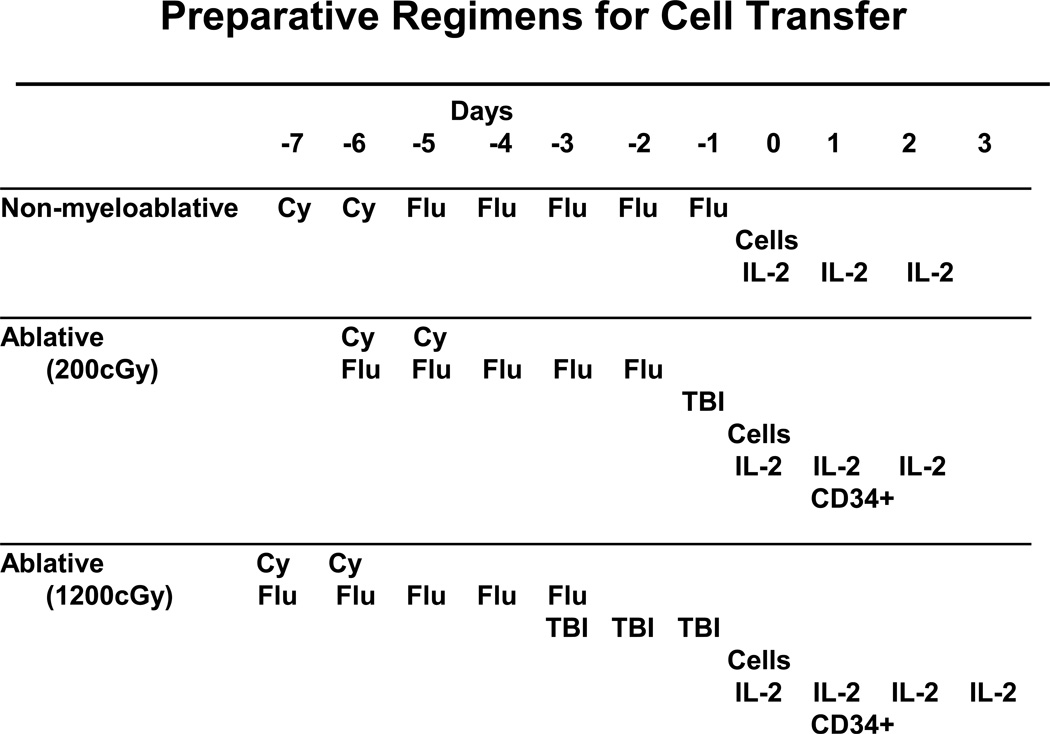
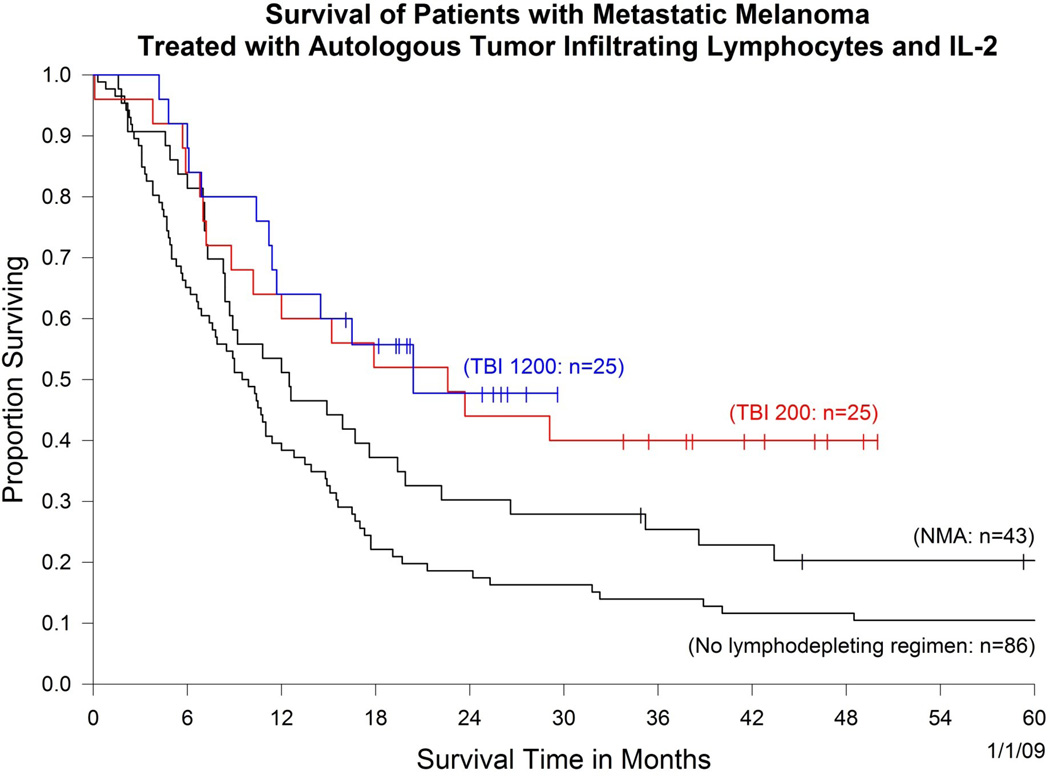
Utilizing this new method of cell preparation, three consecutive protocols were performed using increasing levels of lymphodepletion (Figure 1). In the first protocol, 43 patients were treated with autologous lymphocytes following the administration of a non-myeloablative chemotherapy regimen consisting of 60 mg/kg cyclophosphamide given on two consecutive days followed by five days of 25mg/m2 fludarabine. A second trial was then conducted in 25 patients in which the same chemotherapy was given (but condensed to a five day period) followed by 200cGy whole body irradiation the day before cell administration. In a third trial in 25 patients the total body irradiation was intensified by giving 200cGy twice a day for three consecutive days for a total of 1200cGy. In the latter two trials, circulating CD34+ hematopoietic stem cells were administered. In all protocols 720,000IU/kg IL-2 was administered to tolerance. The objective response rates by RECIST criteria in the three sequential protocols were 49%, 52% and 72% respectively (Table 1). Responses were often durable and many patients have ongoing responses beyond three years, and in the earliest trial beyond five years. All but one of the 12 complete responses are ongoing from 18 to 75 months. Objective cancer regressions were seen at all sites in the body including lung, liver, brain, lymph nodes, subcutaneous tissues and bone. Examples of these responses are shown in Figure 2. The overall survival of patients in these three trials is shown in Figure 3 which also includes the survival of the original 86 patients who received T cell transfer without major lymphodepletion. It should be emphasized that these clinical trials are consecutive rather than randomized and thus, although it appears that increasing lymphodepletion has led to improvement in survival, this conclusion must be drawn with caution.
Figure 1.
Schema of the lymphodepleting preparative regimens used in the adoptive cell transfer protocols in the Surgery Branch, NCI.
Table 1.
Cell Transfer Therapy*
| (1/1/09) | ||||
|---|---|---|---|---|
| Treatment | Total | PR | CR | OR (%) |
| No TBI | 43 | 17 | 3 | 21 (49%) |
| (77+,45+,34+,2928,14,13,11,8,8,7,4,3,3,2,2,2) | (75+,70+,60+,59+) | |||
| 200 cGy TBI | 25 | 11 | 2 | 13 (52%) |
| (45+.41+.35+.1410,6,5,5,4,3,3) | (49+,38+) | |||
| 1200 cGy TBI | 25 | 11 | 7 | 18 (72%) |
| (26+,19+,19+,19+,13,7,6,6,5,4,3) | (29+,19,25+,25+,19+,19+,18+) |
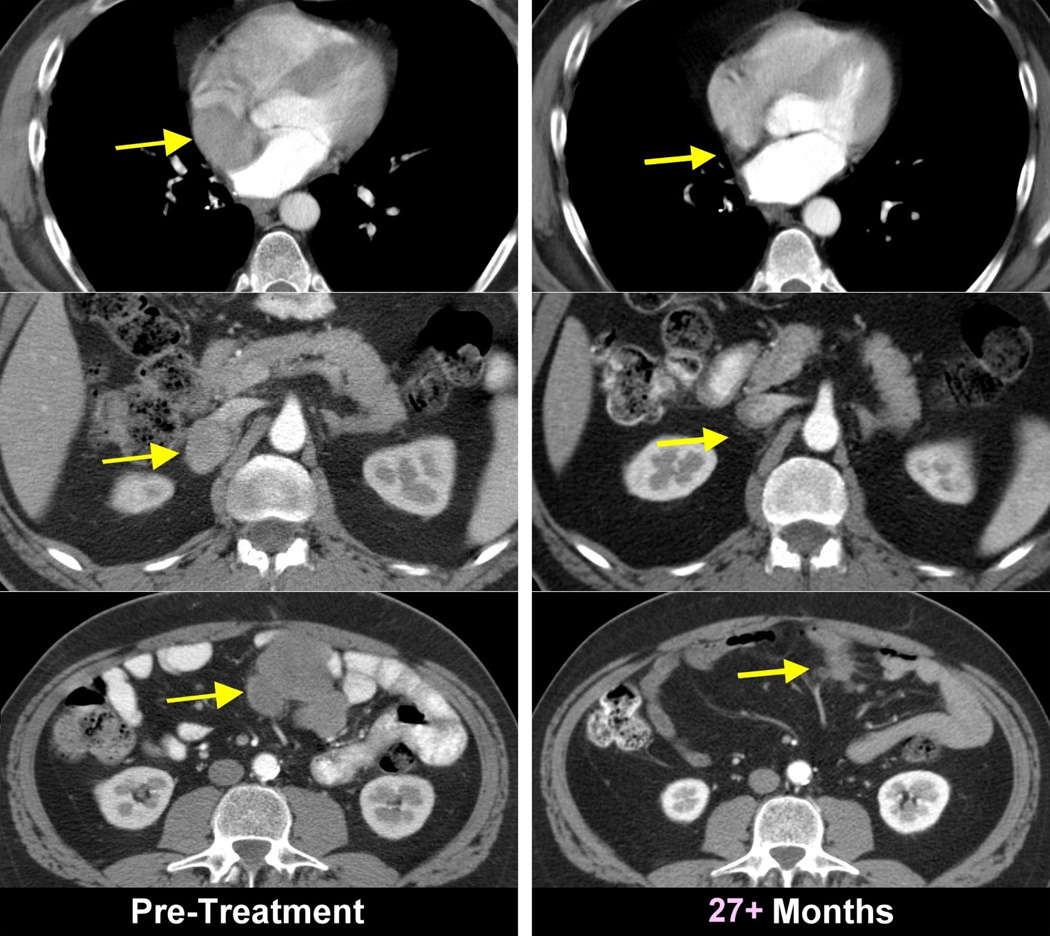
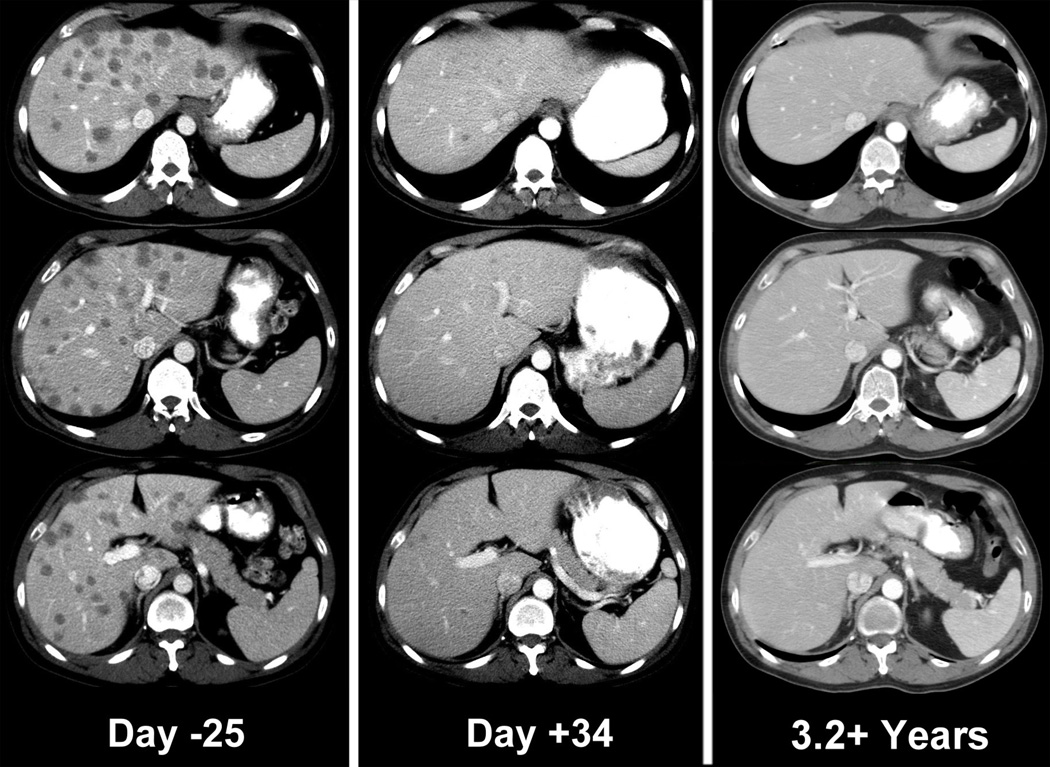
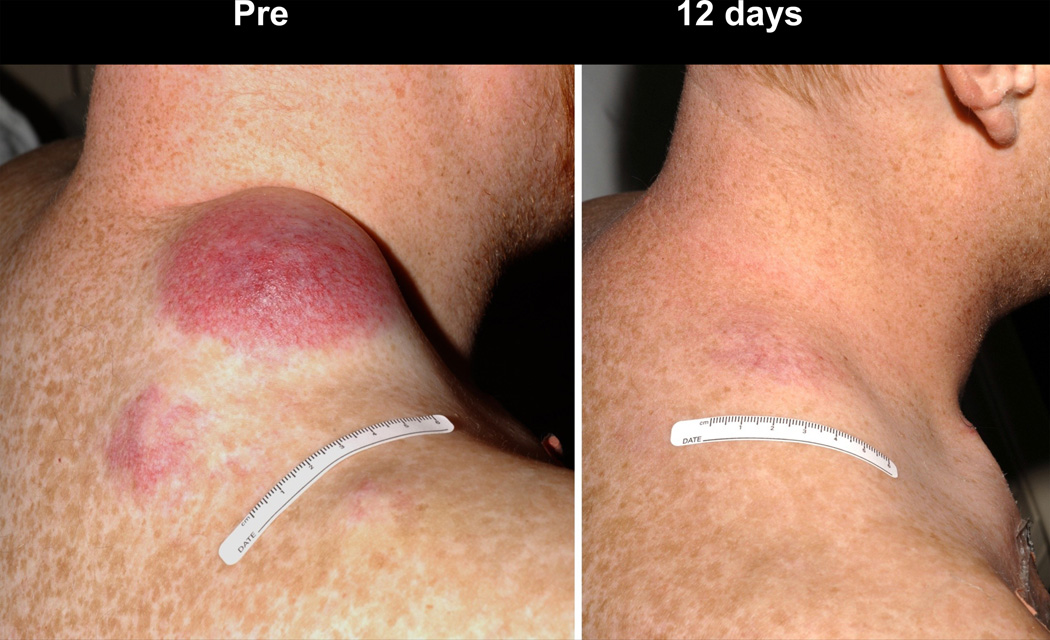
Figure 2.
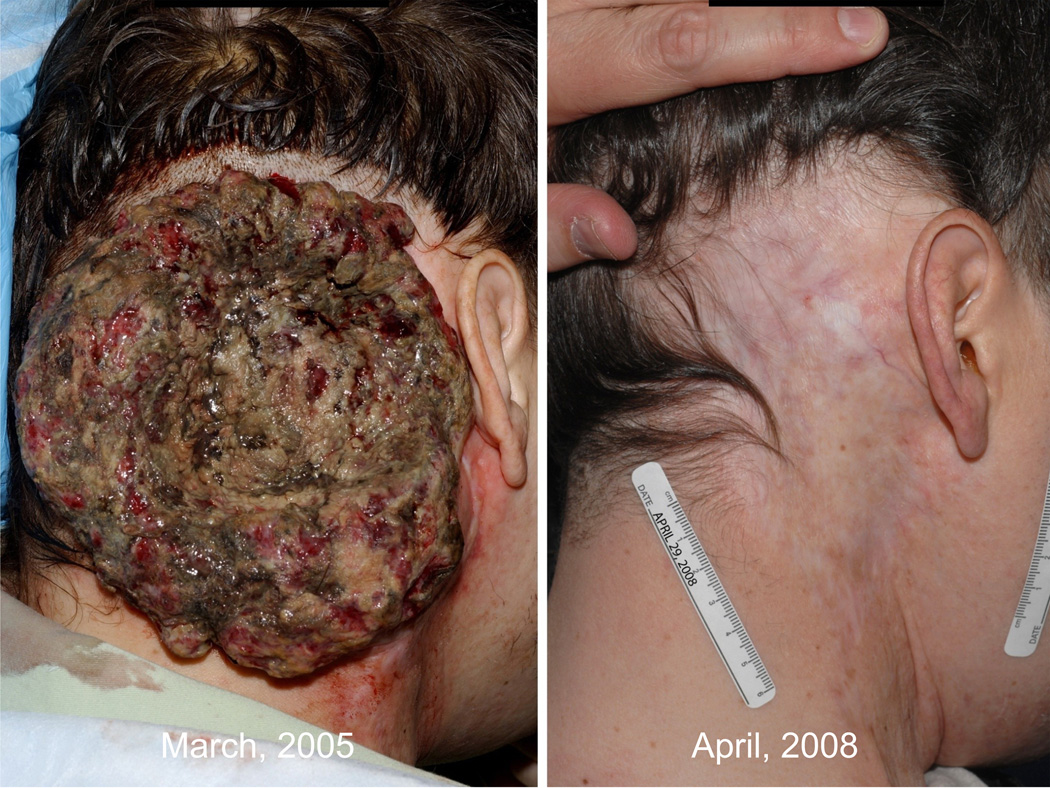
Objective clinical regressions in patients with metastatic melanoma treated with cell transfer therapy. A. Regression of melanoma metastases in the heart (upper), adrenal (middle) and peritoneal cavity (lower) now ongoing at 34 months in a 53 year old male. B. Regression of multiple liver metastases now ongoing at 60 months in a 45 year old male. C. Rapid regression of multiple subcutaneous and nodal metastases now ongoing at 35 months in a 29 year old male. D. Regression of a large fungating scalp mass now ongoing at 34 months in a 40 year old male.
Figure 3.
Survival of patients treated with cell transfer therapy in four consecutive clinical trials using increasing regimens of a lymphodepleting preparative regimen prior to adoptive cell transfer (NMA, non-myeloablative chemotherapy; TBI, total body irradiation). The number in parentheses is the number of patients in each trial.
These studies have shown that T cell based immunotherapy is capable of mediating the regression of large vascularized invasive metastatic melanoma in humans. It should be emphasized that the widely held belief that immunotherapy can only affect minimal disease in the adjuvant setting is thus not correct.
Mechanisms of action of ACT in humans
Extensive studies of the mechanisms of action of ACT in humans have been performed. Based on animal models it appears that the lymphopenic environment acts predominantly by eliminating T regulatory cells and by eliminating competition for homeostatic cytokines such as IL-7 and IL-15 that are vital for T cell survival23–25. Other factors may be involved as well such as the impact of chemotherapy and whole body irradiation on increasing extravasation of bacteria from the intestinal tract and thus result in toll like receptor stimulation26. Evidence for a role of IL-15 in our ACT clinical trials comes from studies demonstrating that there are no circulating levels of IL-15 detectable in the serum of patients prior to lymphodepleting chemoradiation but high levels exist in all patients at the time of cell infusion following the chemoradiation5.
Extensive studies have been performed to identify both patient and cell factors that are associated with objective clinical responses. Persistence of the infused cells was highly associated with objective response (p>0.001) 22,27. In contrast to prior studies in which the persistence of the administered T cells was very limited, many patients in the current trials exhibited high levels of persistence, sometimes reaching up to 75% of all of the circulating CD8+ cells. It was unusual for patients to exhibit a response unless there was some persistence of the administered cells although persistence alone was not a sufficient criteria to ensure responsiveness. The median percent of the administered cells in PBMC at 1–2 months was 18.5% in responders compared to only 1% in non-responders.
Studies in the pmel mouse model predicted that the state of differentiation of the transferred cells was inversely related to the effectiveness of these cells in ACT28,29. Our studies have suggested that this is the case in humans as well. Two cell characteristics that appear to correlate with the ability of cells to mediate an anti-tumor response are long telomere length (p<0.01) and cells that highly express CD27 (p<0.0001)30,31. Both are markers of less differentiated cells. The response rate was 11% when mean telomere length was less than 5kb which contrasted with a 58% response rate in patients who received cells with mean telomere greater than 5kb.
Improvements in ACT that are being studied
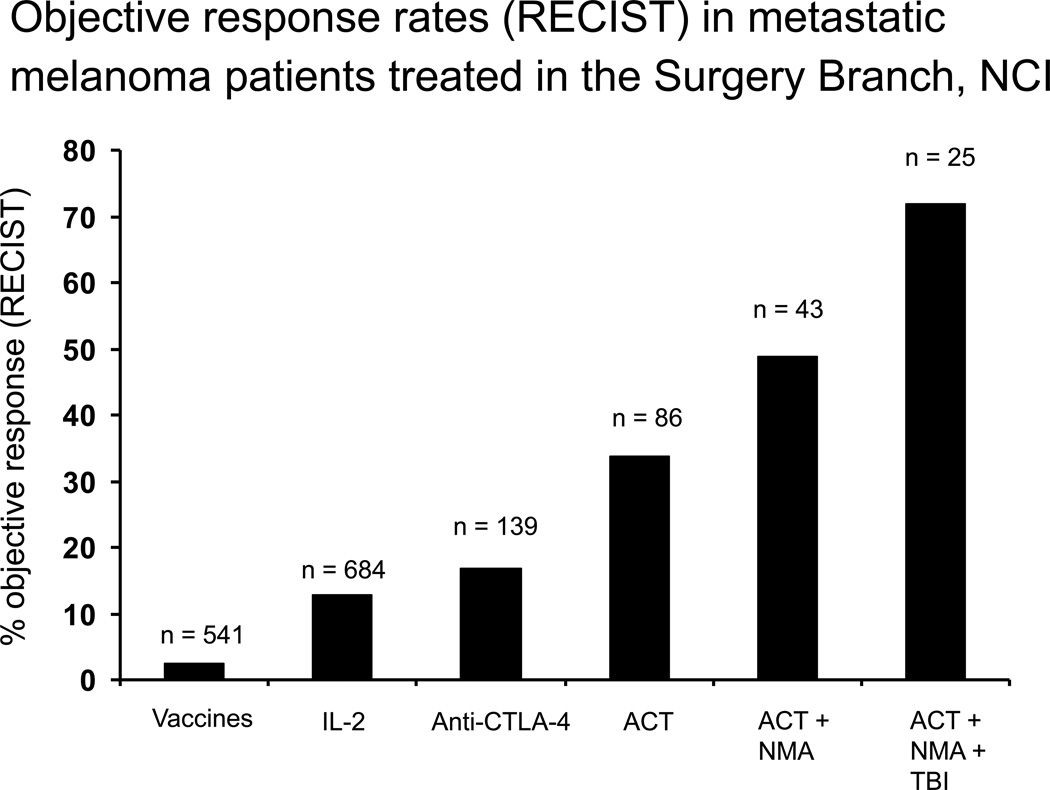
The 49–72% objective response rates in patients with metastatic melanoma indicate that ACT represents the best available treatment for patients with this disease4–6. A summary of Surgery Branch, NCI immunotherapy efforts for the treatment of patients with metastatic melanoma is shown in Figure 4. Objective response rates to treatment vaccines are about 3%, IL-2 and anti CTLA-4 about 15%, and ACT has increased response rates to 72% with increasing lymphodepletion prior to cell transfer.
Figure 4.
Objective response rates using RECIST criteria in patients with metastatic melanoma treated in the Surgery Branch, NCI using different therapeutic strategies. Overall response rates in patients treated with vaccines is about 3% and with IL-2 or anti-CTLA4 is about 15%. With increasing levels of lymphodepletion, adding total body irradiation (TBI) and nonmyeloablative chemotherapy (NMA) to adoptive cell transfer (ACT) can achieve response rates as high as 72%.
Efforts to both improve ACT treatment as well as simplify it to make more widely available are underway. Simplified techniques for generating very “young” TIL have been developed32. The use of low dose outpatient IL-2 (125,000IU/kg qd) 33 is also being explored as a method to potentially reduce some of the cytokine related toxicities seen with the use of high dose IL-2.
Several approaches that may enhance the effectiveness of ACT in humans are summarized in Table 2. Perhaps the single most important factor yet to be explored is the addition of vaccines to the treatment regimen. In animal models the administration of a vaccine encoding the antigen recognized by the transferred cells substantially improves the anti-tumor efficacy of treatment34. This represents a high priority for testing in the human.
Table 2.
Opportunities for Improving ACT for the Treatment of Human Cancer*
| Approach | Selected Examples |
|---|---|
| 1. Genetic modification of lymphocytes to introduce new recognition specificities | α-β TCRchimeric TCR |
| 2. Genetic modification of lymphocytes to alter function functions of T cells | costimulatory molecules (CD8, 41BB)cytokines (IL-2, IL-12, IL-15)homing molecules (CD62L, CCR7)prevention of apoptosis (Bcl-2) |
| 3. Modify host lymphodepletion | selective depletion of CD4+ cells or T regulatory cells |
| 4. Block inhibitory signals on reactive lymphocytes | antibodies to CTLA-4 or PD-1 |
| 5. Administer vaccines to stimulate transferred cells | recombinant virus, peptides, dendritic cells |
| 6. Administer alternative cytokines to support cell growth | IL-15, IL-21, IL-12 |
| 7. Stimulate antigen presenting cells | toll-like receptor agonists |
| 8. Generate less differentiated lymphocytes | select effector memory cells; alter growth promoting cytokines in vitro |
| 9. Overcome antigen escape variants | NK cells |
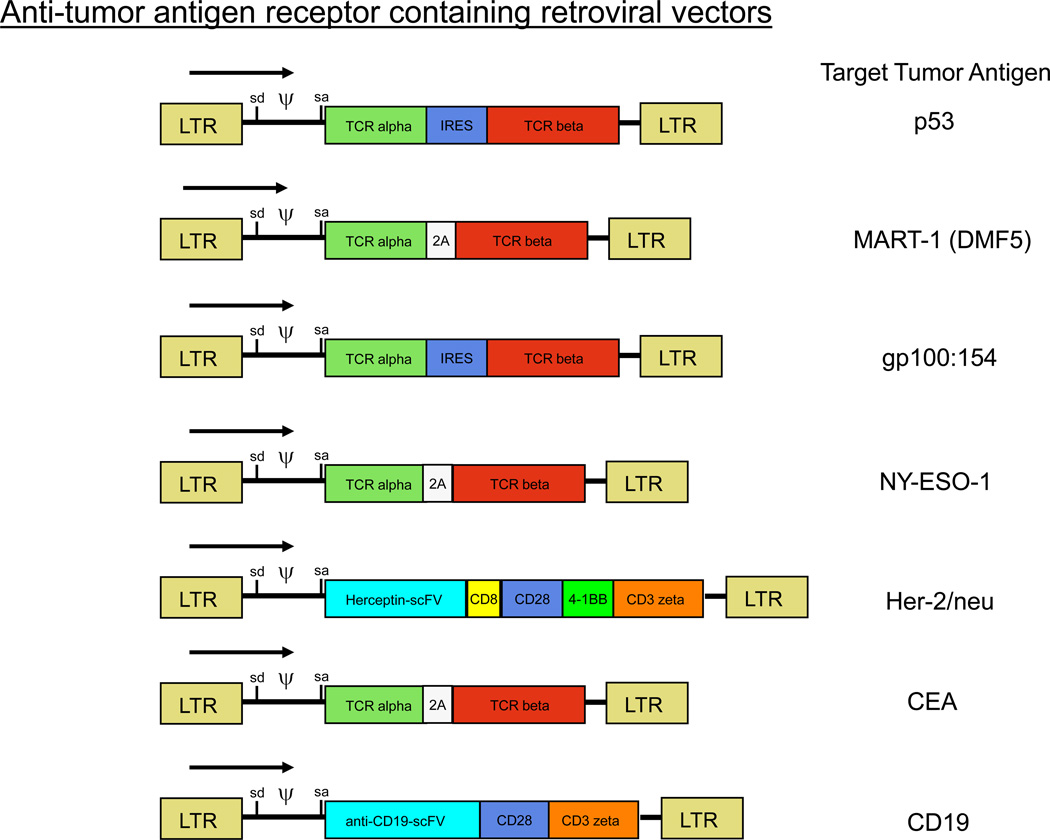
Some patients with metastatic melanoma do not have easily resectable lesions that can give rise to TIL or have lesions with too low a number of infiltrating lymphocytes to provide TIL for therapy. These considerations led us to perform trials exploring the genetic modification of autologous lymphocytes with genes encoding anti-tumor T cell receptors6. In an initial clinical trial, circulating lymphocytes from patients with melanoma were transduced with retroviruses encoding an anti-MART-1 T cell receptor8,35. These lymphocytes were administered to patients following the cyclophosphamide/fludarabine non-myeloablative chemotherapy. In our initial report two of 17 patients responded to this therapy both of whom are disease free three years later. Retroviruses have now been constructed encoding T cell receptors with higher avidity for recognition of melanoma antigens and those are now being evaluated in a clinical trial36. T cell receptors that recognize antigenic epitopes from p53, gp100, carcinoembryonic antigen and the cancer-testes antigen, NY-ESO-1 are being clinically tested as well (Figure 4) 37–39. In addition, chimeric antigen receptors have been constructed utilizing the variable region of the heavy and light chains of monoclonal antibodies against Her2/neu and against CD19 (Figure 5). These chimeric receptors utilize signaling chains from CD28, 41BB and CD3 zeta and when retrovirally inserted into normal lymphocytes can confer the recognition capability of these antibodies40. The ability to convert normal lymphocytes into cells with anti-tumor activity has the potential to extend ACT to patients with common cancers and these clinical trials have begun.
Figure 5.
Schematic diagrams of the structure of retroviruses encoding conventional T cell receptors and chimeric T cell receptors prepared for use in adoptive cell transfer trials.
References
- 1.Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J. Immunol. 1987;138:989–995. [PubMed] [Google Scholar]
- 2.Van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with anti-tumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. Three lymphodepleting regimens were combined with tumor reactive TIL administration and compared for safety and efficacy in sequential ACT clinical trials. Overall 52 of 93 patients (56%) experienced an objective response
- 6.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. This is the first study to show that patients can be successfully treated with lymphocytes engineered by retroviral transduction to express a new TCR conferring recognition of a tumor antigen. Tumor reactive lymphocytes were shown to persist after transfer by PCR and tetramer FACS analysis, and two patients responded to the cell and gene therapy
- 9.Topalian SL, Muul LM, Rosenberg SA. Growth and immunologic characteristics of lymphocytes infiltrating human tumor. Surgical Forum. 1986;37:390–391. [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J. Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, et al. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. Preliminary report. N. Engl.J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SA, et al. Treatment of patients with metastatic melanoma using autologous tumor-infiltrating lymphocytes and interleukin-2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 14.Aebersold P, et al. Lysis of autologous melanoma cells by tumor infiltrating lymphocytes: Association with clinical response. J. Natl. Cancer Inst. 1991;13:932–937. doi: 10.1093/jnci/83.13.932. [DOI] [PubMed] [Google Scholar]
- 15.Schwartzentruber DJ, et al. In vitro predictors of therapeutic response in melanoma patients receiving tumor infiltrating lymphocytes and interleukin-2. J Clin Oncol. 1994;12:1475–1483. doi: 10.1200/JCO.1994.12.7.1475. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, et al. Tumor localization of adoptively transferred Indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J. Clin. Oncol. 1989;7:250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- 17.Griffith KD, et al. In vivo distribution of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J. Natl. Cancer Inst. 1989;81:1709–1717. doi: 10.1093/jnci/81.22.1709. [DOI] [PubMed] [Google Scholar]
- 18.Pockaj BA, et al. Localization of Indium-111-labelled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy: augmentation with cyclophosphamide in association with response. Cancer. 1994;73:1731–1737. doi: 10.1002/1097-0142(19940315)73:6<1731::aid-cncr2820730630>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA, et al. Gene transfer into humans: Immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N. Engl. J. Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 20.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Selective growth, in vitro and in vitro, of individual T cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J. Immunol. 2004;173:7622–7629. doi: 10.4049/jimmunol.173.12.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J. Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antony PA, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrzesiniski C, et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J. Clin. Invest. 2007;117:492–501. doi: 10.1172/JCI30414. Genetic and immunological techniques were used in a transgenic Pmel mouse model to uncover an unexpected mechanism for the promotion of ACT effectiveness by intensive ablative regimens. Introduced hematopoietic stem cells improved ACT efficacy by aiding CD8+ cell engraftment and persistence
- 26.Paulos CM, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. A mechanisms for the improvement of ACT by radiation induced lymphodepletion was explored in the Pmel mouse model. Antigen presenting cells activated by translocated gut microflora stimulated transferred tumor reactive CD8+ cells, suggesting methods for improving clinical ACT therapy
- 27.Robbins PF, et al. Cutting edge: Persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J. Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Shen X, Hodes RJ, Rosenberg SA, Robbins P. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J. Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J. Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran KQ, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J. Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang JC, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J. Clin. Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes MS, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum. Gene. Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson LA, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumorinfiltrating. J. Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theoret MR, et al. Relationship of p53 overexpression on cancers and recognition by anti-p53 T cell receptor-transduced T cells. Hum Gene Ther. 2008;19:1219–1231. doi: 10.1089/hum.2008.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkhurst M, et al. Characterization of genetically modified T cell receptors that recognize the CEA: 691-699 peptide in the context of HLA-A2.1 on human colorectal cancer cells. Clin. Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins PF, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. Site directed mutagenesis was used to engineer modifications in presumptive MHC- and antigen-contact positions of alpha and beta TCR genes. Increased avidity and antigen-specific T cell functionality was observed when modified TCRs were transduced into lymphocytes
- 40.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes. Proc Natl Acad Sci. 1993 doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]