Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome (original) (raw)
Activation of the NLRP3-inflammasome contributes to Staphylococcus aureus PVL-associated diseases, such as necrotizing pneumonia.
Keywords: Panton-Valentine leukocidine, monocytes, macrophages, IL-1β and IL-18 expression, inflammasome
Abstract
The Staphylococcus aureus pore-forming toxin PVL is most likely causative for life-threatening necrotizing infections, which are characterized by massive tissue inflammation and necrosis. Whereas the cytotoxic action of PVL on human neutrophils is already well established, the PVL effects on other sensitive cell types, such as monocytes and macrophages, are less clear. In this study, we used different types of human leukocytes (neutrophils, monocytes, macrophages, lymphocytes) to investigate cell-specific binding of PVL subunits and subsequent proinflammatory and cytotoxic effects. In all PVL-sensitive cells, we identified the binding of the subunit LukS-PV as the critical factor for PVL-induced cytotoxicity, which was followed by binding of LukF-PV. LukS-PV binds to monocytes, macrophages, and neutrophils but not to lymphocytes. Additionally, we showed that PVL binding to monocytes and macrophages leads to release of caspase-1-dependent proinflammatory cytokines IL-1β and IL-18. PVL activates the NLRP3 inflammasome, a signaling complex of myeloid cells that is involved in caspase-1-dependent IL-1β processing in response to pathogens and endogenous danger signals. Specific inhibition of this pathway at several steps significantly reduced inflammasome activation and subsequent pyronecrosis. Furthermore, we found that PAMPs and DAMPs derived from dying neutrophils can dramatically enhance this response by up-regulating pro-IL-1β in monocytes/macrophages. This study analyzes a specific host signaling pathway that mediates PVL-induced inflammation and cytotoxicity, which has high relevance for CA-MRSA-associated and PVL-mediated pathogenic processes, such as necrotizing infections.
Introduction
S. aureus is an important human pathogen that can cause a wide variety of infections, ranging from superficial skin infections to invasive diseases, such as soft tissue infections, pneumonia, osteomyelitis, or sepsis [1]. To establish infections, S. aureus expresses a multitude of virulence factors, including secreted toxins, enzymes, and peptides (e.g., PSMs), as well as surface proteins (e.g., protein A) and wall components (e.g., LTA), that have been described to contribute to disease development [1, 2]. Particularly, the pore-forming toxins, α-toxin [3] and PVL [4], have been implicated in the pathogenesis of severe S. aureus infections. These proteins are secreted as monomers, which insert into host cell membranes and assemble into heptameric structures that penetrate cell membranes [5]. In general, α-toxin is supposed to be a crucial toxin of S. aureus, which is expressed by the majority of clinical isolates, and its contribution to virulence has been demonstrated in various invasive disease models, such as pneumonia, soft tissue infections, and sepsis [6–8]. By contrast, PVL is a bicomponent leukotoxin composed of two distinct proteins—LukS-PV and LukF-PV—which target defined cells of the immune system, mainly neutrophils from humans and rabbits [5, 9, 10]. Only a small percentage of S. aureus isolates (<3%) carries the gene for PVL [11], but PVL-positive S. aureus strains have been associated with severe necrotizing diseases, such as necrotic cutaneous lesions and necrotizing hemorrhagic pneumonia [12, 13]. In this respect, a rapidly progressive hemorrhagic and necrotizing pneumonia with severe leukopenia in children and young adults has been reported. This type of S. aureus pneumonia seems to be a specific disease entity with a high lethality rate. Because of the inflammatory and necrotic histopathological appearance of the lung, the illness was designated “S. aureus hemorrhagic necrotizing pneumonia” [12, 14].
Since the early 1990s, the number of MRSA strains has risen dramatically, which emerged as a leading cause of hospital-acquired infections. Additionally, there has been an alarming increase in the number of CA-MRSA infections—mainly severe skin infections—worldwide [15]. In the United States, both MRSA clones that are most closely associated with community outbreaks, USA300 and USA400, contain pvl genes, as do other successfully CA-MRSA clones, e.g., an ST80 clone in Europe and an ST30 clone in Australia and the United States [16–19]. Despite a clear epidemiological association between the pvl genes and severe, often recurrent primary skin infections or necrotizing pneumonia [12, 13], a definite pathogenic role for PVL in disease development could not be determined, as murine models are not adequate to study the pathogenicity of PVL [9]. Whereas recent work in PVL-sensitive disease models (human cells, rabbits) clearly supports a pathogenic function of PVL in severe necrotizing diseases [9, 20, 21], the precise mechanisms that underlie the PVL-induced inflammation and tissue destruction are largely unknown.
During necrotizing diseases, there are massive inflammation and influx of immune cells to the infection side [12, 22] that cannot be explained by PVL-induced cytotoxicity on neutrophils alone [22]. PVL induces the release of proinflammatory mediators from neutrophils such as histamine, leukotriene B4, and IL-8 [23–25], but research of proinflammatory processes has been focused mostly on neutrophils, whereas there is binding to monocytes and macrophages as well [10]. A recent study comparing the release of proinflammatory mediators from PBMCs by PVL and other staphylococcal toxins indicated a role of all different cell types [26]. This is of great importance, as the activation of monocytic cells can lead to a strong proinflammatory reaction with the secretion of cytokines, such as IL-1β.
IL-1β has multiple biologic effects including the ability to increase the expression of proinflammatory cytokines (IL-1R antagonist, TNF, IL-6, and IL-37), mediators (iNOS, type-2 COX-2 and phospholipase A2, G-CSF, and GM-CSF), and adhesion molecules (ICAM-1, VCAM-1, and VEGF). Moreover, IL-1β mediates the activation of neutrophils to eliminate invading pathogens [27, 28]. The secretion of IL-1β is tightly regulated, and two separate signals are necessary to induce the full range of activation [29]. IL-1β mRNA is induced via activation of PRRs (triggered by pathogen- and host-derived molecules) and leads to synthesis of pro-IL-1β protein. A second signal, e.g., a K+-ionophore, activates caspase-1, a protease that cleaves pro-IL-1β into the biologically active, mature IL-1β [29]. The activation of caspase-1 is mediated by a cytosolic protein complex, the inflammasome [30]. So far, different inflammasomes have been described, including the NLRP3 inflammasome [31]. Compounds that activate the NLRP3 receptor include pore-forming toxins, e.g., nigericin and S. aureus α-toxin [32, 33]. Activation leads to the oligomerization and recruiting of caspase-1 through the adaptor protein ASC, thus forming the active NLRP3 inflammasome complex [31]. NLRP3 receptors are activated by diverse stimuli with variable structures, suggesting that the NLRP3 receptor senses them indirectly. Therefore, cellular stress signals could serve as intermediate steps in NLRP3 activation [31, 34]. For instance, the K+-ionophore nigericin induces an activation of the inflammasome that depends on the release of the lysosomal cysteine protease CTSB [35, 36]. CTSB is not only an activator of the NLRP3 inflammasome but also induces a caspase-1-independent programmed necrotic cell death [36].
It has already been shown that living S. aureus and α-toxin from S. aureus can activate the NLRP3 inflammasome [32, 33], but the role of PVL in inflammasome activation has not been elucidated yet. Here, we demonstrate that PVL is a strong inducer of IL-1β secretion via a CTSB-mediated activation of the NLRP3 inflammasome, which likely contributes to the severe inflammation associated with necrotizing infections.
MATERIALS AND METHODS
Ethics statement
Taking of blood samples from humans and animals and cell isolation were conducted with approval of the local ethics committee (Ethik-Komission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms-Universität Münster; Az. 2008-034-f-S). Human blood samples were taken from healthy blood donors, who provided written, informed consent for the collection of samples and subsequent neutrophil isolation and analysis. All animals were handled in strict accordance with good animal practice and animal keeping, according to European guidelines (86/609/EWG), and in strict accordance with the German Protection of Animal Act (Deutsches Tierschutzgesetz). Taking of blood samples was supervised by the veterinary office of Münster (Veterinäramt der Stadt Münster; Az. 39.32.7.1).
Preparation and culture of professional phagocytes
Buffy coats were obtained from healthy human donors (Red Cross Blood Service, Münster, Germany). PBMCs and lymphocytes were isolated from buffy coats using a Ficoll-Paque density gradient (Amersham Biosciences, GE Healthcare Biosciences, Piscataway, NJ, USA), as described previously [37]. PBMCs were cultivated from McCoy's medium supplemented with 2 mM L-glutamine, penicillin/streptomycin, and 15% FCS in Teflon bags and allowed to rest for 24 h prior to stimulation. Human MDMs were obtained from PBMCs. Briefly, PBMCs were cultured in Teflon bags in RPMI 1640 supplemented with 2 mM L-glutamine, penicillin/streptomycin, and 10% human serum for 7 days with fresh medium changed every 2 days. After 7 days, cells were plated, and 24 h later, nonadherent cells were removed by washing with complete medium before the experiment. Human polymorphonuclear cells (neutrophils) were freshly isolated from Na citrate-treated blood of healthy donors and isolated by dextran sedimentation and Ficoll-Paque density gradient. Neutrophils were resuspended at a final density of 1 × 106 cells/0.5 ml in RPMI 1640 supplemented with 10% heat-inactivated FCS and used immediately for the experiments. All incubations were performed at 37°C in humidified air with 5% (monocytes) or 7% CO2 (all other cell types).
Stimulation of phagocytes
Primary monocytes, macrophages, or neutrophils were cultured on 24-well or six-well plates and subjected to PVL for 15 min–16 h at +37°C under 5% (monocytes) or 7% CO2 (all other cell types). Where indicated, 100 ng/ml ultrapure LPS from Escherichia coli (InvivoGen, San Diego, CA, USA; serotype 0111:B4), 25 μM caspase-1 inhibitor zYVAD-fmk (Enzo Life Sciences, Germany), 30 μM CTSB inhibitor CA-074Me (Enzo Life Sciences), or 130 mM KCl was added to the incubation medium. For the costimulation experiments, HKSA from strain 6850 (80°C for 30 min), LTA (Sigma-Aldrich, St. Louis, MO, USA), MRP8, or S100A12, manufactured at our institute [38, 39], was used. After the incubations, culture media were collected for analysis of their cytokine contents, and cells were washed and lysed for RNA analysis or Western blots.
Staphylococcal virulence factors
The six-His-tagged LukS-PVL and LukF-PVL proteins from E. coli were purified by nickel-nitrilotriacetic acid affinity resin (Qiagen, Germany), as described previously [9]; α-toxin and Protein A (P3838) were obtained from Sigma-Aldrich Chemie GmbH (Germany). PSMα1 and PSMα2 were synthesized by Genosphere Biotechnology (France).
Labeling of PVL
LukS-PV and LukF-PV were labeled with fluorescein (LukS-FITC and LukF-FITC) as follows. The proteins were dialyzed against PBS and dialyzed further against 0.1 M NaCO3, pH 9, at 4°C. Protein concentration was measured, and proteins were incubated further with a tenfold excess of FITC (Sigma-Aldrich Chemie GmbH) for 2 h at room temperature. NH4Cl was added to a final concentration of 50 mM and incubated for 2 h at room temperature. The FITC-coupled PVL subunits were separated from free FITC by a G25 column. The solution was again dialyzed against PBS for 3 h at 4°C. Absorption was measured at 280 nm and 495 nm. The coupling rate had to be between 0.3 and 1.0 and was calculated as follows: molar FITC/PVL = MWPVL/MWFITC × [A495/195/(A280−0.35×A495/εPVL)].
Analysis of cytokine secretion
Human IL-1β, IL-18, TNF-α, and p20 subunit of caspase-1 were quantified in the culture media samples using commercial ELISA according to the manufacturer's protocols (IL-1β and TNF-α ELISAs were from BD Biosciences, Heidelberg, Germany; IL-18 and caspase-1 from R&D Systems, Minneapolis, MN, USA). Concentrations of MRP8/14 and S100A12 were determined by an in-house ELISA, as described previously [37].
Analysis of cell death
Cells were stained with PI (Sigma-Aldrich Chemie GmbH) to detect necrosis-like membrane damage by flow cytometry. Cell death was quantified further by determination of lactate dehydrogenase activity in the medium at the end of exposure periods as described earlier [40].
Analysis of CTSB activation
Monocytes were incubated with or without PVL as described above. All cells were then incubated with Magic Red CTSB substrate (ImmunoChemistry Technologies, Bloomington, MN, USA) for 15 min. The levels of fluorescent Magic Red CTSB substrate present in the cells were quantified using flow cytometry as described previously [41].
Western blotting
IL-1β processing was analyzed in a serum-free culture medium sample corresponding to 2 × 106 cells. After 3 h of treatment supernatants (McCoy's without FCS) were harvested and precipitated with TCA, protein pellets were resuspended in SDS-PAGE buffer. Cell lysates were prepared for Western blot analysis as described earlier [42]. Western blot analysis was performed by standard procedures with the following antibodies: human anticleaved-IL-1β antibody (2021S; Cell Signaling Technology, Danvers, MA, USA) was used at 1/1000 dilution, anti-IL-1β antibody (R&D Systems) was used at 1/500 dilution, and β-actin antibody (Sigma-Aldrich Chemie GmbH) was used at 1/500 dilution. Cell lysates of THP1-knockdown cells were analyzed with human anti-ASC (AL177; Enzo Life Sciences), dilution 1/1000; anti-NLRP3 (Cryo-2; Enzo Life Sciences), dilution 1/1000; anti-CTSB (Enzo Life Sciences), dilution 1/1000; and anti-HMGB1 (4C3; Abcam, Cambridge, MA, USA), dilution 1/1000.
Quantitative RT-PCR
After stimulation, RNA was isolated from the cells, and expression of IL-1β and TNF-α RNA was analyzed by real-time RT-PCR, as described previously [42]. Each measurement was set up in duplicate, and three independent experiments were performed. After normalization to the endogenous housekeeping control gene GAPDH, the relative expression was calculated. The primers used for PCR analysis were: IL-1 forward, 5′-GCGGCCAGGATATAACTGACTTC-3′, and IL-1 reverse, 5′-TCCACATTCAGCACAGGACTCTC-3′; TNF forward, 5′-CTTCTCGAACCCCGAGTGAC-3′, and TNF reverse, 5′-TGAGGTACAGGCCCTCTGATG-3′; GAPDH forward, 5′-TGCACCACCAACTGCTTAGC-3′, and GAPDH reverse, 5′-GGCATGGACTGTGGTCATGAG-3′.
Stable, transfected THP-1 cells
THP1 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI, 10% FCS. shRNAs for knockdown of ASC (shASC), NLRP3 (shNLRP3), CTSB, or an EV and shASCmut and shGFP as negative controls were stably introduced using retrovirus as described previously [43–45]. Efficient knockdown of targeted proteins was confirmed by Western blotting. To assess effects of knockdown, cells were plated at 106 cells/ml. Equal amounts of LukS-PV and LukF-PV were added to yield a final concentration of 2–10 nM, and cells were incubated for 24 h. Cell supernatants were collected and assayed for IL-1β levels. Results represent the average plus sd of triplicate wells and are representative of three independent experiments.
Plasmid construction and transformation
To create Staphylococcus carnosus strains that express virulence factors of S. aureus, we used two basic vectors: the xylose-inducible pXR100 and the pNXR100, which is a noninducible derivate of the pXR100. For the expression of LukS-PV and LukF-PV in E. coli TG1, the commercial IPTG-inducible pQE30UA was used. For creation of the expression vectors, the respective genes were amplified by PCR, purified, and digested. The basic vectors were also digested corresponding to the genes. After ligation, S. carnosus TM300 and E. coli were transformed by protoplast transformation or the CaCl method.
Bacterial strains and cultures
TM300 strains were characterized for the presence of genes encoding PVL by PCR and by Western blots, as described recently [9, 46]. For cell culture experiments with live staphylococci, bacteria were grown overnight at 37°C in Müller-Hinton medium (containing antibiotics/xylose, if mutants are used) without shaking. Bacteria were washed in PBS and resuspended in PBS with 1% HSA. Monocytes were incubated with bacterial suspensions, resulting in a MOI as indicated. Bacterial supernatants were prepared by growing bacteria in 5 ml brain-heart infusion broth (Merck, Rahway, NJ, USA) in a rotary shaker (160 rpm) at 37°C for 12–14 h and pelleted for 10 min at 3350 g. Supernatants were sterile-filtered through a Millex-GP filter unit (0.22 μm; Millipore, Billerica, MA, USA) and used for the experiments. For PVL isolation, E. coli TG1 strains containing expression vectors for LukS-PV and LukF-PV were grown in LB media with IPTG (1 mM) and ampicillin (100 mg/ml), and cell lysates were used to purify PVL as described recently [9].
Mice
Eight- to 12-week-old C57BL/6 mice were obtained from Harlan Winkelmann (Borchen, Germany). Animals were maintained under specific pathogen-free conditions and according to the institutional guidelines in the animal facility of the University of Münster, Institute of Immunology.
Generation of BMDMs
BMDMs were differentiated in vitro from BM cells, as described previously [47]. Briefly, to generate macrophages, BM cells were cultured in DMEM (Invitrogen, Karlsruhe, Germany) containing 2 mM L-glutamine (Invitrogen), 1 mM nonessential amino acids (Invitrogen), 100 mg/ml penicillin/streptomycin (Biochrom, Berlin, Germany), 10% heat-inactivated FCS (Biochrom), and 50 ng/ml M-CSF for 6 days.
Stimulation of BMDMs
Macrophages (1×106/ml) were seeded into 96-well tissue-culture plates (Greiner Bio One, Frickenhausen, Germany) and treated with different stimuli: 1 μg/ml ultrapure LPS from E. coli (InvivoGen; serotype 0111:B4), 4 μg/ml PVL, and 20 μM nigericin. After 6 h, supernatants were harvested, and cytokine production was determined using CBA technology (BD Biosciences), according to the manufacturer's instructions. CBA FlexSets were used for IL-1 and TNF.
Statistical analysis
Statistical analyses were performed by using Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). Analyses between two groups were performed by using an unpaired two-tailed Student's t test. Comparisons among three or more groups were performed by using one-way ANOVA, followed by Dunnett's multiple means test for comparing all columns versus control or by Bonferroni's multiple means test for comparing all pairs of columns. Differences were considered statistically significant at a P value of <0.05. All experiments were done three times or more.
RESULTS
Binding of LukS-PV and LukF-PV to human leukocytes and subsequent cytotoxic effects
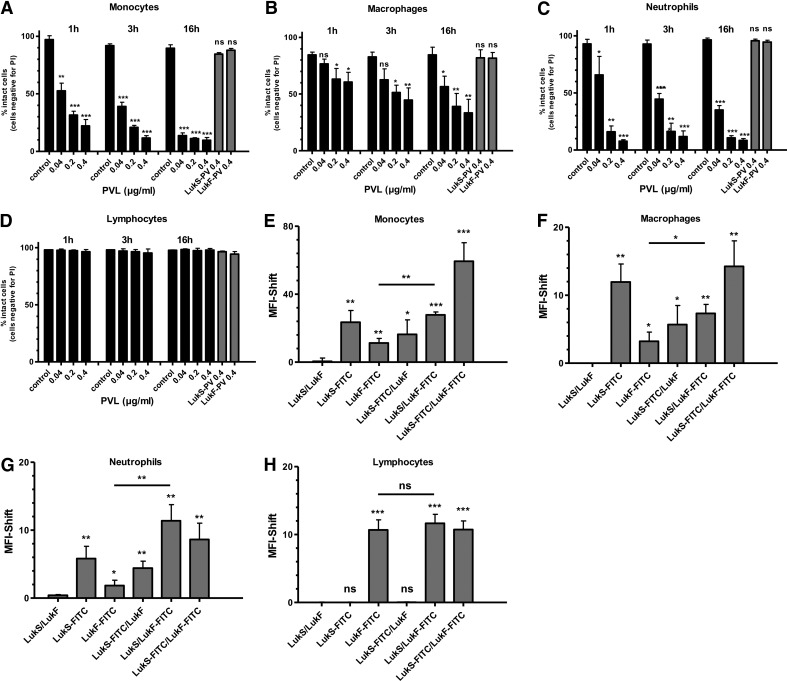
To investigate the differential sensitivity of human leukocytes toward PVL, we challenged primary isolated human monocytes, macrophages, neutrophils, and lymphocytes with increasing doses of PVL. Doses ≥0.04 μg/ml were sufficient to induce cell damage in professional phagocytes (Fig. 1A–C). Cell death occurred rapidly within 1 h and increased further within 16 h. In all sensitive cell types, measured cell death was a result of necrosis, as we could not detect Annexin V-positive cells at any time or dose tested (data not shown). Whereas monocytes and neutrophils showed a comparable rate of cell death, macrophages were less sensitive to PVL-mediated cytotoxicity. No significant cell death was observed in cells incubated with one or the other single subunit—LukS-PV or LukF-PV—even after 16 h incubation. In contrast, PVL did not cause cell death in lymphocytes (Fig. 1D). To detect the reason for this differential sensitivity toward PVL, we incubated leukocytes with FITC-labeled and unlabeled LukS-PV and/or LukF-PV components as indicated and analyzed binding of the PVL components to the cell surface by flow cytometry (Fig. 1E–G). In general, we detected significant binding of LukS-PV to cells that were tested sensitive to PVL, namely, neutrophils, monocytes, and macrophages, whereas there was no binding of LukS-PV to lymphocytes. In lymphocytes, we only found binding of the LukF-PV component, which did not result in cell death (Fig. 1D and H). In neutrophils, monocytes, and macrophages there was also some binding of the LukF-PV component, but this binding was enhanced significantly by pretreatment with the LukS-PV component (Fig. 1E–G). Only binding of both components to the cell surface induced cell death. These findings indicate that binding of the LukS-PV is the crucial factor for cell specificity, which is followed by binding of LukF-PV and subsequent death of the host cell.
Figure 1. Cytolytic effects and binding of purified PVL to human leukocytes.
Primary blood-derived monocytes (A), macrophages (B), neutrophils (C), and lymphocytes (D; 1×106×0.5 ml−1 cells) were incubated with increasing doses of PVL [0.04–0.4 μg/ml (1–10 nM)]. Cells were stimulated for 1, 3, or 16 h, respectively, or with LukS-PV and LukF-PV for 16 h and then were washed and stained with PI, and cell death was measured by flow cytometry. Statistical differences between control and stimulated cells were determined by Student's t test. Leukocytes [monocytes (E), macrophages (F), neutrophils (G) and lymphocytes (H)] (1×106×0.5 ml−1 cells) were incubated with untreated and FITC-conjugated LukS and/or LukF (1.0 μg/ml) for 1 h and washed, and binding of toxin was quantified by flow cytometry. Rate of binding was calculated by the MFI shift between untreated control cells and toxin-treated cells. The values represent the mean ± sd of at least three independent experiments. Statistical differences were determined by ANOVA comparing the MFI between control and treated cells (*_P_≤0.05; **_P_≤0.01; ***_P_≤0.001).
PVL induces a proinflammatory response in professional phagocytes
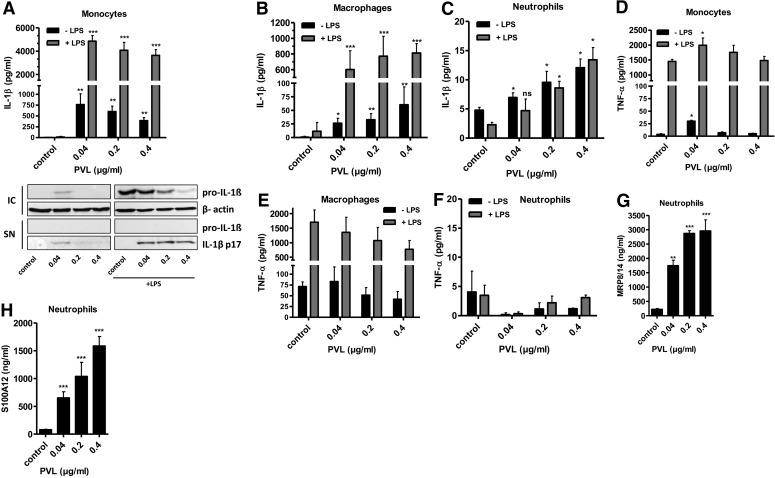
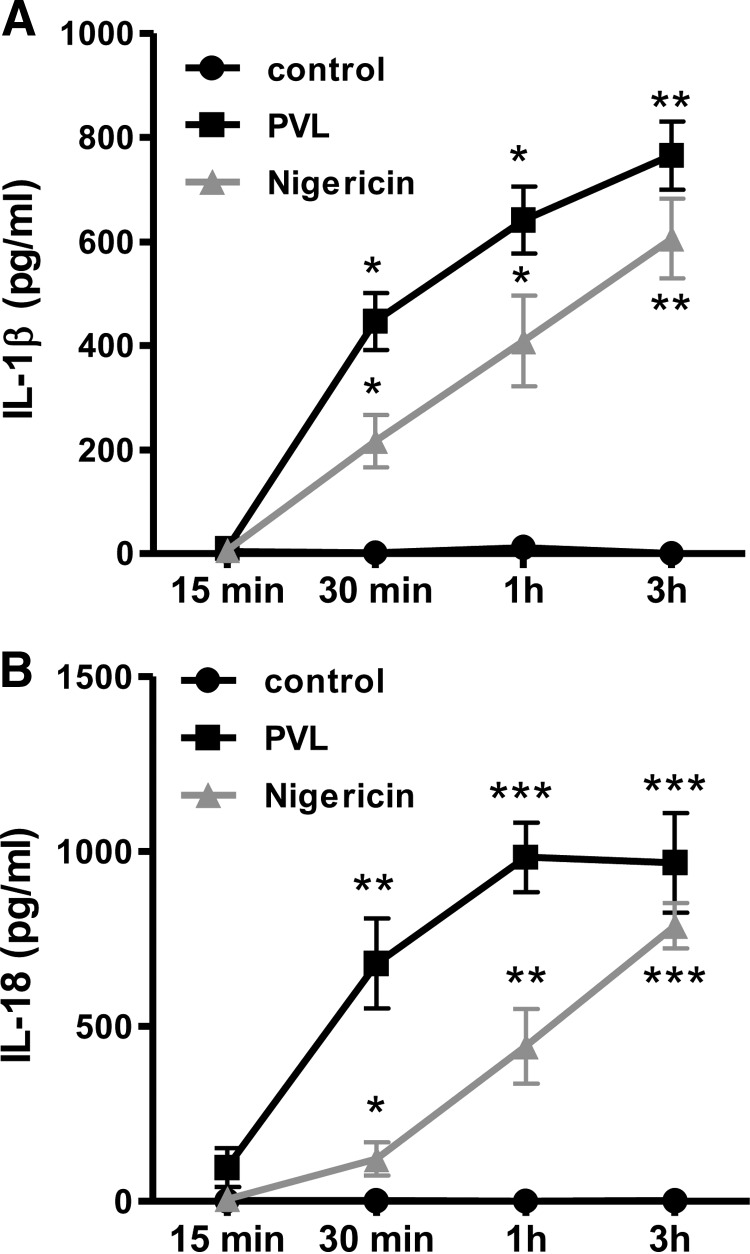
To analyze the proinflammatory IL-1β response induced by PVL binding to human leukocytes, we incubated the different cell types with increasing PVL doses (0.04–0.4 μg/ml) for 3 h. Ultrapure LPS (100 ng/ml) was used as a costimulant and was added 30 min before PVL treatment. PVL induced a strong increase of IL-1β release in monocytes (Fig. 2A) and macrophages (Fig. 2B) that was even more pronounced after LPS prestimulation. Western blot analysis of cell supernatants revealed that mature IL-1β was secreted by activated monocytes in the absence of pro-IL-1β, confirming the processing of IL-1 induced by PVL. Furthermore, in nonprimed cells treated with low doses of PVL, pro-IL-1β was induced. In monocytes, increasing concentrations of PVL did not further enhance IL-1β secretion (Fig. 2A), whereas in macrophages, the IL-1β production was augmented dose-dependently, which can be explained by lower sensitivity of macrophages to PVL. In contrast to IL-1β secretion, TNF-α levels were only marginally/not increased after PVL treatment (Fig. 2D and E). In general, both PVL-induced proinflammatory effects (IL-1β and TNF release) were much less pronounced in neutrophils, and we could not detect an enhancing effect of prestimulation with LPS (Fig. 2C and F). Yet, after PVL treatment of neutrophils, we found a massive release of the DAMPs MRP8/14 (Fig. 2G) and S100A12 (Fig. 2H), which are known to act as endogenous amplifiers of innate immunity [48]. Based on these findings, we performed a kinetic of IL-1β and IL-18 release (Fig. 3). Both inflammasome-regulated proteins showed a quick release with a climax after 1 h, indicating that the secretion of these proteins is a fast mechanism.
Figure 2. Human phagocytes respond to PVL by IL-1β secretion and release of DAMPs.
Primary monocytes (A and D), macrophages (B and E), and neutrophils (C and F–H; 1×106×0.5 ml−1 cells) were incubated with PVL (0.04–0.4 μg/ml) for 3 h. Ultrapure LPS (100 ng/ml) was used as a costimulant for the primary cells and was added 30 min before PVL treatment. Concentrations of IL-1β, TNF-α, MRP8/14, and S100A12 were subsequently determined from cell culture supernatants. The inset in A verifies the presence of mature 17 kDa IL-1β and absence of pro-IL-1β in cell culture supernatants (SN) of primary monocytes, as well as the induction of intracellular pro-IL-1β (IC) by Western blotting. The values represent the means ± sd from at least three experiments. Statistical differences were determined by Student's t test (*_P_≤0.05; **_P_≤0.01; ***_P_≤0.001 compared with untreated cells).
Figure 3. PVL induces a quick secretion of inflammasome-dependent proteins IL-1β and IL-18.
Primary monocytes (1×106×0.5 ml−1 cells) were incubated with 0.04 μg/ml PVL for the indicated time-points; the K+-ionophore nigericin (20 μM) served as a positive control. Release of IL-1β (A) and IL-18 (B) was quantified by ELISA in the supernatants. The values represent the means ± sd from at least three experiments. Statistical differences were determined by Student's t test. (*_P_≤0.05; **_P_≤0.01; ***_P_≤0.001 compared with untreated cells).
PVL-induced IL-1β secretion is mediated by the NLRP3 inflammasome
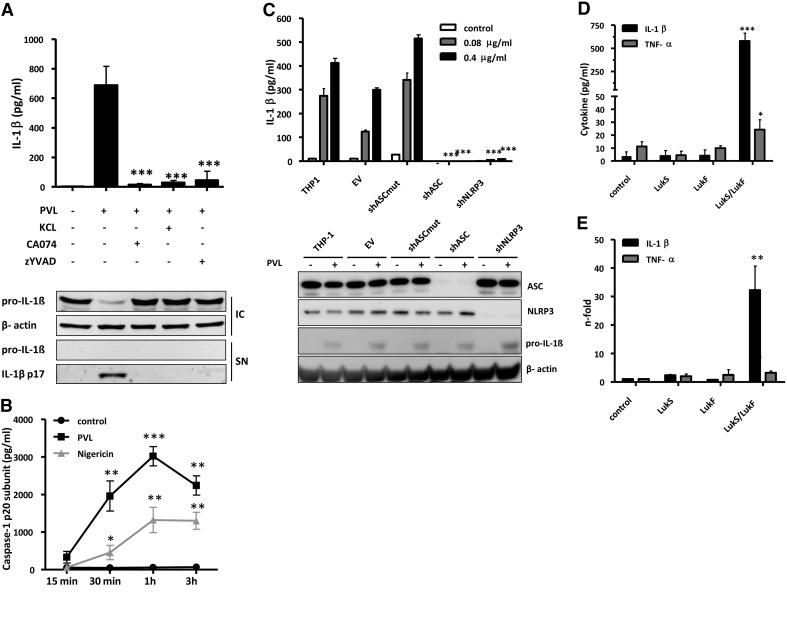
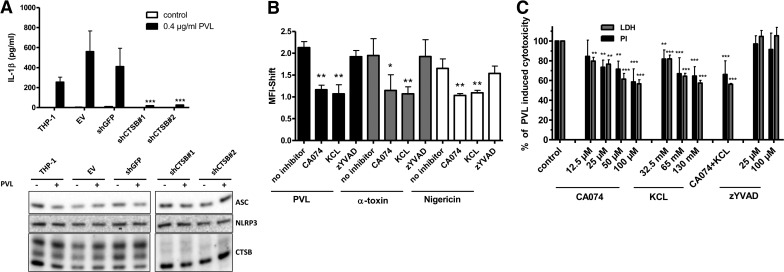
Next, we studied the involved mechanism of PVL-induced IL-1β secretion in human monocytes (Fig. 4A). Firstly, we found that the caspase-1 inhibitor zYVAD-fmk abrogated PVL-induced IL-1β secretion, confirming that IL-1β maturation is indeed caspase-1-dependent. As PVL is a known K+-ionophore, and K+-efflux has been linked to activation of NLRP3 and NLRP1 inflammasomes [49], we incubated cells with high extracellular K+-concentrations that prevented the IL-1β response to PVL as well. Furthermore activation of the lysosomal cysteine protease cathepsin has been associated with activation of the NLRP3 inflammasome. The CTSB inhibitor CA-074Me completely abolished the IL-1β response to PVL. All of our results could be confirmed by Western blots of cell pellets and supernatants showing the inhibition of secreted, mature IL-1β in the presence of high intracellular pro-IL-1β levels, suggesting a major role for CTSB and NLRP3 in PVL-induced inflammasome activation. Cleavage of caspase-1 after PVL treatment was confirmed by measuring the p20 subunit in the supernatant of PVL- or nigericin-treated cells. Caspase-1 levels increased fast in a time-dependent manner (Fig. 4B). Our hypothesis was strengthened further by using stable THP-1-derived cell lines with expression of the inflammasome components NLRP3 or ASC “knocked down” by expression of shRNAs targeting the mRNA encoding those proteins. Western blot analysis indicates that levels of NLRP3 and ASC proteins are reduced by >90% in these cells (Fig. 4C). Testing the effect of PVL on both cell lines revealed a strong reduction of IL-1β secretion when compared with control cell lines with intact expression of NLRP3 and ASC (Fig. 4C). The functionality of the model was confirmed by treatment with LPS and ATP (Supplemental Fig. 1A). Interestingly, THP-1 cells show a high resistance against PVL-induced cytoxicity, demonstrated by the inability of the toxin to induce PI permeability or release of HMGB1 into the culture supernatant in PVL-treated cells (Supplemental Fig. 1B and C). In the absence of cell death, we could rule out passive release of pro-IL-1β to the supernatant but also, could not study cell death mechanisms in this model.
Figure 4. Mechanism of PVL-induced IL-1β secretion.
Primary monocytes (1×106×0.5 ml−1 cells; A) were incubated with 0.04 μg/ml PVL in the absence or presence of KCl (130 mM), CTSB inhibitor CA-074Me (30 μM), or caspase-1 inhibitor zYVAD-fmk (25 μM). After the incubation, IL-1β (average response to PVL, 690 pg/ml) was analyzed by ELISA. The values represent the means ± sd from at least three experiments. The inset in A verifies the inhibition of processing and secretion of mature 17 kDa IL-1β and the absence of pro-IL-1β in cell culture supernatants of LPS-primed primary monocytes (5×106×2.5 ml−1 cells) and the presence of intracellular pro-IL-1β by Western blotting. Statistical differences were determined by ANOVA comparing PVL-treated cells with PVL-treated cells + inhibitors (***_P_≤0.001). Further, cells were treated with PVL (0.04 μg/ml), and p20 caspase-1 subunit was analyzed by ELISA in the supernatant after different time-points; the K+-ionophore nigericin (20 μM) served as a positive control. Statistical differences were determined by Student's t test (*_P_≤0.05; **_P_≤0.01; ***_P_≤0.001 compared with untreated cells; B). THP-1-derived cell lines (C) stably transduced with shRNA expressing retrovirus were treated with 0.08 or 0.4 μg/ml PVL, and cell culture supernatants were analyzed for IL-1β after 24 h. The shRNAs are directed to knock down expression as follows: EV, negative control; shASCmut, negative control; shASC, shRNA directed against ASC protein; shNLRP3, shRNA directed against NLRP3. Knockdown of ASC and NLRP3 and expression of pro-IL-1β were confirmed by Western blot. Results represent the means ± sd of triplicate wells and are representative of three independent experiments. Primary monocytes (5×106×2.5 ml−1 cells) were treated with LukS or LukF or both for 3 h, and cell culture supernatants were analyzed for IL-1β or TNF-α (D). Induction of IL-1β and TNF-α RNA was determined by RT-PCR (E) and expressed as n-fold compared with untreated control. The values represent the means ± sd from at least three experiments. Statistical differences were determined by ANOVA comparing PVL-treated cells to control (*_P_≤0.05; **_P_≤0.01; ***_P_≤0.001).
Furthermore, we tested whether inflammasome activation depends on pore-formation of PVL or whether it is mediated by LukS-PV or LukF-PV components alone. Only the combination of LukS-PV and LukF-PV was able to induce a strong IL-1β release, whereas the single components did not show any effect (Fig. 4D). On the transcriptional level, LukS-PV or LukF-PV alone did not cause any IL-1β gene expression as well. Treatment with the combination of LukS-PV and LukF-PV induced expression of the IL-1β gene (Fig. 4E) that correlated with increased pro-IL-1β protein found after PVL stimulation, as measured by Western blot and ELISA (Fig. 2A). As IL-1β expression can be activated by NF-κB, we analyzed NF-κB activation by PVL. In comparison with LPS, PVL was not able to activate NF-κB (Supplemental Fig. 2A and B).
PVL-mediated K+-efflux leads to CTSB activation and pyronecrosis
In further experiments, we aimed to analyze the inflammasome activation in more detail. For this, we used several inhibitors/compounds interfering at different stages of the inflammasome pathway. IL-1β secretion and cell death induced by the K+-ionophore nigericin are blocked by inhibitors of the cysteine proteinase CTSB. Additionally, NLRP3-dependent pyronecrosis, which does not require caspase-1 activity, can be abrogated by CTSB inhibitors [36]. As we could block IL-1β secretion by the CTSB inhibitor CA-074Me, we investigated the impact of CTSB activation on IL-1β secretion by PVL and its role in cell death. Firstly, we used stable THP-1-derived cell lines with CTSB knocked down by expression of shRNAs targeting the mRNA encoding those proteins. Western blots demonstrate that the levels of CTSB protein are reduced by >90% in these cells without affecting expression of NLRP3 and ASC. These cell lines exhibited a large reduction in IL-1β secretion after stimulation with PVL when compared with control cell lines with intact expression of CTSB (Fig. 5A). Furthermore, we found that 15 min stimulation of monocytes with PVL was followed by cleavage of the fluorescent CTSB substrate, Magic Red-(RR)2, and this could be reduced significantly by high extracellular K+-concentrations, indicating a direct link between K+-efflux and CTSB activation. CA-074Me was used as a positive control for this assay, whereas inhibition of caspase-1 downstream of CTSB activation did not influence the assay. These findings could be reproduced with the inflammasome activators α-toxin and nigericin (Fig. 5B). Finally, we quantified PVL-induced necrotic cell death by PI staining and LDH activity in the presence of increasing doses of CA-074Me, KCl, and zYVAD-fmk. With high doses of CA-074Me and KCl, we were able to inhibit cell death by 40%, whereas zYVAD-fmk did not show any effect (Fig. 5B). These results confirm that CTSB-mediated pyronecrosis contributes partially to PVL-induced cytotoxicity.
Figure 5. PVL-mediated K+-efflux leads to CTSB activation and pyronecrosis.
THP-1-derived cell lines (A), stably transduced with shRNA expressing retrovirus, were treated with 0.4 μg/ml PVL, and cell culture supernatants were analyzed for IL-1β after 24 h. The shRNAs are directed to knockdown expression as follows: EV, negative control; shGFP, negative control; shCTSB#1 and #2, two shRNAs directed against CTSB. Knockdown of CTSB and the presence of ASC and NLRP3 were confirmed by Western blot. Results represent the means ± sd of triplicate wells and are representative of three independent experiments. Statistical differences were determined by ANOVA comparing knockdown cells with control (***_P_≤0.001). CTSB activity was measured via degradation of the fluorescent Magic Red CTSB substrate (ImmunoChemistry Technology). Magic Red CTSB substrate was added to primary monocytes (1×106×0.5 ml−1 cells) treated with PVL (0.4 μg/ml), α-toxin (10 μg/ml), or nigericin (20 μM) for 15 min, with or without pretreatment with KCl (130 mM), CA-074Me (30 μM), or zYVAD-fmk (25 μM) (B). Degradation of the Magic Red substrate was measured via flow cytometry on a BD FACSCalibur. Analysis of the data was accomplished with detection of the optimal Magic Red substrate fluorescence emission in fluorescence-2 on a logarithmic scale. The values represent the means ± sd from three experiments. Statistical differences were determined by ANOVA comparing the MFI between control and treated cells (*_P_≤0.05; **_P_≤0.01). Primary monocytes (1×106×0.5 ml−1 cells; C) were incubated with 0.04 μg/ml PVL for 3 h in the absence or presence of CTSB inhibitor CA-074Me (12.5–100 μM), KCl (32.5–130 mM), or caspase-1 inhibitor zYVAD-fmk (25 or 100 μM). LDH was measured in the supernatants, and cells were washed and stained with PI. The values represent the means ± sd from at least three experiments. Statistical differences were determined by ANOVA comparing PVL-treated cells with PVL-treated cells + inhibitors (**_P_≤0.01; ***_P_≤0.001).
PVL-expressing bacteria cause cytotoxicity and IL-1β secretion
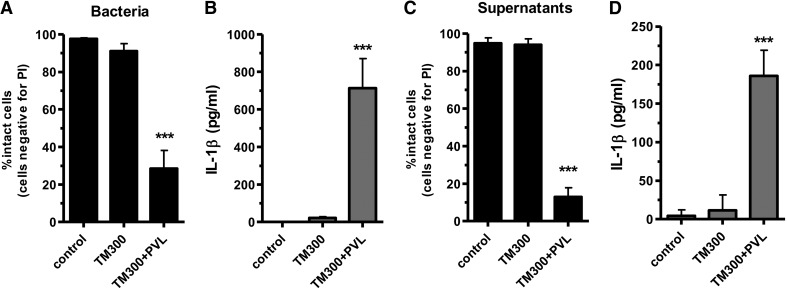
To investigate the impact of PVL expression, we used the strain S. carnosus TM300 (lacking most S. aureus virulence factors) that has been transformed with a plasmid encoding the genes for PVL. Infecting monocytes with live bacteria of TM300 + PVL or with live bacteria of the parent strain TM300 revealed that the expression of PVL is clearly responsible for cell death induction (Fig. 6A) and IL-1β expression (Fig. 6B) in monocytes. As PVL is a bacterial exotoxin, which is rapidly released and acts on cells at the site of infection, we additionally challenged monocytes with sterile-filtered bacterial supernatants from overnight cultures. Culture media from strain TM300 + PVL induced rapid cell death within 1 h (Fig. 6C) and secretion of IL-1β (Fig. 6D), whereas supernatants from the control strain TM300 did not show any effects.
Figure 6. The cytotoxic effect and induction of IL-1β secretion of TM300 and its supernatant are dependent on PVL expression.
Primary monocytes (5×106×2.5 ml−1 cells) were incubated with 10 MOI of bacteria (A and B) for 3 h or for 1 h with bacterial supernatants (C and D), which were prepared from overnight cultures and used for stimulating cells (10% vol/vol) or were left untreated. In this experiment, the heterologous expression strain TM300 + PVL and its WT strain were used. After incubation of the cells with bacterial supernatants or whole bacteria, cells were washed and stained with PI for analysis by flow cytometry. IL-1β was measured in the cell supernatants by ELISA. The values represent the means ± sd of at least three independent experiments. Statistical differences were determined by Student's t test (***_P_≤0.001 compared with TM300).
Costimulation with PAMPs and DAMPs increases PVL-induced IL-1β secretion
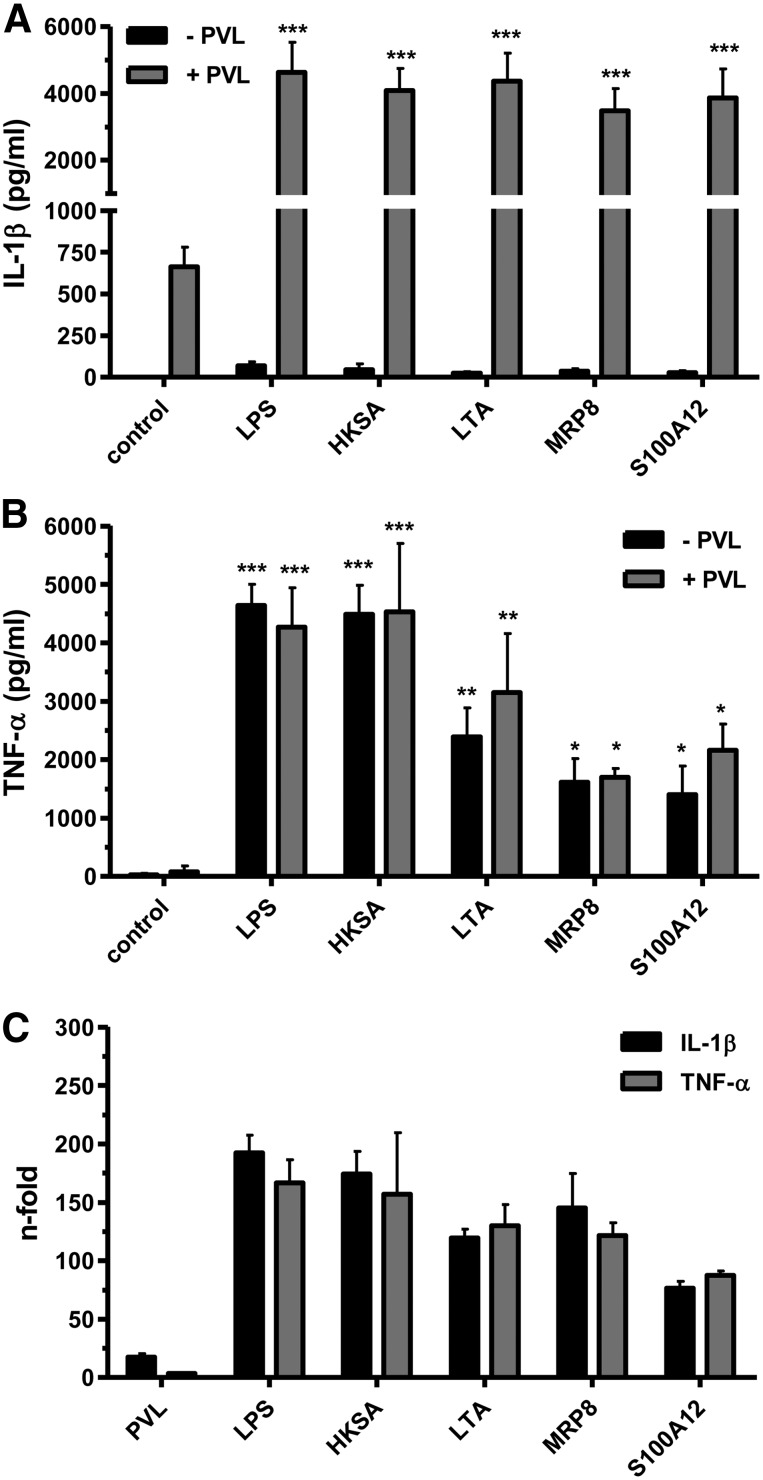
LPS is a common experimental costimulus to increase IL-β secretion by induction of pro-IL-1β [50]. However, LPS is restricted to Gram-negative bacteria and is not present during an infection with Gram-positive S. aureus. Therefore, we analyzed other compounds that are supposed to accumulate at the S. aureus infection side for their potential to prime monocytes to generate pro-IL-1β. In this respect, we analyzed costimulatory effects of PAMPs, which are associated with groups of pathogens and are recognized by cells of the innate immune system, mostly via TLRs, and DAMPs, which are highly released from neutrophils after PVL challenge (see Fig. 2). In our experiments, we costimulated monocytes with the PAMPs HKSA or the cell wall component LTA, a known TLR2 agonist, followed by incubation with PVL. Furthermore, we used the DAMPs MRP8, the active subunit of the MRP8/14 complex, and S100A12, which are released by PVL-damaged neutrophils (Fig. 2G and H), to preactivate monocytes. The stimulation with DAMPs and PAMPs alone was not sufficient to induce IL-1β secretion, whereas the addition of PVL leads to massive release of IL-β (Fig. 7A). By contrast, the activation with PAMPs and DAMPs induced TNF-α secretion in monocytes, which was not further increased by PVL (Fig. 7B). All costimulants were capable of inducing IL-1β gene expression (Fig. 7C), leading to increased production of pro-IL-1β, which resulted in enhanced processing and a strong IL-1β release after PVL stimulation. Therefore, DAMPs and PAMPs are capable of amplifying IL-1β release by priming monocytes before activation by PVL.
Figure 7. Costimulation with PAMPs and DAMPs increases PVL-induced IL-1β secretion.
Primary monocytes (1×106×0.5 ml−1 cells) were incubated with 0.04 μg/ml PVL for 3 h or were left untreated. Ultrapure LPS (100 ng/ml), HKSA (100 MOI), and LTA (10 μg/ml) were used as costimulatory PAMPs, and MRP8 and S100A12 (each 5 μg/ml) served as DAMPs and were added 30 min before PVL treatment, or cells were left untreated as controls. After incubation, cell culture supernatants were analyzed for IL-1β (A) or TNF-α (B). Statistical differences were determined by ANOVA (*_P_≤0.05; **_P_≤0.01; ***P ≤0.001 compared with controls). Furthermore, primary monocytes (5×106×2.5 ml−1 cells) were treated with the above-mentioned PAMPs and DAMPS or PVL (0.04 μg/ml), and induction of IL-1β and TNF-α RNA was determined by RT-PCR (C) and expressed as n-fold compared with untreated controls.
Induction of IL-1β secretion by different staphylococcal virulence factors
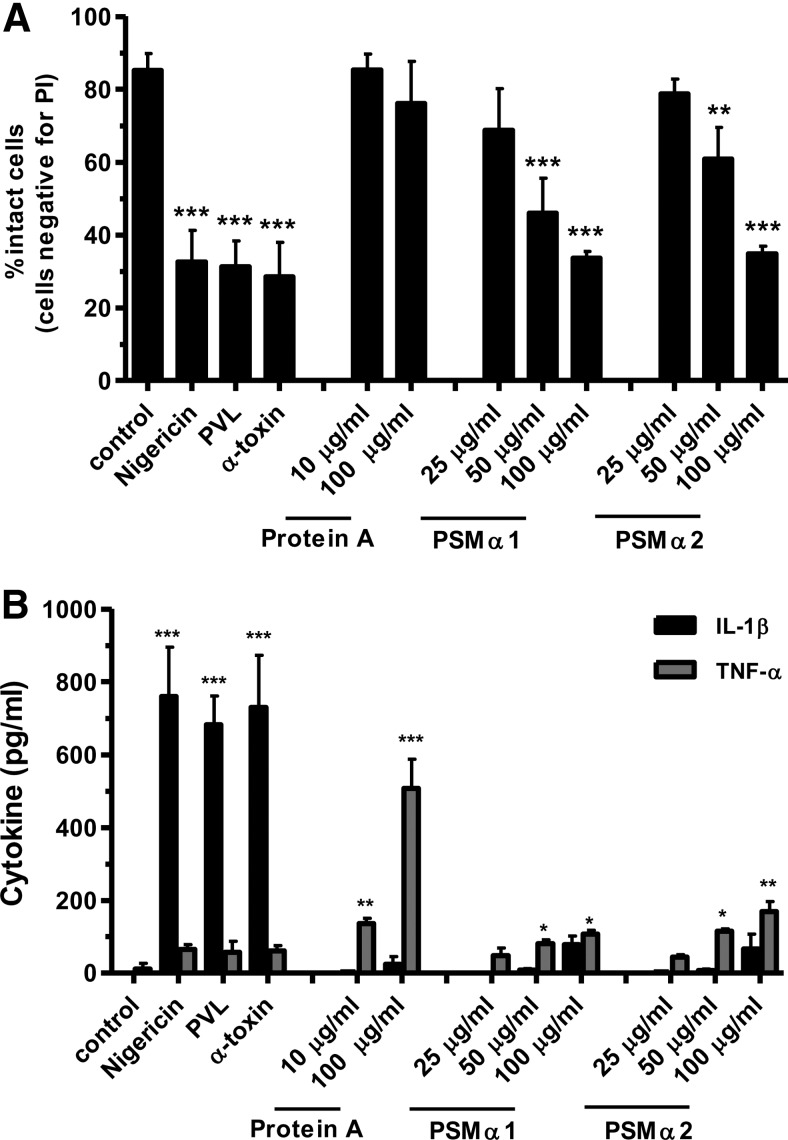
As PVL is not the only important S. aureus virulence factor that can induce a proinflammatory reaction, we compared PVL with α-toxin (which is known to be an inflammasome activator) [32], with protein A (which has been demonstrated to activate TNFR1) [51], and with the PSMα1 and -2 (which have chemoattractive activity for immune cells) [52]. All agents, despite protein A, caused a strong cytotoxic effect in monocytes (Fig. 8A), which is in accordance with previously published work. Interestingly, only the pore-forming toxins PVL, α-toxin, and nigericin, which we used as a positive control, were able to induce a strong IL-1β release (Fig. 8B). By contrast, PSMs only induced weak IL-1β secretion at high concentrations, indicating that cytotoxicity alone is not the responsible factor for IL-1β release but rather, the specific formation of a K+-ionophore.
Figure 8. Induction of IL-1β and TNF-α secretion by staphylococcal components.
Primary monocytes (1×106×0.5 ml−1 cells) were incubated with PVL (0.04 μg/ml), α-toxin (10 μg/ml), Protein A (10 and 100 μg/ml), and PSMα1 and -2 (25–100 μg/ml) for 3 h or were left untreated; the K+-ionophore nigericin (20 μM) served as positive control. After incubation, cells were washed and stained with PI for flow cytometry (A), and cell culture supernatants were analyzed for IL-1β or TNF-α by ELISA (B). The values represent the means ± sd from at least three experiments. Statistical differences were determined by ANOVA (*_P_≤0.05; **_P_≤0.01; ***_P_≤0.001 compared with control).
PVL has no effect on murine BMDMs
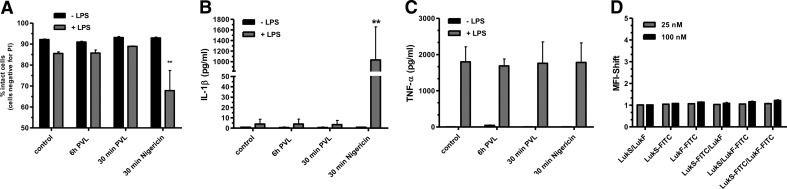
Recently, we could show the species specificity of PVL and excluded its cytotoxic and proinflammatory effects on murine neutrophils [9]. To analyze a potential proinflammatory action of PVL on other murine cell types, we challenged BMDMs with high concentrations of PVL that have been reported to be effective in murine cells before [53]. Despite costimulation with LPS and different time periods of incubation, we could not find any effect of PVL with respect to cytotoxicity (Fig. 9A), IL-1β secretion (Fig. 9B), or TNF-α release (Fig. 9C), whereas nigericin was highly effective to cause cytotoxicity and IL-1β secretion. LukS-PV and LukF-PV are not able to bind to murine BMDMs, which could be demonstrated by FACS analysis with FITC-labeled components (Fig. 9D). These experiments underlined the strict species specificity of PVL.
Figure 9. PVL has no effect on murine BMDMs.
BMDMs were prestimulated with ultrapure LPS (1 μg/ml) for 6 h and then incubated further with PVL (4 μg/ml) for 30 min or costimulated for 6 h with LPS and PVL. Thirty minutes of treatment with nigericin served as a positive control. After incubation, cells were washed and stained with PI (A), and cell culture supernatants were analyzed for IL-1β (B) or TNF-α (C) by CBA. The values represent the means ± sd from at least three experiments. Statistical differences were determined by ANOVA (**_P_≤0.01 compared with control). BMDMs were incubated with unlabeled and FITC-conjugated LukS and/or LukF (1.0 or 4.0 μg/ml) for 1 h and washed, and binding of toxin was quantified by flow cytometry. Rate of binding was calculated by the MFI shift between untreated control cells and toxin-treated cells. The values represent the mean ± sd of at least three independent experiments (D).
DISCUSSION
The pathogenic mechanisms of S. aureus necrotizing infections that lead to massive inflammation and tissue destruction are largely unknown. According to epidemiological data [12] and to results from an in vivo model in rabbits [22], PVL could be identified as a crucial, responsible factor. Yet, the exact molecular mechanisms that are induced by PVL at the cellular level are not fully elucidated. Previous work largely focused on PVL-induced cytotoxicity of human neutrophils, whereas there is binding to monocytes and macrophages as well [10]. It has already been shown that binding of LukS-PV is a critical factor for LukF-PV binding [54–56], but to our knowledge, no previous study has given a comprehensive overview of PVL components binding to all different types of human leukocytes and the subsequent impact on cell viability. In this work, we could confirm the binding of LukS-PV as the critical factor for PVL-induced cytotoxicity, and we performed the first binding studies with human macrophages, demonstrating that LukS-PV binds to monocytes, macrophages, and neutrophils but not to lymphocytes.
Furthermore, we present a novel, specific mechanism of how S. aureus PVL induces a proinflammatory response in primary human monocytic cells. Monocytes are mobile cells that can move quickly to sites of infection to elicit an early immune response. Therefore, the PVL-induced proinflammatory effect on monocytes could play an important role to initiate an infection and to attract further immune cells, such as neutrophils and lymphocytes, which are usually present in large numbers during necrotizing infections. In our experiments, we demonstrate that PVL is capable of inducing activation of caspase-1 through the NLRP3 inflammasome. Pore formation of PVL leads to K+-efflux and the consecutive activation of CTSB, which mediates programmed necrosis and activation of NLRP3. By performing siRNA silencing experiments, we confirmed the NLRP3 receptor as the PVL-responsive element in monocytes. Previous work revealed that S. aureus, lacking α-toxin or β- or γ-hemolysin, was still capable of activating IL-1β secretion, indicating that other staphylococcal factors cause inflammasome activation as well [33]. Our findings demonstrate that PVL is a strong inducer of NLRP3 activation. This effect was independent of other S. aureus virulence factors, as the strain S. carnosus TM300 dramatically increased cytotoxicity and IL-1β secretion in monocytes upon complementation with a plasmid containing the genes for PVL. Additionally, we could show that this ability is not shared by all S. aureus toxins. We confirmed the α-toxin-mediated IL-1β secretion that was reported previously [32], but we also provide evidence that PSMs fail to activate the inflammasome. Accordingly, the ability to activate the inflammasome is restricted to pore-forming toxins and is not shared by other S. aureus components, such as PSMs, protein A, or LTA. Furthermore, we could show that another inflammasome-dependent protein IL-18 [57] is released by PVL stimulation. In contrast to pro-IL-1β, pro-IL-18 is constitutively expressed in cells, independently of a primary stimulus [58], but it shares the same effects of IL-1β and is especially involved in T cell activation [59].
With respect to IL-1β secretion, primary stimuli, such as LPS, induce synthesis of pro-IL-1β and may provide signals that enhance the effect of the secondary stimulus [50]. In this work, we demonstrate that PVL does not require a LPS-priming step, as PVL alone was sufficient to induce pro-IL-1β and activate caspase-1 and mature IL-1β release. PVL treatment is capable of inducing IL-1β gene expression, independent from an early NF-κB activation, and might be a result of an intermediate step by released DAMPs (e.g., MRPs) or cytokines. Nevertheless, priming with LPS enhanced IL-1β release significantly. As LPS is restricted to Gram-negative bacteria, we tested other factors that are usually present during a S. aureus infection. Firstly, we used HKSA and the bacterial wall component LTA as primary stimuli provided by bacteria as PAMPs. For both components, we could demonstrate a priming effect for IL-1β secretion, which is in line with previously published work [32, 60]. In addition, our data point to a novel, positive feedback mechanism by which PVL may induce overwhelming inflammation. We demonstrate for the first time that PVL induces release of the endogenous TLR4 agonists MRP8/14 [39] and the receptor for advanced glycation endproducts agonist S100A12 [38] by neutrophils, which we have previously shown to drive inflammation via activation of endothelial cells and phagocytes [38, 39, 61]. Endogenous DAMPs as well as the PAMPs of S. aureus were all capable of inducing IL-1β mRNA and consecutively enhancing PVL-mediated IL-1β release, indicating that IL-1β secretion in vivo can be enhanced strongly by host and bacterial factors.
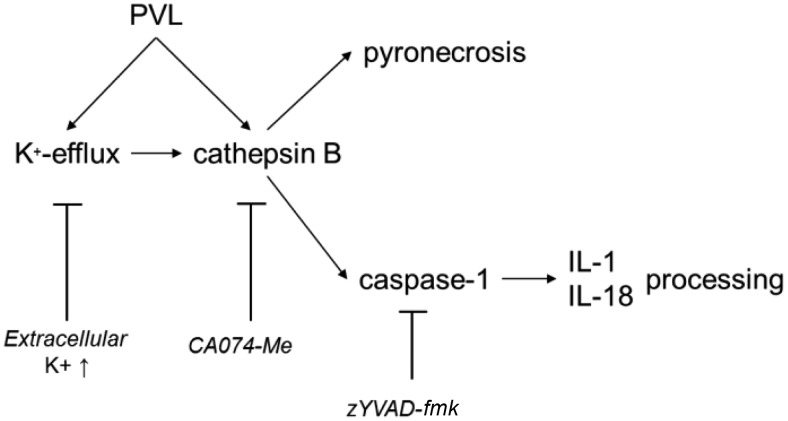
A common feature of secondary stimuli is their ability to induce cell death concomitant with the release of IL-1β [62, 63]. The ability of PVL to activate programmed cell death in host cells has been the subject of different studies [9, 64]. Recently, we demonstrated that PVL induces rapid necrosis in neutrophils without any signs of apoptosis [9]. In this study, we were not able to detect apoptosis in all investigated cell types as well. Pretreatment with CA-074Me and elevated extracellular K+-concentrations significantly reduced PVL-induced cell death. Moreover, we could reveal that although the caspase-1-specific inhibitor zYVAD-fmk effectively blocks the secretion of mature IL-1β from monocytes, it does not protect cells from PVL-induced necrosis. This indicates that PVL-induced necrosis is independent from caspase-1 activation. Nevertheless, CTSB inhibition only partially reduced cell death, and therefore, programmed necrosis, so-called pyronecrosis [65], is not the only pathway leading to PVL-mediated necrosis. Our study was limited by the resistance of THP-1 cells against PVL-induced cytotoxicity, and therefore, our findings are based on experiments with specific inhibitors. Our findings support the hypothesis that PVL leads to K+-efflux and activation of CTSB, which induces necrosis and activation of caspase-1 (Fig. 10). This CTSB-dependent activation of caspase-1 was already shown for different inflammasome activators, such as the pore-forming toxin nigericin, serum amyloid A, and cholesterol crystals [36, 66, 67]. By performing siRNA silencing experiments, we confirmed the crucial role of CTSB for PVL-induced inflammasome activation. K+-efflux [49] and activation of CTSB [68] seem to be essential intermediate steps in activation of the NLRP3 receptor, but the connection between these processes is poorly understood [34]. Our findings suggest that both K+-efflux and CTSB activity contribute to PVL-mediated activation of the NLRP3 inflammasome. Nevertheless, further studies are necessary to identify a possible interaction of these cellular factors in PVL-induced NLRP3 activation, but our findings indicate that the K+-efflux is upstream of CTSB activation.
Figure 10. Proposed mechanism of PVL-induced inflammasome activation.
PVL leads to K+-efflux and CTSB activation, which consecutively activates the inflammasome and induces pyronecrosis. Activated caspase-1 processes IL-1β and IL-18. All steps can be blocked by specific inhibitors.
In conclusion, our study provides the first evidence that PVL activates the NLRP3-inflammasome in a CTSB-dependent manner. This finding is of high biological relevance with regard to severe infections caused by PVL-expressing S. aureus strains. PVL induces cell death in monocytes, macrophages, and neutrophils, which is most likely a strategy of the pathogen to overcome the cellular innate immune system. In addition, our findings regarding priming of phagocytes for PVL responses by endogenous DAMPs may be of relevance for the tissue damage associated with overwhelming disease activity during PVL-driven inflammation and may thus represent a novel molecular target for future adjuvant therapy to appropriate antibiotic use in severe S. aureus infections.
Supplementary Material
Supplemental Data
ACKNOWLEDGMENTS
This work was supported by Deutsche Forschungsgemeinschaft of Germany (DFG) grants (HA3177/2-1 and SFB1009) to B.L., a German Federal Ministry of Education and Research (BMBF) grant (Flu-Bak Signaling) to B.L., and grants from the Interdisciplinary Centre for Clinical Research (Löf2/030/10 and Ro2/004/10) to B.L. and J.R. at the University of Münster. D.H. was supported by an Innovative Medizinische Forschung (IMF) grant of the University of Münster (HO220912). P.M.B. is an awardee of the University of North Carolina STD and HIV Training Program (U.S. National Institutes of Health T32AI007001). J.A.D. is supported by the Burroughs Wellcome Fund through the Career Award for Medical Scientists and U.S. National Institutes of Health (AI088255). We thank Melanie Saers, Heike Berheide, Brigitte Schuhen, and Michaela Brück for excellent technical assistance.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
α-toxin
α-hemolysin
ASC
apoptosis-associated speck-like protein
BMDM
bone marrow-derived macrophage
CA-MRSA
community-acquired methicillin-resistant Staphylococcus aureus
CTSB
cathepsin B
DAMP
danger-associated molecular pattern
EV
empty vector
HKSA
heat-killed Staphylococcus aureus
HMGB1
high-mobility group protein B1
LTA
lipoteichoic acid
LukF-PV
subunit F (“F” for “fast”) of Panton-Valentine leukocidin
LukS-PV
subunit S (“S” for “slow”) of Panton-Valentine leukocidin
MFI
mean fluorescence intensity
MRP8/14
myeloid-related protein complex 8/14
NLRP3
nucleotide-binding domain and leucine-rich repeat-containing gene family, pyrin domain containing 3 protein
PAMP
Pathogen associated molecular pattern
PRR
pattern recognition receptors
PSM
phenol soluble modulins (expressed by Staphylococcus aureus)
PVL
Panton-Valentine leukocidin (Staphylococcus aureus pore-forming toxin)
sh
short hairpin
shASCmut
scrambled sequence with base content equal to short hairpin apoptosis-associated speck-like protein
shGFP
short hairpin RNA against GFP
siRNA
small interfering RNA
zYVAD-fmk
z-Tyr-VAD-fmk
AUTHORSHIP
D.H., J.R., G.P., and B.L. conceived of and designed the experiments. D.H., L.G., V.M., N.N., D.J.T., K.M., T.V., D.F., S.N., J.A., J.A.D., and P.M.B. performed the experiments. D.H. and B.L. analyzed the data and wrote the manuscript. J.R. and G.P. initiated the research, supervised the program, and analyzed and discussed data.
REFERENCES
- 1.Lowy F. D. (1998) Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 [DOI] [PubMed] [Google Scholar]
- 2.Gordon R. J., Lowy F. D. (2008) Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 46 (Suppl. 5), S350–S359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakdi S., Martin E. (1991) Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect. Immun. 59, 2955–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodin A. M. (1960) Purification of the two components of leucocidin from Staphylococcus aureus. Biochem. J. 75, 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko J., Kamio Y. (2004) Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 68, 981–1003 [DOI] [PubMed] [Google Scholar]
- 6.Bubeck S. S., Cantwell A. M., Dube P. H. (2007) Delayed inflammatory response to primary pneumonic plague occurs in both outbred and inbred mice. Infect. Immun. 75, 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoshima I., Inoshima N., Wilke G. A., Powers M. E., Frank K. M., Wang Y., Bubeck Wardenburg J. (2011) A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 17, 1310–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A. H., Nowlan P., Weavers E. D., Foster T. (1987) Virulence of protein A-deficient and α-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 55, 3103–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loffler B., Hussain M., Grundmeier M., Bruck M., Holzinger D., Varga G., Roth J., Kahl B. C., Proctor R. A., Peters G. (2010) Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6, e1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meunier O., Falkenrodt A., Monteil H., Colin D. A. (1995) Application of flow cytometry in toxinology: pathophysiology of human polymorphonuclear leukocytes damaged by a pore-forming toxin from Staphylococcus aureus. Cytometry 21, 241–247 [DOI] [PubMed] [Google Scholar]
- 11.Prevost G., Cribier B., Couppie P., Petiau P., Supersac G., Finck-Barbancon V., Monteil H., Piemont Y. (1995) Panton-Valentine leucocidin and γ-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 63, 4121–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillet Y., Issartel B., Vanhems P., Fournet J. C., Lina G., Bes M., Vandenesch F., Piemont Y., Brousse N., Floret D., Etienne J. (2002) Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359, 753–759 [DOI] [PubMed] [Google Scholar]
- 13.Lina G., Piemont Y., Godail-Gamot F., Bes M., Peter M. O., Gauduchon V., Vandenesch F., Etienne J. (1999) Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29, 1128–1132 [DOI] [PubMed] [Google Scholar]
- 14.Gillet Y., Vanhems P., Lina G., Bes M., Vandenesch F., Floret D., Etienne J. (2007) Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin. Infect. Dis. 45, 315–321 [DOI] [PubMed] [Google Scholar]
- 15.Chambers H. F. (2005) Community-associated MRSA—resistance and virulence converge. N. Engl. J. Med. 352, 1485–1487 [DOI] [PubMed] [Google Scholar]
- 16.Fridkin S. K., Hageman J. C., Morrison M., Sanza L. T., Como-Sabetti K., Jernigan J. A., Harriman K., Harrison L. H., Lynfield R., Farley M. M. (2005) Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352, 1436–1444 [DOI] [PubMed] [Google Scholar]
- 17.Kazakova S. V., Hageman J. C., Matava M., Srinivasan A., Phelan L., Garfinkel B., Boo T., McAllister S., Anderson J., Jensen B., Dodson D., Lonsway D., McDougal L. K., Arduino M., Fraser V. J., Killgore G., Tenover F. C., Cody S., Jernigan D. B. (2005) A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 352, 468–475 [DOI] [PubMed] [Google Scholar]
- 18.Otter J. A., French G. L. (2010) Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect. Dis. 10, 227–239 [DOI] [PubMed] [Google Scholar]
- 19.Vandenesch F., Naimi T., Enright M. C., Lina G., Nimmo G. R., Heffernan H., Liassine N., Bes M., Greenland T., Reverdy M. E., Etienne J. (2003) Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9, 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diep B. A., Otto M. (2008) The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16, 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipinska U., Hermans K., Meulemans L., Dumitrescu O., Badiou C., Duchateau L., Haesebrouck F., Etienne J., Lina G. (2011) Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS One 6, e22864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diep B. A., Chan L., Tattevin P., Kajikawa O., Martin T. R., Basuino L., Mai T. T., Marbach H., Braughton K. R., Whitney A. R., Gardner D. J., Fan X., Tseng C. W., Liu G. Y., Badiou C., Etienne J., Lina G., Matthay M. A., DeLeo F. R., Chambers H. F. (2010) Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. USA 107, 5587–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensler T., Konig B., Prevost G., Piemont Y., Koller M., Konig W. (1994) Leukotriene B4 generation and DNA fragmentation induced by leukocidin from Staphylococcus aureus: protective role of granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF for human neutrophils. Infect. Immun. 62, 2529–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konig B., Koller M., Prevost G., Piemont Y., Alouf J. E., Schreiner A., Konig W. (1994) Activation of human effector cells by different bacterial toxins (leukocidin, alveolysin, and erythrogenic toxin A): generation of interleukin-8. Infect. Immun. 62, 4831–4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konig B., Prevost G., Piemont Y., Konig W. (1995) Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J. Infect. Dis. 171, 607–613 [DOI] [PubMed] [Google Scholar]
- 26.Pichereau S., Moran J. J., Hayney M. S., Shukla S. K., Sakoulas G., Rose W. E. (2012) Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J. Antimicrob. Chemother. 67, 123–129 [DOI] [PubMed] [Google Scholar]
- 27.Dinarello C. A. (1996) Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147 [PubMed] [Google Scholar]
- 28.Dinarello C. A. (2011) A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 41, 1203–1217 [DOI] [PubMed] [Google Scholar]
- 29.Fenton M. J. (1992) Review: transcriptional and post-transcriptional regulation of interleukin 1 gene expression. Int. J. Immunopharmacol. 14, 401–411 [DOI] [PubMed] [Google Scholar]
- 30.Martinon F., Mayor A., Tschopp J. (2009) The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 [DOI] [PubMed] [Google Scholar]
- 31.Tschopp J., Schroder K. (2010) NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 [DOI] [PubMed] [Google Scholar]
- 32.Craven R. R., Gao X., Allen I. C., Gris D., Bubeck Wardenburg J., McElvania-Tekippe E., Ting J. P., Duncan J. A. (2009) Staphylococcus aureus α-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4, e7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 34.Latz E. (2010) The inflammasomes: mechanisms of activation and function. Curr. Opin. Immunol. 22, 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujisawa A., Kambe N., Saito M., Nishikomori R., Tanizaki H., Kanazawa N., Adachi S., Heike T., Sagara J., Suda T., Nakahata T., Miyachi Y. (2007) Disease-associated mutations in CIAS1 induce cathepsin B-dependent rapid cell death of human THP-1 monocytic cells. Blood 109, 2903–2911 [DOI] [PubMed] [Google Scholar]
- 36.Hentze H., Lin X. Y., Choi M. S., Porter A. G. (2003) Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 10, 956–968 [DOI] [PubMed] [Google Scholar]
- 37.Frosch M., Strey A., Vogl T., Wulffraat N. M., Kuis W., Sunderkotter C., Harms E., Sorg C., Roth J. (2000) Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 43, 628–637 [DOI] [PubMed] [Google Scholar]
- 38.Vogl T., Propper C., Hartmann M., Strey A., Strupat K., van den Bos C., Sorg C., Roth J. (1999) S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J. Biol. Chem. 274, 25291–25296 [DOI] [PubMed] [Google Scholar]
- 39.Vogl T., Tenbrock K., Ludwig S., Leukert N., Ehrhardt C., van Zoelen M. A., Nacken W., Foell D., van der Poll T., Sorg C., Roth J. (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 [DOI] [PubMed] [Google Scholar]
- 40.Rammes A., Roth J., Goebeler M., Klempt M., Hartmann M., Sorg C. (1997) Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J. Biol. Chem. 272, 9496–9502 [DOI] [PubMed] [Google Scholar]
- 41.Duncan J. A., Gao X., Huang M. T., O'Connor B. P., Thomas C. E., Willingham S. B., Bergstralh D. T., Jarvis G. A., Sparling P. F., Ting J. P. (2009) Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 182, 6460–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viemann D., Goebeler M., Schmid S., Klimmek K., Sorg C., Ludwig S., Roth J. (2004) Transcriptional profiling of IKK2/NF-κ B- and p38 MAP kinase-dependent gene expression in TNF-α-stimulated primary human endothelial cells. Blood 103, 3365–3373 [DOI] [PubMed] [Google Scholar]
- 43.Huang M. T., Taxman D. J., Holley-Guthrie E. A., Moore C. B., Willingham S. B., Madden V., Parsons R. K., Featherstone G. L., Arnold R. R., O'Connor B. P., Ting J. P. (2009) Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. J. Immunol. 182, 2395–2404 [DOI] [PubMed] [Google Scholar]
- 44.Taxman D. J., Livingstone L. R., Zhang J., Conti B. J., Iocca H. A., Williams K. L., Lich J. D., Ting J. P., Reed W. (2006) Criteria for effective design, construction, and gene knockdown by shRNA vectors. BMC Biotechnol. 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willingham S. B., Bergstralh D. T., O'Connor W., Morrison A. C., Taxman D. J., Duncan J. A., Barnoy S., Venkatesan M. M., Flavell R. A., Deshmukh M., Hoffman H. M., Ting J. P. (2007) Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2, 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haslinger-Loffler B., Kahl B. C., Grundmeier M., Strangfeld K., Wagner B., Fischer U., Cheung A. L., Peters G., Schulze-Osthoff K., Sinha B. (2005) Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell. Microbiol. 7, 1087–1097 [DOI] [PubMed] [Google Scholar]
- 47.Ehrchen J., Helming L., Varga G., Pasche B., Loser K., Gunzer M., Sunderkotter C., Sorg C., Roth J., Lengeling A. (2007) Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 21, 3208–3218 [DOI] [PubMed] [Google Scholar]
- 48.Ehrchen J. M., Sunderkotter C., Foell D., Vogl T., Roth J. (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 86, 557–566 [DOI] [PubMed] [Google Scholar]
- 49.Petrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 [DOI] [PubMed] [Google Scholar]
- 50.Mehta V. B., Hart J., Wewers M. D. (2000) ATP-stimulated release of IL-1β and IL-18 requires priming by LPS and is independent of caspase-1 cleavage. J. Biol. Chem. 276, 3820–3826 [DOI] [PubMed] [Google Scholar]
- 51.Gomez M. I., Lee A., Reddy B., Muir A., Soong G., Pitt A., Cheung A., Prince A. (2004) Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10, 842–848 [DOI] [PubMed] [Google Scholar]
- 52.Kretschmer D., Gleske A. K., Rautenberg M., Wang R., Koberle M., Bohn E., Schoneberg T., Rabiet M. J., Boulay F., Klebanoff S. J., van Kessel K. A., van Strijp J. A., Otto M., Peschel A. (2010) Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7, 463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zivkovic A., Sharif O., Stich K., Doninger B., Biaggio M., Colinge J., Bilban M., Mesteri I., Hazemi P., Lemmens-Gruber R., Knapp S. (2011) TLR 2 and CD14 mediate innate immunity and lung inflammation to staphylococcal Panton-Valentine leukocidin in vivo. J. Immunol. 186, 1608–1617 [DOI] [PubMed] [Google Scholar]
- 54.Colin D. A., Mazurier I., Sire S., Finck-Barbancon V. (1994) Interaction of the two components of leukocidin from Staphylococcus aureus with human polymorphonuclear leukocyte membranes: sequential binding and subsequent activation. Infect. Immun. 62, 3184–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gauduchon V., Werner S., Prevost G., Monteil H., Colin D. A. (2001) Flow cytometric determination of Panton-Valentine leucocidin S component binding. Infect. Immun. 69, 2390–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer F., Girardot R., Piemont Y., Prevost G., Colin D. A. (2009) Analysis of the specificity of Panton-Valentine leucocidin and γ-hemolysin F component binding. Infect. Immun. 77, 266–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinarello C. A. (1999) IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 103, 11–24 [DOI] [PubMed] [Google Scholar]
- 58.Puren A. J., Fantuzzi G., Dinarello C. A. (1999) Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1β are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc. Natl. Acad. Sci. USA 96, 2256–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dinarello C. A. (1999) Interleukin-18. Methods 19, 121–132 [DOI] [PubMed] [Google Scholar]
- 60.Lotz S., Aga E., Wilde I., van Zandbergen G., Hartung T., Solbach W., Laskay T. (2004) Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J. Leukoc. Biol. 75, 467–477 [DOI] [PubMed] [Google Scholar]
- 61.Viemann D., Strey A., Janning A., Jurk K., Klimmek K., Vogl T., Hirono K., Ichida F., Foell D., Kehrel B., Gerke V., Sorg C., Roth J. (2005) Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 105, 2955–2962 [DOI] [PubMed] [Google Scholar]
- 62.Le Feuvre R. A., Brough D., Iwakura Y., Takeda K., Rothwell N. J. (2002) Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J. Biol. Chem. 277, 3210–3218 [DOI] [PubMed] [Google Scholar]
- 63.Perregaux D., Gabel C. A. (1994) Interleukin-1 β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203 [PubMed] [Google Scholar]
- 64.Genestier A. L., Michallet M. C., Prevost G., Bellot G., Chalabreysse L., Peyrol S., Thivolet F., Etienne J., Lina G., Vallette F. M., Vandenesch F., Genestier L. (2005) Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Invest. 115, 3117–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ting J. P., Willingham S. B., Bergstralh D. T. (2008) NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 8, 372–379 [DOI] [PubMed] [Google Scholar]
- 66.Niemi K., Teirila L., Lappalainen J., Rajamaki K., Baumann M. H., Oorni K., Wolff H., Kovanen P. T., Matikainen S., Eklund K. K. (2011) Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J. Immunol. 186, 6119–6128 [DOI] [PubMed] [Google Scholar]
- 67.Rajamaki K., Lappalainen J., Oorni K., Valimaki E., Matikainen S., Kovanen P. T., Eklund K. K. (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5, e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data