Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux (original) (raw)
. Author manuscript; available in PMC: 2012 Oct 26.
Abstract
Background
Endoscopic screening has been proposed for patients with symptoms of gastro-oesophageal reflux disease (GERD) in the hope of reducing mortality from oesophageal adenocarcinoma. Assessing the net benefits of such a strategy requires a precise understanding of the cancer risk in the screened population.
Aim
To estimate precisely the association between symptoms of GERD and oesophageal adenocarcinoma.
Methods
Systematic review and meta-analysis of population-based studies with strict ascertainment of exposure and outcomes.
Results
Five eligible studies were identified. At least weekly symptoms of GERD increased the odds of oesophageal adenocarcinoma fivefold (odds ratio = 4.92; 95% confidence interval = 3.90, 6.22), and daily symptoms increased the odds sevenfold (random effects summary odds ratio = 7.40, 95% confidence interval = 4.94, 11.1), each compared with individuals without symptoms or less frequent symptoms. Duration of symptoms was also associated with oesophageal adenocarcinoma, but with very heterogeneous results, and unclear thresholds.
Conclusions
Frequent GERD symptoms are strongly associated with oesophageal adenocarcinoma. These results should be useful in developing epidemiological models of the development of oesophageal adenocarcinoma, and in models of interventions aimed at reducing mortality from this cancer.
INTRODUCTION
Oesophageal adenocarcinoma is a particularly deadly neoplasm, and its incidence is rising rapidly in Westernized countries.1, 2 Risk factors for oesophageal adenocarcinoma include symptoms of gastro-oesophageal reflux disease, as well as white race, male gender, abdominal obesity and tobacco use.3–7 Some subspecialty guidelines have advocated endoscopic screening of patients with gastro-oesophageal reflux disease in an attempt to reduce mortality from oesophageal adenocarcinoma.8–10 The United States Institute of Medicine recently published a list of priorities for comparative-effectiveness research; within the top 25 priorities was to compare the effectiveness of upper endoscopy utilization in patients with gastro-oesophageal reflux disease on the diagnosis of oesophageal adenocarcinoma.11 In addition to knowledge regarding the efficacy and adverse effects of a screening and endoscopic prevention strategy, assessing the net benefits of a screening strategy requires a precise understanding of the risk of cancer in the screened population, in this case patients with gastro-oesophageal reflux disease. Therefore, we aimed to estimate precisely the association between symptoms of gastro-oesophageal reflux disease and oesophageal adenocarcinoma by conducting a systematic review of the published literature and a meta-analysis of results.
METHODS
We performed a systematic literature search in MED-LINE (1950–August 2008), EMBASE (1947–August 2008), Web of Science (1900–August 2008), Cochrane Central Register of Controlled Trials (3rd Quarter 2008), BIOSIS preview (1926-August 2008), Data Abstracts of Review of Effect (3rd Quarter 2008) and ACP Journal Club (1991- August 2008) to identify studies evaluating the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux disease, without regard to language of the publication. Medical subject headings for our literature review included [‘gastroesophageal reflux’, or ‘GERD’, or ‘oesophageal reflux’, or ‘oesophagitis’, or ‘heartburn’, or ‘pyrosis’, or ‘regurgitation’] and [‘oesophageal neoplasm’, or ‘adenocarcinoma’, or ‘carcinoma’, or ‘Barrett*’, or ‘metaplasia’, or ‘metaplastic’]. This process included electronic searching of supplemental abstracts published in Gastroenterology and Gut. Supplemental abstracts from the American Journal of Gastroenterology between the years 2000 and 2008 were manually searched for relevant studies. Both American and British spellings were used in all search terms. Titles were reviewed by a single author (JBT) and abstracts of interest were reviewed independently by two authors (JBT and JHR). To be eligible, studies had to examine cancer diagnosis and include data regarding gastro-oesophageal reflux disease. Cases and controls were required to be obtained through population-based sampling, and histological confirmation of oesophageal adenocarcinoma was required. Gastro-oesophageal reflux symptoms were required to be assessed either by a questionnaire or interview; studies relying on assessment of gastro-oesophageal reflux disease by medical chart review, administrative diagnosis codes, or prescription databases were excluded. Symptoms of gastro-oesophageal reflux disease were defined as retrosternal burning discomfort and/or effortless regurgitation. Relevant manuscripts were cross-referenced to identify additional potential studies.
Although each study matched controls to cases based on at least age, crude odds ratios from each study were obtained using an unconditional analysis. We estimated the odds ratios for individuals with weekly symptoms of gastro-oesophageal reflux disease vs. those without symptoms or those with symptoms less than weekly and also for individuals with daily symptoms vs. those without symptoms or those with symptoms less than weekly. For meta-analysis of duration of symptoms, effects were examined for duration greater than 20 years, and for duration less than 10–15 years. One study did not present results for duration.7 One presented data for categories of 20–30 years and greater than 30 years, not permitting the ability to use the adjusted effects for both categories combined; for that study, we used the crude data for that stratum.4 Meta-analysis was performed using MIX v.2.0 software (Leon Bax, Kitasato University, Tokyo, Japan).12, 13 Fixed-effects models were tested for heterogeneity, and exclusion sensitivity analysis was performed to try to explain heterogeneous findings. As the subjects in the identified studies were overwhelmingly classified as of white race and as no estimates of the risk of gastro-oesophageal reflux disease were provided stratified by race or ethnicity, the results only reflect a white, non-Hispanic population.
RESULTS
Systematic review
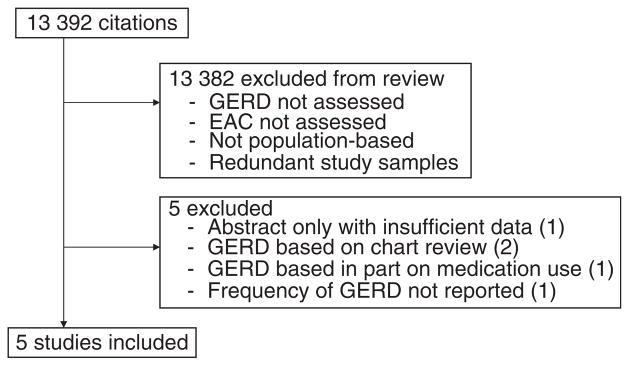
Our systematic review identified 13 392 citations; titles and abstracts were manually evaluated for relevancy. Of these, we found five studies eligible for inclusion (Figure 1 and Table 1).3–7 Additional potential studies were excluded because gastro-oesophageal reflux disease was assessed by chart review,14, 15 or because gastro-oesophageal reflux disease was considered present if subjects consumed antacids regardless of specific symptoms.16 One study was excluded because the definition of gastro-oesophageal reflux disease did not include regurgitation and the frequency of symptoms was not reported.17 One additional potential study had been only presented in abstract;18 attempts to obtain relevant data through personal communication with the authors were unsuccessful. Each study had a retrospective case-control design. In each study design, to avoid reverse causality, symptoms were excluded for a time period (1–10 years) preceding the diagnosis of cancer or the match date. Three studies estimated the association of gastro-oesophageal reflux symptoms with other relevant cancers (such as oesophageal squamous cell carcinoma), finding no relationship with gastro-oesophageal reflux symptoms, thereby making recall bias an unlikely explanation of the relationship between gastro-oesophageal reflux symptoms and oesophageal adenocarcinoma.3–5 In all but one study, the prevalence of weekly gastro-oesophageal reflux symptoms in the subjects with oesophageal adenocarcinoma was less than 50% (Table 1).
Figure 1.
Flow diagram of study selection.
Table 1.
Studies included in meta-analysis
| Reference | Country | Years of OAC diagnosis | Weekly GERD | Daily GERD | ||||
|---|---|---|---|---|---|---|---|---|
| OAC cases Sx−/Sx+ | Controls Sx−/Sx+ | Crude OR (95% CI) | OAC cases Sx−/Sx+ | Controls Sx−/Sx+ | Crude OR (95% CI) | |||
| Lagergren et al.3 | Sweden | 1994–1997 | 76/113 | 685/135 | 7.5 (5.3, 11) | 76/41* | 685/24* | 15 (8.8, 27)* |
| Farrow et al.4 | United States | 1993–1995 | 131/67† | 593/78† | 3.9 (2.7, 5.7)† | 131/42 | 593/40 | 4.8 (3.0, 7.6) |
| Wu et al.5 | United States | 1992–1997 | 108/104 | 1097/250 | 4.2 (3.1, 5.7) | 108/47 | 1097/95 | 5.0 (3.4, 7.5) |
| Anderson et al.6 | Northern Ireland & Republic of Ireland | 2002–2004 | 117/110 | 211/49 | 4.0 (2.7, 6.1) | 117/42 | 211/9 | 8.4 (4.0, 18) |
| Whiteman et al.7 | Australia | 2001–2005 | 210/153 | 1384/184 | 5.5 (4.2, 7.1) | 210/71 | 1384/57 | 8.2 (5.6, 12) |
Meta-analysis of symptom frequency
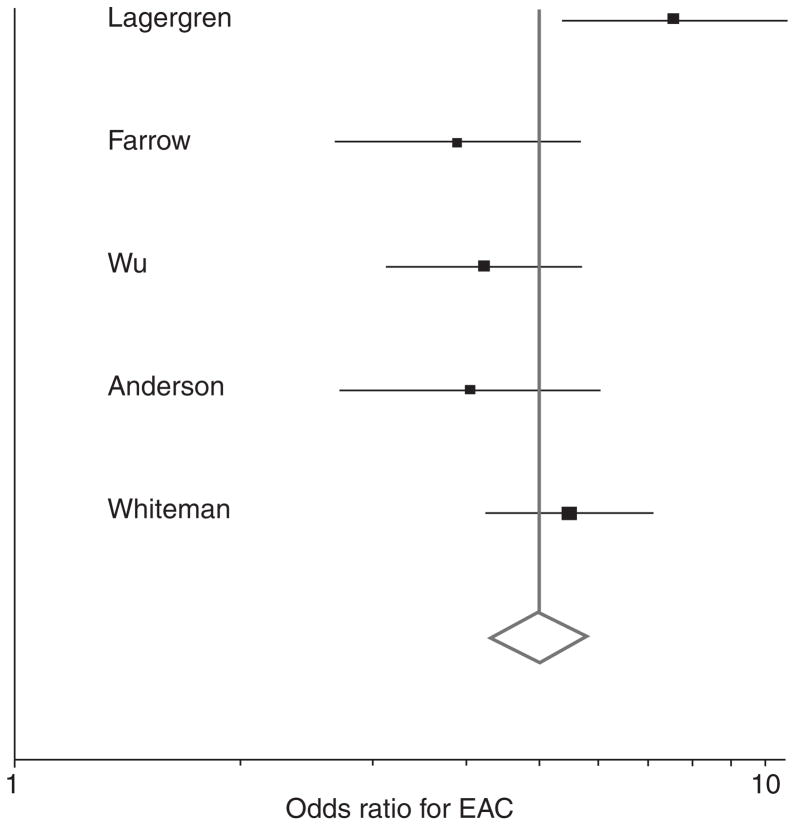
The random effects summary odds ratio for oesophageal adenocarcinoma of weekly gastro-oesophageal reflux symptoms vs. less frequent or no symptoms was 4.92 [95% confidence interval (CI) = 3.90, 6.22], and exhibited moderate heterogeneity (Cochrane’s Q P = 0.04; Inconsistency Index, _I_2 = 60%) (Figure 2). Removing the outlier study by Lagergren et al.3 resolved this heterogeneity (fixed effects odds ratio = 4.57; 95% CI = 3.89, 5.36; Cochrane’s Q P = 0.36, _I_2 = 6%). The random effects summary odds ratio for oesophageal adenocarcinoma of daily gastro-oesophageal reflux symptoms vs. symptoms less than weekly or no symptoms was 7.40 (95% CI = 4.94, 11.1), and also exhibited moderate heterogeneity (Cochrane’s Q P = 0.01; _I_2 = 71%). Removing the Lagergren study partially resolved the heterogeneity (fixed effects odds ratio = 6.20; 95% CI = 4.95, 7.78; Cochrane’s Q P = 0.17; _I_2 = 40%). In the one study where data were available, the association between weekly gastro-oesophageal reflux symptoms and oesophageal adenocarcinoma may have been slightly weaker in women than in men (men: odds ratio = 4.3, 95% CI = 3.3, 5.7; women: odds ratio = 3.5, 95% CI = 1.9, 6.6).7
Figure 2.
Forrest plot of association of weekly GERD symptoms with oesophageal adenocarcinoma. Odds ratios are for symptoms at least weekly vs. no symptoms or symptoms less than weekly. Scale is logarithmic.
Meta-analysis of symptom duration
One study did not collect data on symptom duration.7 The results from the other four studies are summarized in Table 2. For symptoms of at least 20 years, the random effects summary odds ratio was 5.41 (95% CI = 2.45, 11.9), but with very heterogeneous results (Cochranes Q P < 0.01, _I_2 = 89%). Excluding any one study failed to resolve the heterogeneity. For symptoms of shorter duration (less than 10–15 years, depending on the study), the random effects summary odds ratio was 3.05 (95% CI = 1.53, 6.08). Once again, these results were very heterogeneous (Cochranes Q P < 0.01, _I_2 = 84%), and excluding any one study failed to resolve the heterogeneity.
Table 2.
Associations with duration of gastro-oesophageal reflux symptoms
| Reference | GERD <10–15 years vs. no GERD | GERD >20 years vs. no GERD |
|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Lagergren et al.3 | 7.50 (4.20, 13.5) | 16.4 (8.30, 28.4) |
| Farrow et al.4 | 1.60 (1.00, 2.40) | 2.26 (1.47, 3.47)* |
| Wu et al.5 | 2.11 (1.20, 3.69) | 4.89 (3.18, 7.53) |
| Anderson et al.6 | 3.67 (1.94, 6.97) | 5.11 (2.51, 10.4) |
DISCUSSION
We conducted a systematic review and meta-analysis of the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux disease. The first high-quality study demonstrating the association of gastro-oesophageal reflux symptoms with oesophageal adenocarcinoma was published in 1999.3 Since then, four more population-based studies have been published. Although the associations in those studies have been weaker than that described in the initial report, each study has demonstrated strong associations with such symptoms, and each with a positive dose–response relation in terms of frequency of gastro-oesophageal reflux symptoms. Overall, we found that individuals with weekly symptoms have an approximate fivefold increase in the odds of developing oesophageal adenocarcinoma, and those with daily symptoms have an approximate sevenfold increase in the odds of the cancer compared with individuals with symptoms less than weekly or no symptoms. However, most patients with oesophageal adenocarcinoma denied ever having symptoms of gastro-oesophageal reflux at least weekly.
Each study found a strong relationship between oesophageal adenocarcinoma and reflux symptoms of at least 20 years, but the results from the studies were very heterogeneous, with odds ratios ranging from 2.26 to 16.4. Two of the studies found little threshold effect comparing the odds ratios for symptoms less than 10–15 years’ duration and symptoms of greater than 20 years’ duration, both vs. no symptoms. Multiple guidelines describe a strong association between symptom duration and the risk of Barrett’s oesophagus,19, 20 but the association between duration and cancer is not as strong as the data for symptom frequency, and it remains unclear what duration of symptoms would be relevant for cancer screening purposes. Additional population-based studies examining the effect of symptom duration and its interaction with symptom frequency for oesophageal adenocarcinoma are needed.
Although gastro-oesophageal reflux symptoms are clearly strongly associated with the odds of developing oesophageal adenocarcinoma, the absolute risk of that cancer in the population is quite low. The very strong relative risk associated with frequent gastro-oesophageal reflux symptoms should not be confused with the absolute risk of cancer in individuals with these symptoms, which may be quite small, and is not directly addressed in the current study. Nonetheless, these precise estimates of the relative risk of oesophageal adenocarcinoma should prove useful in epidemiological models of the development of oesophageal adenocarcinoma, and in models of interventions to reduce mortality from this type of cancer.
Our study was limited by the available published literature. In particular, all five studies were primarily or entirely conducted in white, non-Hispanic populations therefore the magnitude of the association may differ in other populations. However, given the low baseline incidence of oesophageal adenocarcinoma in some of those other populations (e.g. Asian and African), the association with reflux symptoms in those populations would likely need to be much stronger than among whites for the symptoms to be relevant to the health of individuals of minority populations. Moreover, we were not able to assess the possibility of interactions with other risk factors, such as age, gender, obesity, or tobacco use. For instance, although all of the studies adjusted the effect estimates of gastro-oesophageal reflux symptoms for obesity, Whiteman, et al. further demonstrated a synergistic relationship between reflux and obesity for oesophageal adenocarcinoma.7 Obesity might promote oesophageal adenocarcinoma independent of a mechanical effect promoting reflux, such as via circulating adipokines.21–25
Major strengths of the study include each underlying study was a very high quality study, utilizing population-based sampling and strict ascertainment of both exposures and outcomes, and most adjusting for multiple potential confounders. The reason for the stronger relationship between gastro-oesophageal reflux symptoms and oesophageal adenocarcinoma in the first published study than in later studies is not clear. It may due to differences in the underlying population (Swedish) vs. the other studies (American, Australian and Irish), the questionnaires ascertaining gastro-oesophageal reflux symptoms, or the particular definitions used for oesophageal vs. cardia or junctional adenocarcinomas. Moreover, the Swedish study was the only one to adjust for diet and physical activity.
In summary, we have conducted a systematic review and meta-analysis demonstrating a strong, dose-dependent relationship between frequency of symptoms of gastro-oesophageal reflux and oesophageal adenocarcinoma.
Acknowledgments
We greatly appreciate Anna Wu and David Whiteman for providing crude data from their studies. Declaration of personal interests: None. Declaration of funding interests: JHR is the Damon Runyon – Gordon Family Clinical Investigator. This work is supported by the National Institutes of Health (JHR: K23DK079291). The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
As part of AP&T’s peer-review process, a technical check of this meta-analysis was performed by Dr. P. Collins.
References
- [Accessed July 15, 2008];Fast Stats: Esophagus Cancer. Available at: http://seer.cancer.gov/faststats/selections.php.
- 2.Bollschweiler E, Wolfgarten E, Gutschow C, Holscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–55. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 4.Farrow DC, Vaughan TL, Sweeney C, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–8. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 5.Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer. 2003;98:940–8. doi: 10.1002/cncr.11568. [DOI] [PubMed] [Google Scholar]
- 6.Anderson LA, Watson RG, Murphy SJ, et al. Risk factors for Barrett’s oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–94. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 8.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–32. doi: 10.1111/j.1572-0241.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 9.Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888–95. doi: 10.1111/j.1572-0241.2002.05910.x. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong D, Marshall JK, Chiba N, et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults – update 2004. Can J Gastroenterol. 2005;19:15–35. doi: 10.1155/2005/836030. [DOI] [PubMed] [Google Scholar]
- 11.Sox HC, Greenfield S, editors. Medicine. Washington, DC: The National Academies Press; 2009. Initial national priorities for comparative effectiveness research. [Google Scholar]
- 12.Bax L, Yu L, Ikeda N, Tsuruta H, Moons K. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MIX: Comprehensive Free Software for Meta-Analysis of Causal Research Data – Version 1.7. doi: 10.1186/1471-2288-6-50. Available at: http://www.mix-for-meta-analysis.info. [DOI] [PMC free article] [PubMed]
- 14.Chow WH, Finkle WD, McLaughlin JK, Frankl H, Ziel HK, Fraumeni JF., Jr The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA. 1995;274:474–7. [PubMed] [Google Scholar]
- 15.Crane SJ, Locke GR, III, Harmsen WS, et al. Subsite-specific risk factors for esophageal and gastric adenocarcinoma. Am J Gastroenterol. 2007;102:1596–602. doi: 10.1111/j.1572-0241.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 16.Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–5. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 17.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–8. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tait VK, Birkett NJ, Maziak DE. Dietary iron intake, reflux symptoms and the risk of esophageal adenocarcinoma (EAC): analysis of a case-control study. Proc Am Assoc Cancer Res Ann Meet. 2006;47:945. [Google Scholar]
- 19.Wang KK, Sampliner RE Practice Parameters Committee of the American College of G. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang KK, Wongkeesong M, Buttar NS. American Gastroenterological Association technical review on the role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology. 2005;128:1471–505. doi: 10.1053/j.gastro.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 21.McElholm AR, McKnight A-J, Patterson CC, et al. A population-based study of IGF axis polymorphisms and the esophageal inflammation, metaplasia, adenocarcinoma sequence. Gastroenterology. 2010;139:204–12.e3. doi: 10.1053/j.gastro.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald K, Porter GA, Guernsey DL, Zhao R, Casson AG. A polymorphic variant of the insulin-like growth factor type I receptor gene modifies risk of obesity for esophageal adenocarcinoma. Cancer Epidemiol. 2009;33:37–40. doi: 10.1016/j.canep.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Neale RE, Doecke JD, Pandeya N, et al. Does type 2 diabetes influence the risk of oesophageal adenocarcinoma? Br J Cancer. 2009;100:795–8. doi: 10.1038/sj.bjc.6604908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubenstein JH, Kao JY, Madanick RD, et al. Association of adiponectin multimers with Barrett’s esophagus. Gut. 2009;58:1583–9. doi: 10.1136/gut.2008.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendall BJ, Macdonald GA, Hayward NK, et al. Leptin and the risk of Barrett’s oesophagus. Gut. 2008;57:448–54. doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]