Metformin and the Risk of Cancer: Time-related biases in observational studies (original) (raw)
Abstract
OBJECTIVE
Time-related biases in observational studies of drug effects have been described extensively in different therapeutic areas but less so in diabetes. Immortal time bias, time-window bias, and time-lag bias all tend to greatly exaggerate the benefits observed with a drug.
RESEARCH DESIGN AND METHODS
These time-related biases are described and shown to be prominent in observational studies that have associated metformin with impressive reductions in the incidence of and mortality from cancer. As a consequence, metformin received much attention as a potential anticancer agent; these observational studies sparked the conduction of randomized, controlled trials of metformin as cancer treatment. However, the spectacular effects reported in these studies are compatible with time-related biases.
RESULTS
We found that 13 observational studies suffered from immortal time bias; 9 studies had not considered time-window bias, whereas other studies did not consider inherent time-lagging issues when comparing the first-line treatment metformin with second- or third-line treatments. These studies, subject to time-related biases that are avoidable with proper study design and data analysis, led to illusory extraordinarily significant effects, with reductions in cancer risk with metformin ranging from 20 to 94%. Three studies that avoided these biases reported no effect of metformin use on cancer incidence.
CONCLUSIONS
Although observational studies are important to better understand the effects of drugs, their proper design and analysis is essential to avoid major time-related biases. With respect to metformin, the scientific evidence of its potential beneficial effects on cancer would need to be reassessed critically before embarking on further long and expensive trials.
Time-related biases are common in observational studies of drug effects and have been described extensively in several therapeutic areas. However, these biases have not been described in detail in the field of diabetes. Time-related biases include immortal time bias, a bias introduced with time-fixed cohort analyses that misclassify unexposed time as exposed; time-window bias, a bias introduced because of differential exposure opportunity time windows between subjects; and time-lag bias, a bias introduced by comparing treatments given at different stages of the disease (1,2). These biases are known to exaggerate downward the effect of a drug, thus making a drug seem to be protective when in fact it may have no effect. In this review, we show that several of the observational studies investigating the association between metformin and cancer incidence and mortality are affected by these time-related biases.
Metformin is a drug of choice for the management of type 2 diabetes mellitus (3,4). It reduces insulin resistance, improves glycemic control, and can be combined safely with other antidiabetic drugs (5). In 2005, an observational study using data from Tayside, Scotland, reported a significant 23% reduction in the incidence of any cancer with metformin use, thus putting forward the hypothesis that metformin could lower the risk of cancer onset in patients with diabetes (6). This study generated great interest in metformin as an agent in the prevention and treatment of cancer, and many preclinical studies showed that metformin can inhibit the growth of cancer cells in vitro and in vivo (7,8).
In parallel, a series of observational studies conducted in various databases generally reported similar beneficial results with metformin, thus “confirming” the findings of the 2005 study. Two meta-analyses including some of these observational studies reported a highly significant reduction in cancer incidence or mortality associated with metformin use (risk reductions ranged between 31 and 34%) (9,10). This convergence of evidence from multiple preclinical and epidemiological studies formed the impetus to recommend the conduct of randomized, controlled trials (RCTs) of metformin in the prevention and treatment of cancer (11–14). However, as discussed later, several of these observational studies had important time-related biases that likely exaggerated the potential antitumor effects of metformin.
STUDIES OF CANCER PREVENTION
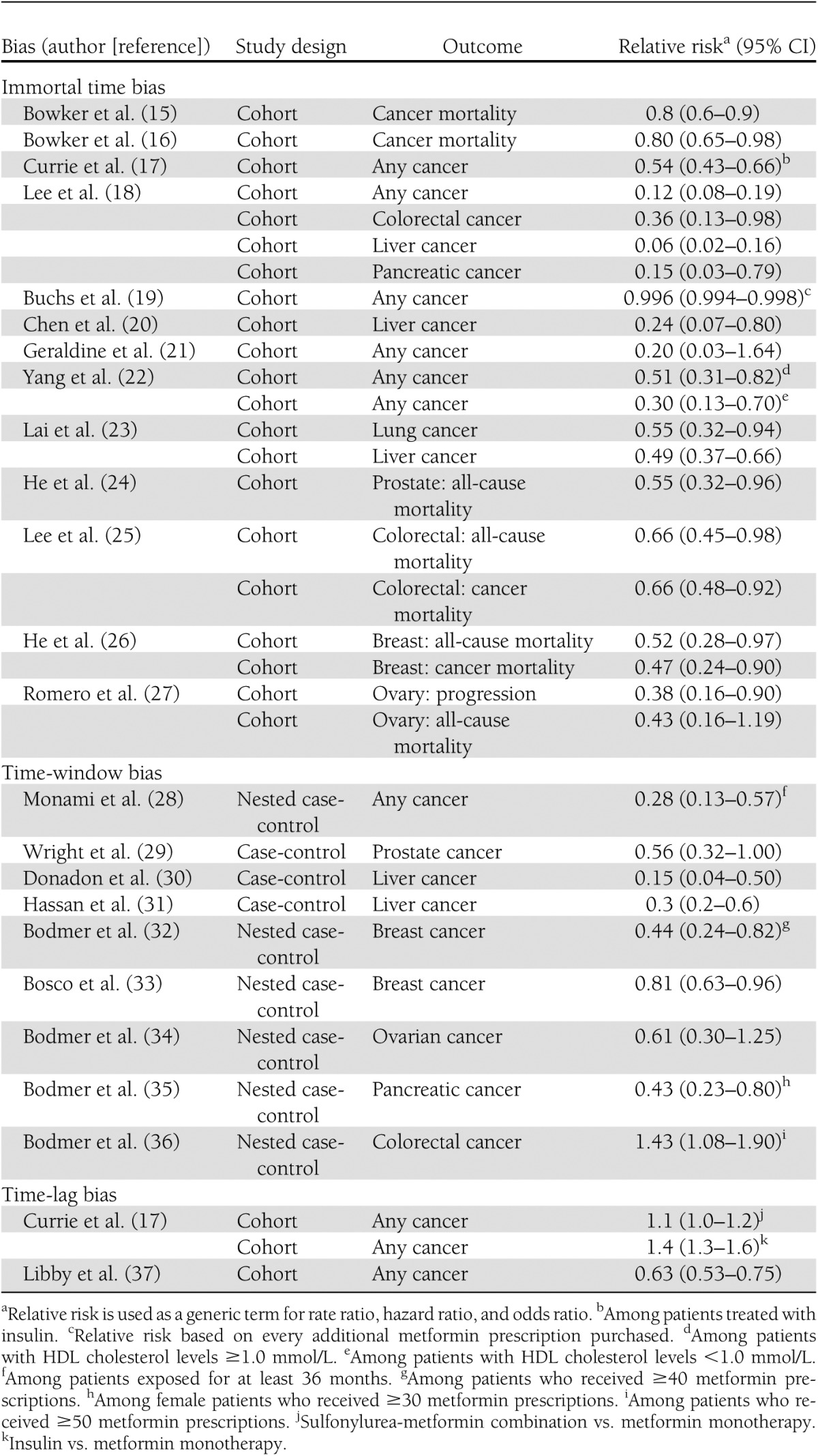
The majority of studies published to date have reported the role of metformin in preventing the occurrence of cancer in general and of some cancers in particular. To describe the different variations of this bias, we use only some of these studies as a model; the list of studies that incurred the same biases is provided in Table 1 (15–37).
Table 1.
Time-related biases in observational studies investigating the effects of metformin on cancer incidence and as a cancer treatment
Immortal time bias
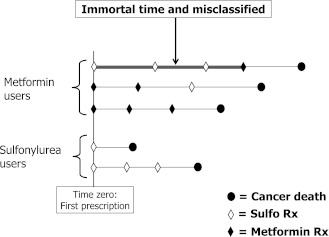
The first published epidemiological study to verify this hypothesis involved a cohort of patients of 10,309 new users of oral hypoglycemic agents (OHAs) identified from the Saskatchewan Health databases (15). Patients entered the cohort at the time of their first OHA prescription during 1991–1996. They were followed through 1999 for death due to cancer, which occurred in 407 patients. Exposure was classified as sulfonylureas only or metformin, with the latter including users of only metformin and those who also used sulfonylureas at some point in time during follow-up. A Cox proportional hazards model was used to estimate the hazard ratio (HR), adjusted for age, sex, insulin use, and a chronic disease score. The adjusted HR of cancer mortality for sulfonylurea versus metformin was 1.3 (95% CI 1.1–1.6), which, when reversed, gives the HR for metformin relative to sulfonylurea as 0.8 (0.6–0.9), suggesting a protective effect of metformin.
Immortal time bias is introduced in this study from its definition of exposure and related analysis. The 3,340 subjects who entered the cohort while using sulfonylureas and remained on these throughout the entire follow-up and the 1,229 who used only metformin from day 1 throughout follow-up pose no problem to the definition of exposure. The bias is introduced by classifying some of the remaining 5,740 who used sulfonylureas and metformin at various times during follow-up as “metformin users.” Although those whose cohort entry prescription was metformin are classified correctly in the metformin-exposed group, those whose cohort entry prescription was a sulfonylurea are not (Fig. 1). Indeed, the time between the sulfonylurea prescription at entry into the cohort and the first metformin prescription during follow-up is called “immortal” (thick gray line in Fig. 1) because the patient must be alive to have received their first metformin prescription (1). Moreover, this immortal person-time should be classified as sulfonylurea-exposed until the start of metformin, at which point the remaining person-time can be classified as metformin-exposed. In this study, the authors misclassified this immortal person-time as metformin-exposed, which will lead to immortal time bias.
Figure 1.
Illustration of immortal time bias using a description of patients exposed to metformin and sulfonylureas who died of cancer according to the definition used in the cohort study by Bowker et al. (15). The top patient initiated and continued treatment with a sulfonylurea and subsequently switched to or added metformin but is classified as a metformin user during the entire follow-up. The time between entry into the cohort and the first metformin prescription thus is immortal (thick line) because the subject must survive to receive this first metformin prescription and is misclassified as exposed to metformin when in fact it is exposed to sulfonylurea, leading to immortal time bias.
Because the data necessary to assess the magnitude of the bias are not provided in the article, for the sake of illustration we assume that the mean delay between cohort entry and the first metformin prescription was 1 year among the 5,740 combined users. This would result in 5,740 immortal person-years of sulfonylurea exposure misclassified as metformin exposure. The reported rates of cancer death for metformin (245/39,026 = 6.3 per 1,000 person-years) and for sulfonylureas (162/16,700 = 9.7 per 1,000 person-years), which were based on misclassified denominators, produced a crude rate ratio of 0.65. By properly reclassifying exposure (per our assumption), the rates would become 245/(39,026–5,740) = 7.4 per 1,000 person-years for metformin and 162/(16,700+5,740) = 7.2 per 1,000 person-years for sulfonylureas, resulting in a corrected crude rate ratio of 1.0. There are more sophisticated ways to estimate the rate ratio while properly accounting for such time-varying exposures, such as Cox proportional hazards models with time-dependent factors. An alternative approach simply is to use an intent-to-treat approach (as is done in RCTs) that classifies exposure according to the first OHA prescription type, irrespective of switching to or adding the other agent, with, of course, the limitations of interpretation inherent to this approach.
In a subsequent publication reanalyzing the same data, the authors recognized the presence of immortal time bias, claiming “we extended our analyses by classifying time-varying exposure of metformin, sulfonylureas and insulin therapy” (16). The reported an adjusted HR of 0.80 (95% CI 0.65–0.98; P = 0.03) for metformin use relative to sulfonylurea monotherapy and the corresponding rates of cancer mortality (6.3 vs. 9.7 per 1,000 person-years) remained identical to those reported previously, which were subject to immortal time bias. This perfect equality of rates is numerically impossible and thus casts doubt on whether the time-varying statistical model was applied correctly to metformin exposure to properly reclassify the misclassified exposure in this second publication.
Immortal time bias also was evident in a study using Taiwanese National Health Insurance data in 2000 (18). It included 12,005 patients with type 2 diabetes who received at least two prescriptions of metformin during 2000–2007; these patients were compared with 4,597 patients who did not use metformin but had other OHAs and 8,643 who did not use any OHA during this period. Cancer events occurring 1 year after entry into the cohort until the end of 2007 were identified. Using the Cox proportional hazards model to adjust for covariates, the use of metformin was associated with lower incidence of cancer with HRs of any cancer of 0.12 (95% CI 0.08–0.19), of colorectal cancer (0.36 [0.13–0.98]), liver cancer (0.06 [0.02–0.16]), and pancreatic cancer (0.15 [0.03–0.79]).
Immortal time bias is introduced in this study from the definition of metformin exposure. Indeed, the 12,005 subjects defined as exposed to metformin during 2000–2007 had to have at least two prescriptions for metformin during this period. The bias is introduced because the time between the first and second metformin prescriptions during follow-up is immortal, that is, the patient must be cancer-free to have received the second metformin prescription, which could have been dispensed much later. Moreover, this person-time should be classified as unexposed until the second metformin prescription, at which point the remaining person-time should be classified as metformin-exposed. Thus, the analysis misclassified all this person-time as metformin exposure, which led to immortal time bias. In contrast, the comparison group of patients who did not use any OHA did not have such a condition of a second prescription.
Another example of this bias is the study using the Hong Kong Diabetes Registry to form a cohort of 2,658 patients diagnosed with type 2 diabetes who did not use metformin when enrolled in the study between 1996 and 2005 or in the 2.5 years before enrollment, with follow-up until 2005 (22). During a median 5.5 years of follow-up, 129 patients developed cancer. A Cox proportional hazards model was used to estimate the HR of cancer, which was adjusted for several covariates. The use of metformin during follow-up was associated with significant reductions in cancer incidence, with HRs of 0.51 (95% CI 0.31–0.82) among patients with HDL cholesterol ≥1.0 mmol/L and 0.30 (0.13–0.70) among patients with HDL cholesterol <1.0 mmol/L.
Immortal time bias also is present in this study because none of the patients were using metformin at the time of entry into the cohort or during the 2.5 years before but were classified in the analysis as metformin users if they received a prescription for metformin during the follow-up. The bias then is introduced by classifying the time between entry into the cohort and the first metformin prescription as metformin-exposed. This person-time is immortal and should have been classified as unexposed until the start of metformin, at which point the remaining person-time should be classified as metformin-exposed. Misclassifying all this person-time as metformin-exposed leads to immortal time bias.
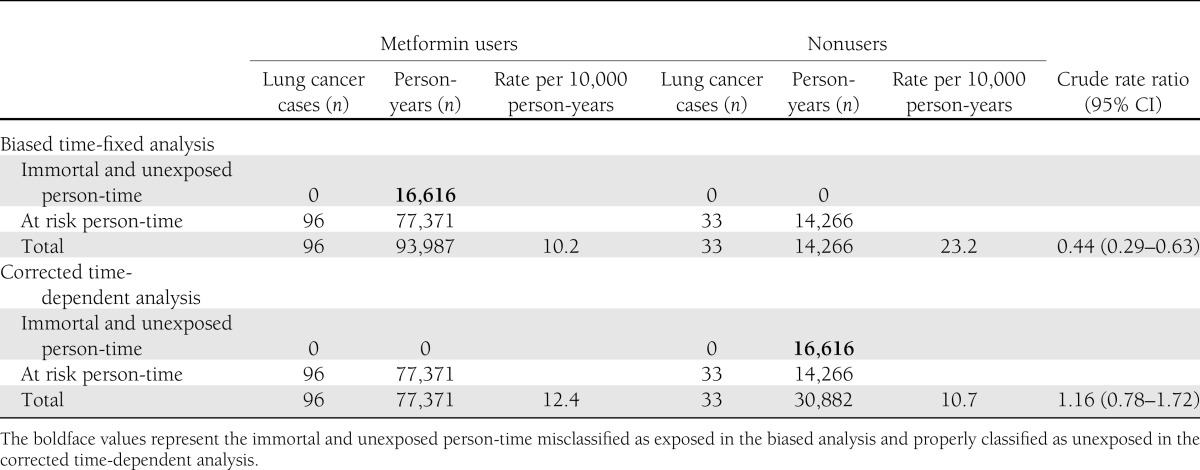
This bias also is present in a recent study investigating the association between metformin and the incidence of lung cancer (23). In that study, the authors used administrative databases from the Taiwanese National Health Insurance program to extract a cohort of 19,624 patients who were treated with antidiabetic agents between 2000 and 2005. In Cox proportional hazards models, the use of metformin was associated with a 45% risk reduction of lung cancer (HR 0.55 [95% CI 0.37–0.82]). Similar results were observed for thiazolidinediones and α-glucosidase inhibitors (0.55 [0.32–0.94] and 0.61 [0.38–0.98], respectively), whereas null results were observed for insulin and sulfonylureas (1.00 [0.68–1.45] and 1.27 [0.75–2.15], respectively). As with the studies described earlier, immortal time bias was introduced by misclassifying unexposed person-time as exposed person-time. Overall, the 19,624 patients generated 108,213 person-years of follow-up. Based on the data presented in the study, the 16,616 patients who ever used metformin generated 93,987 person-years of exposure, an average of 5.7 years per patient. However, the latter included immortal person-time (i.e., time between entry into the cohort and the first metformin prescription, during which no event could have occurred). Assuming an average 1-year delay between entry into the cohort and the first metformin prescription, a total of 16,616 immortal person-years would have been misclassified as exposed. As shown in Table 2, by correctly reclassifying this person-time, the rate of the unexposed group would become 33/(14,266 + 16,616) = 10.7 per 10,000 person-years instead of 23.2 per 10,000 person-years, whereas the rate in the metformin-exposed group would become 96/(93,987 – 16,616) = 12.4 per 10,000 person-years instead of 10.2 per 10,000 person-years, resulting in a corrected crude rate ratio of 1.16. Based on similar calculations, a delay of only 9.6 months between entry into the cohort and the first metformin prescription would have been necessary to bring the crude rate ratio to the null value. Thus, it is important to recognize that in the context of a highly prevalent exposure such as metformin, even small misclassifications of just a few months can result in greatly biased rate ratios.
Table 2.
Comparison between biased time-fixed and corrected time-dependent data analyses for the cohort study of metformin and lung cancer incidence (23)
As summarized in Table 1, immortal time bias affected nine observational studies evaluating the association between metformin and cancer incidence or mortality. Because of important misclassifications of exposure, the risk reductions observed in these studies were greatly exaggerated in favor of metformin. Furthermore, the variations in the reported risk reductions, ranging between 20 and 94% (Table 1), are a direct reflection of the different magnitude of misclassification of immortal time in the studies, leading to bias of different magnitudes.
Time-window bias
A study design that has been used extensively is the nested case-control analysis conducted, in this first study, within a cohort of 22,621 women newly treated with OHAs between 1994 and 2005 who were identified using the U.K. General Practice Research Database (32). During follow-up, 309 women developed breast cancer and were matched to 1,153 controls selected from the cohort on age, general practice, and index date. Overall, the use of metformin was not associated with a decreased risk of breast cancer (odds ratio [OR] 1.03 [95% CI 0.76–1.39]). However, for patients who were prescribed at least 40 prescriptions, metformin was associated with a significant risk reduction (0.44 [0.24–0.82]).
In this study, the authors used risk-set sampling, making metformin exposure time-dependent and thus avoiding immortal time bias. However, the cases and controls were not matched on duration of follow-up or, more specifically, on duration of exposure opportunity time, which can lead to time-window bias (2). Matching on duration of follow-up ensures the same opportunity time of being exposed for cases and matched controls. In this study, the null finding for overall use of metformin likely indicates that cases and matched controls had, on average, similar follow-up times. However, it is possible that in certain subgroups, such as in those who received at least 40 prescriptions, the follow-up may have been different between cases and controls. As such, it is possible that controls had a longer follow-up period than cases and hence a greater opportunity to receive additional prescriptions. The opposite could have occurred as well, and therefore the direction of this bias highly depends on the distribution of follow-up among the randomly selected controls. Indeed, in a subsequent study using similar methods to investigate the effects of metformin on the incidence of colorectal cancer, the same authors reported an increased risk among patients who received at least 50 prescriptions (OR 1.43 [95% CI 1.08–1.90]) (36). This time-window bias also likely was present in other studies by the same authors who used this same approach to investigate the effect of metformin use on the risk of ovarian and pancreatic cancers (34,35).
Another example of time-window bias is a study investigating the association between antidiabetic drugs and the risk of hepatocellular carcinoma (31). This was a single-center, hospital-based, case-control study from Texas, involving 420 cases of hepatocellular carcinoma and 1,104 hospital controls identified between 2000 and 2008. The use of metformin and thiazolidinediones was associated with a decreased risk of hepatocellular carcinoma (OR 0.3 [95% CI 0.2–0.6] and 0.3 [0.1–0.7], respectively). It is interesting that cases were more likely than controls to be controlled with diet (13.1 vs. 2.3%, respectively). A priori, diet-controlled diabetes would have been expected to be associated with the lowest risk of cancer because it is used early in the natural history of the disease primarily in patients with a new diagnosed of type 2 diabetes (based on the assumption that disease severity is associated with an increased risk of cancer). However, it is surprising that diet-controlled diabetes was associated strongly with an increased risk of hepatocellular carcinoma (7.8 [1.5–40.0]). Whereas duration of diabetes was generally similar between the cases and controls, this finding reveals that duration of treated diabetes was largely differential between cases and controls. Indeed, the much lower prevalence of diet-controlled diabetes among the controls suggests that these patients had a longer treatment history than the cases. As such, on the basis of their longer treatment history, controls had a greater opportunity to use metformin and other antidiabetic drugs. This differential treatment time window between cases and controls likely led to the apparent increased risk of hepatocellular carcinoma with diet-controlled diabetes and consequently to an apparent decreased risk with metformin and thiazolidinediones.
As discussed earlier and summarized in Table 1, time-window bias can lead to spurious associations even when proper time-dependent analyses are performed. In some studies, there was an attempt to either adjust or match on duration of diabetes (28,31). Although controlling for this important variable certainly minimizes confounding by disease severity, it does not correct for the differential lengths of the treatment time windows in the cases and controls. Furthermore, it is difficult to predict the direction of the time-window bias because it depends on the distribution of treatment duration in the randomly selected controls compared with the cases. It is fortunate that this bias can easily be avoided at the design stage by properly accounting for duration of treatment in the cases and controls (2).
Bias from time lag and latency
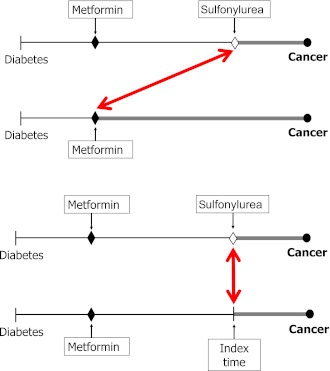
Some studies that have used cohorts of patients treated with antidiabetic drugs have compared the exposure time of metformin use with exposure time of other antidiabetic treatments, including sulfonylureas and insulin. Several methodological aspects have to be considered when a first-line therapy such as metformin is compared with second- or third-line therapies (Fig. 2). First, patients are unlikely to be at the same stage of diabetes, which can induce confounding by disease duration: longer duration of diabetes may be associated with higher cancer incidence, independent of age. In such a case, the proper comparison would require matching on disease duration so that the cohort would be formed as in Fig. 2 (bottom) rather than the time-lagged comparison suggested by Fig. 2 (top). Second, if the first treatment used is associated with an increased incidence of cancer, albeit after a long period of exposure, it is likely that the cancer will occur during exposure to the second-line agent, in which case attribution of the cancer to either treatment is challenging—thus the importance of considering latency time windows.
Figure 2.
Depiction of a time-lagging bias when comparing a first-line diabetic drug (metformin) used at an earlier stage of diabetes with second- or third-line drugs used or added at a later stage (sulfonylurea) (top) and a cohort design that controls for time-lagging bias by comparing two patients at the same stage of diabetes (bottom). The arrows represent the time point where cohort follow-up starts in conducting comparisons: top represents a time-lagged comparison, whereas bottom arrow represents a comparison that accounts for stage of diabetes. (A high-quality color representation of this figure is available in the online issue.)
A study that was affected by confounding by disease stage is one that used The Health Information Network, a U.K. general practice database (17). In this study, the authors identified 62,809 users of OHAs or insulin between 2000 and 2007. The study population was then subdivided into four cohorts: cohort 1 included new users of metformin monotherapy; cohort 2 comprised new users of sulfonylurea monotherapy; cohort 3 included those who switched from monotherapy with metformin or sulfonylurea to a combination of both drugs; and cohort 4 included those who switched from OHAs to insulin. Incidence rates of cancer (specifically breast, pancreas, colorectal, and prostate cancers) were calculated within each of the aforementioned cohorts, and Cox proportional hazards models were used to compare cohorts 2, 3, and 4 with cohort 1 (metformin monotherapy). After a mean follow-up of 2 years, the HRs (95% CIs) were 1.4 (1.2–1.5) for sulfonylurea monotherapy, 1.1 (1.0–1.2) for the metformin-sulfonylurea combination, and 1.4 (1.3–1.6) for insulin. Among patients treated with insulin, the concomitant use of metformin was associated with a statistically significant risk reduction (0.54 [0.43–0.66]). However, this strong inverse association was likely due to immortal time bias because exposure to metformin was assessed at any time during follow-up and thus included person-time during which patients were not yet exposed to this therapy (Table 1). Furthermore, as noted by others, the survival curves of the different cohorts presented by the authors diverged as early as during the first year of treatment, thus arguing against a causal effect, which is biologically inconsistent with the long latencies of cancers (38). Aside from reverse causality and confounding, which are certainly important considerations, such early divergence in the survival curves can also be explained by two other factors. First, comparing the different cohorts, which consisted of second- and third-line treatments, with metformin monotherapy, a first-line treatment, inherently introduces biases related to disease duration and progression that may not be dealt with adequately during analysis. Second, the consideration of cancers diagnosed early after entry into the cohort is problematic, especially for patients with a history of OHA use (i.e., cohorts 3 and 4), because it ignores the fact that previous exposure might have affected future cancer risk. Indeed, patients may remain at risk for an extended period of time long after discontinuation of treatment. For example, former smokers with a history of smoking one pack per day for a period of 1 year have a nearly threefold increased risk of lung cancer after 30 years of cessation (39). One approach to minimize this bias is to apply time-lag periods to exclude cancers diagnosed within a specified time period after entry into the cohort. This method works reasonably well, especially if there is clustering of cancers early during the treatment, as was observed in this study (17).
A study that introduced a variation of bias due to time-lagging of comparison cohorts was that based on the Diabetes Audit and Research in Tayside Study cohort of patients diagnosed with type 2 diabetes in Tayside, Scotland (37). All 4,085 eligible new users of metformin between 1994 and 2003 were matched individually with 4,085 nonusers on year of diabetes diagnosis; each pair was assigned the date of entry into the cohort as the date of the first metformin prescription of the user. Of the subjects who were cancer free at cohort entry, 771 developed cancer during follow-up. Cancer was diagnosed in 7.3% of the metformin users compared with 11.6% of nonusers. A Cox proportional hazards model was used to estimate the HR of cancer, adjusted for several covariates. The adjusted HR of cancer associated with metformin use was 0.63 (95% CI 0.53–0.75), suggesting a strong protective effect of metformin.
Although this study avoided biases associated with prevalent exposures by using a new-user cohort of patients treated with metformin, this approach was not used for the comparison group, which was not based on a specific comparison drug. Because the comparison metformin nonuser does not have a prescription date to define time zero, it was assigned the date of the first metformin prescription of its matched user, a date called the index date. In choosing among potential nonusers for a given user, a variation of bias from time lags was introduced. Indeed, if the potential matched nonuser had a diagnosis of cancer or had died prior to the index date, it was discarded. However, it “was potentially available for a different metformin user…this process was repeated until suitable comparators were identified.”
Nonusers should be alive and cancer free at entry into the cohort just like the matched users, but the net effect of selecting the nonusers with replacement is twofold. First, the final nonuser group had zero subjects excluded because of cancer before the index date, in contrast with the 239 excluded from the metformin group. Second, the nonusers who should have been excluded (and not reused) because of a previous cancer were included as nonusers matched to other users. As a result, cancers that should have been an exclusion criterion were transferred to the follow-up period after the index date and counted as outcome events, thus artificially increasing the rate of cancer in the nonuser group. This phenomenon is noticeable in the Kaplan-Meier curves that suggest a clustering of cancers in the first 2 years of follow-up of the nonuser group. Thus, by forming the nonuser group, the authors artificially induced a cohort that was immortal and cancer free before the index date but had more deaths and cancers after entry into the cohort. As a result of this transfer, the nonuser group had 474 cancers diagnosed during follow-up compared with 297 in the metformin group. If the exclusion of 239 cancers before the first metformin prescription is applied to the nonuser group, the 474 would be reduced to 177.
STUDIES OF CANCER TREATMENT
A number of studies investigated the effects of metformin among patients with cancer. Several of these studies reported significant risk reductions for all-cause mortality, cancer-specific mortality, and cancer progression, ranging between 34 and 62% (24–27). All of the studies reporting these important risk reductions suffered from immortal time bias (summarized in Table 1). We describe below selected studies to illustrate this bias in the setting of cancer treatment.
In the first study, the authors reviewed the records of 233 patients diagnosed with prostate cancer between 1999 and 2008 at a medical institution in Texas (24). They evaluated whether antidiabetic treatments were associated with all-cause mortality in this patient population. Cox proportional hazards models were constructed, with ever-use of insulins and OHAs entered as independent variables, and adjusted for potential confounders. Their results indicate that metformin (HR 0.55 [95% CI 0.32–0.96]) and thiazolidinediones (0.45 [0.21–0.97]) were associated with a significant decrease in all-cause mortality. No statistically significant associations were observed with the other antidiabetic agents, although few patients were exposed in this small dataset. Misclassifying as exposed the time between cohort entry and first prescription of metformin or a thiazolidinedione introduced immortal time bias, thus greatly exaggerating the benefits of these drugs on all-cause mortality. That exposure was defined as any time during the 10-year follow-up period contributed to compound the effect of this misclassification. As explained earlier, the appropriate analysis would have treated exposures to the different antidiabetic agents as time-dependent variables.
The authors of another study identified 595 patients who had been diagnosed with both colorectal cancer and diabetes mellitus and compared the 258 taking metformin any time during follow-up with the 337 nonusers (25). After a median follow-up of 41 months, the adjusted HR of all-cause mortality with metformin use was 0.66 (95% CI 0.45–0.98), whereas for colorectal cancer, mortality was also 0.66 (0.48–0.92). Here again, these mortality reductions are the result of immortal time bias caused by misclassifying as exposed to metformin the time between cohort entry and first prescription, when in fact it is unexposed.
Immortal time bias also was present in a cohort study of 1,983 consecutive patients with HER2+ breast cancer treated between 1 January 1998 and 30 September 2010 (26). All-cause and cancer-specific deaths were identified. Among the patients with diabetes, the HR of all-cause death associated with metformin was 0.52 (95% CI 0.28–0.97), whereas for breast cancer–specific mortality it was 0.47 (0.24–0.90). These mortality reductions again are the result of immortal time bias caused by misclassifying as exposed the time between cancer diagnosis and first prescription of metformin given during follow-up, when in fact it is unexposed to metformin.
CONCLUSIONS
We have shown that a large number of observational studies reporting significant reductions in the incidence of or mortality from cancer associated with metformin use are afflicted with time-related biases such as immortal time bias, time-window bias, and time-lag bias. By not classifying metformin exposure during follow-up or measuring metformin exposure over different time intervals properly, the resulting analyses produced apparent reductions in risk that were created artificially by the misclassification of metformin exposure. These biases, regrettably common in pharmacoepidemiology, have been described extensively in different therapeutic areas (1,2,40–42) but do not seem to have sufficiently penetrated the fields of diabetes and cancer. Although two of the studies recognized the possibility of immortal time bias, the technique of data analysis required to address it was clearly not appropriate (16,18).
Other studies have been conducted with varying results; some have found no association between metformin use and cancer incidence or prognosis (43–48), others have found lower rates of cancer with metformin (49–57), and one study found an increased risk (58). Although these studies were designed and analyzed with no obvious time-related biases, it is of course possible that other epidemiological biases such as selection, information, or confounding could have affected them. This article focused specifically on time-related biases, where metformin was associated with strong risk reductions, ranging from relative risks of 0.80 (20% risk reduction) to 0.06 (94% risk reduction), likely due to flaws in their designs and methods of analysis.
Three recent studies that specifically used the time-dependent techniques needed to avoid both immortal time and time-window biases found no association between metformin use and cancer incidence (59–61). The first study used the U.K. General Practice Research Database and found a rate ratio of prostate cancer incidence of 1.23 (95% CI 0.99–1.52) with metformin use. With more than 36 prescriptions the rate ratio was significantly elevated at 1.40 (1.03–1.89). The second study used the Kaiser Permanente database and found no effect of metformin on the incidence of the 10 different cancers studied, with HRs ranging between 0.8 (0.6–1.1) for melanoma to 1.3 (1.0–1.6) for kidney/renal and pelvic cancers. Finally, no effect of metformin was found on lung cancer incidence (61).
It is also important to recognize that most observational studies focused on the incidence of cancer as it relates to the effects of metformin on carcinogenesis, which is distinct from its effects on proliferation of established tumors. Nevertheless, large RCTs using patients with established tumors currently are being planned or already are in progress. One such study is the MA.32 being conducted by the National Cancer Institute of Canada, a multicenter phase III RCT that includes almost 3,600 women with early breast cancer and is comparing metformin to placebo as adjuvant therapy on the primary end point of invasive, disease-free survival over a 5-year follow-up (12,13). The authors claim that “we believe the science underlying such a trial is strong, the novelty of the intervention is high, and the potential for benefit is large” (12). Although it is possible that the in vitro science is strong and metformin may lead to positive results, we have shown that the pharmacoepidemiological evidence from observational studies is weak at best.
In conclusion, time-related biases are common in the field of diabetes and can be avoided using appropriate study designs and methods of analysis. In the case of the association between metformin and cancer, we believe that methodologically inaccurate studies, even if their results are replicated in different settings, should not be the driving force behind long and expensive trials. At this time, it may be important to question and reassess the scientific evidence of the potential beneficial effects of metformin on cancer. Although the in vitro science seems to be strong, certainly all observational studies identified as potentially biased would need to be redone properly to correct for the bias. Moreover, new observational studies using more rigorous designs and analyses that avoid these biases should urgently be undertaken before more randomized trials are initiated in vain.
Acknowledgments
S.S. is the recipient of the James McGill Chair, and L.A. is the recipient of a Chercheur-Boursier Award from the Fonds de la recherche en santé du Québec.
No potential conflicts of interest relevant to this article were reported.
References
- 1.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008;167:492–499 [DOI] [PubMed] [Google Scholar]
- 2.Suissa S, Dell’aniello S, Vahey S, Renoux C. Time-window bias in case-control studies: statins and lung cancer. Epidemiology 2011;22:228–231 [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusi K, DeFronzo RA. Metformin: a review of its metabolic effects. Diabetes Rev 1998;6:89–131 [Google Scholar]
- 6.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008;27:3576–3586 [DOI] [PubMed] [Google Scholar]
- 8.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007;67:6745–6752 [DOI] [PubMed] [Google Scholar]
- 9.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461 [DOI] [PubMed] [Google Scholar]
- 10.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 2012;7:e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, Decensi A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol Biomarkers Prev 2009;18:701–705 [DOI] [PubMed] [Google Scholar]
- 12.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol 2009;27:3271–3273 [DOI] [PubMed] [Google Scholar]
- 13.Goodwin PJ, Stambolic V, Lemieux J, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat 2011;126:215–220 [DOI] [PubMed] [Google Scholar]
- 14.Higurashi T, Takahashi H, Endo H, et al. Metformin efficacy and safety for colorectal polyps: a double-blind randomized controlled trial. BMC Cancer 2012;12:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29:254–258 [DOI] [PubMed] [Google Scholar]
- 16.Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia 2010;53:1631–1637 [DOI] [PubMed] [Google Scholar]
- 17.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 18.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchs AE, Silverman BG. Incidence of malignancies in patients with diabetes mellitus and correlation with treatment modalities in a large Israeli health maintenance organization: a historical cohort study. Metabolism 2011;60:1379–1385 [DOI] [PubMed] [Google Scholar]
- 20.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol 2011;26:858–865 [DOI] [PubMed] [Google Scholar]
- 21.Geraldine N, Marc A, Carla T, et al. Relation between diabetes, metformin treatment and the occurrence of malignancies in a Belgian primary care setting. Diabetes Res Clin Pract 2012;97:331–336 [DOI] [PubMed] [Google Scholar]
- 22.Yang X, So WY, Ma RC, et al. Low HDL cholesterol, metformin use, and cancer risk in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care 2011;34:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, Chen CC. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer 2012;13:143–148 [DOI] [PubMed] [Google Scholar]
- 24.He XX, Tu SM, Lee MH, Yeung SC. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol 2011;22:2640–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer 2012;131:752–759 [DOI] [PubMed] [Google Scholar]
- 26.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol 2012;23:1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero IL, McCormick A, McEwen KA, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol 2012;119:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monami M, Colombi C, Balzi D, et al. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 2011;34:129–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control 2009;20:1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int 2010;30:750–758 [DOI] [PubMed] [Google Scholar]
- 31.Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer 2010;116:1938–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 2010;33:1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosco JL, Antonsen S, Sørensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev 2011;20:101–111 [DOI] [PubMed] [Google Scholar]
- 34.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: a case-control analysis. Gynecol Oncol 2011;123:200–204 [DOI] [PubMed] [Google Scholar]
- 35.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012;107:620–626 [DOI] [PubMed] [Google Scholar]
- 36.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin is not associated with a decreased risk of colorectal cancer: a case-control analysis. Cancer Epidemiol Biomarkers Prev 2012;21:280–286 [DOI] [PubMed] [Google Scholar]
- 37.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernández-Díaz S, Adami HO. Diabetes therapy and cancer risk: causal effects and other plausible explanations. Diabetologia 2010;53:802–808 [DOI] [PubMed] [Google Scholar]
- 39.Ebbert JO, Yang P, Vachon CM, et al. Lung cancer risk reduction after smoking cessation: observations from a prospective cohort of women. J Clin Oncol 2003;21:921–926 [DOI] [PubMed] [Google Scholar]
- 40.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf 2007;16:241–249 [DOI] [PubMed] [Google Scholar]
- 41.Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. Am J Respir Crit Care Med 2003;168:49–53 [DOI] [PubMed] [Google Scholar]
- 42.Suissa S, Ernst P. Bias in observational study of the effectiveness of nasal corticosteroids in asthma. J Allergy Clin Immunol 2005;115:714–719 [DOI] [PubMed] [Google Scholar]
- 43.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology 2004;127:1044–1050 [DOI] [PubMed] [Google Scholar]
- 44.Chung YW, Han DS, Park KH, Eun CS, Yoo KS, Park CK. Insulin therapy and colorectal adenoma risk among patients with Type 2 diabetes mellitus: a case-control study in Korea. Dis Colon Rectum 2008;51:593–597 [DOI] [PubMed] [Google Scholar]
- 45.Patel T, Hruby G, Badani K, Abate-Shen C, McKiernan JM. Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology 2010;76:1240–1244 [DOI] [PubMed] [Google Scholar]
- 46.Niraula S, Pond G, De WR, Eisenberger M, Tannock IF, Joshua AM. Influence of concurrent medications on outcomes of men with prostate cancer included in the TAX 327 study. Can Urol Assoc J 2011 Nov 2:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh S, Kang M, Kim MY, et al. Korean type 2 diabetes patients have multiple adenomatous polyps compared to non-diabetic controls. J Korean Med Sci 2011;26:1196–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bayraktar S, Hernadez-Aya LF, Lei X, et al. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer 2012;118:1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol 2008;168:925–931 [DOI] [PubMed] [Google Scholar]
- 50.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009;137:482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010;33:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donadon V, Balbi M, Ghersetti M, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol 2009;15:2506–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol 2009;46:279–284 [DOI] [PubMed] [Google Scholar]
- 54.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012;35:299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012;35:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res 2012;18:2905–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garrett CR, Hassabo HM, Bhadkamkar NA, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer 2012;106:1374–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehman DM, Lorenzo C, Hernandez J, Wang CP. Statin use as a moderator of metformin effect on risk for prostate cancer among type 2 diabetic patients. Diabetes Care 2012;35:1002–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011;20:337–344 [DOI] [PubMed] [Google Scholar]
- 60.Ferrara A, Lewis JD, Quesenberry CP, Jr, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care 2011;34:923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smiechowski BB, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and the incidence of lung cancer in patients with type 2 diabetes. Diabetes Care. 24 August 2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]