Clonable progenitors committed to the T lymphocyte lineage in the mouse bone marrow; use of an extrathymic pathway (original) (raw)
Abstract
We searched for clonable committed T cell progenitors in the adult mouse bone marrow and isolated rare (≈0.05%) cells with the Thy-1hiCD2−CD16+CD44hiCD25−Lin− phenotype. In vivo experiments showed that these cells were progenitors committed only to reconstituting the T cell lineage of irradiated Ly5 congenic hosts. Reconstitution of the thymus was minimal compared with that of the bone marrow, spleen, and lymph nodes. At limiting dilutions, donor T cell reconstitution of the spleen frequently occurred without detectable donor cells in the thymus. Progenitors were capable of rapidly reconstituting athymic hosts. In conclusion, the clonable bone marrow progenitors were capable of T cell reconstitution predominantly by means of an extrathymic pathway.
Keywords: T cell progenitor, extrathymic maturation, T cell reconstitution, thymus
Committed T cell progenitors (CTP) that are restricted only to the T cell lineage have been reported to develop in the mouse fetal blood, liver, or spleen before they emigrate to the thymus for further maturation (1–5). However, the presence of multipotent or bipotent early progenitors in the adult and fetal thymus capable of generating non-T lineage cells suggested that commitment to the T lineage occurs only within the thymus (6–12). A recent study indicated that the CD90+/CD117lo/CD3− progenitor cells in the fetal blood and spleen that have been reported to be CTP (1) expressed the NK1.1 marker, and were actually bipotent progenitors capable of generating both T and natural killer (NK) cells despite the expression of such T lineage-associated genes as GATA-3, TCF-1, TCR-Cβ, and pTα (13). The latter study suggested that commitment to the T cell lineage occurred after fetal progenitors emigrate to the thymus and during subsequent rearrangement of the TCR β chain genes (12, 13).
The object of the current study was to search for CTP in the adult bone marrow that are capable of generating only mature T cells in vivo and that can be cloned by in vivo limiting dilution as reported for hematopoietic stem cells and common lymphoid progenitors cells (14–16). In particular, we studied a rare population (≈0.05%) of T cell progenitors in the marrow (Thy-1.2hiCD2−CD16+CD44hiLin−) that are able to generate CD4+ TCRaβ+ and CD8+ TCRaβ+ T cells in vitro (17). The phenotype of these progenitors is similar to that reported for early T cell progenitors in the fetal thymus (18).
Materials and Methods
Mice.
Congenic strains of wild-type C57BL/6 Ly5.2 and C57BL/6 Ly5.1 mice were bred and maintained in the Department of Comparative Medicine animal facility (Stanford University School of Medicine, Stanford, CA). Male and female mice were used at 8 to 12 weeks of age. In addition, C57BL/6-RAG-2−/− mice (B6.SJL; Rag2) and C57BL/6 nu/nu mice expressing the same Ly5.1 allele as the Ly5.1 wild-type mice were purchased from Taconic Farms.
Immunofluorescent Staining and Sorting of Cells.
Bone marrow cells were harvested from the femur and tibia as described (19). Staining and sorting of cell suspensions with fluorochrome or biotin-conjugated mAbs, including FcR blocking and propidium iodide gating, have been described (20). For sorting candidate progenitor cells in the bone marrow, cells were first enriched by incubation with biotin-conjugated anti-Thy-1.2 mAb (5a-8, Caltag, South San Francisco, CA), incubated further with streptavidin-conjugated immunomagnetic beads, and positively selected on MACS-MS magnetic separation columns (Miltenyi Biotech, Auburn, CA) according to the manufacturer's instructions. The enriched cells were stained and sorted thereafter. The following mAbs were conjugated with fluorochromes as described (21): FITC-conjugated anti-Ly5.2 (A20.1.7), biotin-conjugated anti-Ly5.1 (ALI-4A2), and allophycocyanin (APC) conjugated anti-CD44 (IM-781). Conjugated mAbs and reagents were purchased from PharMingen, including FITC and phycoerythrin (PE) anti-CD4 (CT-CD4), PE anti-CD8 (CT-CD8a), PE and FITC anti-TCRαβ (H57–597), PE anti-CD25 (PC61–5.3), FITC and PE anti-Mac-1 (M1/70.15), and Texas red streptavidin. PE and FITC anti-B220 (RA3–6B2), PE anti-Gr-1 (RB6–8C5), PE anti-NK1.1 (PK136), FITC anti-CD16/32 (2.4G2), PE and FITC anti-CD2 (RM2–5), APC anti-CD3 (2Cll), PE anti-Vβ3 (KJ25), FITC anti-Vβ6 (RR4–7), FITC anti-Vβ8 (F23.1), and biotin anti-CD11c (HL3) were purchased from PharMingen. Cells detected by the 2.4G2 mAb are referred to as CD16+ in the text. In some experiments, spleen cells were enriched for Thy-1+ and CD11c+ cells by incubating cells with biotin mAbs directed against these markers, incubated with streptavidin beads, and positively selected on magnetic separation columns (Miltenyi Biotech). In studies of splenic-dendritic cells, spleen cell fragments were treated with collagenase type II (Worthington) and DNase I (Roche Molecular Biochemicals) before making single-cell suspensions according to a protocol designed to optimize the dendritic cell yield as described (22).
Adoptive Transfer of Progenitor Cells and Monitoring.
C57BL/ 6 Ly5.1 host mice were given a single dose of lethal whole-body irradiation (either 800 cGy or 950 cGy) from a 200 kV (20 mA) source (Philips Medical Systems, Milford, CT) at a rate of 72.5 cGy/min. Candidate progenitor cells from Ly5.2 congenic donors mixed with bone marrow cells from RAG-2−/− Ly5.1 donors were injected i.v. into the lateral tail vein within 24 h of irradiation. The lymphoid tissues of recipient mice were harvested at serial time points, and monitored for their content of donor-type (Ly5.2) cells by immunofluorescent staining.
Gene Expression Analysis by Reverse Transcription–PCR.
Total RNA was extracted from sorted cells by using the RNeasy Mini Kit (Qiagen, Santa Clarita, CA). For RNA isolation, between 3 × 104 and 6 × 104 cells were used. RNA was then reverse transcribed by using random hexamer primers followed by PCR amplification. The optimal conditions for PCR amplification for β-actin message, to be used as an internal standard, were established by titration of the number of amplification cycles by using primers specific for β-actin, followed by densitometry analysis to measure ethidium-bromide luminescence of the PCR products. The forward and reverse primers used for β-actin are 5′-TGGGTCAGAAGGACTCCTATG-3′, and 5′-ACCAGACAGCACTGTGTTGGC-3′, respectively. Primers for RAG-1 and RAG-2, as well as the conditions for the PCR, have been described (23). The design of primers specific for pTa gene amplification was based on a sequence found in GenBank (24), accession number U16958 (25). These primers have the following sequences: nested forward, 5′-GGCTCCACCCATCACACTGC-3′; internal forward, 5′-TGCTGGTGGTTTGCCTGGTC-3′; internal reverse, 5′-GGGAGCAGTAGTGTCCAGCATC-3′; and nested reverse, 5′-CCATTTACAAGAGGCAGATCAC-3′.
PCR Analysis for TCR Gene Rearrangements.
TCR Vβ7 and Vβ8 gene rearrangements were detected with a nested PCR technique (26). Primers specific for the Vβ7 locus (first round, 5′-TACCTGATCAAAAGAATGGGAG-3′; and second round, 5′-GAGCATTTCTCCCTGATTCTGG-3′) and for the Jβ2-Cβ2 intronic region (first round, 5′-TCCTGGCTTGCGAGAGAGCG3′; and second round, 5′-TTGAGAGCTGTCTCCTACTATC-3′) have been described (26). The design of primers specific for consensus Vβ8 exon regions was based on a sequence found in GenBank (24), accession number AE000522, and aligned with blast (27). These primers had the following sequences: first round, 5′-CACATGGAGGCTGCAGTCAC-3′; and second round, 5′-CATGTACTGGTATCGGCAGG-3′. The design of primers specific for the intron and Dβ1 exon regions was based on a sequence found in GenBank as above, accession number AE000665. These primers had the following sequences, respectively: first round, 5′-CTTCTTCATAGGGTGGTTCC-3′; and second round, 5′-ACTGTAACATTGTGGGGACAG-3′. Primers specific for the intronic regions flanking the first exon of the TCR Cα gene were used to control for the adequacy of amplification and the integrity of the genomic DNA. These were generously provided by James W. Tung (Stanford University, Stanford, CA); the design was based on a sequence found in GenBank (24), accession number M64239. These primers had the following sequences: first round forward, 5′-AGGCTGTCATTTCCAATGTG-3′; second round forward, 5′-CTTCTA- ACATCAGTCCTCTTG-3′; first round reverse, 5′-CACTCCC- TCCTCCTCATCTG-3′; and second round reverse, 5′-GACCCGTTAGACTCCTTAGTG-3′.
In Vitro Assay for Myeloid/Erythroid Colonies.
Sorted progenitor cells from the C57BL/6 wild-type bone marrow were plated in medium with methyl-cellulose containing stem cell factor, IL-11, Flt3, IL-3, granulocyte/macrophage colony-stimulating factor, erythropoietin, and thrombopoietin. Colonies were counted at day 7 as described (14).
Results
T Cell Reconstitution with Purified Progenitors.
Bone marrow cells were isolated from C57BL/6 Ly5.2 mice and assayed for their capacity to mature into single-positive CD4+ and CD8+ T cells after i.v. injection into lethally irradiated C57BL/6 Ly5.1 hosts. The latter hosts were injected also with a constant number (1 × 106) of marrow cells from C57BL/6 RAG-2−/− Ly5.1 mice. The coinjected marrow cells were unable to develop into mature T or B cells because of their deficient RAG-2 genes (28).
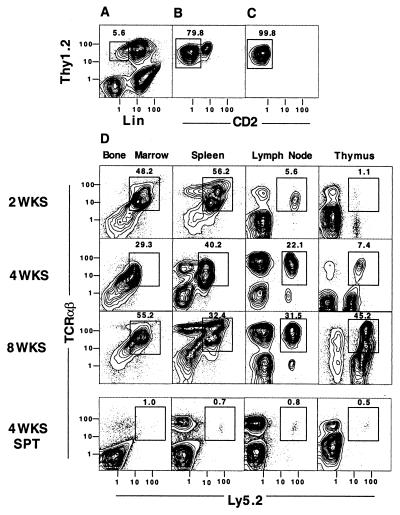
Fig. 1 shows two-color flow cytometric analyses of C57BL/ 6 Ly5.2 marrow cells after enrichment for Thy-1+ cells (expressing Thy-1.2 marker referred to hereafter as Thy-1) by using immunomagnetic beads. The yield of enriched cells was about 1% of the nucleated marrow cells applied to the beads. As shown in Fig. 1A, enriched cells were stained for Thy-1 vs. lineage (Lin)-specific markers (B220, Mac-1, NK1.1, TCRαβ) by using fluorochrome-conjugated mAbs. Approximately 6% of the enriched cells (enclosed in box) were Thy-1hiLin−. The latter cells were gated and analyzed for Thy-1 vs. CD2 markers (Fig. 1B). Of the gated cells, 79.8% were CD2− (enclosed in box) and 21.2% were CD2+. The gated CD2− cells were sorted for subsequent injection into irradiated hosts or analyzed further for their expression of CD16, CD44, and CD25 markers. Reanalysis of sorted cells is shown in Fig. 1C, and 99.8% (enclosed in box) were Thy-1hiCD2−. Further analysis of the gated Thy-1hiCD2− cells used four different fluorochromes in the staining mixture to study CD2 vs. CD16 or CD44, and CD25 vs. CD44. Almost all of the gated cells were CD16+, CD44hi, and CD25−, as reported (17).
Figure 1.
Sorted Thy-1.2hiCD2−Lin− bone marrow cells generate mature T cells. (A) Staining of enriched C57BL/6 Ly5.2 marrow cells for Thy-1.2 vs. lineage-specific (Lin) markers, including B220, Mac-1, TCRαβ, and NK1.1. Box encloses Thy-1.2hiLin− cells, and percentage enclosed in box is shown. (B) Staining for Thy-1.2 vs. CD2 markers on gated cells enclosed in box in A. Cells enclosed in box in B were sorted and reanalyzed in C. (D) Two-color flow cytometric analyses of the lymphoid tissue harvested from lethally irradiated (800 cGy) C57BL/6 Ly5.1 hosts at serial time points after the i.v. injection of 1 × 104 sorted Thy-1hiCD2−Lin− C57BL/6 Ly5.2 bone marrow cells and 1 × 106 whole bone marrow cells from C57BL/6 Ly5.1 RAG-2−/− mice. Staining for TCRαβ vs. Ly5.2 markers is shown. The donor-type (Ly5.2) cells enclosed by boxes are TCRαβ+ T cells. A control group of Ly5.1 hosts was given Ly5.1 RAG-2−/− bone marrow cells and 1 × 104 sorted CD4+ and CD8+ TCRαβ+ single-positive T cells (SPT) instead of sorted progenitor cells from the bone marrow of Ly5.2 donors. Analyses of the latter hosts are shown (Bottom). All images are representative of three to four hosts.
Sorted Thy-1hiCD2−Lin− cells (1 × 104) were injected i.v. into whole-body irradiated (800 cGy) C57BL/6 Ly5.1 wild-type hosts along with 1 × 106 unfractionated marrow cells from C57BL/ 6 Ly5.1 RAG-2−/− donor mice. Fig. 1D shows two-color flow cytometric analyses of the marrow, spleen, peripheral lymph nodes, and thymus from the hosts at 2, 4, or 8 weeks after the cell injections. Boxes enclose the TCRαβ+ T cells that are derived from the sorted Thy-1hiCD2− Ly5.2+ candidate progenitor cells.
At 2 weeks after the cell injection, about half of the marrow and spleen cells were TCRαβ+ T cells derived from the Ly5.2+ progenitors. However, only 5.6% of the lymph node cells were TCRαβ+ Ly5.2+ T cells, and about 1% of thymus cells were TCRαβ+ Ly5.2+. After 4 and 8 weeks, the percentage of TCRαβ+ Ly5.2+ cells in the marrow and spleen remained in the range of about 30–50%, and the percentage of TCRαβ+ Ly5.2+ cells in the lymph nodes increased to 31.5% at 8 weeks. Similarly, the bright TCRαβ+ Ly5.2+ cells in the thymus increased from 1.1% at 2 weeks to 45.2% at 8 weeks. At 8 weeks, more than 90% of thymocytes were donor-type (Ly5.2+) and about 35% were CD4+CD8+. The pattern in all tissues at 8 weeks remained stable at 12 weeks (data not shown).
Table 1 shows the mean ± SE absolute number of Ly5.2+ donor cells in the bone marrow (both femurs), thymus, and spleen at serial time points after the cell injection. The number of lymph nodes obtained from host mice was too variable for accurate analysis. The number of donor cells in the marrow was stable in the range of 5 × 106 to 11 × 106 from 2 to 8 weeks. The mean number of donor cells in the spleen was 2.1 × 106 at 2 weeks, and increased to the range of 4 × 106 to 8 × 106 thereafter. The number of donor cells in the thymus increased about 20-fold from 2 to 3 weeks (0.1 to 2 × 106 cells), and was 0.9 and 3 × 106 thereafter. Comparison of the absolute numbers of donor T cells in the bone marrow of irradiated hosts to that of untreated wild-type mice showed that the reconstituted marrow T cells were 10-fold higher than in untreated mice, but reconstituted spleen cells were about 4-fold lower at 8 weeks (Table 1). Reconstituted thymocytes were about 40-fold lower. Thus, robust reconstitution in the bone marrow was associated with minimal reconstitution of the thymus.
Table 1.
Absolute number of Ly5.2+ cells in adoptive hosts
| Host* | Number of cells per tissue × 106 | ||
|---|---|---|---|
| Bone marrow | Spleen | Thymus | |
| Irradiated host | |||
| 2 weeks | 11 ± 5 | 2.1 ± 0.4 | 0.10 ± 0.01 |
| 3 weeks | 12 ± 5 | 5 ± 2 | 2 ± 1 |
| 4 weeks | 4.7 ± 0.6 | 8 ± 4 | 0.9 ± 0.4 |
| 8 weeks | 10 ± 4 | 4 ± 1 | 3 ± 1 |
| WT C57BL/6 | 1.0 ± 0.3† | 17 ± 2† | 119 ± 27‡ |
Because it was possible that the outgrowth of Ly5.2 T cells in the hosts given purified Ly5.2 progenitor cells and RAG-2−/− Ly5.1 marrow cells may have been derived from a small number of mature bone marrow T cells contaminating the progenitor cells, a control group was set up in which hosts were given RAG-2−/− Ly5.1 marrow cells and 1 × 104 sorted CD4+ and CD8+TCRaβ+ T cells from the bone marrow of Ly5.2 donors instead of 1 × 104 purified progenitor cells. Fig. 1D shows that the expansion of the mature T cells in the lymphoid tissues of the hosts was minimal after 4 weeks and that the bone marrow, spleen, lymph nodes, and thymus contained 1% or less of TCRαβ+Ly5.2 T cells. Thus, contaminating mature T cells from the bone marrow had little capacity to expand in the host lymphoid tissues. This result stands in contrast to previous reports that show dramatic expansions of wild-type or transgenic donor CD4+ or CD8+ T cells after transfer to T cell-deficient hosts (29–32). However, all of these studies used T cells obtained from the lymph nodes and spleen (29–32) that did not have the unusual phenotypic and functional characteristics of T cells in the marrow (19).
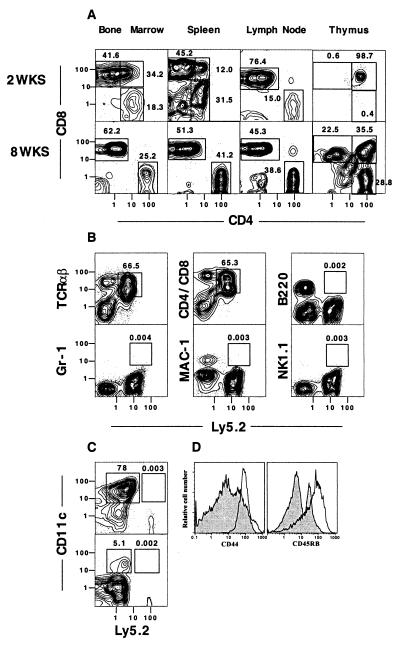
To study the expression of CD4 vs. CD8 markers on Ly5.2+ donor cells in the tissues of the hosts coinjected with Ly5.2 purified progenitors and Ly5.1 RAG-2−/− marrow cells, gated Ly5.2+ cells from each tissue were examined at 2 and 8 weeks. Fig. 2A shows that at least 90% of gated cells in the marrow and lymph nodes at 2 weeks were single-positive T cells with CD8+ cells predominating over CD4+ cells by a ratio of about 4:1 or 5:1. Almost all of the CD8+ T cells in the spleen and lymph nodes expressed the CD8β phenotype (data not shown). About 75% of donor spleen cells were single-positive T cells with a predominance of CD8+ cells. At 2 weeks, CD4+CD8+ double-positive T cells accounted for 12% and 34% of donor cells in the spleen and marrow, respectively. The Ly5.2+ cells in the thymus at 2 weeks were almost all CD4+CD8+ double-positive T cells. At 8 weeks, the intensity of staining of Ly5.2+ CD4+CD8− cells in the marrow, spleen, lymph node, and thymus was similar and bright, and double-positive T cells disappeared from the spleen.
Figure 2.
Sorted Thy-1.2hiCD2−Lin− bone marrow cells generate CD4+ and CD8+ T cells in Ly5 congenic hosts. (A) Two-color analyses of the gated Ly5.2+ donor cells in the lymphoid tissues harvested from lethally irradiated C57BL/6 Ly5.1 hosts 2 and 8 weeks after injection of 1 × 104 sorted Thy-1hiCD2−Lin− C57BL/6 Ly5.2 marrow cells and 1 × 106 marrow cells from C57BL/6 Ly5.1 RAG-2−/− mice, as shown in Fig. 1D. Staining for CD4 vs. CD8 is shown. (B) Two-color flow cytometric analyses of the spleen from hosts at 4 weeks with T cell (TCRαβ or combined CD4 and CD8), B cell (B220), granulocyte (Gr-1), macrophage/myeloid cell (Mac-1), and NK cell (NK1.1) vs. Ly5.2 markers. Boxes enclose donor-type (Ly5.2) cells that stain for the different cell lineages. (C) Staining of the spleen after enrichment of the cells with anti-CD11c mAb and immunomagnetic beads. (Upper) CD11c vs. Ly 5.2 markers. CD11c+Ly5.2− and CD11c+Ly5.2+ cells are enclosed in boxes. (Lower) CD11c vs. Ly5.2 after depletion of Mac-1+ and B220+ cells by gating. (D) One-color analysis of gated Ly5.2+TCRαβ+ donor cells in the host spleen stained for CD44 and CD45RB markers at 8 weeks (open profiles). Shaded profiles show the same analysis of gated TCRαβ+ T cells from the spleen of untreated wild-type donors.
The splenic single-positive TCRαβ+ T cells at 8 weeks expressed very high levels of both CD44 and CD45RB in comparison with the levels expressed by most splenic T cells in untreated wild-type mice (Fig. 2D). The phenotype of these progenitor-derived T cells differed also from the CD44hi CD45RBlo activated-memory phenotype of expanded mature spleen and lymph node CD4+ T cells transferred to T cell-deficient hosts (30–32). The unique CD44hiCD45RBhi phenotype of T cells derived from progenitors in reconstituted hosts was found amongst both CD4+ and CD8+ T cells (data not shown). CD4−CD8− cells persisted in the lymph nodes, spleen, and marrow (Fig. 2A); the latter cells were either TCRαβ+ or expressed the Thy-1hiCD44hiCD16hiCD2−Lin− phenotype of the progenitors (data not shown).
The high percentage of TCRαβ+ cells amongst Ly5.2+ cells in hosts given RAG-2−/− marrow cells as shown in Fig. 1 indicated that the Ly5.2+ progenitors were biased toward the T cell lineage. A more detailed analysis of a variety of lineage markers vs. the Ly5.2 marker in the spleen at 4 weeks is shown in Fig. 2B. Whereas TCRαβ+Ly5.2+ and CD4+ and CD8+ Ly5.2+ cells accounted for 66.5% and 65.3%, respectively, of all spleen cells, the percentage of B220+, Gr-1+, Mac-1+, or NK1.1+ Ly5.2+ cells accounted for less than 0.01% of spleen cells. The non-T cell lineage donor cells accounted for less than 0.01% of cells in the bone marrow, lymph nodes, and thymus also (data not shown). Thus, few if any B cells, granulocytes, macrophages, or NK cells, respectively, developed from the injected Ly5.2+ progenitor cells that seem to be CTP. The inability of the latter progenitors to generate myeloid/erythroid cells was also shown in vitro, because no outgrowth of methyl-cellulose colonies at day 7 was detected after plating 200 CTP in cultures containing stem cell factor, IL-11, Flt3, IL-3, granulocyte/macrophage colony-stimulating factor, erythropoietin, and thrombopoietin. In contrast, 59 myeloid, 3 erythroid, 2 megakaryocytic, and 11 mixed colonies were detected at the same time point after plating 100 sorted Thy-1loSca-1hic-Kitlo hematopoietic stem cells.
Because NK cells represent only a few percent of cells in the normal spleen or marrow, it was possible that a small percentage of the progeny of the purified progenitors were members of the latter lineages and could be detected only after appropriate enrichment. Accordingly, bone marrow cells from adoptive hosts were enriched for Thy-1+ cells by using immunomagnetic beads, because NK cells express this marker (33). The enriched cells were analyzed for NK1.1+ cells; these accounted for 3.2% of all harvested cells, but progenitor-derived Ly5.2+NK1.1+ cells accounted for only 0.01% of enriched cells (data not shown).
To determine whether Ly5.2 dendritic cells were reconstituted by using 1 × 104 purified progenitors, spleen fragments from adoptive hosts were harvested at 4 weeks. Single-cell suspensions were enriched for dendritic cells with anti-CD11c immunomagnetic beads, because the latter cells express this marker (34). Fig. 2C Upper shows the staining pattern of the enriched cells for CD11c vs. Ly5.2. Whereas 78% of CD11c+Ly-5.2− cells were present, 0.003% of enriched cells were CD11c+Ly5.2+ (Ly5.2 progenitor-derived) cells. Because CD11c+ cells contain B cells, macrophages, and dendritic cells, the immunomagnetic bead-enriched spleen cells were gated to remove B220+ and Mac-1+ cells (B cells and macrophages), and CD11c vs. Ly5.2 marker were analyzed thereafter (Fig. 2C Lower). The residual dendritic cells accounted for about 5.1% of the gated spleen cells, and were almost all Ly5.2− cells. CD11c+B220−Mac-1−Ly5.2+ cells were 0.002% of enriched spleen cells. Thus B, myeloid, and dendritic cells derived from the Ly5.2 progenitors were not clearly detected (as judged by flow cytometry). These experiments with Ly5.2 progenitors were repeated to improve the yield of dendritic cells by treating host spleen fragments with collagenase before preparing the single-cell suspensions (22). After immunomagnetic bead enrichment, the percentage of Ly5.2+ CD11c+ cells in the host spleen was still less than 0.1% (data not shown).
Limiting Dilution Assays.
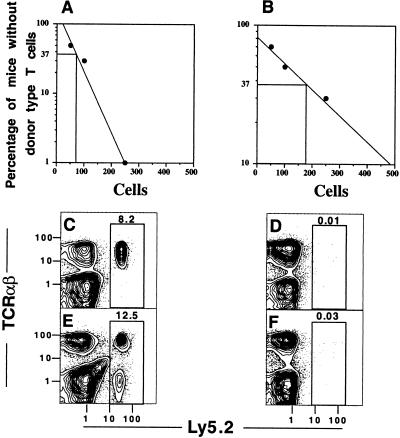
Limiting dilution assays were used to determine the frequency of progenitors in the sorted Thy-1+CD2−Lin− bone marrow cells that were capable of reconstituting mature T cells in the lethally irradiated congenic hosts. Instead of injecting 1 × 104 sorted Ly5.2 cells along with 1 × 106 RAG-2−/− Ly5.1 marrow cells, graded numbers of sorted cells (500, 250, 100, and 50) were injected i.v. with the constant number of RAG-2−/− marrow cells into groups of 10 irradiated mice for each sorted-cell dose. After 4 weeks, the spleen and thymus cells from each host were harvested and stained for TCRαβ vs. Ly5.2 markers. The percentage of hosts without detectable (<0.05%) TCRαβ+Ly5.2+ T cells in the spleen was plotted against the number of sorted cells injected (Fig. 3A). Poisson statistics predict that two-thirds of the hosts in a group will be reconstituted by an average of one progenitor cell per mouse. Thus, for reconstitution of spleen T cells, there was approximately 1 progenitor cell in 75 sorted cells that were injected. A similar analysis for reconstitution of the thymus was approximately 1 progenitor in 180 sorted cells (Fig. 3B).
Figure 3.
Limiting dilution analysis of donor T cell reconstitution of lethally irradiated (950 cGy) C57BL/6 Ly5.1 hosts 4 weeks after i.v. injection of graded numbers of sorted C57BL/6 Ly5.2 Thy-1hiCD2−Lin− (donor) bone marrow cells coinjected with 1 × 106 C57BL/6 Ly5.1 RAG-2−/− whole bone marrow cells. (A and B) Poisson analyses of reconstitution of spleen and thymus, respectively, with groups of 10 hosts for each cell dose. (C and D) Two-color analysis of TCRαβ vs. Ly5.2 markers of the spleen and thymus, respectively, of a single 4-week host given 100 sorted Ly5.2 marrow progenitor cells i.v. This pattern was observed in three of seven reconstituted mice. (E and F) Similar analyses of a single 4-week host given 250 sorted cells. This pattern was observed in 3 of 10 mice. Boxes in C_–_F enclose donor-type (Ly5.2) cells.
Near unit dilution (100 sorted cells injected), three of seven mice with detectable donor T cells showed reconstitution of the spleen without detectable donor Ly5.2+ cells in thymus (Fig. 3 C and D, respectively). At that dilution, four of seven mice showed donor cell reconstitution of both the spleen and the thymus. When 250 sorted cells were injected, 3 of 10 mice showed reconstitution of the spleen (Fig. 3E) without detectable donor cells in the thymus (Fig. 3F), and 7 of 10 mice showed reconstitution of the spleen and thymus.
These experiments indicated that the sorted progenitors can use an extrathymic pathway for reconstitution of the spleen. Extrathymic reconstitution was tested also by injecting 250 sorted cells into a group of irradiated athymic C57BL/6 Ly5.1 nu/nu hosts instead of euthymic hosts. Reconstitution of TCRαβ+ T cells was detected in the latter hosts, and the mean ± SE of the absolute number of progenitor-derived TCRαβ+ Ly5.2+ T cells in the spleen and lymph nodes was 21 ± 8 and 3.8 ± 0.2 × 105, respectively, at 8 weeks (n = 4). The absolute number of donor T cells in the same tissues of irradiated euthymic hosts given 250 sorted progenitors was 12 ± 4 and 5.2 ± 0.8 × 105, respectively, at 8 weeks (n = 3). Ratios of CD4+:CD8+ T cells were similar in the two types of hosts (data not shown).
TCR β Chain Gene Rearrangements and Vβ Repertoire.
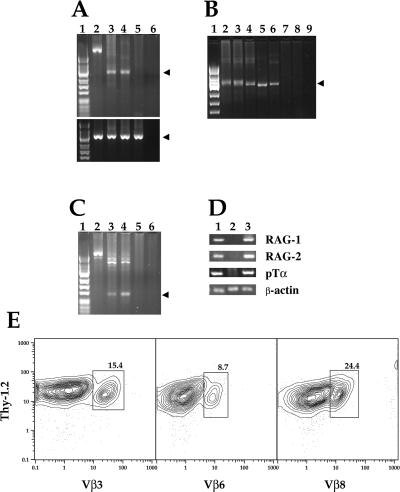
Purified Thy-1+CD2−Lin− marrow cells from untreated wild-type mice were obtained by using anti-CD3 mAb instead of anti-TCRaβ to gate on Lin− cells (see Materials and Methods). The sorted Thy-1+CD2−Lin− marrow cells failed to show rearrangement of the Vβ8 gene segment as determined by a PCR analysis of amplified genomic DNA (Fig. 4A Upper). Primers were chosen to hybridize with the Vβ8 gene segment and with an intron located between the Jβ2 gene cluster and the Cβ2 exon. Whereas amplified, genomic DNA from untreated wild-type spleen cells showed a clear band of amplified DNA of appropriate size, no amplified DNA was detected by using the same amount of genomic DNA from sorted marrow cell progenitors. However, genomic DNA from sorted donor-type (Ly5.2+) spleen cells obtained 4 weeks after the injection of 1 × 104 sorted Ly5.2 marrow progenitor cells into irradiated Ly5.1 hosts showed an amplified band of DNA with primers for Vβ8 (Fig. 4A Upper), which is indicative of gene rearrangement. The sensitivity of the PCR analysis for cells with rearrangements of the Vβ8 gene segment was studied by making mixtures of a cloned BALB/c B cell lymphoma line (BCL1) without Vβ8 gene rearrangements and untreated wild-type C57BL/6 spleen cells. All mixtures contained a constant number (1 × 106) of B cells and graded numbers of spleen cells with 10-fold decrements from 1 × 106 to 1 × 100, such that the ratio of spleen cells:BCL1 cells varied from 1:1 to 1:1 × 106. Fig. 4B shows that an amplified band of Vβ8 DNA was still detected at the cell ratio of 1:1 × 104, but was not detected thereafter. Thus, the PCR analysis can detect about one Vβ8 rearranged cell amongst 0.5 × 105 cells, because Vβ8+ T cells account for about 20% of normal spleen T cells (20). The same analysis was performed to study the rearrangement of the Vβ7 gene with primers that hybridized specifically with the Vβ7 gene segment. Again, the sorted progenitor cells failed to show an amplified Vβ7 band, but the appropriate size band was observed in the host spleen 4 weeks after the progenitor cell injection (data not shown).
Figure 4.
Vβ gene rearrangements, T cell gene expression, and Vβ surface expression in sorted donor (Ly5.2) bone marrow cells and/or their progeny. (A Upper) PCR amplification of genomic DNA isolated from ≈1 × 104 sorted or unfractionated cells by using primers that hybridize with the Vβ8 exon and Jβ2-Cβ2 intron gene segments. Lane 1, size markers from φX174 digested with _Hin_fI; lane 2, DNA from sorted Thy-1hiCD2−Lin− marrow cells; lane 3, DNA from sorted Ly5.2+ cells from the spleen of C57BL/6 Ly5.1 lethally irradiated hosts injected with 1 × 104 sorted Ly5.2 marrow cells and 1 × 106 whole marrow cells from C57BL/6 RAG-2−/− Ly5.1 mice 4 weeks earlier; lane 4, DNA from C57BL/6 Ly5.2 wild-type unfractionated spleen cells; lane 5, DNA from control tk− L cell line; lane 6, no DNA added to PCR. Arrows show amplified bands of appropriate size. (A Lower) PCR amplification of the same DNA samples as in A Upper with primers that hybridize with a Cα exon gene segment. (B) PCR amplification of the same gene segments in A with genomic DNA from cell mixtures with a constant number (1 × 106) of BALB/c B cell lymphoma (BCL1) cells and graded numbers of C57BL/6 spleen cells. Lane 1, size markers; lanes 2–8, 10-fold decrements of added spleen cells starting from 1 × 106 and ending at 1 × 100, respectively; lane 9, BCL1 cells without added spleen cells. (C) PCR amplification of the Dβ1 and Jβ2-Cβ2 intron gene segments with genomic DNA from sorted or unfractionated cells. Lanes are labeled as in A. (D) Reverse transcription–PCR analysis of mRNA from sorted or unfractionated cells for RAG-1, RAG-2, pTα, and β-actin genes. Lane 1, sorted Thy-1hiCD2−Lin− marrow cells; lane 2, sorted CD4+ and CD8+ TCRαβ+ T cells from the spleen; lane 3, sorted CD4−CD8−TCRαβ thymocytes. (E) Two-color flow cytometric analyses of gated Ly5.2+ cells from the spleen of lethally irradiated Ly5.1 hosts given 250 sorted Thy-1hiCD2−Lin− Ly5.2 marrow cells and 1 × 106 C57BL/6 RAG-2−/− Ly5.1 marrow cells 4 weeks earlier. Staining of Thy-1.2 vs. Vβ3, Vβ6, and Vβ8 is shown. Boxes enclose Vβ+Thy-1.2+ cells.
Because rearrangements of the Dβ-Jβ gene segments can precede rearrangements of the Vβ gene segment during early T cell maturation, PCR analysis for Dβ gene segment rearrangement was performed with a primer that hybridized with the Dβ1 segment, and the Jβ2-Cβ2 intron primer was the same as described above. Fig. 4C shows that an amplified DNA band was not detected with genomic DNA from sorted progenitor cells, but a band of the appropriate size was detected by using either normal spleen cells or spleen cells from irradiated hosts 4 weeks after the progenitor cell injection. Fig. 4A Lower shows positive control PCR amplifications of the Cα exon 1 gene segment for all genomic DNA samples used in Fig. 4 A and C.
The bipotent T and NK progenitors in the fetal blood and spleen reported elsewhere (13) expressed the RAG-1, RAG-2, and pTα genes that precede Vβ gene rearrangement. To compare similar gene expression in sorted progenitors in the adult bone marrow to those reported in the fetus, RNA was extracted from sorted progenitors from the bone marrow of wild-type C57BL/6 mice, and reverse transcription–PCR analysis was performed. Fig. 4D shows that the sorted progenitors expressed the RAG-1, RAG-2, and pTα genes but that sorted CD4+ and CD8+ TCRαβ+ spleen cells from normal C57BL/6 mice did not. On the other hand, sorted CD4−CD8− thymocytes from the same mice expressed all three genes as expected (Fig. 4D). β-Actin controls were obtained to assure adequacy of the RNA yields.
In further experiments, the spleen cells from 10 hosts injected with 250 sorted cells (receiving an average of about three progenitor cells per mouse) were stained to determine the percentage of donor (Ly5.2+) T cells expressing three different Vβ receptors (Vβ3, Vβ6, and Vβ8). The two-color flow cytometric analyses of each Vβ vs. Thy-1.2 with gated Ly5.2+ cells are shown in Fig. 4E (boxes enclose Vβ+Thy-1.2+ cells). The percentages of the three Vβ T cell subsets (8.7% to 24.4%) were similar to the percentages amongst T cells in the normal C57BL/6 spleen (20). Thus, limited numbers of progenitor cells can reconstitute the Vβ subsets without obvious skewing toward a predominant type.
Discussion
Our recent study showed that the sorted Thy-1hiCD2−Lin− marrow cells generated CD4+ and CD8+ single-positive T cells in vitro by means of double-positive intermediary cells (17). Injection of 1 × 104 of these sorted marrow cells from Ly5.2 donor mice into lethally irradiated Ly5.1 congenic hosts along with 1 × 106 marrow cells from C57BL/6 RAG-2−/− Ly5.1 donors was able to reconstitute the bone marrow, spleen, lymph nodes, and thymus with Ly5.2+ donor-type TCRαβ+ T cells as early as 2 weeks later. The earliest and largest expansion of donor-type single-positive T cells occurred in the bone marrow, and the latest and least occurred in the thymus.
Whereas the absolute number of donor T cells in the host marrow exceeded the absolute number of T cells in the bone marrow of untreated wild-type mice, the number in the thymus was about 40-fold lower. In contrast, reconstitution of irradiated hosts with 1 × 103 Thy-1loSca-1hic-KitloLin− hematopoietic stem cells (15, 16) resulted in an absolute number of thymocytes and bone marrow T cells that was similar to that of untreated mice (data not shown). This result suggested that progenitors used in the present study may have reconstituted T cells in the marrow and peripheral lymphoid tissues predominantly through an extrathymic pathway, and that thymus reconstitution was severely restricted. The ability of progenitors to reconstitute by means of an extrathymic pathway was confirmed by using athymic nu/nu hosts. The latter hosts rapidly developed similar numbers of donor T cells in the spleen and lymph nodes as compared with euthymic hosts, given equal limiting numbers (250 per host) of progenitor cells. It is unlikely that mature marrow T cells that contaminate the sorted progenitor cells were the source of donor T cells in the host lymphoid tissues, because injection of 1 × 104 mature marrow TCRαβ+ T cells failed to reconstitute hosts. Reconstituted CD4+ and CD8+ T cells had an unusual CD44hiCD45RBhi phenotype.
The Thy-1hiCD2−Lin− marrow progenitors were committed to the T cell lineage and failed to reconstitute detectable B (B220+) cells, granulocytes (Gr-1+), macrophages (Mac-1+), NK (NK1.1+) cells, or dendritic (CD11c+B220−Mac-1−) cells in the bone marrow or spleen of the hosts. The inability to differentiate into myeloid and erythroid cells was confirmed by in vitro culture experiments. The T cell commitment of the progenitors occurred in the marrow before the rearrangement of the TCR β chain genes, because PCR analysis of genomic DNA obtained from the purified progenitors failed to detect rearrangements of the Dβ1, Vβ7, or Vβ8 gene segments. The CTP expressed the RAG-1, RAG-2, and pTα genes as did the bipotent NK and T cell progenitors reported elsewhere (13), but the CTP have a more restricted developmental potential.
In conclusion, the study shows that commitment of progenitors to the T cell lineage occurs in the bone marrow before the rearrangement of the TCR genes. Furthermore, these unusual progenitors are capable of only limited reconstitution at the thymus of the adoptive hosts, and the bone marrow and peripheral lymphoid tissues can be reconstituted by means of an extrathymic pathway.
Acknowledgments
We thank Drs. T. Miyamoto and I. L. Weissman, Department of Pathology, Stanford University for assays of myeloid/erythroid colonies and for help in the design and analysis of experiments. We are grateful to Dr. J. W. Tung for providing Cα primers, A. Mukhopadhyay for technical assistance, and V. Cleaver and M. Hansen for preparation of the manuscript. The studies were supported by National Institutes of Health Grants AI-43013, HL-58250, and HL-57443, and by Grant AI-37683S1 (to M.E.G.-O.).
Abbreviations
CTP
committed T cell progenitors
TCR
T cell antigen receptor
PE
phycoerythrin
NK
natural killer
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rodewald H R, Kretzschmar K, Takeda S, Hohl C, Dessing M. EMBO J. 1994;13:4229–4240. doi: 10.1002/j.1460-2075.1994.tb06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodewald H R. Curr Opin Immunol. 1995;7:176–187. doi: 10.1016/0952-7915(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 3.Kawamoto H, Ohmura K, Katsura Y. Int Immunol. 1997;9:1011–1019. doi: 10.1093/intimm/9.7.1011. [DOI] [PubMed] [Google Scholar]
- 4.Kawamoto H, Ohmura K, Fujimoto S, Katsura Y. J Immunol. 1999;162:2725–2731. [PubMed] [Google Scholar]
- 5.Kawamoto H, Ohmura K, Katsura Y. J Immunol. 1998;161:3799–3802. [PubMed] [Google Scholar]
- 6.Wu L, Antica M, Johnson G R, Scollay R, Shortman K. J Exp Med. 1991;174:1617–1627. doi: 10.1084/jem.174.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodewald H R, Moingeon P, Lucich J L, Dosiou C, Lopez P, Reinherz E L. Cell. 1992;69:139–150. doi: 10.1016/0092-8674(92)90125-v. [DOI] [PubMed] [Google Scholar]
- 8.Carlyle J R, Michie A M, Furlonger C, Nakano T, Lenardo M J, Paige C J, Zuniga-Pflucker J C. J Exp Med. 1997;186:173–182. doi: 10.1084/jem.186.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shortman K, Wu L. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzaki Y, Gyotoku J, Ogawa M, Nishikawa S, Katsura Y, Gachelin G, Nakauchi H. J Exp Med. 1993;178:1283–1292. doi: 10.1084/jem.178.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardavin C, Wu L, Li C L, Shortman K. Nature (London) 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 12.Carlyle J R, Zuniga-Pflucker J C. Immunol Rev. 1998;165:63–74. doi: 10.1111/j.1600-065x.1998.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 13.Carlyle J R, Zuniga-Pflucker J C. Immunity. 1998;9:187–197. doi: 10.1016/s1074-7613(00)80601-9. [DOI] [PubMed] [Google Scholar]
- 14.Kondo M, Weissman I L, Akashi K. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 15.Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 16.Uchida N, Weissman I L. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dejbakhsh-Jones S, Strober S. Proc Natl Acad Sci USA. 1999;96:14493–14498. doi: 10.1073/pnas.96.25.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodewald H R, Awad K, Moingeon P, D'Adamio L, Rabinowitz D, Shinkai Y, Reinherz E L. J Exp Med. 1993;177:1079–1092. doi: 10.1084/jem.177.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, Ojeda-Garcia M, Sibley R, Strober S. J Exp Med. 1999;189:1073–1081. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Ojeda M E, Dejbakhsh-Jones S, Weissman I L, Strober S. J Exp Med. 1998;187:1813–1823. doi: 10.1084/jem.187.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akashi K, Weissman I L. Immunity. 1996;5:147–161. doi: 10.1016/s1074-7613(00)80491-4. [DOI] [PubMed] [Google Scholar]
- 22.Vremec D, Shortman K. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 23.Taubenberger J K, Reid A H, Izon D, Boehme S A. Cell Immunol. 1996;171:41–47. doi: 10.1006/cimm.1996.0170. [DOI] [PubMed] [Google Scholar]
- 24.Benson D A, Boguski M S, Lipman D J, Ostell J, Ouellette B F. Nucleic Acids Res. 1998;26:1–7. doi: 10.1093/nar/26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saint-Ruf C, Ungewiss K, Groettrup M, Bruno L, Fehling H J, von Boehmer H. Science. 1994;266:1208–1212. [PubMed] [Google Scholar]
- 26.Schmidt-Wolf I G, Liang O, Dejbakhsh-Jones S, Wang H, Cheng L, Holm B, Bell R, Strober S. J Immunol. 1993;151:5348–5353. [PubMed] [Google Scholar]
- 27.Altschul S F, Gish W, Miller W, Myers E W, Lipman E W. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Shinkai Y, Rathbun G, Lam K P, Oltz V, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A M, et al. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 29.Goldrath A W, Bevan M J. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beutner U, MacDonald H R. Int Immunol. 1998;10:305–310. doi: 10.1093/intimm/10.3.305. [DOI] [PubMed] [Google Scholar]
- 31.Kieper W C, Jameson S C. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muranski P, Chmielowski B, Ignatowicz L. J Immunol. 2000;164:3087–3094. doi: 10.4049/jimmunol.164.6.3087. [DOI] [PubMed] [Google Scholar]
- 33.Herberman R B, Nunn M E, Holden H T. J Immunol. 1978;121:304–309. [PubMed] [Google Scholar]
- 34.Metlay J P, Witmer-Pack M D, Agger R, Crowley M T, Lawless D, Steinman R M. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]