Exosomes from IDO+ DC are therapeutic in CIA and DTH disease models (original) (raw)
. Author manuscript; available in PMC: 2012 Nov 7.
Published in final edited form as: Arthritis Rheum. 2009 Feb;60(2):380–389. doi: 10.1002/art.24229
Abstract
Objective
We have demonstrated previously that dendritic cells (DC), modified with immunosuppressive cytokines, and exosomes derived from the DC can suppress the onset of murine CIA and reduce the severity of established arthritis. Indoleamine 2,3-dioxygenase (IDO) is a tryptophan degrading enzyme important for immune regulation and tolerance maintenance. DC expressing functional IDO can inhibit T cells by either depleting them of essential tryptophan and/or by producing toxic metabolites, as well as by generating regulatory T cells. In this study, we examined the immunosuppressive effects of bone marrow derived DC, genetically modified to express IDO, and IDO+-DC-derived exosomes.
Methods
Bone marrow derived DC were adenovirally transduced with IDO or CTLA4-Ig (an inducer of IDO), and the resulting DC and exosomes were tested for their immunosuppressive ability in the collagen-induced arthritis and delayed type hypersensitivity murine models.
Results
We demonstrate that both DC and exosomes derived from DC overexpressing IDO are anti-inflammatory in collagen-induced arthritis and delayed type hypersensitivity murine models. The suppressive effects were partially dependent on B7 costimulatory molecules. In addition, gene transfer of CTLA4-Ig to DC resulted in induction of IDO in the DC and exosomes able to reduce inflammation in an IDO-dependent manner.
Conclusion
These results demonstrate that both IDO expressing DC and DC-derived exosomes are immunosuppressive and anti-inflammatory, and are able to reverse established arthritis. Therefore, exosomes from IDO+ DC may represent a novel therapy for rheumatoid arthritis.
Keywords: Dendritic cells, Exosomes, IDO, Arthritis, Inflammatory disease
Exosomes are small membrane vesicles, approximately 50–100 nm in diameter, that are produced within the multivesicular bodies of the late endocytic compartment of hematopoietic and non-hematopoietic cells. Exosomes are then secreted into the extracellular space by fusion of the limiting membrane of mutivesicular bodies with the plasma membrane. Exosomes originating from B cells, T cells, dendritic cells (DC), and mast cells, can confer immunoregulatory signals between cells in either an immuno-stimulatory or suppressive manner. Indeed, exosomes derived from APCs contain many of the important regulatory molecules to carry out this function, such as MHC Class I and II, CD80 (B7-1), and CD86 (B7-2), as well as various adhesion molecules that may target exosomes to their acceptor cells (1). Previously we demonstrated that exosomes derived from immature DC treated with IL-10 produce anti-inflammatory exosomes able to suppress the onset on murine collagen-induced arthritis (CIA) and reduce the severity of established arthritis (2). Moreover, DC transduced with an adenoviral vector expressing FasL or IL-4 produce exosomes that suppress inflammation in a murine model of delayed type hypersensitivity (DTH) and partially reverse established CIA through a MHC Class II-dependent, but MHC Class I-independent mechanism (3, 4). Interestingly, the DC-derived exosomes are as or more immunosuppressive than their parental DC.
Indoleamine 2,3-dioxygenase (IDO) is a tryptophan degrading enzyme that is also important in host defense and immunosuppression. Only certain subsets of cells appear capable of producing functional IDO, including CD19+ plasmacytoid DC (5) and CD8α+, B220+, CD19+ splenic DC (6). IDO is transcriptionally induced by a variety of inflammatory stimuli, such as IFN-γ, IFN-α, CD40L, GITR, and TNFα (7). Cellular infection with viruses and microbes can also induce IDO (7). In the cases of CD40L and GITR, downstream signaling appears to involve a non-canonical NF-κB mediated induction of IDO (8, 9) Interestingly, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) on T regulatory cells or soluble CTLA-4-immunoglobulin (CTLA4-Ig) induces functional IDO in DC by binding to and inducing signaling though B7 molecules (10).
The immunosuppressive ability of IDO was initially described as being important for maternal tolerance of the fetus, as mice treated with the IDO inhibitor, 1-methyl-tryptophan (1-MT) underwent spontaneous abortion (11, 12). More recently it has been demonstrated that endogenous IDO is involved in maintaining tolerance in a number of settings (7), including rheumatoid arthritis (13, 14), cancer (15), transplantation (16), diabetes (16, 17), and experimental autoimmune encephalitis (EAE) (18, 19). Elucidation of the mechanism by which IDO inhibits the T cell response is still being investigated. One possible mechanism is that IDO depletes T cells of essential tryptophan, causing activation of GCN2 kinase and rendering the T cells anergic (20). IDO also produces metabolites of tryptophan, collectively termed kynurenines, that can regulate T cells through a poorly understood mechanism (21). However, these two possible mechanisms are not mutually exclusive, and recent data suggests that a combination of the two mechanisms may be working together to suppress CD8+ effector T cells and to activate T regulatory cells (22, 23).
IDO appears to have an immunosuppressive role in arthritis based on studies showing that inhibition of IDO accelerates CIA (13), and that orally tolerizing mice to collagen induces an IDO+ suppressor DC population (24). Also, treatment with an agonistic monoclonal antibody to anti-4-1BB, a T-cell costimulatory receptor, can inhibit CIA through an IDO dependent pathway (14). Finally, CTLA4-Ig upregulates IDO in certain populations of cells and has recently been approved for the treatment of rheumatoid arthritis (25–28). Even though tryptophan degradation is enhanced in rheumatoid arthritis (29), apparently it is not enough to suppress disease (30).
Due to the immunosuppressive activity of IDO+ DC, we have examined whether IDO+ DC and exosomes derived from IDO-expressing DC would be therapeutic in treating established CIA and blocking inflammation in a footpad DTH model of antigen-specific inflammation. Herein we document that both IDO-expressing DC and DC-derived exosomes are anti-inflammatory and therapeutic in both CIA and DTH models. The suppressive effects in the DTH model were partially dependent on B7-1 and B7-2 costimulatory molecules, as exosomes from B7-1 and B7-2 double knockout mice were less able to confer the therapeutic effect. Finally, exosomes from CTLA4-Ig expressing DC also reduced inflammation in an IDO dependent manner. These results suggest that exogenous expression of IDO in bone marrow derived DC or induction of endogenous IDO renders them immunosuppressive. Moreover, these results demonstrate that IDO expression in DC results in the generation of immunosuppressive exosomes.
MATERIALS AND METHODS
Mice
Female C57BL/6 (H-2Kb) mice and male DBA/1LacJ (H-2Kq) mice, all 7–8 wk of age, were purchased from the Jackson Laboratory (Bar Harbor, ME). B7 knockout mice, DKO= B6.129S4-Cd80tm1Shr Cd86tm2Shr/J B7-1 = B6.129S4-Cd80tm1Shr/J B7-2 = B6.129S4-Cd86tm1Shr/J, were also purchased from Jackson Laboratory. Animals were maintained in a pathogen-free animal facility at the University of Pittsburgh Biotechnology Center (Pittsburgh, PA).
Vector construction and adenovirus generation
ΔE1,E3 first generation adenoviruses expressing murine IDO (Ad.IDO) and CTLA4-Ig (Ad.CTLA4-Ig) under the regulation of the CMV promoter were constructed by Cre-Lox recombination, propagated, and titered according to standard protocols as previously described (31). Briefly, the recombinant adenoviruses were generated by homologous recombination in 293 cells expressing Cre recombinase (CRE8 cells), after co-transfection of DNA, an adenovirus 5-derived, E1-and E3-deleted adenoviral backbone (psi5) and pAdlox, the adenoviral shuttle vector. The inserted cDNA sequences are expressed under the human CMV promoter. The recombinant adenoviruses were purified by CsCl gradient ultracentrifugation, dialyzed in sterile virus storage buffer, aliquoted and stored at −80°C until use. The CRE8 cells were grown and maintained in DMEM (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal calf serum.
DC generation
Murine bone marrow (BM)-derived DC were prepared using the bulk culture method as adapted from Y.I. Son et al. (32). Briefly, BM was collected from tibias and femurs of six-to seven-week old mice. Contaminating erythrocytes were lysed with ACK cell lysing buffer (Mediatech, Herdon, VA). Monocytes were collected from the interface after centrifuging on Nycoprep (NycoMed, Roskilde, Denmark) at 600 × g for 20 min at RT. Cells were then cultured for 24 h in complete media (CM; RPMI 1640 containing 10% FBS, 50 μM 2-ME, 2 mM glutamine, 0.1 mM nonessential amino acids, 100 μg/ml streptomycin, and 100 IU/ml penicillin) to remove the adherent macrophages. The non-adherent cells then were placed in fresh CM containing 1000 U/ml of mGM/CSF and mIL-4 to generate DC. Cells were cultured for 4 days and harvested for Ad transduction. For Ad infection, 1 × 106 DC were mixed with 5 × 107 PFU of the viruses in total volume of 1 ml serum-free media. After a 2 hr incubation with virus, 10 ml complete media was added to the cells. In certain experiments, the cells were also treated with 1-methyl-D-tryptophan (1-MT)(Sigma)(200μM) or L-Trp (245 μM) at this time. After incubation for 24 hr, DC were washed intensively three times and cultured for a further 48 h. On day 8, culture supernatant was collected for exosome purification and recovery of the Ad-transduced DC. This infection method routinely gives us ~70–80% transfection efficiency using Ad.eGFP as a control (33). There was no toxic effect of 1-MT or L-Trp on the cells as observed by trypan blue exclusion and overall cell count.
Exosome isolation
Exosomes were isolated as previously described using differential centrifugation (2). Collected culture supernatants were centrifuged at 300 g for 10 min, 1200 g for 20 min, and 10,000 g for 30 min. The supernatant from the final centrifugation was ultra-centrifuged at 100,000 g for 1 h in the ultra-centrifuge. The exosome pellet was washed in saline, again centrifuged at 100,000 g for 1 h, and resuspended in 120 μl of PBS for further studies. The exosomal protein content was quantified by a micro Bradford protein assay (Bio-Rad, CA). Each batch was standardized by protein content and 1 μg was suspended in 20μl of PBS for in vivo mouse studies. This method of exosome isolation routinely gives us a relatively pure population of nanovesicles that are <100nm (as visualized by EM), and enriched in exosomal proteins (as determined by WB and FACS), such as hsc70, CD81, CD80/86 and MHC I/II.
Exosome administration into the DTH model
C57BL/6 mice were sensitized by subcutaneously injecting 100 μg of Keyhole Limpet Hemocyanin (KLH) antigen emulsified 1:1 in Freund’s complete adjuvant (FCA) at a single dorsal site. Ten days later, one hind footpad of the immunized mice was injected intradermally with 106 DC or 1 μg of DC-derived exosomes plus KLH antigen (20μg) in 50μl total volume. The contra-lateral footpad received an equal volume of saline plus Ag (20μg in 50μl) without DC or exosomes. Footpad swelling was measured using a spring-loaded caliper (Dyer). Results were expressed as the difference in swelling (X 0.01 mm), before and after Ag boost injection. The in vivo experiments were performed with 5 mice/group and repeated at least twice to ensure reproducibility.
Murine CIA model
Male DBA/1lacJ (H-2q) mice, 7 to 8 week of age were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained in a pathogen-free animal facility at the University of Pittsburgh Biotechnology Center. Bovine Type II collagen (Chondrex) in 0.05 M acetic acid at a concentration of 2 mg/ml was emulsified in an equal volume of Freund’s Complete Adjuvant (FCA) and 150 μg injected into the base of the tail. For the treatment of established arthritis, mice were injected with 20 μg of lipopolysaccharide (LPS) intra-peritoneally at day 28 to induce synchronous disease onset. Four days after LPS injection (day 32), DC or exosomes from Ad.IDO-transduced or non-transduced DC were i.v. injected into the mice with evidence of disease. The in vivo experiments were performed with 12 mice/group and repeated twice to ensure reproducibility.
The mice were monitored by established macroscopic system ranging from 0 to 4: 0, normal; 1, detectable arthritis with erythema; 2, significant swelling and redness; 3, severe swelling and redness from joint to digit; and 4, maximal swelling and deformity with ankylosis. The average of macroscopic score was expressed as a cumulative value for all paws, with a maximum possible score of 16 per mouse.
Western blot analysis
For western blot, exosomal proteins (3–10μg) were separated by 12% or 15% SDS-PAGE, semi-dry transferred onto PVDF and detected by western blotting using an enhanced chemiluminescence detection kit. Primary antibodies used for western blot were: rabbit polyclonal anti-GFP (Santa Cruz), mouse monoclonal anti-hsc70 (Santa Cruz), rabbit polyclonal anti-beta actin (abcam), mouse monoclonal anti-IDO (Upstate), and rat monoclonal anti-IDO (Santa Cruz). Semi-quantitative analysis of the protein density bands was performed using the NIH Image J program.
Measurement of kynurenine concentrations
Kynurenine was measured in exosomes (1 μg) or exosome-free supernatants (60μl) using a spectrophotometric assay. Samples were mixed 2:1 with 30% trichloroacetic acid, vortexed, and centrifuged at 3716 x g for 10 minutes. 75μl of supernatant was then added to an equal volume of Ehrlich reagent (2% 4-(dimethylamino)benzaldehyde in glacial acetic acid) in a 96 well microtiter plate. Samples (in triplicate) were analyzed against a standard curve of L-kynurenine (Sigma)(0–5 mM). Absorbance was measured at 490nm using a microplate reader.
Flow cytometry
For phenotypic analysis of DC, the cells were blocked with normal goat serum and then were stained with PE- or FITC-conjugated monoclonal antibodies (BD Pharmingen) against murine CD11b, CD11c, CD80, CD86, H-2Kb, I-Ab. The DC were analyzed by FACSCalibur (Becton Dickenson, CA) and the CellQuest software analysis program. Isotype-matched irrelevant mAbs were used as negative controls.
Statistical analysis
Results were compared by analysis of variance (ANOVA) with Fisher’s Least Significant Difference post-hoc test. When appropriate, the Kruskal-Wallis non-parametric test was used to compare means between groups. P values less than 0.05 were considered statistically significant, and all tests were conducted using SPSS statistical software (SPSS, Chicago IL).
RESULTS
Administration of DC/IDO or Exo/IDO reduces severity of murine CIA
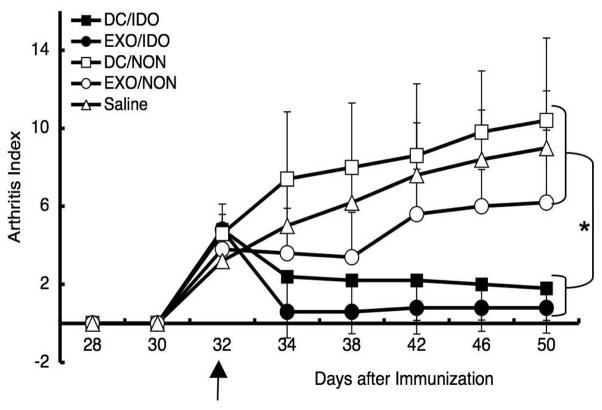
Since overexpression of IDO can result in immunosuppression and tolerance in vitro and in vivo in certain animal models such as a transplant tolerance (10), we examined the ability of DC genetically modified to overexpress IDO and the exosomes derived from the modified DC, to treat CIA. BM-DC were infected with a recombinant adenoviral (Ad) vector expressing IDO (Ad.IDO), or non-infected, and the exosomes and cells were harvested three days later. The DC (106 cells) or exosomes (1 μg) were injected i.v. on day 32 post immunization into DBA1 mice with early stage arthritis. A single injection of either DC/IDO or exosomes from DC/IDO (Exo/IDO) was able to reverse arthritis progression, while disease progressed normally in the saline or non-infected DC injected control groups (Fig. 1). The non-infected exosome group was also slightly therapeutic as previously observed (4). This is likely due to our previous analysis showing that there may be a significant number of still immature and thus anti-inflammatory and tolerogenic BM-DC in the preparation of mock/non-infected DC (Table 1) (33). Western blot analysis confirmed the expression of IDO in the infected DC/IDO lysate, as well as in the exo/IDO (Fig. 4B). No IDO was detectable in the non-infected cells or exosomes by western blot. Also, as shown by FACS analysis in Table 1, there was no significant change in the maturation status of the IDO-infected cells. Since exosomes were as or more therapeutic as the DC in the CIA model, and likely more stable (34–36), most of our following experiments were carried out using exosomes alone.
Figure 1.
IDO+ DC and DC-derived exosomes are therapeutic in murine CIA. Exosomes were isolated from DBA1 BM-DC that were previously infected with Ad.IDO or non-infected. The purified exosomes as well as DC were injected i.v. at day 32 into DBA1 mice that were immunized with bovine type II collagen and received LPS at day 28 to synchronize disease onset. Mice were monitored periodically by established macroscopic scoring system on a 0 to 4 scale: 0= normal, 1= detectable arthritis with erythma, 2= significant swelling and redness, 3= severe swelling and redness from joint to digit, 4= maximal swelling and deformity with ankylosis. The macroscopic score (mean ± SD) was expressed as a cumulative value for all paws, with a maximum possible score of 16 (n = 12). Arrows indicate the day of treatment. * denotes significance at _P_=0.001 compared to DC/Non and Saline groups and P<0.05 compared to Exo/Non groups.
Table 1.
FACS analysis of DC used in study.
| % cells PE-labeled | ||||
|---|---|---|---|---|
| CD80 | CD86 | MHC-I | MHC-II | |
| DC-Non | 23.65 | 27.89 | 74.84 | 34.87 |
| DC-Psi5 | 29.86 | 45.28 | 54.66 | 45.38 |
| DC-IDO | 24.26 | 36.60 | 61.18 | 35.10 |
| DC-CTLA4-Ig | 31.55 | 49.39 | 53.61 | 48.90 |
| DC-CTLA4-Ig+1-MT | 30.85 | 48.62 | 52.56 | 48.31 |
| DC-CTLA4-Ig+L-trp | 27.81 | 42.15 | 51.81 | 42.10 |
Figure 4.
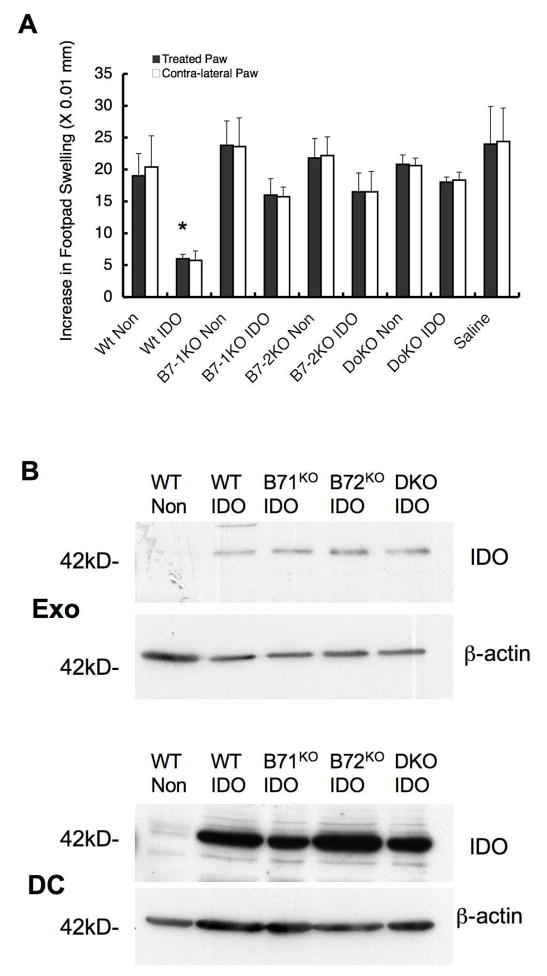
DTH suppression by exosomes is dependent on B7-1 and B7-2. Exosomes were isolated from BM-DC from wild type C57BL/6 mice or B7-1KO, B7-2KO, or double knockout (DKO) mice previously transduced with Ad.IDO or non-infected. (A) The exosomes from the genetically-modified DC were tested in wild type recipient mice. The purified exosomes were injected into the right footpad of KLH-immunized mice concurrently with injection of KLH antigen into both hind footpads, and the extent of swelling was measured at 24 h. Dark bars represent difference of footpad thickness in the treated paws and white bars in the non-treated contra-lateral paws (n=5). * denotes significance of _P_=0.05 compared to all other groups for both treated and contralateral paws. (B) Five μg of total DC or exosome protein extract was run on a 15% SDS-PAGE gel and immunoblotted for IDO or β-actin.
Exosomes from DC/CTLA4-Ig are also therapeutic in a murine CIA model
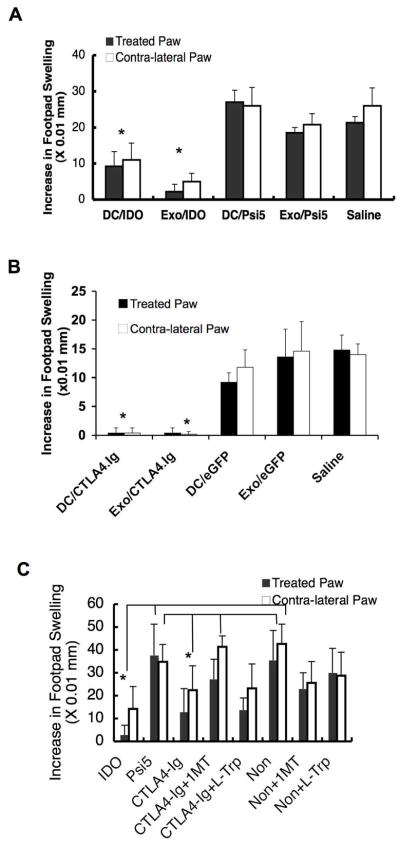
CTLA4-Ig is a synthetic fusion protein that binds with high affinity to B7-1 and B7-2, resulting in upregulation of IDO in certain DC subsets and immunosuppression (25–28). Our previous studies have shown that DC genetically engineered to express CTLA4-Ig plus NF-κB oligodeoxyribonucleotide decoys, to prevent DC maturation, could significantly prolong heart allograft survival (37). To determine whether DC derived exosomes from DC expressing CTLA4-Ig would be immunosuppressive as well, BM-DC were infected with Ad.CTLA4-Ig, or non infected, and the exosomes were collected three days later. Western blot analysis confirmed an increased expression of IDO in the DC following Ad.CTLA4-Ig infection (Fig. 2A). As with exosomes from the DC infected with Ad.IDO, a single injection of exosomes from DC/CTLA4-Ig (Exo/CTLA4-Ig) was able to reverse arthritis progression, while disease progressed normally in the saline injected control group (Fig. 2B). This immunosuppression was dependent on IDO mediated deprivation of tryptophan, as addition of the competitive IDO inhibitor, 1-MT, or excess L-Trp to the DC was able to reduce the effect. It is important to note that the IDO inhibitors were added to the DC and removed prior to the exosome isolation procedure. Although there is CTLA-4-Ig in the exosomes from the Ad.CTLA4-Ig infected DC (Fig. 2C), the ability of 1-MT and L-Trp to abolish the therapeutic effect of the exosomes suggests that their suppressive effects are dependent upon IDO activity in the DC.
Figure 2.
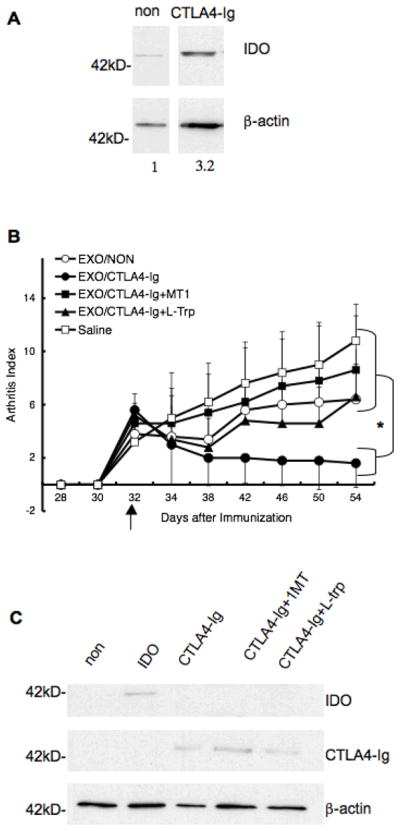
CTLA4-Ig+ DC and DC-derived exosomes are therapeutic in murine CIA in an IDO dependent manner. Exosomes were isolated from DBA1 BM-DC that were previously infected with Ad.CTLA4-Ig and treated with ±1-MT or L-Trp, or non- infected. (A) DC extracts (2 μg) from non-infected Ad.CTLA4-Ig cells were run on a 12% SDS-PAGE gel and immunoblotted for IDO or β-actin. Numbers indicate relative semi-quantitative expression of IDO after normalization to βactin using the NIH software program Image J. (B) The purified exosomes as well as DC were injected i.v. at day 32 into DBA mice that were immunized with bovine type II collagen and received LPS at day 28 for synchronous disease onset. Mice were monitored periodically by established macroscopic scoring system on a 0 to 4 scale: 0= normal, 1= detectable arthritis with erythma, 2= significant swelling and redness, 3= severe swelling and redness from joint to digit, 4= maximal swelling and deformity with ankylosis. The macroscopic score (mean ± SD) was expressed as a cumulative value for all paws, with a maximum possible score of 16 (n = 12). Arrows indicate the day of treatment. * denotes significance at _P_=0.05 of Exo/CTLA-4 group compared to all other groups. (C) Five μg of total exosome protein was run on a 15% SDS-PAGE gel and immunoblotted for IDO, CTLA4-Ig, or β-actin.
Local administration of DC/IDO or Exo/IDO inhibits the DTH response
To determine if the exosomes from DC genetically modified to express IDO were therapeutic in an Ag-specific model of inflammation more amenable to analysis of mechanism, a DTH mouse model was utilized. In this model, mice were immunized to a specific Ag, keyhole limpet hemocyanin (KLH), and then a Th1-mediated inflammatory response was induced two weeks post-immunization by intradermal injection of the specific Ag into the hind footpads. We have used this model previously to demonstrate that Ad.vIL-10, Ad.IL-4 and Ad.FasL-transduced DC and DC-derived exosomes were anti-inflammatory. The KLH-immunized mice received either 106 DC or 1 μg of exosomes injected into one hind paw at the same time as a KLH boost injection into both hind paws. Local injection of DC/IDO and Exo/IDO (Fig. 3A) significantly suppressed paw swelling not only in the treated paw, but also in the untreated contralateral paw at 24 h, 48 h (shown in 3A) and 72 h post-injection of Ag. In contrast, injection of DC/Ψ5 or exosomes derived from the control DC was unable to inhibit the DTH response. These results demonstrate that a single, local footpad injection of genetically modified DC expressing IDO as well as exosomes derived from the DC/IDO are able to suppress the DTH response in not only the treated paw, but also in the contralateral, untreated paw, suggesting a systemic effect following local injection. This is not due to systemic injection of DC or exosomes, as they were injected intradermally into the hind footpad. Exosomes from DC/CTLA4-Ig were also able to suppress inflammation in both the treated and contralateral paw, and were as effective as the DC/CTLA4-Ig (Fig. 3B). The addition of 1-MT, but not L-Trp, to the DC was able to inhibit the anti-inflammatory effect of CTLA-4 (Fig. 3C). These results show that Exo/IDO and Exo/CTLA-Ig can both be used therapeutically to suppress inflammation in the DTH model.
Figure 3.
Suppression of DTH by IDO+ DC and DC-derived exosomes. (A) IDO+ DC/Exo are suppressive in the DTH model. Exosomes were isolated from BM- DC that were either infected with the Ad.Ψ5 (control) or Ad.IDO. The purified DC or exosomes were injected into the right footpad of KLH-immunized C57 BL/6 mice concurrently as KLH was injected into both hind footpads and the extent of swelling was measured at 48 h. Dark bars represent difference of footpad thickness in the treated paws and white bars in the non-treated contra-lateral paws (n=5). * denotes significance of P=0.001 compared to DC/Psi5, Exo/Psi5, and Saline controls for both treated and contralateral paws. (B–C). CTLA4-Ig+ DC/Exo are also suppressive in the DTH model in an IDO dependent manner. Exosomes were isolated from BM-DC that were either infected with the Ad.Ψ5 or eGFP (controls) or Ad.CTLA4-Ig and treated with ± 1-MT or L-Trp. The purified DC or exosomes were injected into the right footpad of KLH- immunized C57 BL/6 mice concurrently as KLH was injected into both hind footpads and the extent of swelling was measured at 24 h (C) or 48 h (B). Note that (C) is exosomes only. Dark bars represent difference of footpad thickness in the treated paws and white bars in the non-treated contra-lateral paws (n=5). (B)* denotes significance of P=0.001 compared to DC/eGFP, Exo/eGFP, and Saline Control Groups for both treated and contralateral paws (C) * denotes significance of P=0.05 of CTLA4-Ig compared to controls Psi5, CTLA4-Ig+1MT and Noninfected controls for the treated paws, as well as significance of P=0.05 of IDO group compared to all groups excepting the CTLA-4-Ig and CTLA-4Ig+1MT for both treated and contralateral paws.
Anti-inflammatory effect of Exo/IDO is dependent on B7 molecules
To determine whether the anti-inflammatory effect of Exo/IDO was dependent on the IDO present in the exosomes or due to modification of the immunosuppressive factors on the exosomes, vesicles deficient in factors important for conferring the suppressive effects of exosomes in other experiments were used. We have previously shown that the immunosuppressive activity of exosomes in the DTH model is MHC Class II, FasL, and B7-1/B7-2 dependent (3, 4)(and unpublished data). Thus we examined the ability of exosomes from B7 deficient, IDO+ DC to suppress the DTH response. Bone marrow derived DC from wt, B7-1−/−, B7-2−/−, and double knockout mice were transduced with Ad.IDO or a control vector and exosomes isolated. Interestingly, loss of either or both of the B7 co-stimulatory molecules significantly reduced the anti-inflammatory effect of the Exo/IDO (Fig. 4A). This effect was not due to differing levels of IDO in the exosomes (Fig. 4B). Therefore, the immunosuppressive effect of Exo/IDO depends, at least partially, on the B7-1/2 molecules consistent with our previous observation using DC treated with IL-10 as well as exosomes derived from the DC/IL-10 (unpublished data).
Exo/IDO do not contain tryptophan metabolites
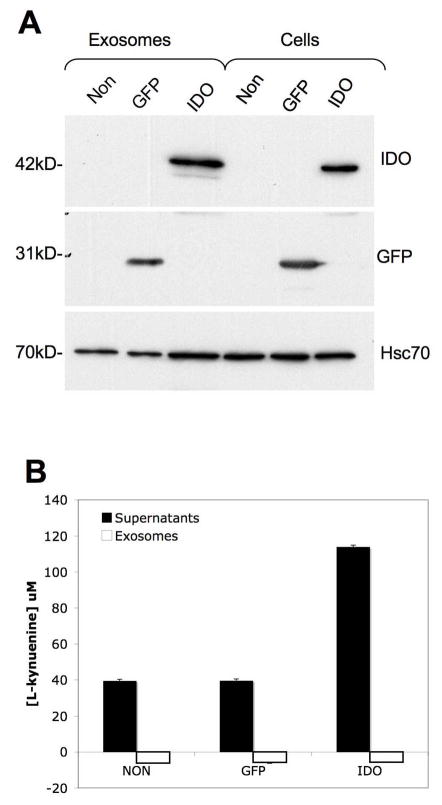
IDO can deplete T cells of tryptophan, and also produce cytotoxic metabolites of tryptophan, collectively termed kynurenines, that can regulate T cells (21). To determine whether Exo/IDO could be conferring their immunosuppressive effects through delivery of cytotoxic kynurenines to T cells, we assayed for the presence of L-kynurenine in exosomes and in the exosome-free supernatants. For this experiment, we used TA3 Hauschka cells, a mouse mammary carcinoma line, because of their ease of infection and their ability to produce large quantities of exosomes, without the use of limited primary cells. The cells were infected with Ad.IDO or Ad.GFP as a control or were non-infected. Western blot analysis confirmed the expression of IDO or GFP in the cells and exosomes (Fig. 5A). The exosomes or exosome-free supernatants were then analyzed for L-kynurenine. Only the exosome-free supernatants from Exo/IDO contained L-kynurenine (Fig. 5B). Thus, exosomes appear not to carry detectable levels of the cytotoxic tryptophan metabolites.
Figure 5.
Exosomes from IDO+ cells do not contain Trp metabolites. (A) Overexpression of IDO in TA3 cells results in the release of exosomes containing IDO. TA3 cells were either non infected (non) or infected with rAd containing expression cassettes for either eGFP or IDO. Exosomes were collected from the culture supernatant, and analyzed by western blot along with cell lysates for levels of IDO, eGFP, and hsc70. Seven μg of total protein extract from both exosomes and cells was loaded on the gel. (B). Exosomes (1 μg) or exosome-free supernatants (60μl) were assayed for levels of L-kynurenine.
DISCUSSION
IDO is an immuno-modulatory protein that has gained significant research interest in the last decade due to its ability to induce or maintain peripheral tolerance and immunosuppression in pregnancy, autoimmune disease, cancer, asthma, and transplantation (13, 15). IDO-expressing DC can suppress the T effector response and activate T regulatory cells by either depleting the local area of tryptophan, by producing toxic metabolites, or both (23). Here we have examined the immunosuppressive activity of DC genetically modified to express IDO and exosomes derived from the DC in both CIA and DTH mouse models. Our results demonstrate that both DC/IDO and Exo/IDO can reverse established CIA and reduce inflammation in the DTH footpad model.
The mechanism of IDO-mediated immunosuppression in general is still poorly understood. It has been reported that both IDO mediated local deprivation of essential Trp, and cytotoxic Trp metabolites may be working together to suppress CD8+ effector T cells and to activate T regulatory cells (22, 23). Surprisingly, the Exo/IDO were as suppressive in the CIA and DTH models as the DC/IDO. Since the Exo/IDO contain exogenous IDO protein, they may be functioning by delivering functional IDO to IDO− DC or T cells, rendering the DC tolerogenic and/or causing T cell anergy. We could not detect any L-kynurenine metabolic product in the Exo/IDO samples, only in the exosome free supernatants, suggesting that delivery of toxic metabolites is not the mechanism. There was also no significant change in the maturation status of the IDO-DC, suggesting that the therapeutic effect was not due to a change in DC maturity. We hypothesize that IDO expression in the DC modifies the DC-derived exosomes in some other way(s) to render them tolerogenic. Indeed, we were able to demonstrate a role for components of exosomes in conferring the suppressive effects of Exo/IDO. In particular, we demonstrated that the co-stimulatory molecules B7-1 and B7-2, which are required for the suppressive effects of DC and exosomes, are partially required for the suppressive effects of Exo/IDO. This result suggests that the Exo/IDO could be directly interacting with T cells. However, it is also possible that the exosomes interact with endogenous APCs to alter their function through a B7 dependent mechanism. Consistent with this hypothesis is the fact that we have previously demonstrated the requirement for MHC Class II in both the exosomes and in the recipient mice for exosomes to regulate T cells responses in vivo (3).
We have also demonstrated that exosomes derived from DC/CTLA4-Ig are immuno-suppressive in the CIA and DTH models. CTLA-4 on T regulatory cells or soluble CTLA4-Ig can induce functional IDO in DC by binding to B7 molecules (10). In our BM-DC, we semi-quantitatively measured an approximate 3-fold increase in IDO expression after CTLA4-Ig infection, but not in the exosomes. We demonstrated that the suppressive activity of the DC/CTLA4-Ig derived exosomes was dependent upon IDO activity in the DC by using 1-MT and L-Trp in the CIA model. In the DTH model, only 1-MT was able to block CTLA-4 suppression, while L-Trp had no effect. Thus, the mechanism may be slightly different in the two models. We do not believe the difference is due to any toxic effect of 1-MT, since there is never any increase in cell death during treatment (data not shown).
Overall, this study highlights the potential therapeutic use of exosomes from DC genetically engineered to overexpress IDO. Moreover, the results demonstrate that IDO activity can modify the activity of DC-derived exosomes, rendering them more immunosuppressive. The use of exosomes, instead of DC, allows for a more stable delivery method, without loss of activity (34–36). While this study focuses on arthritis, it is also likely that DC/IDO and/or Exo/IDO may also have therapeutic effects in other models of autoimmunity in which IDO has been shown to have immunosuppressive effects.
Acknowledgments
We would like to thank Ms. Joan Nash for technical assistance and Dr. Maliha Zahid for help with statistical analysis.
This work was supported by NIH grant AI56374 and JDRF grant 7-2005-1154 to P.D.R.
References
- 1.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE, Robbins PD. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective Treatment of Inflammatory Disease Models with Exosomes Derived from Dendritic Cells Genetically Modified to Express IL-4. J Immunol. 2007;179:2242–2249. doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- 5.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manlapat AK, Kahler DJ, Chandler PR, Munn DH, Mellor AL. Cell-autonomous control of interferon type I expression by indoleamine 2,3-dioxygenase in regulatory CD19+ dendritic cells. Eur J Immunol. 2007;37:1064–1071. doi: 10.1002/eji.200636690. [DOI] [PubMed] [Google Scholar]
- 7.King NJ, Thomas SR. Molecules in focus: Indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007 doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Tas SW, Vervoordeldonk MJ, Hajji N, Schuitemaker JH, van der Sluijs KF, May MJ, Ghosh S, Kapsenberg ML, Tak PP, de Jong EC. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- 9.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 10.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 12.Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 13.Szanto S, Koreny T, Mikecz K, Glant TT, Szekanecz Z, Varga J. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res Ther. 2007;9:R50. doi: 10.1186/ar2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4–1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 15.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor- induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander AM, Crawford M, Bertera S, Rudert WA, Takikawa O, Robbins PD, Trucco M. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Diabetes. 2002;51:356–365. doi: 10.2337/diabetes.51.2.356. [DOI] [PubMed] [Google Scholar]
- 17.Grohmann U, Fallarino F, Bianchi R, Vacca C, Orabona C, Belladonna ML, Fioretti MC, Puccetti P. Tryptophan catabolism in nonobese diabetic mice. Adv Exp Med Biol. 2003;527:47–54. doi: 10.1007/978-1-4615-0135-0_5. [DOI] [PubMed] [Google Scholar]
- 18.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 19.Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 20.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, Schwarcz R, Fallarino F, Puccetti P. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 22.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Fioretti MC, Puccetti P. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl Immunol. 2006;17:58–60. doi: 10.1016/j.trim.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 24.Park MJ, Min SY, Park KS, Cho YG, Cho ML, Jung YO, Park HS, Chang SH, Cho SG, Min JK, Park SH, Kim HY. Indoleamine 2,3-dioxygenase-expressing dendritic cells are involved in the generation of CD4+CD25+ regulatory T cells in Peyer’s patches in an orally tolerized, collagen-induced arthritis mouse model. Arthritis Res Ther. 2008;10:R11. doi: 10.1186/ar2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH, Mellor AL. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH, Sharma MD, Mellor AL. Ligation of B7–1/B7–2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 27.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 28.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 29.Schroecksnadel K, Kaser S, Ledochowski M, Neurauter G, Mur E, Herold M, Fuchs D. Increased degradation of tryptophan in blood of patients with rheumatoid arthritis. J Rheumatol. 2003;30:1935–1939. [PubMed] [Google Scholar]
- 30.Zhu L, Ji F, Wang Y, Zhang Y, Liu Q, Zhang JZ, Matsushima K, Cao Q, Zhang Y. Synovial autoreactive T cells in rheumatoid arthritis resist IDO-mediated inhibition. J Immunol. 2006;177:8226–8233. doi: 10.4049/jimmunol.177.11.8226. [DOI] [PubMed] [Google Scholar]
- 31.Kim SH, Evans CH, Kim S, Oligino T, Ghivizzani SC, Robbins PD. Gene therapy for established murine collagen-induced arthritis by local and systemic adenovirus-mediated delivery of interleukin-4. Arthritis Res. 2000;2:293–302. doi: 10.1186/ar104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Son YI, Egawa S, Tatsumi T, Redlinger RE, Jr, Kalinski P, Kanto T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–157. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Kim S, Oligino TJ, Robbins PD. Effective treatment of established mouse collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express FasL. Mol Ther. 2002;6:584–590. [PubMed] [Google Scholar]
- 34.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, Salles JP, Perret B, Bonnerot C, Record M. Mast cell-and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33:522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 36.Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, Gauldie J, Bramson J, Wan Y. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J Immunol. 2007;179:5024–5032. doi: 10.4049/jimmunol.179.8.5024. [DOI] [PubMed] [Google Scholar]
- 37.Bonham CA, Peng L, Liang X, Chen Z, Wang L, Ma L, Hackstein H, Robbins PD, Thomson AW, Fung JJ, Qian S, Lu L. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol. 2002;169:3382–3391. doi: 10.4049/jimmunol.169.6.3382. [DOI] [PubMed] [Google Scholar]
- 38.Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. 2005;105:1574–1581. doi: 10.1182/blood-2004-06-2089. [DOI] [PubMed] [Google Scholar]