GLP-1 Receptor Activation Inhibits VLDL Production and Reverses Hepatic Steatosis by Decreasing Hepatic Lipogenesis in High-Fat-Fed APOE*3-Leiden Mice (original) (raw)
Abstract
Objective
In addition to improve glucose intolerance, recent studies suggest that glucagon-like peptide-1 (GLP-1) receptor agonism also decreases triglyceride (TG) levels. The aim of this study was to evaluate the effect of GLP-1 receptor agonism on very-low-density lipoprotein (VLDL)-TG production and liver TG metabolism.
Experimental Approach
The GLP-1 peptide analogues CNTO3649 and exendin-4 were continuously administered subcutaneously to high fat diet-fed APOE*3-Leiden transgenic mice. After 4 weeks, hepatic VLDL production, lipid content, and expression profiles of selected genes involved in lipid metabolism were determined.
Results
CNTO3649 and exendin-4 reduced fasting plasma glucose (up to −30% and −28% respectively) and insulin (−43% and −65% respectively). In addition, these agents reduced VLDL-TG production (−36% and −54% respectively) and VLDL-apoB production (−36% and −43% respectively), indicating reduced production of VLDL particles rather than reduced lipidation of apoB. Moreover, they markedly decreased hepatic content of TG (−39% and −55% respectively), cholesterol (−30% and −55% respectively), and phospholipids (−23% and −36% respectively), accompanied by down-regulation of expression of genes involved in hepatic lipogenesis (Srebp-1c, Fasn, Dgat1) and apoB synthesis (Apob).
Conclusion
GLP-1 receptor agonism reduces VLDL production and hepatic steatosis in addition to an improvement of glycemic control. These data suggest that GLP-receptor agonists could reduce hepatic steatosis and ameliorate dyslipidemia in patients with type 2 diabetes mellitus.
Introduction
Type 2 diabetes mellitus (T2DM) has become a major metabolic disorder in both developed and developing countries, with impaired glucose tolerance and insulin resistance as hallmarks [1], [2]. In addition to glucose metabolism, lipid metabolism is disturbed in T2DM patients, reflected by increased plasma levels of low-density lipoprotein, VLDL-TG, and decreased levels of high-density lipoprotein. Moreover, T2DM is strongly associated with fatty liver disease (i.e. hepatic steatosis) [3], for which no effective pharmacotherapeutic options are yet available.
GLP-1 is an incretin hormone produced by intestinal L cells and the brain [4], [5]. GLP-1 is released in response to food intake to stimulate glucose-dependent insulin secretion by the pancreas [4], [6]. Additionally, GLP-1 exerts multiple other effects, including inhibition of food intake [7], slowing gastric emptying [8], and inhibition of glucagon secretion [9]. Thus, GLP-1 was considered as a good target for the treatment of T2DM. However, therapeutic application of GLP-1 is hampered due to its short circulating half-life (<2 minutes), because it is rapidly degraded by dipeptidyl peptidase 4 (DPP-4) that is widely expressed in endothelium and intestinal mucosa [10]. Therefore, pharmaceutical GLP-1 analogues that are resistant to inactivation by DPP-4 have been developed with an improved pharmacokinetic profile related to a longer half-life, of which exenatide (a synthetic version of exendin-4) was approved in 2005 for the treatment of T2DM [11]. We have previously described that CNTO736, a GLP-1 MimetibodyTM receptor agonist that incorporates a GLP-1 peptide analogue genetically fused by a unique linker to a domain that includes the Fc portion of an antibody, has an even longer circulating half-life than exendin-4 and retains the beneficial effects of GLP-1 on glucose metabolism [12]. The long-acting GLP-1 analogue CNTO3649, a more recent version of CNTO736 with two point mutations introduced to improve protein solubility, retains this advantageous pharmacokinetic profile.
In addition to improving glucose metabolism, preliminary studies suggested that GLP-1 receptor agonism decreases plasma TG levels in patients with T2DM [13], [14]. However, the mechanism underlying these beneficial effects on TG metabolism remains unclear. Therefore, the objective of the present study was to evaluate the effects of GLP-1 receptor agonism via CNTO3649 and exendin-4 on VLDL-TG production and liver TG metabolism, and further to explore the underlying mechanisms, in APOE*3-Leiden (E3L) transgenic mice fed a high fat diet (HFD) [15].
Materials and Methods
Animals
For all experiments, 8–10 weeks old male E3L mice [16] were used, housed in a temperature and humidity-controlled environment with free access to food and water. Experiments were performed after 7 h of fasting at 14 ∶00 pm with food withdrawn at 7∶00 am. Body weight was measured weekly during experiments. All animal experiments were performed in accordance with the regulations of Dutch law on animal welfare, and the Institutional Ethics Committee for Animal Procedures from the Leiden University Medical Center, Leiden, The Netherlands, approved the protocol. All surgery was performed under isoflurane anesthesia
Experiments
Two experiments were conducted, each of which was designed to investigate a specific aspect of the overall hypothesis.
In the first experiment, mice were fed a HFD (44 energy% fat, derived from bovine fat; Hope Farms, Woerden, The Netherlands) for 22 weeks. After 18 weeks of HFD feeding, mice were divided into 5 groups, matched for fasting body weight and plasma glucose levels. An osmotic minipump (model 1004, Alzet DURECT Corp., Cupertino, CA) was implanted subcutaneously in the left back region under light isoflurane anesthesia for the continuous delivery of CNTO3649 (1.0 or 3.0 mg/kg/day, dissolved in PBS), exendin-4 (15 or 50 µg/kg/day, dissolved in PBS) or PBS as a control for up to 4 weeks, while continuously feeding mice the HFD. Additionally, one group of mice received PBS while being fed a chow diet throughout the whole experiment as a control for HFD feeding. After 4 weeks of drug treatment, hepatic VLDL-TG and VLDL-apoB production were determined.
In the second experiment, mice were fed the HFD for 13 weeks. After 9 weeks of HFD feeding, mice were divided into 5 groups, matched for fasting body weight and plasma glucose levels. Osmotic minipumps were implanted subcutaneously for the continuous delivery of CNTO3649 (0.3 or 1.0 mg/kg/day, dissolved in PBS), exendin-4 (15 or 50 µg/kg/day, dissolved in PBS) or PBS as a control for up to 4 weeks, while continuously feeding the mice the HFD. Additionally, one group of mice received PBS while being fed a chow diet as a control for HFD feeding. After 4 weeks of drug treatment, mice were perfused with ice-cold PBS via the heart, and livers were isolated to investigate hepatic lipid content and determine expression of selected genes involved in lipid metabolism. In addition, skeletal muscles from the hind leg were isolated to determine expression of selected genes involved in thermogenesis and fatty acid oxidation.
Compounds
CNTO3649 (molecular weight = 68,000 g/mol) was constructed by fusing a GLP-1 peptide analogue to a flexible Gly/Ser linker and a fragment of a V region heavy chain (VH) domain linked directly to the CH2 and CH3 domains of an Fc as described previously for CNTO736 [17]. Exendin-4 (molecular weight = 4186.6 g/mol) was purchased from Sigma (St. Louis, MO).
Plasma glucose and insulin analysis
Blood was collected by tail bleeding into chilled capillary tubes. The tubes were placed on ice and centrifuged, and the obtained plasma was snap-frozen in liquid nitrogen and stored at −20°C for further measurements. Plasma was assayed for glucose using a commercially available enzymatic kit according to the manufacturer's protocol (Instruchemie, Delfzijl, The Netherlands), and insulin was measured by ELISA (Mercodia AB, Uppsala, Sweden).
Hepatic VLDL-TG and VLDL-apoB production
Mice were fasted for 7 hours, with food withdrawn at 7.00 am and anesthetized by intraperitoneal injection of 6.25 mg/kg acepromazine (Alfasan, Woerden, The Netherlands), 6.25 mg/kg midazolam (Roche, Mijdrecht, The Netherlands), and 0.3125 mg/kg fentanyl (Janssen-Cilag, Tilburg, The Netherlands). Mice received an intravenous (iv) injection of 100 µl PBS containing 100 µCi Tran35S label (MP Biomedicals, Eindhoven, the Netherlands) resulting in incorporation of 35S into newly produced apoB required for hepatic VLDL production. After 30 min, the animals received an iv injection of tyloxapol (500 mg/kg body weight; Triton WR-1339, Sigma), as a 10% (w/w) solution in sterile saline, to prevent systemic lipolysis of newly secreted hepatic VLDL-TG [18]. Blood samples were drawn before (t = 0) and at 15, 30, 60, and 90 min after tyloxapol injection. Plasma was assayed for TG concentration using the commercially available enzymatic kit 11488872 (Roche Molecular Biochemicals, Indianapolis, IN). At 120 min, mice were euthanized, and blood was collected by orbital puncture for isolation of VLDL by density gradient ultracentrifugation [19]. 35S-apoB was measured in the VLDL fraction and VLDL-apoB production rate was calculated as dpm.h−1 [20]. TG and total cholesterol (TC) concentrations in the VLDL fractions were determined using the commercially available enzymatic kits 11488872 and 236691 (Roche) respectively, and phospholipid (PL) concentration was measured using a commercial kit (phospholipids B, Wako Chemicals, Neuss, Germany).
Hepatic lipid content
Liver lipids were extracted according to a modified protocol from Bligh and Dyer [21]. Briefly, small liver pieces were homogenized in ice-cold methanol. After centrifugation, lipids were extracted by addition of 1800 µl CH3OH: CHCl3 (1∶3 v/v) to 45 µl homogenate, followed by vigorous vortexing and phase separation by centrifugation (5 min at 2,000 rpm). The CHCl3 phase was dried and dissolved in 2% Triton X-100. TG, TC, and PL concentrations were measured using commercial kits as described above. Liver lipids were expressed as nmol per mg protein, which was determined using the BCA protein assay kit.
Hepatic gene expression analysis
Total RNA was extracted from liver pieces using the Nucleospin RNA II kit (Macherey-Nagel, Duren, Germany) or from muscle pieces using the RNeasy Fibrous Tissue Mini kit (Qiagen, Valencia, CA, USA) according to manufacturer's instructions. RNA quality of each sample was examined by the lab-on-a-chip method using Experion Std Sens analysis kit (Biorad, Hercules, CA) and RNA concentration of each sample was determined by Nanodrop technology (Thermo Scientific, Wilmington, USA). Then, total RNA was reverse-transcribed with iScript cDNA synthesis kit (1708891, Bio-Rad), subsequently, obtained cDNA was purified with Nucleospin Extract II kit (636973, Macherey-Nagel, Bioké). Real-time PCR was performed on a CFX96 machine (Bio-Rad), the reaction mixture consisting of SYBR-Green Sensimix (QT615, GC Biotech), cDNA, primers (Biolegio, Nijmegen, The Netherlands), and nuclease-free water in a total reaction volume of 10 µl. mRNA values of each gene were normalized to mRNA levels of cyclophilin (Cyclo) and hypoxanthine ribosyltransferase (Hprt). Primer sequences are listed in Table S1.
Statistical analysis
Differences between groups were determined with the Kruskal-Wallis non-parametric test for k independent samples. When significant differences were found, the Mann-Whitney non-parametric test was used as a post-hoc test to determine differences between two independent groups. A P-value of less than 0.05 was considered statistically significant. Data are presented as means ± SEM.
Results
GLP-1 receptor agonism reduces body weight and fasting plasma glucose and insulin levels in high fat diet-fed E3L mice
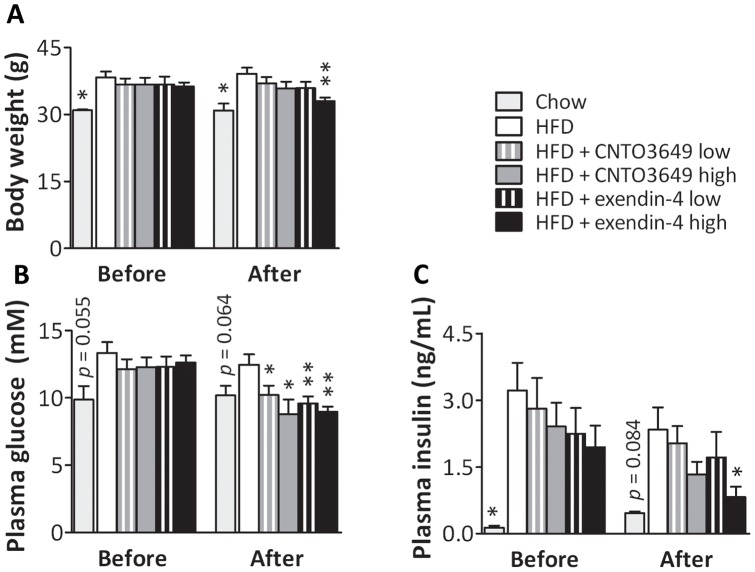
E3L mice were fed a HFD for 18 weeks and thereafter were treated with the GLP-1 receptor agonists CNTO3649 or exendin-4 via subcutaneous osmotic minipumps for 4 weeks while continuing the HFD. Body weight and fasting plasma glucose and insulin levels before and after treatment are shown in Figure 1. Eighteen weeks of HFD feeding increased body weight (+24%, P<0.05) (Fig. 1A), tended to increase fasting plasma glucose (+36%, P = 0.055) (Fig. 1B) and increased fasting insulin levels (13-fold, P<0.05) (Fig. 1C) compared to chow diet feeding. CNTO3649 (1.0 and 3.0 mg/kg/day) and the low dose of exendin-4 (15 µg/kg/day) did not affect body weight, whereas the high dose of exendin-4 (50 µg/kg/day) decreased body weight (-16%, P<0.01) (Fig. 1A) as compared to HFD control mice. Both doses of CNTO3649 and exendin-4 decreased fasting plasma glucose levels (up to −30%, P<0.05 and −28%, P<0.01, respectively) compared to HFD control mice (Fig. 1B). Additionally, the high dose of both CNTO3649 and exendin-4 decreased plasma insulin (−43% and −65%, respectively, P<0.05 for exendin-4 only) compared to HFD control mice (Fig. 1C). Collectively, these data confirm that GLP-1 receptor agonism by either CNTO3649 or exendin-4 improved glycemic control in the HFD-fed E3L mouse model.
Figure 1. GLP-1 receptor agonism reduces body weight and fasting plasma glucose and insulin levels.
APOE*3-Leiden (E3L) mice were fed a high fat diet (HFD) for 22 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (1.0 or 3.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice was included fed a chow diet that received vehicle (chow control). Blood was collected by tail bleeding after 7 h of fasting. Just before drug treatment (week 18) and after treatment (week 22), body weight (A), plasma glucose (B) and plasma insulin (C) levels were determined. Values are means ± SEM for at least 6 mice per group. _*_P<0.05, _**_P<0.01 compared to HFD controls.
GLP-1 receptor agonism reduces hepatic secretion of VLDL particles without affecting particle composition
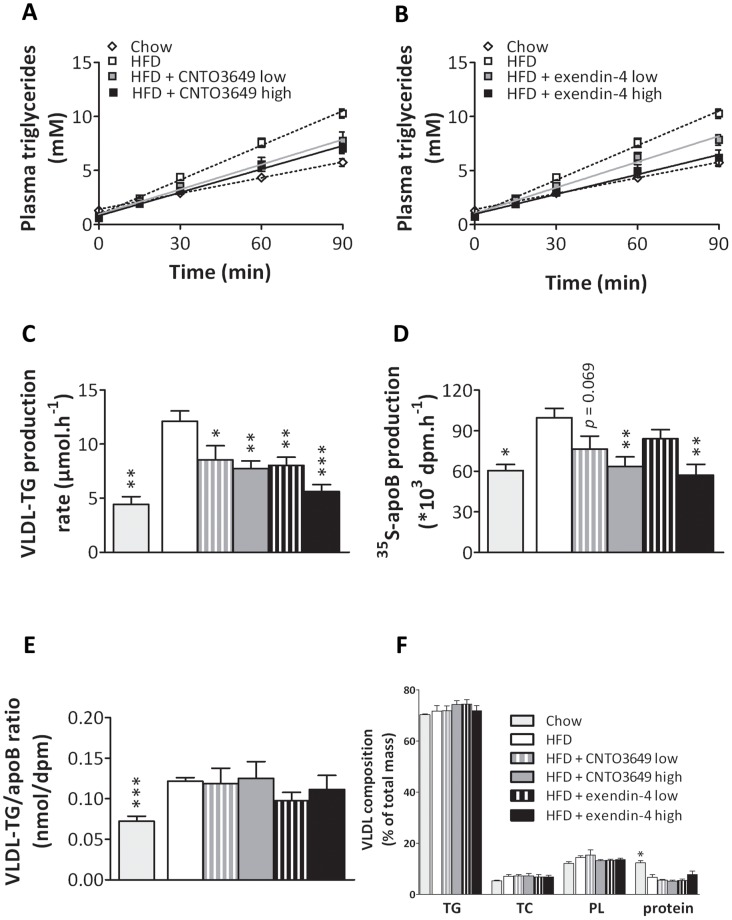
To evaluate the effect of GLP-1 receptor agonism on hepatic VLDL production, mice received an intravenous injection of Tran35S to label newly formed apoB, and tyloxapol to block LPL-mediated lipolysis of newly synthesized VLDL. HFD feeding increased the hepatic production rate of both VLDL-TG (Fig. 2A–C) and VLDL-apoB (Fig. 2D) compared to chow diet, which is in line with a previous study [22]. Interestingly, the VLDL-TG production rate induced by HFD was reduced by both doses of CNTO3649 (up to −36%, P<0.01) (Fig. 2A, C) and exendin-4 (up to −54%, P<0.001) (Fig. 2B, C), as determined from the slope of the curve from the individual mice. Likewise, the VLDL-apoB production rate as induced by HFD was decreased by the high dose of both CNTO3649 and exendin-4 (−36% and −43%, P<0.01, respectively) (Fig. 2D). HFD feeding increased the TG/apoB ratio within VLDL as compared to chow feeding by +68% (P<0.001) (Fig. 2E), indicating that HFD induces the formation of larger lipid-enriched VLDL particles. However, both CNTO3649 and exendin-4 did not affect the VLDL-TG/apoB ratio compared with HFD control group. Since each VLDL particle contains a single apoB molecule, GLP-1 receptor agonism apparently decreases the production rate of VLDL particles rather than decreasing the lipidation of VLDL particles. Accordingly, CNTO3649 and exendin-4 did not affect the composition of VLDL with respect to TG, TC, PL, and protein content as compared with HFD control group (Fig. 2F).
Figure 2. GLP-1 receptor agonism reduces hepatic VLDL-TG and VLDL-apoB production without affecting VLDL particle composition.
E3L mice were fed a HFD for 22 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (1.0 or 3.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice fed a chow diet was included that received vehicle (chow control). After 7 h fasting, mice were injected with Tran35S label (t = −30 min) and Triton WR-1339 (t = 0 min). Blood was drawn at the indicated time points and plasma TG concentrations were determined (A, B). VLDL-TG production rate was calculated as µmol/h from the slopes of the TG-time curves of the individual mice (C). At t = 120 min, mice were exsanguinated, and VLDL was isolated by density gradient ultracentrifugation. 35S-activity was determined, and VLDL-apoB production rate was calculated as dpm.h−1 (D). The VLDL-TG production rate to VLDL-apoB production rate ratio was calculated as nmol/dpm (E). The content of triglycerides, cholesterol, phospholipids and protein in VLDL was determined and calculated as % of total mass (F). Values are means ± SEM for at least 6 mice per group. _*_P<0.05, _**_P<0.01, _***_P<0.001 compared to HFD controls. TG: triglycerides; TC: total cholesterol; PL: phospholipids; Pro: protein.
GLP-1 receptor agonism reverses high fat diet-induced hepatic steatosis
To obtain insight into the mechanism underlying the reduction in hepatic VLDL production induced by GLP-1 agonism, we next determined the effect of the GLP-1 receptor agonists on hepatic lipid content in a second set of mice. Consistent with the first experiment, CNTO3649 and exendin-4 decreased body weight and fasting plasma glucose and insulin levels compared to the HFD control group (Figure S1) to a similar extent as in the first study. HFD feeding induced a marked increase in hepatic TG, TC, and PL content compared to chow diet (Fig. 3), indicating that HFD leads to hepatic steatosis. The high dose of both CNTO3649 and exendin-4 largely reduced hepatic TG (−39%, P<0.05 and −55%, P<0.01, respectively). Hepatic TC was reduced by both doses of CNTO3649 and exendin-4, (up to −32%, P<0.05 and −55%, P<0.01, respectively). Also, both doses of CNTO3649 and exendin-4 reduced hepatic PL (up to −23%, P<0.01 and −36%, P<0.01, respectively). Importantly, hepatic lipid content observed after treatment with the high dosages of CNTO3649 and exendin-4 group did not differ from that of the chow control group (P>0.05), suggesting that GLP-1 receptor agonism completely reversed HFD-induced hepatic steatosis.
Figure 3. GLP-1 receptor agonism reverses high fat diet-induced hepatic steatosis.
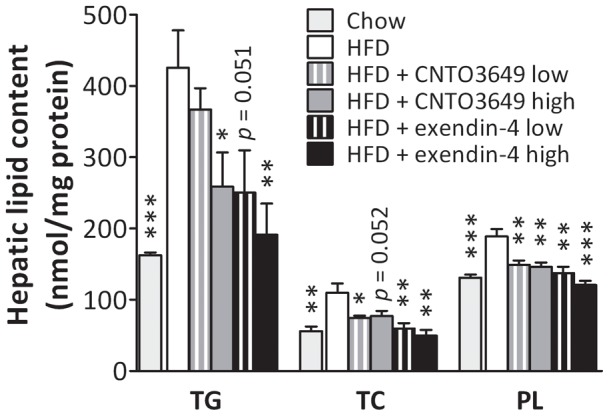
E3L mice were fed HFD for 13 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (0.3 or 1.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice was included fed a chow diet that received vehicle (chow control). Livers were isolated from 7 h fasted mice, liver pieces were homogenized, and triglycerides, cholesterol and phospholipids were determined as nmol per mg protein. Values are means ± SEM for at least 6 mice per group. _*_P<0.05, _**_P<0.01, _***_P<0.001 compared to HFD controls.
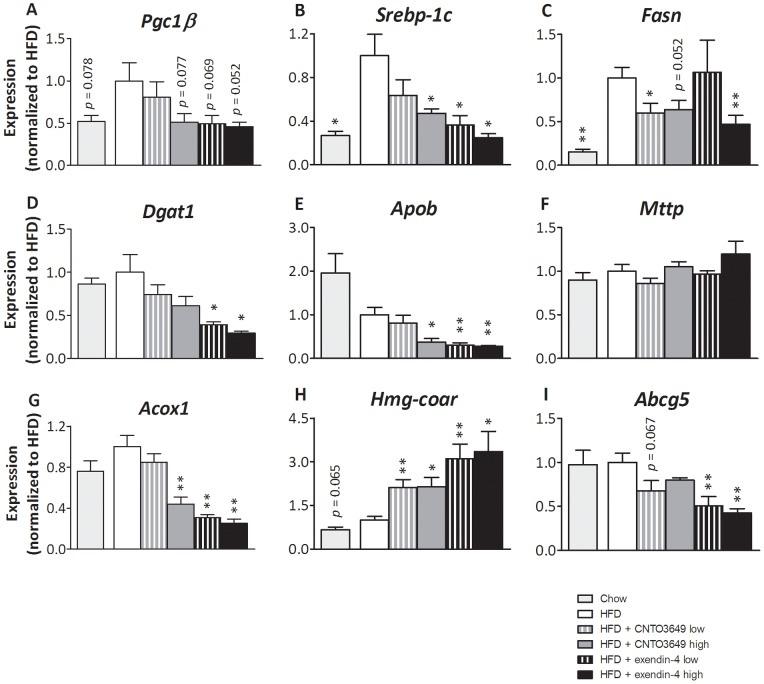
GLP-1 receptor agonism affects hepatic expression of genes involved in VLDL production, lipogenesis and lipid homeostasis
To elucidate the mechanism how GLP-1 receptor agonism reduces liver lipids and VLDL secretion, we investigated the hepatic expression of genes involved in lipid metabolism (i.e. lipogenesis, VLDL secretion, cholesterol metabolism, and FA oxidation) (Fig. 4). HFD feeding strongly tended to increase the expression of peroxisome proliferator-activated receptor gamma coactivator 1-beta (Pgc1β), whereas the high dose of CNTO3649 and both doses of exendin-4 tended to reduce this HFD-induced increase in Pgc1β. Next, HFD feeding increased the expression of the lipogenic transcription factor sterol regulatory element binding protein 1c (Srebp-1c) (3.7-fold, P<0.05) (Fig. 4A) and its target gene FA synthase (Fasn) (6.7-fold, P<0.01) (Fig. 4B), which plays a role in de novo lipogenesis, contributing to HFD-induced hepatic steatosis. The high dose of CNTO3649 and both doses of exendin-4 decreased the expression of Srebp-1c (−53%, P<0.05, and up to −75%, P<0.05, respectively) (Fig. 4A) and the low dose of CNTO3649 and the high dose of exendin-4 decreased Fasn (−40%, P<0.05 and −53%, P<0.01, respectively) (Fig. 4B) compared with the HFD group. Acyl:diacylglycerol transferase 1 (Dgat1), which catalyzes the final step in hepatic TG synthesis, was significantly decreased by exendin-4 only (up to −71%, P<0.05) (Fig. 4C). In addition, the high dose of CNTO3649 and both doses of exendin-4 suppressed expression of apoB (Apob) (−62%, P<0.05 and up to −72%, P<0.01, respectively) (Fig. 4D), without affecting expression of microsomal TG transfer protein (Mttp) (Fig. 4E) that mediates apoB lipidation. In addition, the high dose of CNTO3649 and both doses of exendin-4 suppressed the expression of the FA oxidation gene acyl-CoA oxidase 1 (Acox1) (−56% and up to −75%, P<0.01, respectively) (Fig. 4F). Moreover, both doses of CNTO3649 and exendin-4 increased the expression of HMG-CoA reductase (Hmgcoar) (up to 2.1-fold and 3.4-fold, P<0.05, respectively) involved in de novo cholesterol synthesis (Fig. 4G). Finally, the expression of ATP-binding cassette sub-family G member 5 (Abcg5), involved in bile acid secretion, was significantly decreased for both doses of exendin-4 only (up −58%, P<0.01) (Fig. 4H).
Figure 4. GLP-1 receptor agonism affects hepatic expression of genes involved in VLDL production, lipogenesis, and lipid homeostasis.
E3L mice were fed HFD for 13 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (0.3 or 1.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice was included fed a chow diet that received vehicle (chow control). Livers were isolated from 7 h fasted mice, and mRNA was extracted from liver pieces. mRNA values of indicated genes were normalized to Cyclo and Hprt mRNA levels. Data were calculated as fold difference as compared with the HFD control group. Values are means ± SEM for at least 6 mice per group. *P<0.05, **P<0.01, ***P<0.001 compared to HFD controls.
Collectively, these data indicate that GLP-1 receptor agonism decreases lipogenesis and apoB synthesis, consequently resulting in suppression of VLDL particle production and a compensatory decrease in hepatic FA oxidation. In addition, the reduction in hepatic cholesterol content results in compensatory mechanisms to increase hepatic cholesterol synthesis and decrease secretion of hepatic cholesterol as bile acids.
GLP-1 receptor agonism affects muscle expression of genes involved in fatty acid oxidation
To gain insight into the fate of the FA from the diet we also measured muscle expression of genes involved in thermogenesis and FA oxidation (Figure S2). No differences between groups were found for the thermogenic markers uncoupling protein 1 (Ucp1) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc1α), suggesting that both compounds did not affect energy expenditure by the muscle. However, the expression of Acox1 was significantly increased for the high dose of CNTO3649 and exendin-4 (+33% and +56%, P<0.05, respectively) as compared to HFD controls. Also, the high dose of exendin-4 increased the expression of carnitine palmitoyltransferase 1 (Cpt1) (+73%, P<0.05). These data indicate an increased FA oxidation in muscle.
Discussion
In the present study, we show that GLP-1 receptor agonism both by CNTO3649 and exendin-4 decreases fasting plasma glucose and insulin levels in HFD-fed E3L mice. This is in line with earlier reports that GLP-1 and its analogs ameliorate whole-body glucose intolerance in obese animal models [23], [24] and in T2DM patients [25]. More importantly, to our knowledge, this study is the first to demonstrate that CNTO3649 and exendin-4 reduce hepatic VLDL particle production, evidenced by similarly reduced VLDL-TG and VLDL-apoB production rates. In addition, both GLP-1 receptor agonists largely decrease the hepatic lipid content thereby reversing HFD-induced hepatic steatosis.
Increased plasma VLDL-TG levels are a central feature of T2DM, and are mainly caused by increased hepatic VLDL-TG and VLDL-apoB production [26]. We observed that HFD feeding increased VLDL-TG and VLDL-apoB production, and in addition increased the VLDL-TG/apoB ratio. As each VLDL particle contains a single molecule of apoB, VLDL-apoB reflects particle number, whereas VLDL-TG reflects the major lipid constituent of the particle. An increased VLDL-apoB production rate with a concomitantly increased VLDL-TG/apoB ratio thus indicates that HFD feeding not only results in overproduction of VLDL particles but also in the formation of larger lipid-enriched VLDL particles. Both CNTO3649 and exendin-4 reduced the VLDL-TG and VLDL-apoB production rates without affecting the VLDL-TG/apoB ratio, suggesting that GLP-1 agonism reduces the VLDL particle production without affecting the lipidation of VLDL-apoB. This was indeed confirmed by VLDL composition analysis. Notably, we have observed the same effects in WT mice treated with exendin-4 (data not shown), ruling out a possible impact of the genetic background of the E3L mice on the treatment outcome.
We also observed that both CNTO3649 and exendin-4 completely reversed HFD-induced hepatic steatosis reflected by largely decreased hepatic TG and TC contents to the low levels observed in chow-fed control mice. This corroborates recent studies showing that prolonged infusion of exendin-4 in ob/ob mice reduced hepatic TG accumulation [27]. Hepatic gene expression analysis revealed that the GLP-1 receptor agonists decreased the expression of the nuclear transcription factor Srebp-1c and its targets Fasn and Dgat1, which are involved in de novo FA and TG synthesis, respectively. At the same time, the GLP-1 receptor agonists decreased ApoB expression without affecting the expression of Mttp, of which the gene product MTP is involved in the transfer of TG onto apoB. On the other hand, they decreased the expression of Acox1 and Cpt1a (not shown), both of which are involved in FA oxidation.
Collectively, these data strongly suggest that GLP-1 receptor agonism primarily reduces hepatic lipogenesis, thereby causing a reduction in hepatic TG content, with a compensatory reduction in FA oxidation. Taken together with the concomitantly reduced apoB production, lower hepatic availability of TG results in a reduced production of VLDL particles. It is known that the contribution of de novo lipogenesis to total hepatic VLDL secretion strongly increases from 2–5% under healthy conditions up to 25–30% in T2DM patients with hepatic steatosis [28], [29]. Since HFD feeding of E3L mice similarly induced hepatic steatosis, the contribution of de novo lipogenesis to the increased VLDL production was likely also augmented by HFD feeding, and reversed by GLP-1 receptor agonism concomitant with attenuating hepatic steatosis. Interestingly, the increased expression of Acox and Cpt1 in the muscle suggests an increase in FA oxidation. In addition, by indirect calorimetry (Figure S3) we observed that exendin-4 treatment reduces the respiratory exchange rate, indicating an increased oxidation of fat. Although this effect was transient, it might have contributed to differences in the observed phenotypes after prolonged treatment. Collectively, these data suggest that GLP-1 receptor agonism not only decreases the production of FA, but also upregulates an oxidative pathway in the muscle to deal with the elevated uptake of FA present in the diet.
The mechanism underlying the reduced hepatic cholesterol content is less clear, although it may be expected from the reduced TG content given the tight relationship between hepatic TG and cholesterol levels. The reduction in hepatic cholesterol content evidently results in a compensatory induction of Hmgcoar, involved in de novo cholesterol synthesis, and downregulation of Abcg5, involved in the elimination of hepatic cholesterol as bile acids into the bile.
It is interesting to speculate on the molecular mechanisms that underlie the reduction in hepatic lipogenesis as induced by GLP-1 receptor agonism. Since Srebp-1c plays a crucial role in insulin-mediated de novo lipogenesis in the liver [30], it is well possible that the improved HFD-induced glucose intolerance accompanied by reduced insulin levels resulted in downregulation of hepatic Srebp-1c expression, thereby attenuating the HFD-induced increase in de novo lipogenesis. Beside insulin levels, Pgc1β could also be involved as it impacts on Srebp-1c expression [31]. Therefore, the observed trend towards a reduction in Pgc1β expression might also have contributed to a decreased Srebp-1c and consequently a decrease in de novo lipogenesis. Interestingly, it has recently been established that human hepatocytes [32], [33] as well as rodent hepatocytes [34] express GLP-1 receptors. In fact, incubation of hepatocytes with GLP-1 and exendin-4 in the absence of insulin directly reduces Srebp-1c [34], and reduces hepatocyte steatosis [32]. This indicates that GLP-1 receptor agonism may directly downregulate Srebp-1c and lipogenesis through binding of hepatocytic receptors. Third, GLP-1 receptor agonism by exendin-4 or the DPP-4 inhibitor sitagliptin reduces the intestinal production of chylomicron-TG and apoB in hamsters [35], and the DPP-4 inhibitor vildagliptin reduces postprandial chylomicron-TG and apoB in T2DM patients [36]. Indeed, pilot data from our lab confirmed that exendin-4 reduces postprandial TG excursion in mice (unpublished). Since uptake of TG from the diet eventually contributes to VLDL-TG production [37], [38], reduced chylomicron production may contribute to the observed effect of GLP-1 receptor agonism on hepatic VLDL production. Finally, circulating GLP-1 can cross the blood-brain barrier [39] and GLP-1 receptors are abundantly expressed in many brain areas [40]. Several studies have shown that central GLP-1 receptor signaling mediates the effect of GLP-1 on hepatic glucose output [41] and lipid deposition in white adipose tissue [42]. It is therefore reasonable to postulate that the brain-nerve-liver axis might contribute to the beneficial effects of GLP-1 agonism on hepatic lipid metabolism and VLDL secretion.
In our previous study, in which we administered exendin-4 by daily intraperitoneal injections, we were unable to detect any effect of exendin-4 on VLDL production, albeit that exendin-4 did improve glucose tolerance [12]. In that study, daily injections of CNTO736, a previous version of CNTO3649, with a considerably longer half-life than exendin-4, did reduce VLDL production. Since we now demonstrate that continuous delivery of exendin-4 inhibits VLDL production, it is likely that the ability of GLP-1 agonists to reduce hepatic steatosis and VLDL production is mainly determined by their pharmacokinetic profile. Whereas pulsated exposure of GLP-1 (analogues) is sufficient to improve glucose intolerance, more chronic exposure is required for additional beneficial effects on TG metabolism.
How do the present data obtained in E3L mice translate to clinical practice? Recently, it has been reported that treatment of T2DM patients with exenatide on top of pioglitazone resulted in a greater decrease in both hepatic TG and plasma TG levels compared to treatment with pioglitazone only [43]. Based on our present data, it is likely that these observations can be explained by a reduction in _Srebp-1c_- induced lipogenesis, resulting in attenuation of the hepatic TG content, reduction of VLDL-TG production and thus plasma VLDL-TG levels. Therefore, it would be interesting to determine the effect of GLP-1 analogues and DPP-4 inhibitors on VLDL production in future human intervention studies. In addition, we anticipate that the effects of long-circulating GLP-1 receptor agonists such as liraglutide (duration of action ≥24 hours) on lipid metabolism will prove to be superior to those of exenatide (duration of action <24 hours) and in addition will lead to better tolerability due to the necessity of injecting once daily only as compared to twice daily for exenatide [44]. In addition to the beneficial effects on glucose metabolism, VLDL secretion and hepatic lipid content, GLP-1 receptor agonism also reduces blood pressure and the severity of myocardial infarction, while it concomitantly improves left ventricular ejection fraction after infarction [45], enforcing GLP-1 receptor agonism as a valuable therapy to combat T2DM and associated cardiovascular diseases.
In conclusion, our results show that GLP-1 agonism not only decreases bodyweight and improves glycemic control, but also reduces HFD-induced hepatic steatosis, thereby reducing hepatic VLDL biosynthesis and secretion. Therefore, we anticipate that GLP-1 receptor agonism is a valuable strategy to treat T2DM patients, especially those with disturbed lipid metabolism related to hepatic steatosis.
Supporting Information
Figure S1
GLP-1 receptor agonism reduces fasting glucose and insulin levels. E3L mice were fed a high fat diet (HFD) for 13 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (0.3 or 1.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice fed a chow diet was included that received vehicle (chow control). Just before drug treatment (week 13) and after treatment (week 17), body weight (A), plasma glucose (B) and plasma insulin (C) levels were determined. Values are means ± SEM for at least 6 mice. _*_P<0.05, _**_P<0.01, _***_P<0.001 compared to HFD controls.
(TIF)
Figure S2
GLP-1 receptor agonism affects muscle expression of genes involved in fatty acid oxidation. E3L mice were fed HFD for 13 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (0.3 or 1.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice was included fed a chow diet that received vehicle (chow control). Skeletal muscles were isolated from 7 h fasted mice, and mRNA was extracted from muscle pieces. mRNA values of indicated genes were normalized to Cyclo and Hprt mRNA levels. Data were calculated as fold difference as compared with the HFD control group. Values are means ± SEM for at least 6 mice per group. *P<0.05, **P<0.01 compared to HFD controls.
(TIF)
Figure S3
Exendin-4 treatment reduces respiratory exchange ratio. C57Bl/6 mice were fed a HFD for 3 weeks before they were treated with either vehicle (control) or exendin-4 (50 μg/kg/day). Directly after the initiation of the treatment, indirect calorimetry measurements were started. Individual energy intake (A), activity (B), O2 consumption, and CO2 production rates were monitored. Respiratory exchange rate (C) and total energy expenditure (D) were calculated from the O2 consumption and CO2 production rates. Lines represent the mean values of 8 mice treated with vehicle (solid lines) or exendin-4 (dotted lines). Black areas under the x-axis represent the dark (12 hours) and white areas the light periods (12 hours). *P<0.05 compared to HFD controls.
(TIF)
Table S1
Primer sequences used for RT-qPCR. Abcg5, ATP-binding cassette sub-family G member 5; Acox1, acyl-CoA oxidase 1; Apob, apolipoprotein B; Cpt1, carnitine palmitoyltransferase 1; Cyclo, cyclophilin; Dgat1, acyl:diacylglycerol transferase 1; Fasn, fatty acid synthase; Hmgcoar, HMG-CoA reductase;. Hprt, hypoxanthine ribosyltransferase; Mttp, microsomal TG transfer protein; Pgc1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Pgc1β, peroxisome proliferator-activated receptor gamma coactivator 1-beta; Srebp-1c, sterol regulatory element binding protein 1c; Ucp1, uncoupling protein 1.
(DOC)
Funding Statement
This research was supported by a grant from Janssen Research & Development, the Netherlands Heart Foundation (NHS grant 2007B81 to PCNR), and the Dutch Diabetes Foundation (DFN grant 2007.00.010). PCNR is an Established Investigator of the Netherlands Heart Foundation (grant 2009T038). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stumvoll M, Goldstein BJ, van Haeften TW (2005) Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365: 1333–1346. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840–846. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JR, Mohanty SR (2010) Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci 55: 560–578. [DOI] [PubMed] [Google Scholar]
- 4.Kreymann B, Williams G, Ghatei MA, Bloom SR (1987) Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2: 1300–1304. [DOI] [PubMed] [Google Scholar]
- 5.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C (1997) Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270. [DOI] [PubMed] [Google Scholar]
- 6.Holst JJ, Orskov C, Nielsen OV, Schwartz TW (1987) Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 211: 169–174. [DOI] [PubMed] [Google Scholar]
- 7.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, et al. (1996) A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72. [DOI] [PubMed] [Google Scholar]
- 8.Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, et al. (1993) Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38: 665–673. [DOI] [PubMed] [Google Scholar]
- 9.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, et al. (1996) Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 81: 327–332. [DOI] [PubMed] [Google Scholar]
- 10.Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 11.Exenatide (Byetta) for type 2 diabetes. Med Lett Drugs Ther 47: 45–46. [PubMed] [Google Scholar]
- 12.Parlevliet ET, Schroder-van der Elst JP, Corssmit EP, Picha K, O'Neil K, et al. (2009) CNTO736, a novel glucagon-like peptide-1 receptor agonist, ameliorates insulin resistance and inhibits very low-density lipoprotein production in high-fat-fed mice. J Pharmacol Exp Ther 328: 240–248. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan P, Chaudhuri A, Bhatia R, Al-Atrash F, Mohanty P, et al. (2007) Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract 13: 444–450. [DOI] [PubMed] [Google Scholar]
- 14.Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, et al. (2008) Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 24: 275–286. [DOI] [PubMed] [Google Scholar]
- 15.van Vlijmen BJ, van den Maagdenberg AM, Gijbels MJ, van der Boom H, HogenEsch H, et al. (1994) Diet-induced hyperlipoproteinemia and atherosclerosis in apolipoprotein E3-Leiden transgenic mice. J Clin Invest 93: 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Maagdenberg AM, Hofker MH, Krimpenfort PJ, de B, I, van VB, et al. (1993) Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J Biol Chem 268: 10540–10545. [PubMed] [Google Scholar]
- 17.Picha KM, Cunningham MR, Drucker DJ, Mathur A, Ort T, et al. (2008) Protein engineering strategies for sustained glucagon-like peptide-1 receptor-dependent control of glucose homeostasis. Diabetes 57: 1926–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aalto-Setala K, Fisher EA, Chen X, Chajek-Shaul T, Hayek T, et al. (1992) Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest 90: 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redgrave TG, Roberts DC, West CE (1975) Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem 65: 42–49. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Catalina F, Grundy SM, Patel S (1996) Method to measure apolipoprotein B-48 and B-100 secretion rates in an individual mouse: evidence for a very rapid turnover of VLDL and preferential removal of B-48- relative to B-100-containing lipoproteins. J Lipid Res 37: 210–220. [PubMed] [Google Scholar]
- 21.Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 22.Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, et al. (2009) Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 58: 2198–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gedulin BR, Smith P, Prickett KS, Tryon M, Barnhill S, et al. (2005) Dose-response for glycaemic and metabolic changes 28 days after single injection of long-acting release exenatide in diabetic fatty Zucker rats. Diabetologia 48: 1380–1385. [DOI] [PubMed] [Google Scholar]
- 24.Green BD, Lavery KS, Irwin N, O'harte FP, Harriott P, et al. (2006) Novel glucagon-like peptide-1 (GLP-1) analog (Val8)GLP-1 results in significant improvements of glucose tolerance and pancreatic beta-cell function after 3-week daily administration in obese diabetic (ob/ob) mice. J Pharmacol Exp Ther 318: 914–921. [DOI] [PubMed] [Google Scholar]
- 25.Zander M, Madsbad S, Madsen JL, Holst JJ (2002) Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359: 824–830. [DOI] [PubMed] [Google Scholar]
- 26.Adiels M, Olofsson SO, Taskinen MR, Boren J (2006) Diabetic dyslipidaemia. Curr Opin Lipidol 17: 238–246. [DOI] [PubMed] [Google Scholar]
- 27.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA (2006) Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diraison F, Moulin P, Beylot M (2003) Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab 29: 478–485. [DOI] [PubMed] [Google Scholar]
- 29.Hellerstein MK, Neese RA, Schwarz JM (1993) Model for measuring absolute rates of hepatic de novo lipogenesis and reesterification of free fatty acids. Am J Physiol 265: E814–E820. [DOI] [PubMed] [Google Scholar]
- 30.Ferre P, Foretz M, Azzout-Marniche D, Becard D, Foufelle F (2001) Sterol-regulatory-element-binding protein 1c mediates insulin action on hepatic gene expression. Biochem Soc Trans 29: 547–552. [DOI] [PubMed] [Google Scholar]
- 31.Lin J, Yang R, Tarr PT, Wu PH, Handschin C, et al. (2005) Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell 120: 261–273. [DOI] [PubMed] [Google Scholar]
- 32.Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, et al. (2010) Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 51: 1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De MS, et al. (2011) Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 31: 1285–1297. [DOI] [PubMed] [Google Scholar]
- 34.Tomas E, Stanojevic V, Habener JF (2010) GLP-1 (9–36) amide metabolite suppression of glucose production in isolated mouse hepatocytes. Horm Metab Res 42: 657–662. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, et al. (2010) The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 53: 552–561. [DOI] [PubMed] [Google Scholar]
- 36.Matikainen N, Manttari S, Schweizer A, Ulvestad A, Mills D, et al. (2006) Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia 49: 2049–2057. [DOI] [PubMed] [Google Scholar]
- 37.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, et al. (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrows BR, Parks EJ (2006) Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab 91: 1446–1452. [DOI] [PubMed] [Google Scholar]
- 39.Kastin AJ, Akerstrom V, Pan W (2002) Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18: 7–14. [DOI] [PubMed] [Google Scholar]
- 40.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP (1995) Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7: 2294–2300. [DOI] [PubMed] [Google Scholar]
- 41.Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, Pijl H (2010) GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 299: E318–E324. [DOI] [PubMed] [Google Scholar]
- 42.Nogueiras R, Perez-Tilve D, Veyrat-Durebex C, Morgan DA, Varela L, et al. (2009) Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J Neurosci 29: 5916–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sathyanarayana P, Jogi M, Muthupillai R, Krishnamurthy R, Samson SL, et al. (2011) Effects of combined exenatide and pioglitazone therapy on hepatic fat content in type 2 diabetes. Obesity (Silver Spring) 19: 2310–2315. [DOI] [PubMed] [Google Scholar]
- 44.Garber AJ (2011) Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 34 Suppl 2S279–S284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tahrani AA, Bailey CJ, Del PS, Barnett AH (2011) Management of type 2 diabetes: new and future developments in treatment. Lancet 378: 182–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
GLP-1 receptor agonism reduces fasting glucose and insulin levels. E3L mice were fed a high fat diet (HFD) for 13 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (0.3 or 1.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice fed a chow diet was included that received vehicle (chow control). Just before drug treatment (week 13) and after treatment (week 17), body weight (A), plasma glucose (B) and plasma insulin (C) levels were determined. Values are means ± SEM for at least 6 mice. _*_P<0.05, _**_P<0.01, _***_P<0.001 compared to HFD controls.
(TIF)
Figure S2
GLP-1 receptor agonism affects muscle expression of genes involved in fatty acid oxidation. E3L mice were fed HFD for 13 weeks. The last 4 weeks, mice were treated with either vehicle (HFD control), CNTO3649 (0.3 or 1.0 mg/kg/day) or exendin-4 (15 or 50 μg/kg/day). As a control for HFD feeding, an additional group of mice was included fed a chow diet that received vehicle (chow control). Skeletal muscles were isolated from 7 h fasted mice, and mRNA was extracted from muscle pieces. mRNA values of indicated genes were normalized to Cyclo and Hprt mRNA levels. Data were calculated as fold difference as compared with the HFD control group. Values are means ± SEM for at least 6 mice per group. *P<0.05, **P<0.01 compared to HFD controls.
(TIF)
Figure S3
Exendin-4 treatment reduces respiratory exchange ratio. C57Bl/6 mice were fed a HFD for 3 weeks before they were treated with either vehicle (control) or exendin-4 (50 μg/kg/day). Directly after the initiation of the treatment, indirect calorimetry measurements were started. Individual energy intake (A), activity (B), O2 consumption, and CO2 production rates were monitored. Respiratory exchange rate (C) and total energy expenditure (D) were calculated from the O2 consumption and CO2 production rates. Lines represent the mean values of 8 mice treated with vehicle (solid lines) or exendin-4 (dotted lines). Black areas under the x-axis represent the dark (12 hours) and white areas the light periods (12 hours). *P<0.05 compared to HFD controls.
(TIF)
Table S1
Primer sequences used for RT-qPCR. Abcg5, ATP-binding cassette sub-family G member 5; Acox1, acyl-CoA oxidase 1; Apob, apolipoprotein B; Cpt1, carnitine palmitoyltransferase 1; Cyclo, cyclophilin; Dgat1, acyl:diacylglycerol transferase 1; Fasn, fatty acid synthase; Hmgcoar, HMG-CoA reductase;. Hprt, hypoxanthine ribosyltransferase; Mttp, microsomal TG transfer protein; Pgc1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Pgc1β, peroxisome proliferator-activated receptor gamma coactivator 1-beta; Srebp-1c, sterol regulatory element binding protein 1c; Ucp1, uncoupling protein 1.
(DOC)