Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss (original) (raw)
. Author manuscript; available in PMC: 2013 Nov 1.
Published in final edited form as: Acta Neuropathol. 2012 Sep 2;124(5):665–680. doi: 10.1007/s00401-012-1040-2
Abstract
Gastrointestinal dysfunction is a prominent non-motor feature of Parkinson’s disease (PD) that contributes directly to the morbidity of patients, complicates management of motor symptoms, and may herald incipient PD in patients without motor disability. Although PD has traditionally been considered a disease of dopaminergic neurons in the substantia nigra, analyses of gastrointestinal samples from PD patients have consistently revealed pathology in the enteric nervous system (ENS). The relationship of PD pathology to GI dysmotility is poorly understood, and this lack of understanding has led to limited success in developing treatments for PD-related GI symptoms. We have quantitatively compared myenteric neuron density and relative abundance of NO, VIP, and catecholamine neurons between patients with PD and control individuals along the length of the GI tract. In addition, we have examined the frequency of GI α-synuclein neuritic pathology and its co-localization with the same neuronal markers. We have included a comparison with a small population of patients with incidental Lewy bodies (ILB) found at autopsy. These data indicate there is no neuronal loss in the myenteric plexus in PD. Lewy body pathology parallels parasympathetic autonomic input from the DMV, not the distribution of extrinsic sympathetic input or intrinsic enteric neurons, and is only rarely co-localized with tyrosine hydroxylase. These data provide a critical background to which further analyses of the effect of PD on the GI tract may be compared and suggest that neuropathology in myenteric neurons is unlikely to be a causative factor in PD-related GI dysmotility.
Keywords: enteric, gastrointestinal, nitric oxide, vasoactive intestinal peptide, catecholamine, acetylcholine, constipation, gastroparesis, Lewy body, synuclein
Introduction
Gastrointestinal dysfunction is a prominent non-motor feature of Parkinson’s disease (PD). PD patients experience symptoms that span the entire alimentary tract including abnormal salivation, dysphagia, delayed gastric emptying, constipation, and defecatory dysfunction [41,42]. GI dysmotility contributes directly to the morbidity of PD and complicates the disease’s clinical management. For example, in the stomach, delayed emptying leads to nausea, contributes to weight loss, and adds to fluctuations in motor impairment from variable absorption of medication [30,14,22,21]. In the colon, longer transit time causes harder stools and constipation [4,15,51]. In some cases, GI symptoms may be a herald of incipient PD [1]. The exact mechanism of motility dysfunction in PD is poorly understood, and lack of understanding of changes in the gastrointestinal tract in PD has led to limited success in developing treatments.
Control of GI motility is directed by the intrinsic enteric nervous system (ENS), a semiautonomous neuronal network that consists of a deep myenteric and more superficial submucosal plexus [19,58,28]. The myenteric plexus is the more important of the two in terms of controlling motility. Circuitry in this plexus controls temporal coordination of intestinal smooth muscle upon which effective peristalsis depends. Neurons producing virtually every neurotransmitter seen in the central nervous system have been identified within the ENS, including acetylcholine, nitric oxide (NO), vasoactive intestinal peptide (VIP), and catecholamines [28]. Central modulation of ENS function is mediated by autonomic parasympathetic input primarily from the dorsal motor nucleus of the vagus (DMV) and sympathetic input from para- and prevertebral ganglia.
Although PD has traditionally been considered a disease of dopaminergic neurons in the substantia nigra, analyses of gastrointestinal samples from PD patients have consistently revealed neural pathology. Lewy bodies and AS neuritic pathology have been found in the ENS in nearly every PD patient examined [56,55,54,29,31,52] and may appear early in the disease course [11,6].
Despite the frequency of enteric α-synuclein aggregation and GI symptoms in PD, it is not clear whether or not there is a causal relationship between the two findings, and clinicopathological correlation studies have only begun to be performed [13,32]. In addition, experience from the midbrain suggests that clinical symptoms in PD are driven primarily by neuronal loss rather than aggregation of α-synuclein. As such, quantitative evaluation of neuron populations that control GI motility, such as enteric neurons, is an important step toward determining the pathological underpinnings of GI symptoms in PD.
We have compared myenteric neuron density and relative abundance of NO, VIP, and catecholamine neurons between patients with PD and control individuals along the length of the GI tract. In addition, we have examined the frequency of GI α-synuclein neuritic pathology and its co-localization with the same neuronal markers. We have included a comparison with a small population of patients with incidental Lewy bodies (ILB) found at autopsy. These data indicate there is no neuronal loss in the myenteric plexus in PD. Lewy body pathology parallels parasympathetic autonomic input from the DMV, not the distribution of extrinsic sympathetic input or intrinsic enteric neurons.
Materials and Methods
Tissue samples
Six-micron formalin-fixed paraffin embedded intestinal tissue cross sections from patients with Parkinson’s disease (PD) or incidental Lewy body disease (ILB), or from control individuals with no known neurological condition (Table 1) were obtained from the Arizona Parkinson’s Disease Consortium and the Banner Sun Health Research Institute Brain and Body Donation Program in Sun City, AZ. Areas examined included stomach, duodenum, ileum, transverse colon, and rectum. Results of phosphorylated α-synuclein immunostaining from some of these samples have been previously reported [6]. Hematoxylin and eosin stained sections were examined from each sample to confirm GI tract segment and tissue integrity. All protocols related to the Brain and Body Donation Program were approved by the Banner Sun Health Research Institute Institutional Review Board.
Table 1.
Patient characteristics for postmortem samples
| CASE | Age | Gender | PMI (hrs) | Cause of death |
|---|---|---|---|---|
| Control | ||||
| 1 | 73 | M | 2.5 | acute myeloid leukemia |
| 2 | 88 | F | 2.5 | complications of stroke |
| 3 | 91 | M | 3.2 | stroke, atherosclerotic cardiovascular disease |
| 4 | 91 | M | 2.7 | respiratory arrest |
| 5 | 92 | M | 3.0 | cardiorespiratory failure due to severe anemia |
| 6 | 75 | F | 2.8 | pulmonary embolus, IVC thrombus, malignancy NOS |
| 7 | 87 | M | 2.3 | squamous cell carcinoma |
| 8 | 76 | M | 2.3 | multiple myeloma |
| 9 | 88 | F | 3.0 | intracranial hemorrhage due to thrombocytopenia |
| 10 | 91 | F | 7.3 | congestive heart failure |
| 11 | 82 | M | 3.7 | acute renal failure |
| 12 | 65 | M | 3.5 | metastatic lung cancer |
| PD | ||||
| 13 | 79 | M | 2.5 | Parkinson’s disease |
| 14 | 87 | F | 2.2 | cardiorespiratory arrest |
| 15 | 82 | F | 4.2 | Parkinson’s disease |
| 16 | 63 | M | 18.5 | Parkinson’s disease |
| 17 | 69 | M | 4.0 | Parkinson’s disease |
| 18 | 69 | M | 4.2 | coronary artery disease |
| 19 | 76 | M | 4.8 | complications of hip fracture and surgery |
| 20 | 75 | M | 2.3 | intracranial hemorrhage |
| 21 | 82 | M | 10.3 | Parkinson’s disease |
| 22 | 82 | F | 3.0 | brain cancer |
| 23 | 74 | M | 8.8 | Parkinson’s disease |
| 24 | 75 | M | 2.3 | Parkinson’s disease |
| 25 | 79 | F | 3.2 | cardiac arrest due to arteriosclerotic disease |
| ILB | ||||
| 26 | 82 | F | 2.5 | acute myeloid leukemia |
| 27 | 87 | F | 10.3 | ischemic heart disease, pulmonary embolism |
| 28 | 74 | M | 3.5 | glioblastoma multiforme |
| 29 | 94 | F | 2.8 | ischemic heart disease |
Antibody Characterization
Please see Table 2 for a list of all primary antibodies used. The rabbit anti-NOS antibody recognized a band of 161 kD on western blots of human brain (manufacturer’s data sheet). Staining was eliminated by preincubation of the diluted antibody (1:500) with 25 micrograms/ml of recombinant rat protein. The antibody stained a pattern of cellular morphology and distribution in the human ENS that was comparable to previous reports concerning nitric oxide neurons [9,38].
Table 2.
Primary antibodies
| Antigen | Immunogen | Source | Dilution |
|---|---|---|---|
| Human neuronal protein HuC/HuD (HuC/D) | 12-Residue synthetic peptide representing the carboxy-terminal domain of human HuD from amino acids 240–251 | InvitrogenMouse ponoclonalCat: A21271Lot: 53877A | 1:1000 |
| Neuronal nitric oxide synthase (nNOS) | Recombinant human nNOS | MilliporeRabbit polyclonalCat: AB5380Lot: LV1547827 | 1:1000 |
| Tyrosine hydroxylase (TH) | Denatured TH from rat pheochromocytoma | MilliporeRabbit polyclonalCat: AB152Lot: LV1458671 | 1:500 |
| Vasoactive intestinal peptide (VIP) | Human VIP, aa1-95, N-terminal | Santa Cruz BiotechnologyRabbit polyclonalCat: sc-20727Lot: A0203 | 1:500 |
| Alpha-synuclein (AS) LB509 | Lewy bodies purified from patients | ZymedMouse monoclonalCat: 18-0215Lot: 20369980 | 1:400 (IF)1:1000 (IHC) |
The rabbit anti-VIP antibody recognized a single band of 20 kD on western blots of mouse brain (manufacturer’s data sheets). The antibody stained a pattern of cellular morphology and distribution in the human ENS that was comparable to previous reports concerning VIP neurons [3,38].
The rabbit anti-TH antibody recognized a band of 60 kD on western blots of rat brain (manufacturer’s data sheets). The antibody stained a pattern of cellular morphology and distribution in the human ENS that was comparable to previous reports concerning catecholaminergic neurons [3,38].
The mouse anti-HuC/D antibody recognized bands of 36, 40, and 42 kD on western blots of rat brain [40]. The antibody stained a pattern of cellular morphology and distribution in the human ENS that was comparable to previous reports [43,25,35,9,38].
The mouse anti-α-synuclein antibody recognized a band of 18 kD on western blots of human brain and recognized α- but not β-synuclein (manufacturer’s data sheet). The antibody stained a pattern of Lewy bodies in the human ENS that was comparable to previous reports [6,7].
Immunostaining
After de-waxing and washing the sections in distilled water, antigen retrieval was performed using boiling 1 mM sodium citrate solution (pH 8.5) for 10 minutes. Sections were cooled to room temperature, and washed with distilled water and 1% tris-buffered saline pH 7.3 (TBS). When staining for Lewy bodies with LB509, incubation with 5–12 μg/ml proteinase K (Enzo Life Science; Cat. 33802; Lot 28BEA0) for 20–30 min 37 °C. Otherwise, the next step was a one-hour blocking incubation in 5% normal donkey serum (NDS). Sections were incubated in primary antibody solution overnight at 4 °C.
The following day, sections were washed with TBS and incubated with secondary antibody for 1–2h. For co-immunofluorscence, rabbit primary antibodies against nNOS, VIP, and TH were visualized with AlexaFluor488-conjugated goat anti-rabbit secondary antibody (1:500, Invitrogen). Mouse anti-HuC/D and LB509 were visualized with Cy3-conjugated donkey- or goat anti-mouse secondary (1:200 or 1:500, Jackson Immunolabs). Immunofluorescent samples were washed with TBS and coverslipped using Aquamount. For immunohistochemistry of Lewy bodies, after incubation with LB509, endogenous peroxidases were blocked by incubation with 3% H2O2 for 10 min, sections were incubated with biotin-conjugated goat anti-mouse secondary antibody (1:1000, Jackson), washed with TBS, and exposed to ABC solution (Vector Labs) for 30 min at room temperature. They were then incubated with 3, 3′-diaminobenzidine (DAB) tetrachloride for 30 minutes, washed, and coverslipped.
No labeling was observed when protocols were completed without exposure to primary antibody. Control experiments were performed to ensure there was no cross-reactivity of secondary antibodies in co-labeling experiments. All sections were evaluated by blinded observers in pseudorandom order.
Neuron counting
Counting was performed by a blinded observer on an upright Olympus BX-60 CDU spinning-disc confocal microscope at 20X magnification. Neuronal cell body and nuclear diameters were measured using Slidebook imaging software (Intelligent Imaging Innovations).
For evaluation of total myenteric neurons per ganglion area, ten serial sections from each GI segment collected at 60 μm intervals were stained for the pan-neuronal marker HuC/D for every case. In every section, photomicrographs of every myenteric ganglion along the ridge separating longitudinal from circular intestinal smooth muscle were captured using a Hamamatsu ORCA-ER High Resolution Digital Monochrome CCD camera. Ganglion area was measured using Slidebook, and neuronal profiles were counted manually. In order to avoid underestimation of neuronal number, both nucleated and non-nucleated profiles were counted [20]. For each case, neuronal profiles and ganglion area were both summed within segments. The number of neurons per ganglion area (mm2) was calculated for each case as Σ neuron profiles ÷ Σ area measurements [26].
For evaluation of relative proportions of individual neurochemical phenotypes (NOS, VIP, TH) using double-label immunofluorescence, three sections were examined per segment in each case. All positive neurons in each segment were counted and divided by the total number of HuC/D-positive cells in each segment [3,38].
For both per area and relative proportion data, values for each case were averaged together and are presented as mean ± standard error (N = number of cases). Statistical comparisons among individual GI tract segments between controls and patients were made using mixed-factor repeated measures ANOVA with post-hoc Bonferroni tests. A p-value < 0.05 was considered significant.
Analysis of α-synuclein staining
For evaluation of α-synuclein aggregation, one section was examined per segment in each case after IHC with the LB509 antibody. Samples in which synuclein aggregates were detected were subsequently evaluated with co-labeling protocols for LB509 with NOS, VIP, and TH using one additional section for each neurochemical phenotype. Analysis of IHC stained sections was performed by a blinded observer on an upright Olympus BX-60 light microscope. For co-localization studies, slides were examined on an upright Olympus BX-60 CDU spinning-disc confocal microscope at 20X and 60X magnifications.
IHC-stained slides were analyzed for the presence or absence of α-synuclein aggregates. The gut layer (myenteric plexus, submucosa, etc.) in which aggregates were detected was noted as was the frequency of myenteric and submucosal ganglia containing aggregates. In the two slides in which co-localization was observed, the Slidebook software was used to count the proportion of α-synuclein-positive objects that were also positive for TH.
Results
Qualitative examination confirmed integrity of intestinal layers from mucosa to serosa, low levels of background staining, and discernable immunostaining for all sections and labeling techniques.
NOS, VIP, TH antibodies all labeled neuronal cell bodies in the myenteric plexus (Figs 1 and 8). Perikaryal TH and VIP staining tended to have a granular pattern, whereas NOS immunoreactivity was more diffuse in the cell body. Staining of neuronal processes was robust for all three antibodies.
Fig. 1. Quantitative assessment of myenteric neurons.
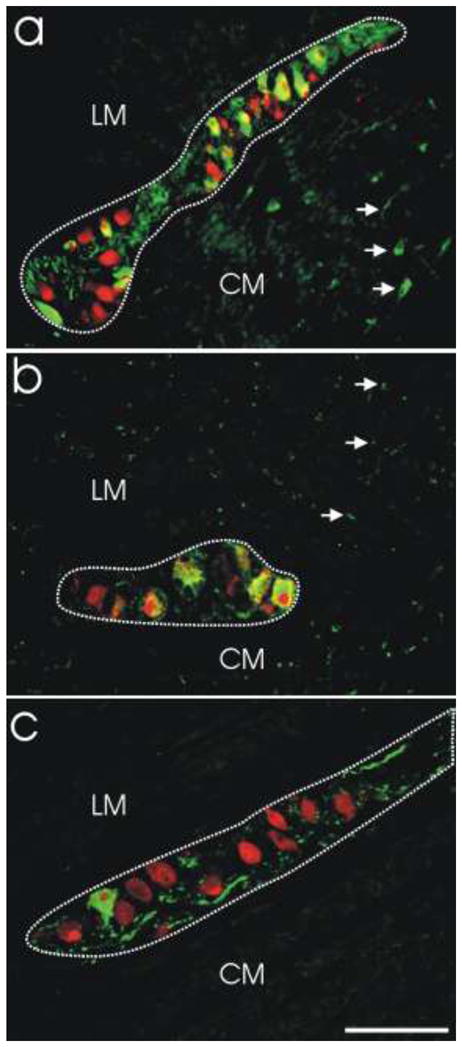
Absolute numbers (neurons/mm2) and relative proportions of myenteric neuron phenotypes were assessed using double-label immunofluorescence. a. Myenteric ganglion from colon demonstrating double-label immunofluorescence for NOS (green) and HuC/D (red). Double-labeled NOS-positive neurons are yellow/orange. NOS-negative neurons (HuC/D only) are red. NOS-positive processes are green (arrows). b. Myenteric ganglion from stomach demonstrating double-label immunofluorescence for VIP (green) and HuC/D (red). Double-labeled VIP-positive neurons are yellow/orange. VIP-negative neurons (HuC/D only) are red. VIP-positive processes are green (arrows). c. Myenteric ganglion from stomach demonstrating double-label immunofluorescence for TH (green) and HuC/D (red). Double-labeled TH-positive neurons are yellow/orange. TH-negative neurons (HuC/D only) are red. TH-positive processes are green. Dotted lines outline ganglion area. LM, longitudinal muscle layer; CM, circular muscle layer. Scale bar = 100 μm
Fig. 8. Lewy bodies in gut from PD patients were not observed to co-localize with NOS or VIP, and only very rarely with TH.
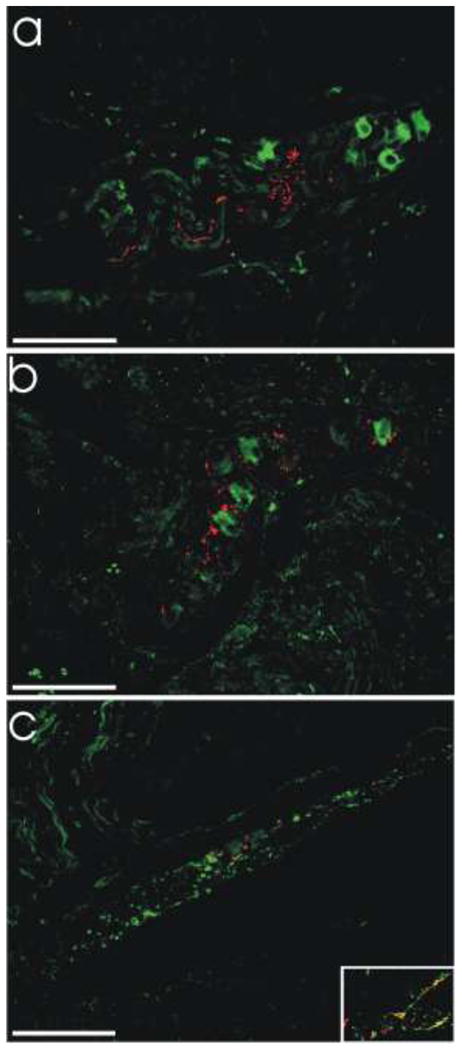
a. LB509 (red) and NOS (green). b. LB509 (red) and VIP (green). c. LB509 (red) and TH (green). All scale bars = 100 μm. Inset in c shows a small area of double-labeling of LB509-positive neurites with TH.
The pan-neuronal antibody HuC/D was used to label all neurons in the myenteric plexus as the basis for relative quantification of neurochemical phenotype. For NOS, VIP, and TH, double-label immunofluorescence was used to quantify the proportion of each subtype (Fig 1). All neurons positive for NOS, VIP, or TH were also HuC/D-positive. The total number of neuron profiles counted per section and per mm2 for individual samples was comparable for all co-labeling protocols (not shown). Table 3 demonstrates neuronal cell body and nuclear diameters were essentially identical for all neuronal subtypes in the myenteric plexus.
Table 3.
Average ± SEM diameter (μm)
| Neuron type | Soma | Nucleus |
|---|---|---|
| NOS | 23 ± 1.6 | 10 ± 0.4 |
| VIP | 23 ± 0.8 | 11 ± 0.3 |
| TH | 23 ± 1.4 | 11 ± 0.6 |
| HuC/D | 23 ± 0.8 | 11 ± 0.3 |
Table 4 shows the number of myenteric neuron profiles counted and area measurements for each segment in individual cases. On average (± SEM) across all cases there were 474 ± 51 neuron profiles counted in the stomach, 707 ± 54 in the duodenum, and 1594 ± 200 in the colon. As can be seen in Table 5, the average density of myenteric neurons across all cases was fairly similar in all three segments (470 ± 23 neurons per mm2 in the stomach, 404 ± 17 in the duodenum, and 526 ± 22 in the colon).
Table 4.
Neuron profile counts and myenteric ganglion area measurements in post-mortem samples
| Stomach | Duodenum | Colon | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CASE | Neurons | Area (mm2) | Neurons/mm2 | Neurons | Area (mm2) | Neurons/mm2 | Neurons | Area (mm2) | Neurons/mm2 |
| Control | |||||||||
| 1 | 131 | 0.33 | 400 | 433 | 1.17 | 371 | 1365 | 2.52 | 542 |
| 2 | 395 | 0.89 | 446 | 520 | 1.50 | 346 | 1663 | 4.61 | 361 |
| 3 | 263 | 0.71 | 370 | 713 | 2.37 | 300 | 1765 | 4.19 | 421 |
| 4 | 384 | 1.22 | 315 | 1270 | 3.21 | 396 | 2251 | 4.38 | 513 |
| 5 | 139 | 0.18 | 771 | 1020 | 2.70 | 378 | 1482 | 2.38 | 623 |
| 6 | 470 | 1.01 | 466 | 966 | 2.33 | 415 | 1203 | 2.15 | 559 |
| 7 | 724 | 2.14 | 339 | 540 | 1.29 | 418 | 417 | 0.91 | 458 |
| 8 | 896 | 1.33 | 674 | 451 | 0.99 | 456 | 3048 | 3.92 | 778 |
| 9 | 465 | 1.43 | 325 | 609 | 1.31 | 463 | 248 | 0.37 | 669 |
| 10 | 1195 | 3.66 | 326 | 699 | 2.00 | 350 | 1648 | 3.34 | 494 |
| 11 | 453 | 0.76 | 594 | 722 | 1.28 | 565 | 872 | 1.70 | 513 |
| 12 | 927 | 1.87 | 495 | 896 | 1.67 | 536 | 1223 | 2.22 | 552 |
| PD | |||||||||
| 13 | 852 | 1.78 | 479 | 708 | 1.92 | 369 | 1569 | 3.24 | 484 |
| 14 | 645 | 1.18 | 545 | 689 | 1.17 | 591 | 1121 | 2.20 | 509 |
| 15 | 671 | 1.54 | 435 | 477 | 1.05 | 456 | 1123 | 2.39 | 470 |
| 16 | 334 | 0.92 | 362 | 375 | 1.83 | 205 | 1778 | 4.69 | 379 |
| 17 | 110 | 0.16 | 671 | 637 | 1.51 | 423 | 468 | 0.76 | 615 |
| 18 | 341 | 0.64 | 535 | 509 | 1.20 | 426 | 1041 | 2.68 | 389 |
| 19 | 241 | 0.73 | 331 | 387 | 1.43 | 270 | 4700 | 8.02 | 586 |
| 20 | 638 | 1.32 | 483 | 1432 | 3.63 | 395 | 2611 | 4.43 | 589 |
| 21 | 314 | 0.88 | 358 | 716 | 2.02 | 355 | 491 | 1.12 | 438 |
| 22 | 525 | 0.97 | 540 | 1153 | 2.44 | 473 | 3647 | 5.93 | 615 |
| 23 | 459 | 1.22 | 376 | 263 | 0.79 | 335 | 155 | 0.58 | 267 |
| 24 | 667 | 1.54 | 433 | 437 | 1.57 | 278 | 825 | 1.19 | 691 |
| 25 | 559 | 1.39 | 401 | 1119 | 3.71 | 302 | 2365 | 4.20 | 564 |
| ILB | |||||||||
| 26 | 250 | 0.51 | 495 | 1033 | 2.69 | 384 | 2109 | 3.82 | 552 |
| 27 | 55 | 0.07 | 756 | 506 | 0.87 | 582 | 974 | 1.34 | 729 |
| 28 | 458 | 1.06 | 431 | 534 | 1.21 | 443 | 794 | 2.19 | 363 |
| 29 | 196 | 0.42 | 469 | 691 | 1.57 | 440 | 3261 | 6.27 | 520 |
| Mean ± SEM | |||||||||
| Control | 537 ± 96 | 1.29 ± 0.3 | 460 ± 43 | 736 ± 74 | 1.82 ± 0.2 | 416 ± 23 | 1432 ± 219 | 2.7 ± 0.4 | 540 ± 32 |
| PD | 489 ± 61 | 1.1 ± 1.8 | 458 ± 28 | 685 ± 101 | 1.86 ± 0.3 | 375 ± 29 | 1684 ± 385 | 3.2 ± 0.6 | 507 ± 34 |
| ILB | 238 ± 83 | 0.5 ± 0.2 | 538 ± 74 | 691 ± 121 | 1.58 ± 0.4 | 462 ± 42 | 1784 ± 572 | 3.4 ± 1.1 | 540 ± 75 |
| All | 474 ± 51 | 1.1 ± 0.13 | 470 ± 23 | 707 ± 54 | 1.81 ± 0.1 | 404 ± 17 | 1594 ± 200 | 3.0 ± 0.3 | 526 ± 22 |
Table 5.
Clinical and pathological characteristics of PD cases
| PD case | Age at onset | Disease duration (years) | H & Y stage | SN depigmentation | DMV Lewy pathology |
|---|---|---|---|---|---|
| 13 | 75 | 4 | n/a | severe | 3 |
| 14 | 78 | 9 | 3 | severe | 3 |
| 15 | 63 | 19 | 3 | severe | 3 |
| 16 | 49 | 14 | 3 | severe | 4 |
| 17 | 47 | 22 | 4 | severe | 4 |
| 18 | 59 | 10 | 2.5 | severe | 3 |
| 19 | 67 | 9 | 3 | severe | 4 |
| 20 | 54 | 21 | 3 | severe | 4 |
| 21 | 67 | 15 | 4 | severe | 4 |
| 22 | 73 | 9 | 2.5 | severe | 4 |
| 23 | 57 | 17 | 3 | severe | 3 |
| 24 | 59 | 16 | 3 | severe | 2 |
| 25 | 65 | 14 | 4 | n/a | 3 |
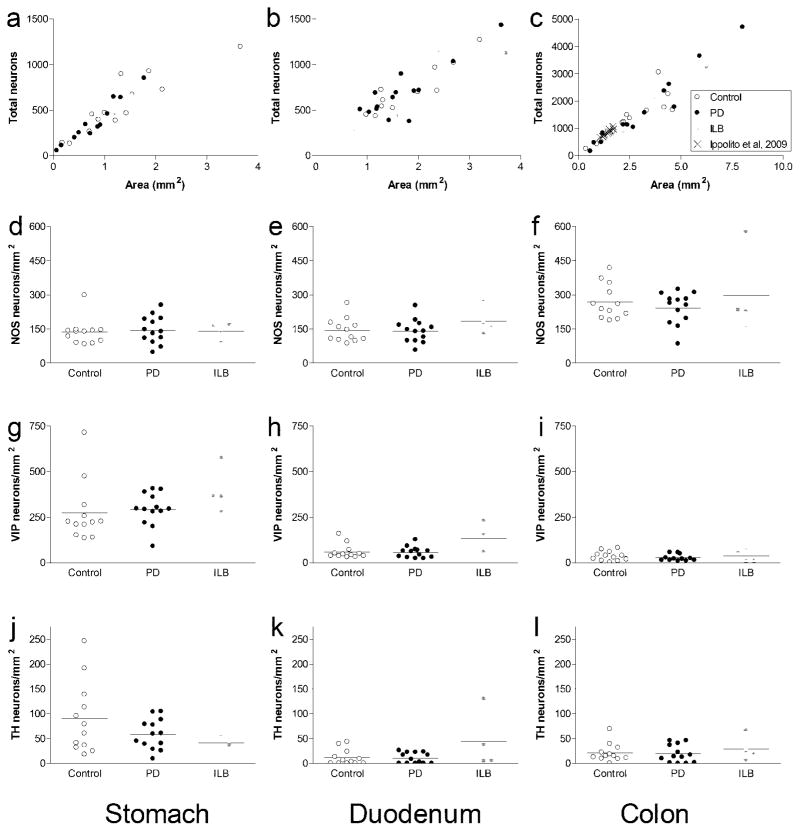
As expected, there was a linear relationship between neuron number and ganglion area (Fig 2a–c). When compared to the calculated averages, the slopes of linear regression lines (Fig 2a–c) give comparable neuronal density values in each segment (408 ± 17 neurons per mm2 in the stomach, 381 ± 14 in the duodenum, and 533 ± 18 in the colon). Not surprisingly, correlation between the two methods was closer when more neuronal profiles were counted. Superimposing colon data from Ippolito et al., 2009 onto our colon graph revealed very close agreement between the two studies (Fig 2c).
Fig. 2. Numbers of myenteric neurons (per mm2 ganglion area) in stomach, duodenum, and colon.
a-c. Scatter plots of total neurons (HuD-positive) counted per mm2 from all cases for (a) stomach (R2 = 0.84), (b) duodenum (R2 = 0.77), and (c) colon (R2 = 0.90). d–f. Dot plots of NOS neurons counted per mm2 for (d) stomach, (e) duodenum, and (f) colon. g–i. Dot plots of VIP neurons counted per mm2 for (g) stomach, (h) duodenum, and (i) colon. j–l. Dot plots of TH neurons counted per mm2 for (j) stomach, (k) duodenum, and (l) colon
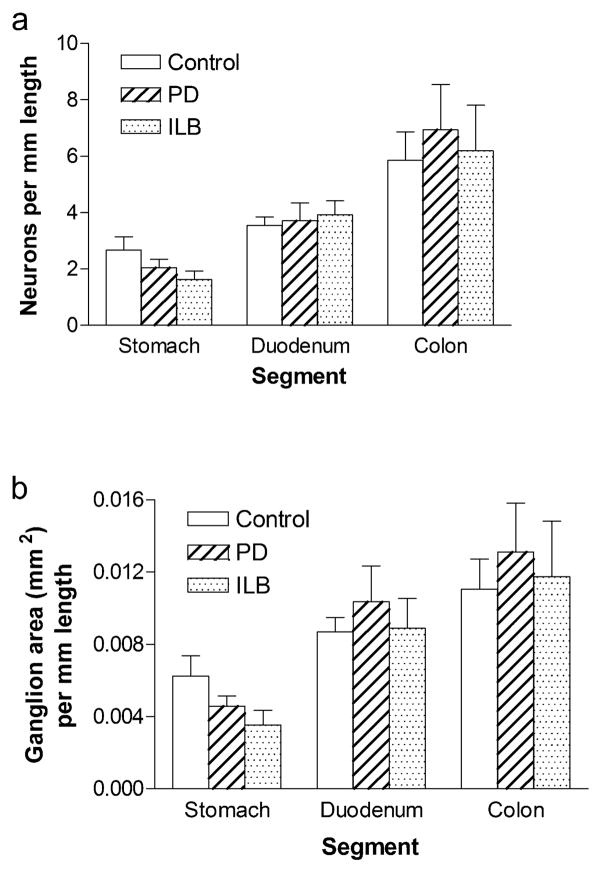
As expected from prior reports, while density per ganglion area was fairly comparable in different segments, neurons per mm length (Fig 3a) increased in a proximal to distal gradient reflecting greater total myenteric ganglion area in colon than in stomach (Fig 3b). As with the neuronal density per area data, the data concerning neuronal density per mm colonic length was in close agreement with previous reports [9].
Fig. 3. Myenteric neuron number and ganglion area (per mm intestinal length) in PD and ILB.
a. HUD neurons per mm length b. Ganglion area per mm length. Bars are mean ± SEM from 12 control individuals, 13 PD patients, and 4 ILB patients. There is no difference between controls and patients in any segment. Density of ganglia per mm length varies based on GI segment examined
There were no differences in total myenteric neuron density between controls or PD patients in any segment examined (Table 5, Fig 2a–c, and Fig 3a). There were also no differences in individual myenteric neurochemical phenotypes (NOS, VIP, TH); that data was more variable due to smaller numbers of neurons counted (Fig 2d–l).
Proportion of neuron phenotypes in the myenteric plexus
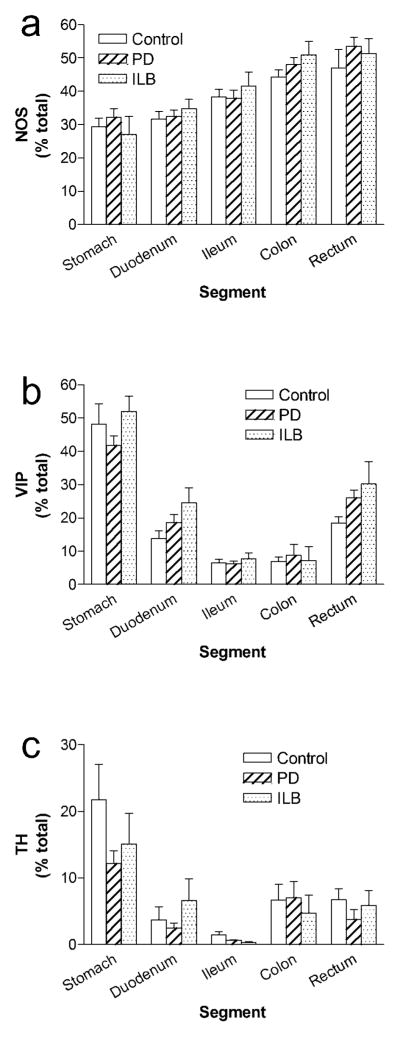
As we and others have previously described, there are striking overall patterns in the neurochemical coding of the normal human myenteric plexus that vary based on GI tract segment (Fig 4). In general, the proportion of NO neurons in the human myenteric plexus is higher in distal (47 ± 6% in rectum) than proximal segments (29 ± 3% in stomach; Fig 4a). There is a progressive increase in abundance of NO neurons (R2 = 0.28, F = 22.3, p < 0.0001 for slope > 0) along the length of the gut. VIP neurons are most prominent in the stomach where they account for nearly half (48 ± 6%) of myenteric neurons; other segments have a significantly lower number of VIP-positive myenteric neurons (p < 0.01 vs. stomach, repeated measures ANOVA with post-hoc Dunnett test; Fig 4b). A small but significant proportion of myenteric neurons were TH-positive (22 ± 5% in stomach and 1–7% in other regions; Fig 4c). Similar to VIP, TH neurons were more prominent in the stomach than any other region (p < 0.01, repeated measures ANOVA with post-hoc Dunnett test).
Fig. 4. Relative proportions of major myenteric neuron subtypes in PD and ILB.
a. NOS b. VIP c. TH. Bars are mean ± SEM from 12 control individuals, 13 PD patients, and 4 ILB patients. There is no difference in the proportion of any neurochemical phenotype between controls and patients in any segment. The relative proportions of individual neurochemical subtypes vary based on GI tract segment
There were no differences in relative proportions of individual myenteric neuron phenotypes between controls or patients in any segment examined. Specifically, for NOS, two-way repeated measures ANOVA revealed no interaction between disease state and segment [F(8,104) = 0.4, p = 0.9] and no main effect of disease state [F(2,104) = 1.5, p = 0.2]; as expected the main effect of segment was significant [F(4,104) = 18.7, p < 0.0001]. For VIP, there was no interaction between disease state and segment [F(8,104) = 1.3, p = 0.3] and no main effect of disease state [F(2,104) = 2, p = 0.2]; as expected the main effect of segment was significant [F(4,104) = 64.7, p < 0.0001]. For TH, there was no interaction between disease state and segment [F(8,104) = 1.3, p = 0.3] and no main effect of disease state [F(2,104) = 1.1, p = 0.4]; as expected the main effect of segment was significant [F(4,104) = 16.6, p < 0.0001]. Post-hoc comparisons were not performed since there was no effect of disease state on neuron proportions.
Assessment of α-synuclein pathology in the gut from PD and ILB patients
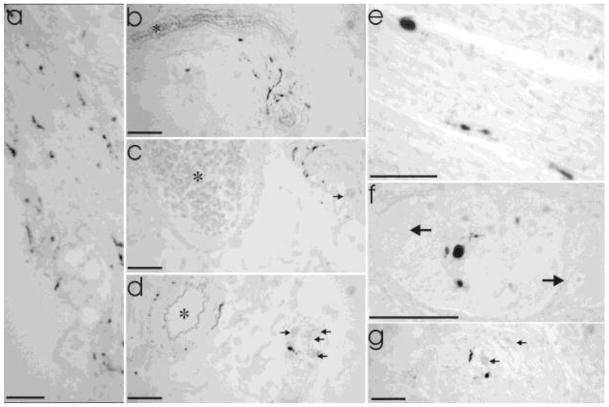
Alpha-synuclein neuritic pathology or cytoplasmic Lewy bodies were not found in any samples from control individuals, but were detected in at least one GI segment in 12/13 PD cases and 2/4 ILB cases after examination of a single section from each segment (5 sections per case) with immunohistochemistry. Extending examination of PD and ILB cases to three sections per segment revealed alpha-synuclein neuritic pathology in 13/13 PD cases and 2/4 ILB cases. Representative examples are presented in Fig 5. In PD and ILB, pathology was detected in every segment of the gut in at least one case, but the frequency differed based on segment and disease (PD v. ILB) (Fig 6). In general, α-synuclein neuritic pathology was more frequent in the proximal than distal GI tract and most prominent in the stomach.
Fig. 5. Examples of Lewy pathology in gut from PD and ILB patients in different layers from different segments of the gut.
a. Mucosal staining in the stomach (lumen on lower left). b–d demonstrate submucosal staining in stomach (b), duodenum (c), and colon (d). Asterisks (*) indicate submucosal blood vessels. Arrows in c and d indicate neurons in submucosal ganglia. e. Synuclein staining in circular muscle (stomach). Staining in muscle layers was extremely rare with this the only observable example in the study. f–g. Myenteric ganglia staining in ileum (f, high magnification) and colon (g, lower magnification). Arrows indicate myenteric neurons in ganglia surrounded by intestinal smooth muscle. All scale bars = 50 μm
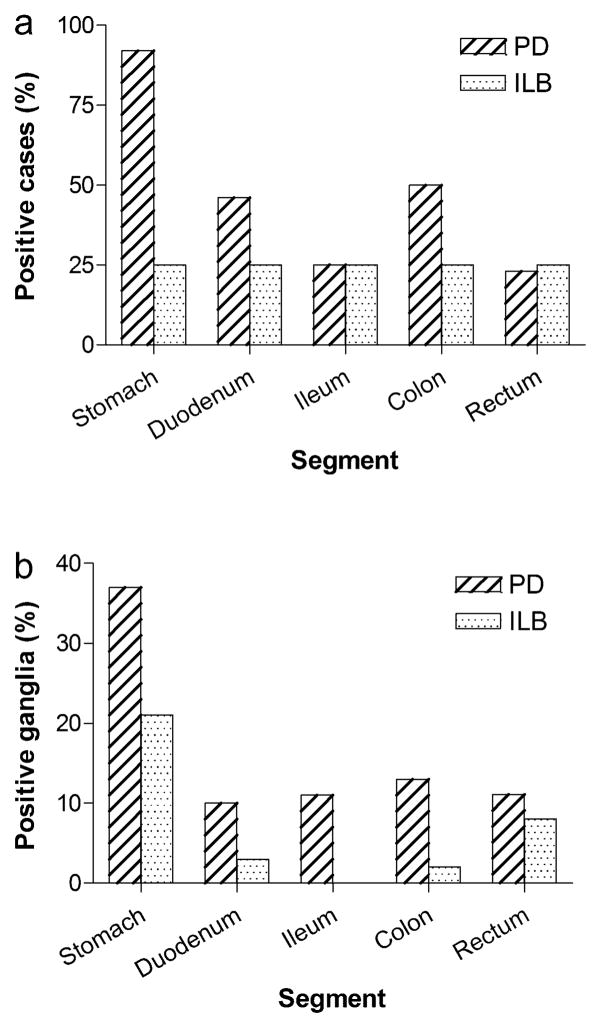
Fig. 6. Distribution of Lewy pathology by gut segment in PD and ILB patients.
a. The percent of cases in which Lewy bodies were detected in any layer of any gut segment after examination of one section per segment (a total of 5 sections per case). N = 13 for PD and 4 for ILB. No Lewy bodies were detected in control samples (not shown). b. The percent of the total number of myenteric ganglia counted that contained Lewy bodies for each patient group. Total number of ganglia examined: stomach (123 PD and 24 ILB), duodenum (104 PD and 33 ILB), ileum (221 PD and 62 ILB), colon (214 PD and 64 ILB), and rectum (27 PD and 37 ILB)
LB509-positive aggregates were identified most often in the myenteric plexus, but were also frequently seen in the submucosal plexus and surrounding submucosal blood vessels. Staining in other gut layers (serosa, smooth muscle, mucosa) was observed, but rarely (Figs 5 and 7).
Fig. 7. Distribution of Lewy pathology by gut layer in PD patients.
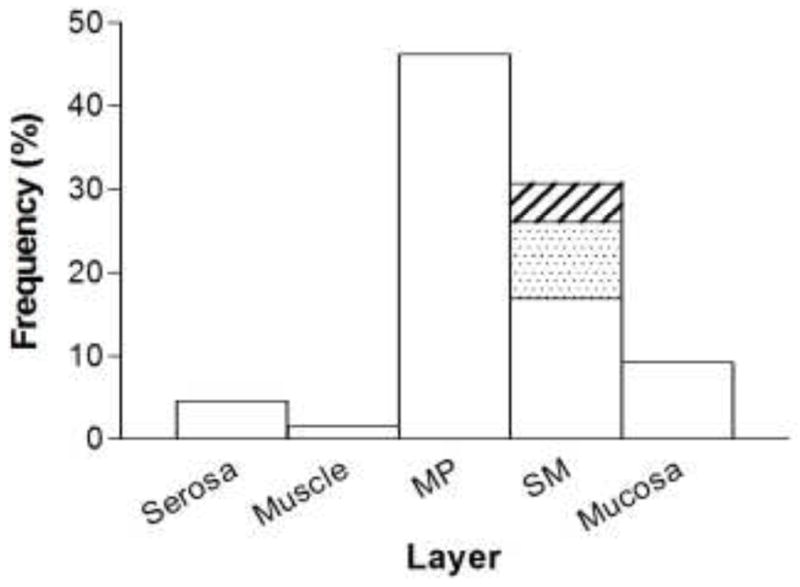
Distribution of Lewy pathology in PD patients by layer across all gut segments. MP, myenteric plexus; SM, submucosa. Submucosal (SM) staining is broken out into 3 categories: submucosal blood vessels only (white), submucosal plexus only (shaded), and staining in both structures (hatched)
Thirty tissue blocks from PD and ILB cases in which LB509-positive aggregates were identified using the immunohistochemical method were re-evaluated using dual-label immunofluorescence. Of 90 sections examined, we detected fluorescent LB509-positive aggregates in 66. Co-localization experiments revealed no labeling of α-synuclein pathology (areas of LB509-positivity) with NOS or VIP, with the exception of a single cytoplasmic Lewy body observed in one VIP-positive myenteric neuron (Fig 8). Of 21 intestinal sections examined with TH immunofluorescence, four showed a small degree of co-localization with LB509 (Fig 8c). Even in these sections, only a minority of LB509-positive aggregates were TH-positive (39 out of 245; 15.9%). Taken together, these data allow us to estimate that less than 3% of α-synuclein aggregates in the GI tract from these cases were TH-positive.
Correlation of GI pathology with brain pathology and clinical data from PD and ILB patients
All patients had advanced clinical PD and advanced brain pathology (Table 5). There was no correlation between any clinical parameter (age, age at onset, disease duration, Hoehn and Yahr stage) and any enteric histopathological parameter (neuron density, neuronal proportions, Lewy body frequency, or Lewy body density). Given the advanced nature of the brain pathology in this population of cases, there was no effective way to correlate brain neuropathology with enteric neuropathology; both were present in every PD case examined.
Some clinical data concerning GI function and medications was available for all patients; however, this data was obtained from retrospective review of patient records not by specific questioning. As such, reliable correlation analyses could not be performed.
Discussion
The present study provides the first comprehensive and quantitative neuropathological assessment of the myenteric plexus in Parkinson’s disease.
The main findings of this study are: 1) there is no absolute or proportional loss of myenteric neurons in Parkinson’s disease; 2) gastrointestinal Lewy body pathology parallels parasympathetic autonomic input from the DMV; 3) Lewy pathology in the GI tract is rarely located in catecholaminergic fibers and almost never found in VIP or NO neural elements. In addition, this study provides a comprehensive assessment of neuronal density and neurochemical coding in the myenteric plexus across multiple segments of the normal human GI tract.
Myenteric neuron numbers in PD
Quantification of myenteric neuron number has been a problematic technical hurdle. Unlike brain nuclei that are anatomically well-demarcated, the fishnet-like architecture of the MP does not lend itself to traditional quantitative techniques such as stereology. Most of the available data have come from ‘whole mount’ preparations of surgically obtained specimens [19]. There are several limitations to this approach, including limited availability of and access to tissue; time-sensitive, labor-intensive, technically challenging preparation techniques; difficulties associated with immunostaining in thick sections of GI tissue; and lack of clarity as to how to appropriately report results (neurons per ganglia, neurons per square mm, etc.). The first two issues have been particularly limiting.
In contrast, using paraffin-embedded material allows tissue specimens to be easily stored and handled without excessive care and to be made quickly available from archival collections, such as the Banner Sun Health Research Institute Brain and Body Donation Program used for this study. In addition, routine hematoxylin-eosin staining can be used to screen out samples not appropriate for analysis due to pathologies unrelated to the subject of investigation. Several studies have been performed on paraffin sections to evaluate the organization of the myenteric plexus [9,5,24] and to obtain relative proportions of myenteric neurochemical phenotypes in humans and other mammals [3,38]. The quantitative method used in this study was described recently to provide accurate detection and reliable quantification of immunolabeled myenteric ganglion cells in human colon [26,10,57]. There was very close agreement between our results and those of Ippolito et al (2009) indicating this method is valid, reproducible, and provides a reliable indication of myenteric neuron density in the colon [26]. Our results also indicate it is generalizable to other segments of the GI tract. In addition to the colon, there was a clear linear relationship between neuron number and ganglion area in the stomach and duodenum.
There was no difference in myenteric neuron density between PD patients and controls. More specifically, there was no difference in either total myenteric neurons or individual neurochemical phenotypes (NO, VIP, TH) in stomach, duodenum, or colon. Furthermore, there was no relative difference in NO, VIP, or TH neurons in any GI segment, indicating that there is no selective vulnerability of any individual myenteric neuron subtype in PD. Although cholinergic neurons were not specifically measured in this study, given their high density in the MP, any alteration would have been reflected in total and relative neuron counts.
Importantly, there was no difference in myenteric neuron density between groups regardless of whether it was measured in neurons per ganglion area or neurons per length of intestine. It is unclear how a myenteric ganglion is remodeled in the face of neurodegeneration. Hypothetically, if a myenteric neuron degenerates and the ganglion remains the same size, density per ganglion area would decrease. Conversely, if the ganglion area shrinks in a compensatory manner after neuronal death, there would be no alteration in density per area and a resulting erroneous conclusion that neurons were unaffected. Our similar results regardless of method indicate that myenteric neurons are truly preserved in this sample of PD patients.
As with every study of this type, our ability to detect a statistical difference was limited by available samples and inherent variability of the data. Based on sample size and a coefficient of variation in the data of 20–25%, the power to detect a 30% difference in myenteric neuron density in these experiments was greater than 90%; the power to detect a 10% difference was about 30%. The variability observed in this series is consistent with that expected for biological samples. A larger sample size is always desirable; however, samples were limited by the tissue collection biases that the Banner Sun Health Research Institute Brain and Body Donation Program was created to address. Given the labor-intensive nature of these experiments and analyses, expectation for future studies using a greater number of samples should be tempered. Fortunately, since there was essentially complete overlap of values from PD and control cases, the chance that even a small physiologically relevant difference was missed (type II error) is low.
The preservation of myenteric neurons was in marked contrast to extensive loss of dopamine neurons in the substantia nigra from the same PD individuals. The finding that myenteric neuron density is normal even in this population with advanced PD strongly suggests that myenteric neuron loss is not a feature of PD.
On the surface, this contradicts a widely cited paper by Singaram and collaborators that reported massive dopamine neuron loss in the colonic myenteric plexus of PD patients [50]. However, that conclusion was based primarily on dopamine immunohistochemistry, which is not a valid technique for identification of dopamine or dopamine neurons in situ. In the same study, there was no change in tyrosine hydroxylase immunohistochemistry. Based on that and the results of the present study, it is highly likely that the dopaminergic neuronal loss they reported was an experimental artifact.
One other study has investigated the ENS quantitatively from PD patients. The main focus of the study was the feasibility of detecting α-synuclein neuritic pathology in colon biopsy samples from living subjects, but a secondary analysis included counting neurons in the submucosal plexus and found a 15% decrease in SMP neurons per ganglion in PD patients [33]. The study is very intriguing and suggests method that could allow longitudinal pathological studies in PD patients. The neurons per ganglion calculation is one frequently used in studies of this type but whether or not it is a valid quantification method is not entirely clear. There are potential confounding variables to the technique such as significant tissue manipulation and subjective determination of ganglion borders, so replication of the results is critical. Despite those technical concerns, the study was well done, and interpreting our results in its context suggests that if neuron loss in any division of the ENS is causally responsible for the GI symptoms observed in PD, it is more likely to be the SMP [18]. Again, when compared to the devastation seen in the substantia nigra, even though there may be some loss of submucosal neurons in PD, the extent is minimal, raising a question as to whether or not it is clinically relevant. In addition, it is important to remember the neurochemistry and neuroanatomy of the ENS is adaptive, meaning a decrease in neurons may be a result, as opposed to a cause, of GI symptomatology.
GI α-synuclein pathology in PD
The method used for detection of α-synuclein pathology in this study was very sensitive, detecting proteinase K-resistant immunoreactivity in at least one segment in every PD patient. This is comparable to other series in which Lewy pathology has been detected in the vast majority of cases examined [56,55,6,33,52].
Despite the high frequency of GI α-synuclein pathology detected in this study, the overall burden of GI Lewy pathology was quite low. In the majority of patients, Lewy bodies were rare and detected in a small minority of enteric neural elements. Of course, this is a post-mortem, cross-sectional study, so we cannot comment on whether there are any quantitative changes in pathology over time. It is possible that following degeneration of processes, Lewy bodies disappear in a fashion analogous to that hypothesized for the cardiac sympathetic innervation [39].
LB pathology was most commonly detected in the myenteric plexus, with the submucosal layer (SMP and blood vessels) being the next most common. As mentioned above, Lewy pathology has recently been detected in submusosal colon biopsies in living PD patients [33,49]. Detection has also been demonstrated in gastric and duodenal biopsies [47]. In conjunction with prior results, the present data suggest that evaluating both enteric plexuses may provide an even higher yield [6,11,56]. Given that full-thickness intestinal biopsies and analysis of the myenteric plexus from them is now possible, that may provide a fruitful area of study for PD research and a reliable longitudinal pathological metric for evaluating PD progression. [13,37].
We have confirmed the proximal to distal gradient of Lewy pathology that has previously been reported in the GI tract of PD patients [6,56]. This gradient indicates a correlation between Lewy pathology in the myenteric plexus and innervation by the vagus nerve in that GI segments known to have dense innervation from the DMV (e.g., stomach) had higher frequencies of PD pathology. In contrast, the densities of extrinsic sympathetic and intrinsic enteric innervation are more consistent throughout the GI tract. This relationship suggests that either a proportion of α-synuclein neuritic pathology is located in vagal efferents, vagal innervation increases the propensity to develop damage in PD, or both.
Immunofluorescent co-labeling experiments revealed no overlap between α-synuclein neuritic pathology and NOS-positivity, a single cytoplasmic Lewy body in one VIP-positive myenteric neuron, and a low frequency of overlap (~3%) with TH immunoreactivity. Co-localization with TH or VIP has been previously reported, although in both instances at much higher percentages (> 50% each) with conflicting results between reports [33,54].
There are several possible interpretations consistent with the minimal co-localization observed here. First, the majority of α-synuclein neuritic pathology may be located in other neuronal phenotypes, such as cholinergic processes, either intrinsic to the ENS or extrinsic to the GI tract, such as DMV efferents. Second, there may be neurochemical plasticity in the ENS induced by synuclein deposition. Third, synuclein aggregation may cause downregulation of expression of neuronal markers. Finally, synuclein aggregates may physically displace the neurochemical markers, such that even though the neurons as a whole continue to express them, they are ‘crowded out’ of neurites in discrete areas. A combination of any or all of these possibilities may exist. In particular, changes in marker expression or physical displacement may be variable enough to be responsible for disparate results to date. Further investigation using additional markers and longitudinal sampling would be very helpful to help answer the question as to exactly which neural (or non-neural) compartment contains enteric α-synuclein neuritic pathology.
Neurochemical coding and density of myenteric plexus neurons from control individuals
Even considering control samples alone, the numbers of cases and neurons examined in this series of experiments are an order of magnitude greater than any report in the literature to date concerning neurochemical coding of enteric neurons. Overall, these data are in near-complete agreement with previously published reports concerning relative density of myenteric neurochemical phenotypes generated from more limited sample sets.
As expected, a significant proportion of myenteric plexus neurons express nitric oxide synthase (NOS) in neuronal cell bodies, and fibers innervating circular muscle and myenteric ganglia are dense. There is a progressive increase in the proportion of myenteric neurons expressing NOS from proximal to distal GI segments; NO fibers demonstrate a similar pattern. The quantitative difference is considerable in that just over a quarter of myenteric neurons in the stomach body expresses NOS while nearly half uses NO as a neurotransmitter in the rectum. Previous reports have indicated percentages of NOS myenteric neurons in humans ranging from 27–40% in stomach [44,38] to 43–51% in colon [46,45,35]. Estimates in small intestine have ranged from 20–38% [12,8]. In general, published results suggest that an increase in the proportion of myenteric neurons expressing NOS in a proximal to distal GI gradient is a fairly consistent finding across mammalian species suggesting a conserved function for NOS neurons in GI physiology [16,17]
The distribution of VIP in the ENS is opposite to that of NOS. VIP is expressed in nearly 50% of myenteric neurons in the stomach but a significantly lower percentage in more distal segments. Previous reports also support the conclusion that VIP-positive myenteric neurons are most abundant in the stomach in mammals [44,23,36,38,3]. This anatomical pattern suggests that VIP may be especially important for the modulation of gastric motility and emptying. In contrast to NOS, VIP fibers were frequent in the mucosa and submucosa in addition to innervating muscle and plexus layers. Mucosal and submucosal VIP innervation is likely derived from VIP neurons in the submucosal plexus where VIP-positive neurons have been described to be the majority in humans [3].
Myenteric neurons in humans expressing TH have been previously reported to lack dopamine-β-hydroxylase, indicating they are dopaminergic [34,3]. Conversely, many TH-positive fibers innervating the myenteric plexus and other layers of the GI tract (e.g., submucosal blood vessels) are noradrenergic and represent extrinsic sympathetic innervation [3].
As previously reported by us and others, a significant but variable proportion (approximately 1–20%) of human myentric neurons are TH-positive [3,38,53]. Catecholaminergic innervation of the myenteric ganglia was dense, presumably representing both enteric and extrinsic sympathetic fibers. Similar to VIP, TH neurons were most abundant in the stomach where they constitute nearly one fifth of myenteric neurons. In contrast to NOS and VIP, there are interesting differences between humans and other mammals with regard to TH-positive myenteric neurons. They are very rare (0–2%) in the monkey [38]; reports in rodents have varied from 0.5 to 10% in mouse small intestine [34,48,2] to 15% in rat stomach [23].
Interestingly, our data indicate that myenteric neuron density per ganglion area is comparable across GI tract segments. This is in contrast to myenteric neuron density per mm intestine, which is higher in the colon than in the duodenum and stomach. This indicates that while myenteric neuron density within ganglia is consistent throughout the GI tract, there is relatively more ganglion area (and thus more neurons) in the distal GI tract when compared to the proximal. This is likely secondary to larger ganglion size and greater numbers of ganglia [19,27]. It will be important to bear this finding in mind when interpreting future experiments assessing neuronal numbers and possible neuronal loss in the myenteric plexus.
Summary
These data strongly suggest that myenteric neuron loss is not a feature of Parkinson’s disease. Differences in digestive and storage functions of different segments of the GI tract are reflected in significant differences in neurochemical coding of myenteric neurons, but there is no change in these phenotypes in PD. As previously suggested, Lewy body pathology parallels parasympathetic autonomic input from the DMV, not the distribution of extrinsic sympathetic input or intrinsic enteric neurons. Further investigation will be required to determine the exact compartment in which α-synuclein aggregates in the GI tract of PD patients. Based on available data, neuropathology in the DMV and/or submucosal plexus is more likely than damage to the myenteric plexus to be a causative factor in PD-related GI dysmotility.
Acknowledgments
This work was supported by the Michael J. Fox Foundation for Parkinson’s Research (JGG). We are grateful to the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of the human specimens. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, and 05-901 to the Arizona Parkinson’s Disease Consortium) and the Prescott Family Initiative of the Michael J. Fox Foundation for Parkinson’s Research.
Literature cited
- 1.Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57 (3):456–462. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, Srinivasan S, Greene JG. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Experimental neurology. 2007;207 (1):4–12. doi: 10.1016/j.expneurol.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anlauf M, Schafer MK, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. The Journal of comparative neurology. 2003;459 (1):90–111. doi: 10.1002/cne.10599. [DOI] [PubMed] [Google Scholar]
- 4.Ashraf W, Wszolek ZK, Pfeiffer RF, Normand M, Maurer K, Srb F, Edwards LL, Quigley EM. Anorectal function in fluctuating (on-off) Parkinson’s disease: evaluation by combined anorectal manometry and electromyography. Mov Disord. 1995;10 (5):650–657. doi: 10.1002/mds.870100519. [DOI] [PubMed] [Google Scholar]
- 5.Aube AC, Cabarrocas J, Bauer J, Philippe D, Aubert P, Doulay F, Liblau R, Galmiche JP, Neunlist M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut. 2006;55(5):630–637. doi: 10.1136/gut.2005.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119 (6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Leverenz JB, Roncaroli F, Buttini M, Hladik CL, Sue LI, Noorigian JV, Adler CH. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116 (3):277–288. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belai A, Burnstock G. Distribution and colocalization of nitric oxide synthase and calretinin in myenteric neurons of developing, aging, and Crohn’s disease human small intestine. Digestive diseases and sciences. 1999;44 (8):1579–1587. doi: 10.1023/a:1026658826010. [DOI] [PubMed] [Google Scholar]
- 9.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL, Linden D, Cima RR, Dozois EJ, Larson DW, Camilleri M, Zinsmeister AR, Pozo MJ, Hicks GA, Farrugia G. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21 (7):746–e746. doi: 10.1111/j.1365-2982.2008.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernardini N, Segnani C, Ippolito C, De Giorgio R, Colucci R, Faussone-Pellegrini MS, Chiarugi M, Campani D, Castagna M, Mattii L, Blandizzi C, Dolfi A. Immunohistochemical analysis of myenteric ganglia and interstitial cells of Cajal in ulcerative colitis. Journal of cellular and molecular medicine. 2012;16(2):318–327. doi: 10.1111/j.1582-4934.2011.01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neuroscience letters. 2006;396 (1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Brehmer A, Schrodl F, Neuhuber W. Morphology of VIP/nNOS-immunoreactive myenteric neurons in the human gut. Histochemistry and cell biology. 2006;125 (5):557–565. doi: 10.1007/s00418-005-0107-8. [DOI] [PubMed] [Google Scholar]
- 13.Derkinderen P, Rouaud T, Lebouvier T, Bruley des Varannes S, Neunlist M, De Giorgio R. Parkinson disease: the enteric nervous system spills its guts. Neurology. 2011;77(19):1761–1767. doi: 10.1212/WNL.0b013e318236ef60. [DOI] [PubMed] [Google Scholar]
- 14.Djaldetti R, Ziv I, Melamed E. Impaired absorption of oral levodopa: a major cause for response fluctuations in Parkinson’s disease. Isr J Med Sci. 1996;32 (12):1224–1227. [PubMed] [Google Scholar]
- 15.Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology. 1992;42 (4):726–732. doi: 10.1212/wnl.42.4.726. [DOI] [PubMed] [Google Scholar]
- 16.Ekblad E, Alm P, Sundler F. Distribution, origin and projections of nitric oxide synthase-containing neurons in gut and pancreas. Neuroscience. 1994;63 (1):233–248. doi: 10.1016/0306-4522(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 17.Ekblad E, Mulder H, Uddman R, Sundler F. NOS-containing neurons in the rat gut and coeliac ganglia. Neuropharmacology. 1994;33 (11):1323–1331. doi: 10.1016/0028-3908(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 18.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PloS one. 2011;6(12):e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furness JB. The Enteric Nervous System. Blackwell; Malden: 2006. [Google Scholar]
- 20.Ganns D, Schrodl F, Neuhuber W, Brehmer A. Investigation of general and cytoskeletal markers to estimate numbers and proportions of neurons in the human intestine. Histology and histopathology. 2006;21 (1):41–51. doi: 10.14670/HH-21.41. [DOI] [PubMed] [Google Scholar]
- 21.Goetze O, Nikodem AB, Wiezcorek J, Banasch M, Przuntek H, Mueller T, Schmidt WE, Woitalla D. Predictors of gastric emptying in Parkinson’s disease. Neurogastroenterol Motil. 2006;18 (5):369–375. doi: 10.1111/j.1365-2982.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 22.Goetze O, Wieczorek J, Mueller T, Przuntek H, Schmidt WE, Woitalla D. Impaired gastric emptying of a solid test meal in patients with Parkinson’s disease using 13C-sodium octanoate breath test. Neuroscience letters. 2005;375 (3):170–173. doi: 10.1016/j.neulet.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Experimental neurology. 2009 doi: 10.1016/j.expneurol.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanani M, Fellig Y, Udassin R, Freund HR. Age-related changes in the morphology of the myenteric plexus of the human colon. Auton Neurosci. 2004;113(1–2):71–78. doi: 10.1016/j.autneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K, Ruhl A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. The Journal of comparative neurology. 2008;509 (4):356–371. doi: 10.1002/cne.21769. [DOI] [PubMed] [Google Scholar]
- 26.Ippolito C, Segnani C, De Giorgio R, Blandizzi C, Mattii L, Castagna M, Moscato S, Dolfi A, Bernardini N. Quantitative evaluation of myenteric ganglion cells in normal human left colon: implications for histopathological analysis. Cell and tissue research. 2009;336 (2):191–201. doi: 10.1007/s00441-009-0770-5. [DOI] [PubMed] [Google Scholar]
- 27.Irwin DA. The anatomy of Auerbach’s plexus. Am J Anat. 1931;49:141–166. [Google Scholar]
- 28.Johnson LR. The Mosby Physiology Monograph Series. 6. Mosby, Inc; St. Louis: 2001. Gastrointestinal Physiology. [Google Scholar]
- 29.Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37 (7):1253–1255. doi: 10.1212/wnl.37.7.1253. [DOI] [PubMed] [Google Scholar]
- 30.Kurlan R, Rothfield KP, Woodward WR, Nutt JG, Miller C, Lichter D, Shoulson I. Erratic gastric emptying of levodopa may cause “random” fluctuations of parkinsonian mobility. Neurology. 1988;38 (3):419–421. doi: 10.1212/wnl.38.3.419. [DOI] [PubMed] [Google Scholar]
- 31.Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, Naveilhan P, Galmiche JP, Bruley des Varannes S, Derkinderen P, Neunlist M. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut. 2008;57 (12):1741–1743. doi: 10.1136/gut.2008.162503. [DOI] [PubMed] [Google Scholar]
- 32.Lebouvier T, Chaumette T, Paillusson S, Duyckaerts C, Bruley des Varannes S, Neunlist M, Derkinderen P. The second brain and Parkinson’s disease. The European journal of neuroscience. 2009;30 (5):735–741. doi: 10.1111/j.1460-9568.2009.06873.x. [DOI] [PubMed] [Google Scholar]
- 33.Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N’Guyen JM, Chaumette T, Tasselli M, Paillusson S, Flamand M, Galmiche JP, Damier P, Derkinderen P. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PloS one. 2010;5 (9):e12728. doi: 10.1371/journal.pone.0012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci. 2004;24 (6):1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy EM, Defontgalland D, Costa M, Brookes SJ, Wattchow DA. Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterol Motil. 2007;19 (2):126–134. doi: 10.1111/j.1365-2982.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- 36.Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, Schemann M, Galmiche JP. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52 (1):84–90. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neunlist M, Coquenlorge S, Aubert P, Duchalais-Dassonneville E, des Varannes SB, Meurette G, Coron E. Colonic endoscopic full-thickness biopsies: from the neuropathological analysis of the myenteric plexus to the functional study of neuromuscular transmission. Gastrointestinal endoscopy. 2011;73(5):1029–1034. doi: 10.1016/j.gie.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 38.Noorian AR, Taylor GM, Annerino DM, Greene JG. Neurochemical phenotypes of myenteric neurons in the rhesus monkey. The Journal of comparative neurology. 2011;519 (17):3387–3401. doi: 10.1002/cne.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, Takahashi H. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131 (Pt 3):642–650. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 40.Pascale A, Gusev PA, Amadio M, Dottorini T, Govoni S, Alkon DL, Quattrone A. Increase of the RNA-binding protein HuD and posttranscriptional up-regulation of the GAP-43 gene during spatial memory. Proceedings of the National Academy of Sciences of the United States of America. 2004;101 (5):1217–1222. doi: 10.1073/pnas.0307674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet neurology. 2003;2 (2):107–116. doi: 10.1016/s1474-4422(03)00307-7. [DOI] [PubMed] [Google Scholar]
- 42.Pfeiffer RF, Quigley EM. Gastrointestinal motility problems in patients with Parkinson’s disease: epidemiology, pathophysiology, and guidelines for management. CNS Drugs. 1999;11:435–448. [Google Scholar]
- 43.Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. Journal of neuroscience methods. 2004;133 (1–2):99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Pimont S, Bruley Des Varannes S, Le Neel JC, Aubert P, Galmiche JP, Neunlist M. Neurochemical coding of myenteric neurones in the human gastric fundus. Neurogastroenterol Motil. 2003;15 (6):655–662. doi: 10.1046/j.1350-1925.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 45.Porter AJ, Wattchow DA, Brookes SJ, Costa M. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology. 1997;113 (6):1916–1923. doi: 10.1016/s0016-5085(97)70011-8. [DOI] [PubMed] [Google Scholar]
- 46.Porter AJ, Wattchow DA, Brookes SJ, Costa M. Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut. 2002;51 (1):70–75. doi: 10.1136/gut.51.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pouclet H, Lebouvier T, Coron E, Neunlist M, Derkinderen P. Lewy pathology in gastric and duodenal biopsies in Parkinson’s Disease. Mov Disord. 2012;27(6):708. doi: 10.1002/mds.24993. [DOI] [PubMed] [Google Scholar]
- 48.Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell and tissue research. 2008;334 (2):147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 49.Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27 (6):709–715. doi: 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 50.Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet. 1995;346 (8979):861–864. doi: 10.1016/s0140-6736(95)92707-7. [DOI] [PubMed] [Google Scholar]
- 51.Ueki A, Otsuka M. Life style risks of Parkinson’s disease: association between decreased water intake and constipation. Journal of neurology. 2004;251(Suppl 7):vII18–23. doi: 10.1007/s00415-004-1706-3. [DOI] [PubMed] [Google Scholar]
- 52.Wakabayashi K, Mori F, Tanji K, Orimo S, Takahashi H. Involvement of the peripheral nervous system in synucleinopathies, tauopathies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol. 2010;120 (1):1–12. doi: 10.1007/s00401-010-0706-x. [DOI] [PubMed] [Google Scholar]
- 53.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the Auerbach’s and Meissner’s plexuses of humans. Neuroscience letters. 1989;96 (3):259–263. doi: 10.1016/0304-3940(89)90388-1. [DOI] [PubMed] [Google Scholar]
- 54.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta neuropathologica. 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 55.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta neuropathologica. 1988;76 (3):217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 56.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Lewy bodies in the enteric nervous system in Parkinson’s disease. Arch Histol Cytol. 1989;52(Suppl):191–194. doi: 10.1679/aohc.52.suppl_191. [DOI] [PubMed] [Google Scholar]
- 57.Wedel T, Busing V, Heinrichs G, Nohroudi K, Bruch HP, Roblick UJ, Bottner M. Diverticular disease is associated with an enteric neuropathy as revealed by morphometric analysis. Neurogastroenterol Motil. 2010;22(4):407–414. e493–404. doi: 10.1111/j.1365-2982.2009.01445.x. [DOI] [PubMed] [Google Scholar]
- 58.Wedel T, Roblick U, Gleiss J, Schiedeck T, Bruch HP, Kuhnel W, Krammer HJ. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann Anat. 1999;181 (4):327–337. doi: 10.1016/S0940-9602(99)80122-8. [DOI] [PubMed] [Google Scholar]