Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy (original) (raw)
Abstract
Aims
To investigate whether phospholamban gene (PLN) mutations underlie patients diagnosed with either arrhythmogenic right ventricular cardiomyopathy (ARVC) or idiopathic dilated cardiomyopathy (DCM).
Methods and results
We screened a cohort of 97 ARVC and 257 DCM unrelated index patients for PLN mutations and evaluated their clinical characteristics. PLN mutation R14del was identified in 12 (12 % ) ARVC patients and in 39 (15 % ) DCM patients. Haplotype analysis revealed a common founder, estimated to be between 575 and 825 years old. A low voltage electrocardiogram was present in 46 % of R14del carriers. Compared with R14del– DCM patients, R14del+ DCM patients more often demonstrated appropriate implantable cardioverter defibrillator discharge (47 % vs. 10 % , P < 0.001), cardiac transplantation (18 % vs. 2 % , P < 0.001), and a family history for sudden cardiac death (SCD) at < 50 years (36 % vs. 16 % , P = 0.007). We observed a similar pattern in the ARVC patients although this was not statistically significant. The average age of 26 family members who died of SCD was 37.7 years. Immunohistochemistry in available myocardial samples revealed absent/depressed plakoglobin levels at intercalated disks in five of seven (71 % ) R14del+ ARVC samples, but in only one of nine (11 % ) R14del+ DCM samples (P = 0.03).
Conclusions
The PLN R14del founder mutation is present in a substantial number of patients clinically diagnosed with DCM or ARVC. R14del+ patients diagnosed with DCM showed an arrhythmogenic phenotype, and SCD at young age can be the presenting symptom. These findings support the concept of ‘arrhythmogenic cardiomyopathy’.
Keywords: Arrhythmia, Arrhythmogenic cardiomyopathy, Arrhythmogenic right ventricular cardiomyopathy, Dilated cardiomyopathy, Genetics
Introduction
Idiopathic dilated cardiomyopathy (DCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC) are clinically heterogeneous diseases of the myocardium, associated with mechanical and/or electrical dysfunction.1 Clinically, ARVC is characterized by ventricular arrhythmias, predominantly from the right ventricle, and sudden cardiac death (SCD), often preceding structural changes.2–5 DCM, on the other hand, is characterized by left ventricular (LV) contractile dysfunction and progressive heart failure, with arrhythmias often being present but less prominent.6 The management strategies of ARVC and DCM therefore differ. For ARVC, the aim is to prevent SCD and ventricular tachycardia, by using an implantable cardioverter defibrillator (ICD) or antiarrhythmic pharmacological treatment.3 The management of DCM is mainly directed at treating heart failure symptoms and preventing disease progression and related complications.6,7 ICD implantation as primary prevention is recommended in patients in New York Heart Association (NYHA) functional class II or III, who are receiving optimal medical therapy and have an LV ejection fraction ≤30 % (American College of Cardiology/American Heart Association; ACC/AHA) or ≤35 % (European Society of Cardiology; ESC).7,8
Although considered separate entities by both the AHA and the ESC,1,9 DCM and ARVC have overlapping clinical features. Classic ARVC shows primarily right ventricular (RV) involvement. However, histopathological and functional LV involvement is present in 76–84 % of ARVC patients. Also left-dominant forms exist, leading to the postulation that left-dominant arrhythmogenic cardiomyopathy is a separate entity.10,11 Finally, at the molecular level (desmosomal proteins, gap junctions), both ventricles are affected in a similar way in ARVC.12 The principal discriminating feature of left-dominant arrhythmogenic cardiomyopathy from DCM would be the predisposition to ventricular arrhythmias in early stages of the disease, disproportionate to the morphological abnormalities and impaired systolic function.5,11,13 Conversely, deteriorating RV function is a strong predictor of worse outcome in DCM.14
Comprehensive screening of desmosomal genes in ARVC patients has identified pathogenic mutations in 40–58 % of clearly affected patients.15–17 Given the clinical overlap between ARVC and DCM, we focused on non-desmosomal genes known to lead to DCM with an arrhythmogenic phenotype and which could potentially explain genetically unsolved ARVC cases. So far, mutations in >30 different genes have been reported to cause DCM,6,18 including the gene encoding phospholamban (PLN). PLN is a regulator of the sarcoplasmic reticulum Ca2+- (SERCA2a) pump in cardiac muscle and thus important for maintaining Ca2+ homeostasis.19 In DCM, three different PLN mutations have been identified;20–23 the cardiac phenotype of PLN mutation carriers was characterized by the presence of malignant ventricular arrhythmias and interstitial fibrosis. We therefore screened both DCM and ARVC patients for PLN mutations and compared their clinical and genetic characteristics.
Methods
Patients
A cohort of index patients, referred to the Departments of Clinical Genetics of three university hospitals (University Medical Center Groningen, Academic Medical Center Amsterdam, and University Medical Center Utrecht, The Netherlands), by the attending cardiologist (heart failure specialists or cardiac electrophysiologists), was evaluated. No index patient within the cohort had a known familial relationship with any other index patient in the cohort. Retrospectively, available data on medical history, physical examination, 12-lead electrocardiogram (ECG), echocardiography, Holter monitoring, exercise testing, signal-averaged ECG, nuclear scintigraphy, magnetic resonance imaging, and/or RV angiography studies were collected. Age of presentation was defined as the age when the first symptoms or signs most likely to be attributable to the disease occurred. For DCM, the diagnostic criteria of Mestroni et al. were used.24 For ARVC, recently modified task force criteria were used.25 Two patients who did not fulfil the modified criteria because histomorphometric analyses of myocardial tissue had not been performed were nonetheless considered as having ARVC, since they were previously diagnosed with ARVC, based on original task force criteria. The study complied with the Declaration of Helsinki and was approved by the local institutional review committees, and informed consent was obtained from all participants.
Electrocardiograms
Electrocardiograms from index patients and their relatives were analysed and interpreted in a blinded fashion by two cardiologists (R.A.d.B and M.P.v.d.B.). Low voltage on the ECG was defined as the QRS peak to peak amplitude in leads I, II, and III being <0.5 mV.
Genetic analysis
Genomic DNA was isolated from peripheral blood samples according to standard protocols. Bidirectional direct sequencing of the coding region of PLN was performed in all index patients using a BigDye Terminator DNA sequencing kit (version 2.0) on a 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). To assess the prevalence of PLN mutations in a US cohort, 46 desmosomal gene-negative ARVC index patients were screened. A total of 473 anonymous ethnically matched controls were also screened.
Nine microsatellite markers around PLN were selected for haplotype analysis. Primers and conditions are available upon request. The availability of DNA of relatives enabled the verification of the phase and reconstruction of haplotypes. Haplotype analysis was also performed in previously described DCM families from Greece and Germany.22,23 The method described by Machado et al. was used to calculate the age of the haplotype.26
Besides PLN analysis, DCM index patients were screened for mutations in the most frequently mutated genes in DCM: LMNA, MYH7, and TNNT2. All ARVC index patients were also screened for mutations in PKP2, DSC2, DSG2, DSP, and JUP. Primers and PCR conditions are available upon request.
Immunohistochemistry and histology
Formalin-fixed, paraffin-embedded myocardium samples were available from a subset of the ARVC and DCM patients carrying a PLN mutation and were used for immunohistochemical staining. Samples were analysed and interpreted in a blinded fashion. Information regarding antibodies and immunohistochemical protocols has been described before.12 Available complete hearts from transplant procedures or autopsies were analysed for signs of lipofibromatosis.
Statistical analysis
Mutation rates in patients vs. controls, clinical characteristics, and ECG parameters of mutation-carrying index patients vs. non-carriers were compared by either the Student's _t_-test or Fisher's exact test. Values of P < 0.05 were considered significant. All the data were analysed with PASW 18.0 software (SPSS, Chicago, IL, USA).
Results
Patients and genetic analysis
A total of 354 unrelated index patients were evaluated; 257 diagnosed with DCM and 97 with ARVC. A 3 bp deletion of PLN (c.40_42delAGA; p.R14del) was identified in 39 (15 % ) DCM index patients and 12 (12 % ) ARVC index patients. R14del was found in one of 473 control samples (P < 10−18). None of the 39 R14del+ DCM patients had a mutation in LMNA, MYH7, or TNNT2, and none of the 12 R14del+ ARVC patients had a mutation in PKP2, DSC2, DSG2, DSP, or JUP. In addition, one of 46 (2 % ) ARVC index patients from the USA also carried the PLN R14del mutation.
Haplotype analysis was performed in 36 (71 % ) of the Dutch R14del+ index patients, the patient from the USA, and in published Greek and German DCM families.22,23 A shared haplotype for five markers in a 1.2 Mb region surrounding PLN was found, although in two patients either the size of one or more markers had changed or a recombination had occurred. The parental ancestry of the R14del+ patient from the USA was German/Norwegian. The Greek patients had a different haplotype (see Supplementary material, Table S1). Allowing 25 years per generation, the age of the haplotype containing the mutation is estimated to be between 575 and 825 years old.
Clinical data
Detailed clinical data of all 52 R14del+ patients (51 Dutch, 1 American) are given in the Supplementary material, Table S2. Twenty-nine (56 % ) were female, the mean age at presentation was 44.3 ± 12.6 years, and mean follow-up was 9.2 (range 0–26) years. The R14del+ index patients mainly presented with ventricular tachycardia/fibrillation (VT/VF) (n = 18), heart failure (n = 11), or syncope (n = 3), or were identified after family screening following SCD (n = 7). Notably, three of five patients who presented with VF were younger than 30 years old. Table 1 lists the ARVC and DCM criteria for all 52 R14del+ index patients. When evaluating retrospectively, five R14del+ patients diagnosed with DCM had a borderline diagnosis of ARVC (i.e. one major and one minor or three minor criteria) and 12 had a possible diagnosis of ARVC (two minor criteria) at the time of DCM diagnosis. A criterion for abnormal repolarization was present in 23 (59 % ) and a criterion for arrhythmia in 22 (56 % ). A description of arrhythmias present at baseline in R14del+ DCM patients is given in the Supplementary material, Table S3. In summary, a sustained or non-sustained VT or VF was present at baseline in 18/39 (46 % ). In addition, although this is not an ARVC criterion as such, 15 (38 % ) R14del+ DCM patients showed RV dilatation. At the time of ARVC diagnosis, four R14del+ ARVC patients had impaired LV systolic function; none of them had LV dilatation.
Table 1.
Modified task force criteria for the diagnosis of arrhythmogenic right ventricular cardiomyopathy and Mestroni criteria for the diagnosis of idiopathic dilated cardiomyopathy in phospholamban gene R14del mutation carriers
| Modified task force criteria for ARVC | Mestroni criteria for DCM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Index patient | Function | Tissuea | Repolarization | Depolarization | Rhythm | Family history | TFC (major/minor) | LVEF < 45 % | FS < 25 % | LVEDD > 117 % |
| DCM | ||||||||||
| D01 | 0/0 | ++ | ++ | ++ | ||||||
| D02 | + | + | 0/2 | ++ | ++ | |||||
| D03 | + | + | + | 0/3 | ++ | ++ | ++ | |||
| D04b | + | + | 0/2 | ++ | ++ | + | ||||
| D05 | 0/0 | ++ | ++ | ++ | ||||||
| D06 | + | + | 0/2 | ++ | ++ | |||||
| D07 | + | + | 0/2 | ++ | ++ | ++ | ||||
| D08b | + | 0/1 | ++ | ++ | ||||||
| D09 | + | + | + | 0/3 | ++ | ++ | ++ | |||
| D10 | + | + | 0/2 | ++ | ++ | ++ | ||||
| D11 | 0/0 | ++ | ++ | ++ | ||||||
| D12 | 0/0 | ++ | ++ | ++ | ||||||
| D13 | + | + | 0/2 | ++ | ++ | ++ | ||||
| D14 | + | 0/1 | ++ | ++ | ++ | |||||
| D15 | 0/0 | ++ | ++ | ++ | ||||||
| D16 | + | 0/1 | ++ | ++ | ++ | |||||
| D17 | + | 0/1 | ++ | ++ | ++ | |||||
| D18 | 0/0 | ++ | ++ | ++ | ||||||
| D19 | 0/0 | ++ | ++ | ++ | ||||||
| D20 | 0/0 | ++ | ++ | ++ | ||||||
| D21 | 0/0 | ++ | ++ | ++ | ||||||
| D22 | ++ | + | 1/1 | ++ | + | ++ | ||||
| D23 | + | 0/1 | ++ | ++ | ||||||
| D24 | + | 0/1 | ++ | ++ | ++ | |||||
| D25 | + | + | 0/2 | ++ | ++ | |||||
| D26 | + | 0/1 | ++ | ++ | ||||||
| D27 | + | + | 0/2 | ++ | ++ | ++ | ||||
| D28 | + | + | 0/2 | ++ | ++ | |||||
| D29 | + | 0/1 | ++ | ++ | ++ | |||||
| D30 | 0/0 | ++ | ++ | ++ | ||||||
| D31 | + | 0/1 | ++ | ++ | ++ | |||||
| D32 | + | 0/1 | ++ | ++ | ++ | |||||
| D33 | 0/0 | ++ | ++ | ++ | ||||||
| D34 | + | + | + | 0/3 | ++ | ++ | ++ | |||
| D35 | + | ++ | 1/1 | ++ | ++ | |||||
| D36 | + | + | 0/2 | ++ | ++ | ++ | ||||
| D37 | + | 0/1 | ++ | ++ | ||||||
| D38 | + | + | 0/2 | ++ | ++ | ++ | ||||
| D39 | + | + | 0/2 | ++ | ++ | |||||
| ARVC | ||||||||||
| A01 | ++ | ++ | + | ++ | 3/1 | ++ | ||||
| A02 | ++ | ++ | + | ++ | 3/2 | ++ | ||||
| A03 | + | + | ++ | 1/2 | ||||||
| A04 | ++ | ++ | + | + | 2/2 | |||||
| A05 | ++ | + | + | 1/2 | ||||||
| A06 | ++ | ++ | + | 2/1 | ||||||
| A07 | ++ | ++ | + | 2/1 | ||||||
| A08 | ++ | ++ | + | 2/1 | ++ | |||||
| A09 | ++ | ++ | ++ | 3/0 | ++ | |||||
| A10c | + | + | + | 0/3 | ||||||
| A11 | + | + | ++ | 1/2 | ||||||
| A12c | + | + | + | 0/3 | ||||||
| USA | + | + | ++ | 1/2 |
A clinical diagnosis of DCM was made in family members of 21 of 39 (54 % ) DCM index patients, whereas in family members of 2 of 13 (15 % ) ARVC index patients a clinical diagnosis of ARVC was made. None of the DCM index patients had a family member with the clinical diagnosis of ARVC; one ARVC index patient had a parent diagnosed with DCM. The average age of the 26 family members who died of SCD was 37.7 years. Details on family history are given in the Supplementary material, Table S4.
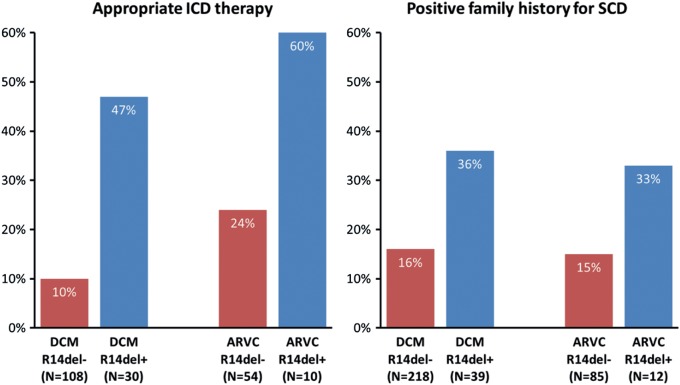
For both the DCM and ARVC groups, R14del+ index patients did not differ from R14del– index patients regarding age at presentation or sex (data not shown). Figure 1 shows their arrhythmia-related characteristics. Compared with R14del– DCM patients, R14del+ DCM patients more often had a positive family history for SCD below age 50 in first- and second-degree relatives and more often experienced an appropriate ICD discharge. Furthermore, R14del+ DCM patients more often underwent cardiac transplantation (18 % vs. 2 % , P < 0.001).
Figure 1.
Arrhythmia-related characteristics for both the idiopathic dilated cardiomyopathy (DCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC) groups. The left panel shows the rates of appropriate implantable cardioverter defibrillator (ICD) discharge in patients carrying an ICD. In the DCM cohort, 108 PLN (phospholamban gene) R14del– patients and 30 PLN R14del+ index patients underwent ICD implantation (48 % vs. 77 % , P = 0.003), and in the ARVC cohort these numbers were 54 and 10 (64 % vs. 83 % , P = 0.21), respectively. The difference in the rates of appropriate ICD discharge reached statistical significance for the DCM cohort (10 % vs. 47 % , P < 0.001), but not for the ARVC cohort (24 % vs. 60 % , P = 0.053). The right panel shows family history for sudden cardiac death < 50 years in first- and second-degree relatives. This difference reached statistical significance for the DCM cohort (16 % vs. 36 % , P = 0.007), but not for the ARVC cohort (15 % vs. 33 % , P = 0.22).
Within the ARVC group, R14del mutation carriers had more severe arrhythmia characteristics, yet these were not statistically significant when compared with non-carriers. Comparing R14del+ DCM patients with R14del+ ARVC patients did not reveal differences in these parameters.
Electrocardiograms
In 46 % of PLN R14del+ index patients, a low voltage ECG at baseline was found. Inverted T waves as listed in the modified task force criteria for ARVC25 were present in 29 (57 % ) patients; 19 of them showed inverted T waves in the left pre-cordial leads (V4–V6) (Supplementary material, Table S5). Holter monitoring was performed in 40 R14del+ index patients; > 500 ventricular extrasystoles per 24 h, another diagnostic ARVC criterion, were present in 26 (65 % ).
Immunohistochemistry and histology
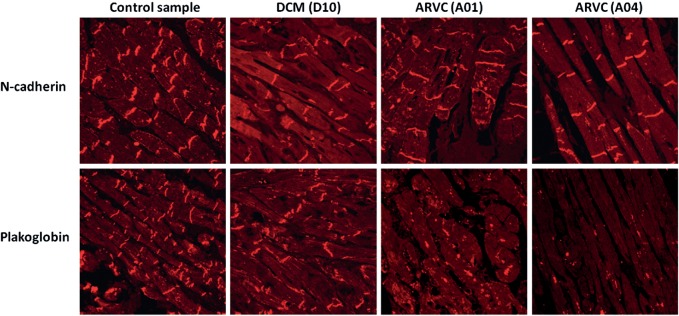
Immunohistochemistry revealed absent or markedly reduced immunoreactive signal levels for the desmosomal protein plakoglobin at intercalated disks in 5 of 7 (71 % ) R14del+ ARVC samples. However, only 1 of 9 (11 % ) R14del+ DCM samples showed depressed signal levels (P = 0.03) (Figure 2, Table 2). An overview of reported histological findings in RV biopsies from PLN R14del index patients are given in the Supplementary material, Table S6.
Figure 2.
Immunofluorescence images of endomyocardial biopsy samples from two patients (A01 and A04) diagnosed with arrhythmogenic right ventricular cardiomyopathy (ARVC) and one (D10) with idiopathic dilated cardiomyopathy (DCM), all carrying the PLN (phospholamban gene) R14del mutation, compared with a control sample. Representative images from a blinded analysis of endomyocardial biopsy samples show that immunoreactive signal levels for plakoglobin in two subjects with ARVC differ from the signal levels in a control subject and a subject with DCM. N-cadherin serves as a tissue control marker. Numbers in parentheses correspond to the subject numbers in the Supplementary material, Table S2.
Table 2.
Immunohistochemical diagnoses in blinded analysis of cardiac specimens from patients with arrhythmogenic right ventricular cardiomyopathy and idiopathic dilated cardiomyopathy carrying the phospholamban gene R14del mutation
| Family ID | Patient | Sample type | Immunofluorescent signal for plakoglobin | Diagnostic test |
|---|---|---|---|---|
| DCM | ||||
| D01 | First-degree relative | RV biopsy | Normal | Negative |
| D03 | Index | RV biopsy | Depressed | Positive |
| D08 | Index | Explanted heart | Normal | Negative |
| D09 | Index | RV biopsy | Normal | Negative |
| D10 | Index | RV biopsy | Normal | Negative |
| D11 | Second-degree relative | RV biopsy | Normal | Negative |
| D15 | Index | RV biopsy | Normal | Negative |
| D16 | First-degree relative | Autopsy | Normal | Negative |
| D20 | Index | RV biopsy | Normal | Negative |
| ARVC | ||||
| A01 | Index | RV biopsy | Absent | Positive |
| A02 | Index | RV biopsy | Absent | Positive |
| A03 | Index | RV biopsy | Absent | Positive |
| A04 | Index | RV biopsy | Absent | Positive |
| A06 | Index | RV biopsy | Normal | Negative |
| A10 | Index | RV biopsy | Normal | Negative |
| A11 | Index | RV biopsy | Very depressed | Positive |
Discussion
The distinction of DCM and ARVC as separate clinical entities, which is made by both the AHA and the ESC,1,9 has important implications for clinical practice, guiding both diagnostics and treatment. However, the considerable overlap encountered between different disease subtypes is an inevitable limitation of any classification.1 The main finding of this study is that patients clinically labelled with different diagnoses (i.e. DCM or ARVC) based upon accepted clinical criteria carry an identical mutation: PLN R14del. This specific mutation has been studied extensively in a mouse model by Haghighi et al.; transgenic mice overexpressing this mutation exhibited depressed cardiac function, histopathological abnormalities (fibrosis), and premature death, recapitulating the phenotype of the family they described.22
As PLN is a Ca2+-ATPase regulator, Ca2+ homeostasis might play an important role in the pathogenesis in R14del+ patients. Ca2+ regulates the assembly and disassembly of the desmosome: it is likely that both extracellular and cytoplasmic Ca2+ levels are important for cell–cell junction formation.27 Superinhibition of SERCA2a activity by mutant PLN leads to reduced Ca2+ uptake into the sarcoplasmic reticulum,22 which could consequently result in desmosomal disassembly due to elevated cytoplasmic Ca2+ levels and/or impaired Ca2+ homeostasis.
Phenotype of R14del mutation carriers
The R14del mutation has previously been associated with a low voltage ECG,23 which was also present in 46 % of the R14del+ index patients described in this study. The substrate for these low voltage ECGs and the observed arrhythmogenic phenotype may be the presence of cardiac fibrosis, which was a frequent finding at histological examination and has also been found in the R14del mouse model.22
When compared with non-carrier DCM patients, patients diagnosed with DCM carrying PLN R14del exhibit an arrhythmogenic phenotype. This is reflected by high rates of VT/VF as a presenting symptom, appropriate ICD interventions, and positive family history for premature SCD. In addition, more R14del+ DCM patients underwent cardiac transplantation compared with R14del– DCM patients, further attesting to the malignancy of this mutation. R14del+ patients diagnosed with ARVC showed a severe arrhythmogenic phenotype comparable with that of non-carrier ARVC patients.
Two different diagnoses or a single entity?
In cardiological practice, the diagnostic label of either DCM or ARVC is often determined by the initial presentation, e.g. heart failure symptoms or arrhythmias, and this was probably also the case in the patients presented in this study. Those presenting with symptomatic ventricular arrhythmias were probably analysed with ARVC in mind, whereas patients with asymptomatic arrhythmias could have progressed towards heart failure and were more likely to be diagnosed as DCM. Ventricular ectopy and non-sustained VT could have been asymptomatic, and not all patients diagnosed with DCM underwent Holter monitoring to detect arrhythmias. Follow-up by either an electrophysiologist in the case of an ARVC diagnosis or a heart failure-oriented cardiologist in the case of a DCM diagnosis may have led to findings that seemed to confirm whichever diagnosis was first considered. However, in retrospect, a clear overlap was already present at the time of diagnosis (Table 1). In addition to similar arrhythmia-related characteristics (Figure 1), five R14del+ DCM patients had a borderline diagnosis of ARVC and 12 had a possible diagnosis of ARVC at the time of DCM diagnosis. Moreover, RV dilatation was present in 15 (38 % ) R14del+ DCM patients. Conversely, four R14del+ ARVC patients had impaired LV systolic function. These clinical findings suggest a single yet variable disease entity rather than two separate diagnoses, which is in agreement with the finding of a single underlying mutation, i.e. PLN R14del.
Taken together, our results support the concept of ‘arrhythmogenic cardiomyopathy’ as an entity encompassing ARVC, including left-dominant arrhythmogenic cardiomyopathy, and arrhythmogenic forms of DCM.5,28
Immunohistochemical differences
Within this PLN R14del-related arrhythmogenic cardiomyopathy spectrum on the immunohistochemical level, samples from PLN R14del+ patients diagnosed with ARVC showed reduced plakoglobin signal levels in the majority (5 of 7) of cases, which is compatible with previous observations in ARVC,12 whereas only one of nine R14del+ patients with a clinical diagnosis of DCM showed identical findings (P = 0.03). Diminished plakoglobin signal at intercalated disks appears to track with the ARVC phenotype rather than genotypes. Other unknown genetic, epigenetic, or environmental factors, such as strenuous exercise, may either cause reduced plakoglobin levels, guiding the phenotype towards ARVC-related manifestations (and diagnosis), or, alternatively, these factors lead to a more arrhythmogenic phenotype resulting in a clinical diagnosis of ARVC and secondary reduced plakoglobin levels. We have excluded the co-occurrence of mutations in desmosomal genes in the ARVC patients as a cause for the reduced plakoglobin levels. Failure of plakoglobin to localize correctly in ARVC suggests a final common pathway in which desmosomal instability, caused by mutations in genes encoding either desmosomal proteins or other proteins such as PLN, leads to a subcellular redistribution of plakoglobin, which is believed to play a pivotal role in altered signalling pathways.5,29
Implications for diagnostics and therapy
By providing evidence for the concept of arrhythmogenic cardiomyopathy, our results may have important diagnostic implications. The modified task force criteria for ARVC diagnosis are not designed to discriminate between different cardiomyopathy subtypes and do not contain a criterion for LV involvement, with the exception of inverted T waves in V4–V6,25 while RV involvement is not included in the criteria for DCM.24. We therefore call for the formulation of criteria for the diagnosis of arrhythmogenic cardiomyopathy, encompassing ARVC and arrhythmogenic forms of DCM. Regarding the therapeutic implications, the ESC's 2008 heart failure guidelines recommended ICD implantation for primary prevention in non-ischaemic cardiomyopathy, to reduce mortality in patients with an LV ejection fraction ≤35 % , in NYHA functional class II or III.8 In our cohort, 44 % of R14del+ DCM patients carrying an ICD had experienced an appropriate shock. This warrants caution with R14del+ DCM patients with a relatively preserved LV function (>30–35 % ).
Study limitations
As mentioned above, in DCM patients, RV function and arrhythmias were not systematically investigated, as was the case for LV function in ARVC patients, reflecting everyday clinical practice. In addition, the included patients had all been referred to tertiary referral centres, thus possibly reflecting the more severe end of the disease spectrum. Family members were not systematically investigated in this study.
Global relevance
We identified the PLN R14del founder mutation in a substantial percentage of Dutch ARVC and DCM patients (12 % and 15 % , respectively), and were able to confirm this in an ARVC patient from the USA. The haplotype containing this mutation was estimated to be >575 years old, showing that all R14del+ mutation carriers are distantly related, and was also present in patients from the USA and Germany. In addition, emigration of Dutch mutation carriers in the 19th and early 20th century, not only to the USA but also to Canada, South-Africa, Australia, and New Zealand, could have resulted in R14del mutation carriers on multiple continents.
Conclusion
This is the first study to describe a role for PLN in ARVC. R14del+ patients diagnosed with DCM showed an arrhythmogenic phenotype. These findings support the concept of ‘arrhythmogenic cardiomyopathy’.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
The Netherlands Heart Foundation (2007B132); the Van Buchem Foundation; the National Institutes of Health (HL102361).
Confict of interest: none declared.
Supplementary Material
Supplementary Data
Acknowledgments
We thank the patients and their relatives who made this work possible, Dr M.G. Posch and Dr E.G. Kranias for providing DNA samples, and Jackie Senior for editing the manuscript.
References
- 1.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–133. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 3.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 4.Marcus FI, Zareba W, Calkins H, Towbin JA, Basso C, Bluemke DA, Estes NA, III, Picard MH, Sanborn D, Thiene G, Wichter T, Cannom D, Wilber DJ, Scheinman M, Duff H, Daubert J, Talajic M, Krahn A, Sweeney M, Garan H, Sakaguchi S, Lerman BB, Kerr C, Kron J, Steinberg JS, Sherrill D, Gear K, Brown M, Severski P, Polonsky S, McNitt S. Arrhythmogenic right ventricular cardiomyopathy/dysplasia clinical presentation and diagnostic evaluation: results from the North American Multidisciplinary Study. Heart Rhythm. 2009;6:984–992. doi: 10.1016/j.hrthm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saffitz JE. The pathobiology of arrhythmogenic cardiomyopathy. Annu Rev Pathol. 2011;6:299–321. doi: 10.1146/annurev-pathol-011110-130151. [DOI] [PubMed] [Google Scholar]
- 6.Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010;375:752–762. doi: 10.1016/S0140-6736(09)62023-7. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 10.Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–1520. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 11.Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710–1720. doi: 10.1161/CIRCULATIONAHA.106.660241. [DOI] [PubMed] [Google Scholar]
- 12.Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 13.Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 14.La Vecchia L, Varotto L, Zanolla L, Spadaro GL, Fontanelli A. Right ventricular function predicts transplant-free survival in idiopathic dilated cardiomyopathy. J Cardiovasc Med. 2006;7:706–710. doi: 10.2459/01.JCM.0000243006.90170.ce. [DOI] [PubMed] [Google Scholar]
- 15.Sen-Chowdhry S, Syrris P, McKenna WJ. Role of genetic analysis in the management of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2007;50:1813–1821. doi: 10.1016/j.jacc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Comprehensive desmosome mutation analysis in North Americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. [en Haan AD, Tan B, Zikusoka M, Ibanez Llado L, Jain R, Daly A, Tichnell C, James C, Amat-Alarcon N, Abraham T, Russell S, Bluemke D, Calkins H, Dalal D, Judge D];Circ Cardiovasc Genet. 2009 2:428–435. doi: 10.1161/CIRCGENETICS.109.858217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox MG, van der Zwaag PA, van der Werf C, van der Smagt JJ, Noorman M, Bhuiyan ZA, Wiesfeld AC, Volders PG, van Langen IM, Atsma DE, Dooijes D, van den Wijngaard A, Houweling AC, Jongbloed JD, Jordaens L, Cramer MJ, Doevendans PA, de Bakker JM, Wilde AA, van Tintelen JP, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype–phenotype follow-up study. Circulation. 2011;123:2690–2700. doi: 10.1161/CIRCULATIONAHA.110.988287. [DOI] [PubMed] [Google Scholar]
- 18.Judge DP, Johnson NM. Genetic evaluation of familial cardiomyopathy. J Cardiovasc Transl Res. 2008;1:144–54. doi: 10.1007/s12265-008-9025-1. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 21.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW, MacLennan DH, Kremastinos DT, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW, MacLennan DH, Kremastinos DT, Kranias EG. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci USA. 2006;103:1388–1393. doi: 10.1073/pnas.0510519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posch MG, Perrot A, Geier C, Boldt LH, Schmidt G, Lehmkuhl HB, Hetzer R, Dietz R, Gutberlet M, Haverkamp W, Ozcelik C. Genetic deletion of arginine 14 in phospholamban causes dilated cardiomyopathy with attenuated electrocardiographic R amplitudes. Heart Rhythm. 2009;6:480–486. doi: 10.1016/j.hrthm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Mestroni L, Maisch B, McKenna WJ, Schwartz K, Charron P, Rocco C, Tesson F, Richter A, Wilke A, Komajda M. Guidelines for the study of familial dilated cardiomyopathies. Eur Heart J. 1999;20:93–102. doi: 10.1053/euhj.1998.1145. [DOI] [PubMed] [Google Scholar]
- 25.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado PM, Brandao RD, Cavaco BM, Eugenio J, Bento S, Nave M, Rodrigues P, Fernandes A, Vaz F. Screening for a BRCA2 rearrangement in high-risk breast/ovarian cancer families: evidence for a founder effect and analysis of the associated phenotypes. J Clin Oncol. 2007;25:2027–2034. doi: 10.1200/JCO.2006.06.9443. [DOI] [PubMed] [Google Scholar]
- 27.Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol. 2004;15:665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Sen-Chowdhry S, Morgan RD, Chambers JC, McKenna WJ. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu Rev Med. 2010;61:233–253. doi: 10.1146/annurev.med.052208.130419. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Swope D, Raess N, Cheng L, Muller EJ, Radice GL. Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling. Mol Cell Biol. 2011;31:1134–1144. doi: 10.1128/MCB.01025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data