Adiponectin Trajectories Before Type 2 Diabetes Diagnosis: Whitehall II study (original) (raw)
Abstract
OBJECTIVE
The role of adiponectin in the natural history of diabetes is not well characterized. We set out to characterize prediagnosis trajectories of adiponectin in individuals who develop type 2 diabetes.
RESEARCH DESIGN AND METHODS
In a case-cohort study (335 incident diabetes case and 2,474 noncase subjects) nested in the Whitehall II study, serum adiponectin was measured up to three times per participant (1991–1993, 1997–1999, and 2003–2004). Multilevel models adjusted for age and ethnicity were fitted to assess 13-year trajectories of log-transformed adiponectin preceding diabetes diagnosis or a randomly selected time point during follow-up (year0) based on 755/5,095 (case/noncase) person-examinations.
RESULTS
Adiponectin levels were lower in diabetes case than in noncase subjects (median 7,141 [interquartile range 5,187–10,304] vs. 8,818 [6,535–12,369] ng/mL at baseline, P < 0.0001). Control subjects showed a modest decline in adiponectin throughout follow-up (0.3% per year, P < 0.0001) at higher levels in women than in men (difference at year0: 5,358 ng/mL, P < 0.0001). Female case and early-onset case (age at diagnosis <52 years) subjects had a steeper decline than control subjects (slope difference −1.1% per year, P = 0.001 in females, −1.6% per year in early-onset case subjects, P = 0.034). In men, adiponectin slopes for case and noncase subjects were parallel. The slope differences by diabetes onset were largely attenuated after adjustment for changes in obesity, whereas the sex-specific slope differences were independent of obesity.
CONCLUSIONS
Lower adiponectin levels were observed already a decade before the diagnosis of diabetes. The marked sex difference in trajectories suggests that sex-specific mechanisms affect the association between adiponectin levels and diabetes development.
Adiponectin is an adipose tissue–derived insulin sensitizer. Adiponectin modifies glucose homeostasis and exhibits anti-inflammatory and antiatherogenic effects (1,2). Epidemiological data link lower adiponectin levels to disease states including type 2 diabetes, metabolic syndrome, hypertension, cardiovascular disease, and cancer (3).
Insulin resistance is one of the major pathophysiological factors of diabetes, and adiponectin, given its strong association with insulin sensitivity, may be centrally involved in the events leading to diabetes (3–5). This is supported by the fact that adiponectin has independently predicted diabetes in longitudinal studies (6–15).
Time-to-event analysis based on single biomarker measurements is essential for individual risk prediction and public health planning but gives limited information on the natural history of a given disease. To provide new insights into the series of events leading to diabetes onset, we used repeated measures of diabetes-related variables and described trajectories of glycemia and interleukin-1 receptor antagonist before diabetes diagnosis (5,16). However, studies with repeat data on adiponectin in relation to diabetes development are scarce (10,17–22). In spontaneously diabetic Rhesus monkeys, adiponectin trajectories until diabetes manifestation were declining (21). Human studies (based on two measurement points per individual) have suggested decreasing adiponectin to be associated with an increase in insulin resistance or obesity (20,22). Diabetes prevention trials reported increasing adiponectin levels in intervention groups with parallel weight loss (10,17).
To overcome the limitations of the previous studies (i.e., lack of well-defined incident diabetic and control groups and insufficient number of repeat measures), we conducted up to three clinical examinations per individual to investigate adiponectin trajectories in a middle-aged British population separately among persons who developed incident diabetes and those who remained normoglycemic during follow-up. In addition to adjustments for age and ethnicity, we took into account factors related to insulin resistance, such as sex, age at onset of diabetes, and obesity.
RESEARCH DESIGN AND METHODS
We present results from a nested case-cohort study within the Whitehall II prospective cohort. The cohort was established between 1985 and 1988 (phase 1) and included 10,308 (6,895 men) nonindustrial British civil servants aged 35–55 years working in London offices of 20 departments (23). Study phase 3 (1991–1993) when glucose tolerance was first assessed by a 75-g oral glucose tolerance test (OGTT) serves as the baseline for the current analysis (men/women: n = 6,058/2,758). Participants were followed through postal questionnaires at approximately 2.5-year intervals (phases 4–8), and further clinical examinations (including an OGTT) were performed in 1997–1999 (phase 5: n = 5,444/2,358) and 2003–2004 (phase 7: n = 4,894/2,074) (23). The study was approved by the University College London Medical School Committee on the Ethics of Human Research. Informed consent was obtained at baseline and renewed at each contact.
The present case-cohort study is based on a random sample from the source population who attended the phase 3 examination and were followed up to phase 7 (n = 8,816) (16). We excluded participants with prevalent diabetes at baseline (n = 42), missing follow-up data on diabetes (n = 552), and missing data for key variables (weight, waist circumference, cholesterol, triglycerides, fasting glucose, fasting insulin, and C-reactive protein [additionally limited to subjects with C-reactive protein <10 mg/L]) at baseline (n = 2,018) or during follow-up (phases 5 and 7; n = 3,049), leading to a case-cohort population of 2,810 subjects (335 with incident type 2 diabetes and 2,475 without diabetes).
Measurements
Adiponectin.
Adiponectin serum concentrations were measured with the Quantikine ELISA kit (R&D Systems, Wiesbaden, Germany). Blood collection, processing, and storage followed the same standard operating procedures during all study phases. Venous samples were taken into native tubes in the fasting state (≥5 h of fasting) before a standard 2-h OGTT. Samples were centrifuged on-site within an hour. Serum was immediately removed from the monovette tubes into microtubes and stored at −80°C. All assays were performed consecutively in the same laboratory (German Diabetes Center), and samples from different study phases of the same participant were measured using the same ELISA plate in order to minimize assay imprecision. Mean intra- and interassay CVs were 3.3–5.1 and 12.8–13.8%, respectively. The limit of detection was 3.9 ng/mL. All samples gave values above the limit of detection.
Blood glucose and diabetes.
Venous samples for glucose determination were taken into fluoride monovette tubes. Blood glucose was measured using glucose oxidase method (5). Diabetes was defined by a fasting glucose ≥7.0 mmol/L or a 2-h postload glucose ≥11.1 mmol/L using a 75-g OGTT (4). Participants reporting doctor-diagnosed diabetes (13.1% of incident case subjects) or use of glucose-lowering medication (30.4%) were classified as having diabetes regardless of OGTT results. The date of diagnosis was assigned according to the interval method as the midpoint between the first visit with a diabetes diagnosis and the last visit without diabetes.
Other covariates.
The following variables were included as time-invariant covariates: sex, ethnicity (white vs. nonwhite), early-onset diabetes (<52 years of age at the time of diagnosis: yes/no), and age at the end of follow-up. BMI, waist circumference (both assessed at each clinical examination contemporaneously with blood draws), and fasting serum insulin (measured by human insulin radioimmunoassay at phase 3 and by insulin ELISA [Dako] at later phases) (5) were included as time-varying covariates.
Statistical analysis
Statistical analyses were undertaken using SPSS 14.0 statistical software (SPSS, Chicago, IL), and statistical significance was inferred at a two-tailed P < 0.05. Owing to the skewed distribution of adiponectin values, all analyses use log2-transformed adiponectin. We compared the characteristics of case subjects (those who developed type 2 diabetes) and noncase subjects (those who did not develop diabetes) using t tests and χ2 tests as appropriate.
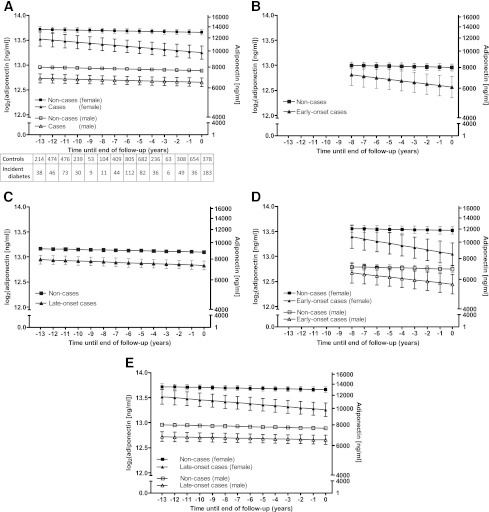
For the subsequent longitudinal analysis, we centered time around the date of diabetes diagnosis for case subjects and at a randomly selected time point for noncase subjects to approximate the follow-up time distribution of case subjects (i.e., year0) (16). Participants were then tracked backward (retrospectively) to the first clinical screening when adiponectin measurement was obtained (phase 3 [the baseline]). For example, a participant who reported diagnosed diabetes at phase 6 has his time 0 at the midpoint of phases 5 and 6 (estimated time of diagnosis) and has two adiponectin measurements: one at phase 5, ~1 year prior to the diagnosis, and another at phase 3, ~6 years prior to the diagnosis. Of a total of 8,233 measurements (964 in case and 7,269 in control subjects), 2,383 measurements were taken after year0 and were excluded from further analysis. The analysis was based on 755 measurements in 335 case subjects (136 with 3, 148 with 2, and 51 with 1 measurement point) and 5,095 measurements in 2,474 control subjects (743 with 3, 1,135 with 2, and 596 with 1 measurement point). As indicated in the table associated in Fig. 1, adiponectin measurements were well distributed throughout the 13-year time window of the study.
Figure 1.
Model-predicted log-transformed adiponectin trajectories before the diagnosis of diabetes or end of follow-up in 335 incident diabetes case and 2,475 control subjects. A: Adiponectin trajectories by sex and incident diabetes status. B: Adiponectin trajectories by incident diabetes status in case subjects with early-onset diabetes. C: Adiponectin trajectories by incident diabetes status in case subjects with late-onset diabetes. D: Adiponectin trajectories by sex and incident diabetes status in case subjects with early-onset diabetes. E: Adiponectin trajectories by sex and incident diabetes status in case subjects with late-onset diabetes. Multilevel longitudinal modeling using linear growth models. All models are adjusted for age at the end of follow-up and for ethnicity (white/nonwhite). A shows a model additionally adjusted for sex, B and C are additionally adjusted for age at onset (early onset: <52 years of age at diagnosis), and D and E are adjusted for both sex and age at onset. Estimated for a hypothetical subject population 72% male, 92% white, and aged 63 years (A, C, and E) or 50 years (B and D) at year0. Error bars show 95% CIs for the fixed effects. The tables show the number of measurements for each year at and before diabetes diagnosis and end of follow-up.
We used multilevel longitudinal modeling to estimate 13-year adiponectin trajectories before diabetes onset or until year0 (5). Data were structured so that the repeated measurements (person-observations) of adiponectin were nested within subjects and the nonindependence of the person-observations (the same individuals contributing to more than one observation in the dataset) was taken into account in estimating SEs. Differences in adiponectin trajectories between case and noncase subjects were modeled using a popular form of multilevel model, i.e., linear growth curves adjusted for age at year0 and ethnicity (both time-invarying covariates). Estimated marginal means predicted by these linear growth models were used for graphical representation of adiponectin trajectories.
Nonlinearity in adiponectin trajectories was checked by adding quadratic and cubic terms of time-by-caseness interaction to the models; they were all nonsignificant (P > 0.1); thus, we describe trajectories only with linear time terms. This is also in agreement with locally weighted scatterplot smoothers displaying unadjusted associations without making assumptions as to the functional form of the association (data not shown).
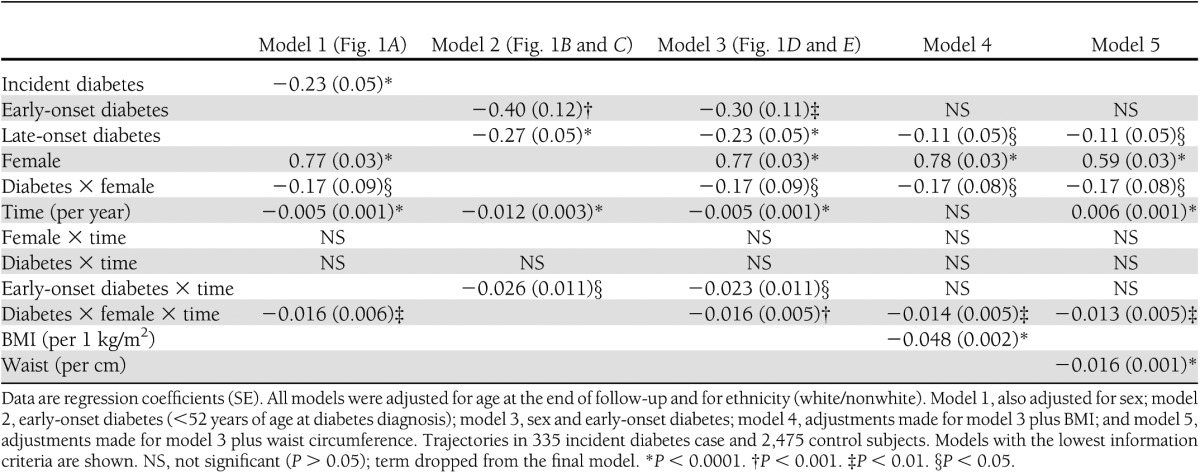
We investigated the effects of sex and onset of diabetes (early versus late), first separately and then together, on the trajectories by adding their main effects and time interactions to the model. The terms in the upper part of Table 3 (incident diabetes, early-onset diabetes, late-onset diabetes, female, and diabetes × female) refer to the intercept differences between the groups described by the term and their respective control subjects at year0. The terms that include time (or × time) refer to slopes (and slope differences between the above-described groups). The terms for BMI and waist represent the cross-sectional associations between adiponectin and the given obesity measure. Finally, we further added the main effect of BMI (and in a separate model, waist circumference) to the model as time-varying covariate.
Table 3.
Fixed effects for multilevel models of change over time of log2(adiponectin) concentrations before diabetes diagnosis or end of follow-up
Since large ethnic differences were previously described in adiponectin levels (10) and ethnicity may bias our results, we ran a sensitivity analysis restricted to white participants (n = 270 incident case and n = 2,299 control subjects). A further sensitivity analysis was done investigating whether the adjustment for fasting insulin levels (as a time-varying covariate) would abolish the effect of sex or onset of diabetes on adiponectin trajectories.
RESULTS
Participants excluded from analysis (n = 6,006) were generally younger; had a smaller waist circumference; had higher systolic blood pressure and fasting and postload blood glucose; and were more likely to be female, of lower socioeconomic position, and current smokers at baseline (Table 1).
Table 1.
Characteristics of participants excluded and included in the current analysis at study baseline
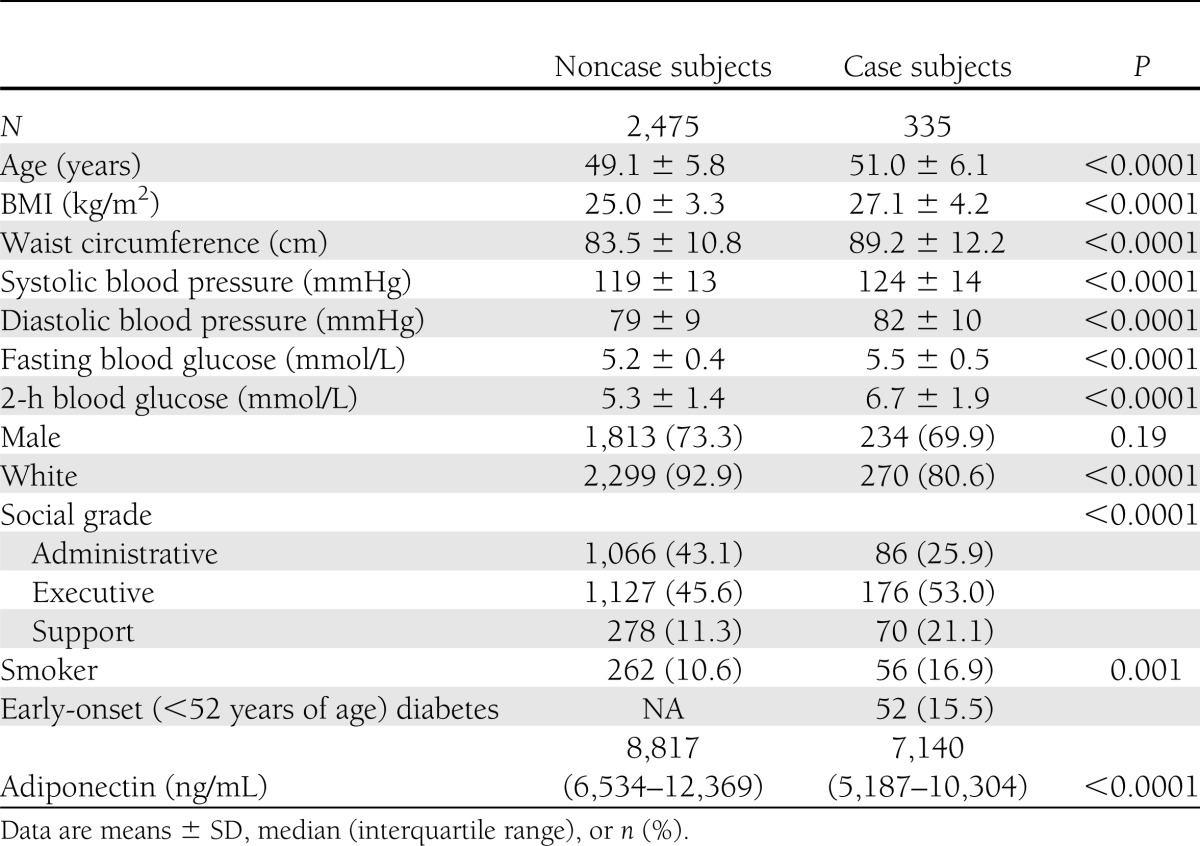
Incident case subjects (n = 335) were older and more obese, had higher blood pressure and fasting and postload glucose, and had lower adiponectin levels than noncase subjects (n = 2,475). Case subjects were less frequently white and more frequently of lower socioeconomic status and smokers (Table 2).
Table 2.
Baseline characteristics of incident diabetes case and noncase subjects
Adiponectin trajectories by sex
Noncase subjects had a slight decrease in adiponectin levels over time (−0.34% per year) without significant sex differences (P = 0.658). However, males had substantially lower adiponectin levels than women (mean difference: 5,358 ng/mL at year0, P < 0.0001) (Fig. 1_A_ and Table 3).
Both male and female diabetic case subjects had lower adiponectin levels than their same-sex control subjects throughout follow-up. At diagnosis, the difference was 1,131 ng/mL in male and 3,181 ng/mL in female subjects (both P < 0.0001). While trajectories in case and control subjects were parallel in males (P = 0.84), female case subjects had a steeper decline than female control subjects (slope difference −1.1% per year, P = 0.004), resulting in diverging adiponectin trajectories between case and noncase subjects over time (Fig. 1_A_ and Table 3).
Adiponectin trajectories by age at diabetes onset
Both early-onset (<52 years of age) and late-onset (≥52 years of age) diabetes case subjects had lower adiponectin levels at diagnosis compared with control subjects (difference 1,907 ng/mL, P = 0.001, and 1,486 ng/mL, P < 0.0001, respectively). In addition, early-onset case subjects had a steeper decline of adiponectin levels than control subjects (slope difference: −1.78% per year, P = 0.016), while late-onset case subjects had a slope parallel that of control subjects (P = 0.13 for case-by-time interaction) (Fig. 1_B_ and C and Table 3).
Adiponectin trajectories by sex and age at onset
The contemporaneous adjustment for sex and age at onset provided similar results. Male and female control subjects had similar (P = 0.66) modest decline in adiponectin over time (−0.34% per year, P < 0.0001). Male control subjects had lower adiponectin levels compared with female control subjects throughout follow-up (difference at year0: 5,355 ng/mL, P < 0.0001) (Fig. 1_D_ and E and Table 3).
All incident diabetes case subjects had lower adiponectin levels than control subjects throughout follow-up. At year0, this difference between case and control subjects was 1,121 ng/mL for late-onset males (P < 0.0001), 1,295 ng/mL for early-onset males (P = 0.005), 3,146 ng/mL for late-onset females (P < 0.0001), and 3,284 ng/mL for early-onset females (P < 0.0001) (Fig. 1_D_ and E and Table 3).
Adiponectin trajectories were parallel for late-onset male case and control subjects (P = 0.87). In contrast, late-onset female case subjects showed a steeper decline compared with control subjects (slope difference: −1.07% per year, P = 0.001) (Fig. 1_E_ and Table 3). Early-onset case subjects of both sexes had steeper declines compared with control subjects and the respective late-onset case subjects (−1.58% per year, P = 0.034) (Fig. 1_D_ and Table 3).
Adjustment for BMI and waist circumference
The downward slope of the adiponectin trajectory in control subjects was attenuated to nonsignificance after adjustment for BMI, although the sex difference remained almost the same (5,537 ng/mL at year0, P < 0.0001). Adjustment for waist circumference in a separate model changed the slope from a modest decrease to a slight increase (0.38% per year, P < 0.0001) in control subjects, and the sex difference was somewhat attenuated (4,107 ng/mL at year0, P < 0.0001) (Table 3).
Adjustment for obesity measures attenuated the difference in adiponectin levels between case and control subjects at year0. In early-onset males, the attenuation was substantial with adjustment for BMI or waist circumference (from a difference of 18.8% to 10.5 and 11.1%, respectively); neither of the adjusted differences were statistically significant. In late-onset males, the attenuations were also substantial (from 14.7% to 7.3 and 7.3%, respectively); however, the differences remained statistically significant. The differences also remained significant for women with early- and late-onset diabetes. This was explained by the generally larger difference in adiposity between female case and control subjects (diabetes × female interaction) compared with male case and control subjects and not by differential adiposity trajectories between women and men (Table 3).
After adjustment for either BMI or waist, the slope difference between early-onset male case and control subjects was attenuated to nonsignificant (P > 0.1), while the steeper decline among female case subjects compared with control subjects remained almost the same (−0.95% per year for BMI and −0.92% per year for waist adjustment) (Table 3).
Sensitivity analysis
Our sensitivity analysis restricted to white case and control subjects largely confirmed the findings in our main analysis, suggesting that ethnicity did not confound our findings (Supplementary Table 1). The model adjusted for time-varying insulin level showed results similar to those after adjustment for obesity measures: the slope difference between early-onset case and control subjects was attenuated to nonsignificant (Supplementary Table 2).
CONCLUSIONS
In this 13-year longitudinal study of middle-aged British civil servants, we found higher adiponectin levels in females compared with males. In people who remained free of diabetes during the study, a modest age-adjusted decrease in adiponectin levels was largely accounted for by increases in obesity. For diabetes case subjects, the estimated adiponectin levels at diagnosis were lower compared with those in sex-specific controls. This difference was partly explained by obesity. Early-onset diabetes case subjects of both sexes and late-onset female case subjects had a steeper decline in prediagnosis adiponectin levels compared with sex-specific control subjects, while parallel declines were observed for males with late-onset diabetes and control subjects. The slope difference in early-onset diabetes compared with control and late-onset case subjects was largely explained by changes in obesity, whereas the sex-specific slope differences were independent of obesity.
While it is widely accepted that adiponectin is an independent predictor of type 2 diabetes (13), the actual changes in adiponectin levels are not well described in humans. A study in spontaneously diabetic Rhesus monkeys described trajectories of obesity and adipokines using several repeat measures (21), but that study lacked nondiabetic control subjects. In agreement with our findings, a decreasing linear trajectory in adiponectin was observed in this animal model in relation to the development of obesity and diabetes. Among Rhesus monkeys, the changes in adiponectin and insulin sensitivity were parallel and the observed cross-sectional covariates of adiponectin were insulin sensitivity, weight, fat weight, and plasma insulin. Adiponectin was not related to age or insulin secretion (21). Our findings suggesting a modest decrease in adiponectin levels with age and that obesity explained part of the cross-sectional differences between case and control subjects are in line with the study in monkeys.
Previous human reports on adiponectin changes were based only on two time points and thus unable to investigate adiponectin trajectories (20,22). Reports from population-based cohorts found changes in adiponectin to be inversely associated with weight and postprandial glucose and positively associated with change in HDL cholesterol (20,22). However, these studies did not investigate the changes separately for diabetes case and control subjects. Our finding that the slope differences between early- and late-onset diabetes case subjects are partially explained by changes in BMI and waist circumference supports an inverse association between changes in obesity and adiponectin levels.
Reports from randomized trials also suggest that changes in adiponectin levels are related to weight changes (10,17). After 2 years of lifestyle intervention, increasing adiponectin levels were found with a decrease in BMI and inflammatory markers (17). In the Diabetes Prevention Program, intensive lifestyle intervention resulted in an increase in adiponectin levels compared with the metformin and placebo groups. Both baseline level and change in weight predicted adiponectin at the end of the follow-up. Higher baseline adiponectin levels (such as those in females) were associated with larger decreases (steeper declines) in adiponectin, corresponding with the observations in our study (10).
The larger separation between incident case and noncase subjects among women compared with men during the 13-year follow-up of our study is in agreement with the sex-by-adiponectin interaction observed in some studies investigating the risk of diabetes during a similar or shorter follow-up (8,11). However, several investigators have not reported (or tested) such interaction effects (6,9,12,14,15). Furthermore, our findings suggest that women go through a larger deterioration in adiponectin levels than men preceding diabetes onset, and this is not explained by sex-specific obesity trajectories. Our finding is in line with observations that suggest greater relative excess of CVD risk factors among diabetic women compared with diabetic men (24).
That the slope differences in adiponectin trajectories between early-onset versus late-onset case and control subjects were largely explained by changes in obesity means that people with early-onset diabetes are more obese and have a steeper increase in their obesity compared with later-onset case and control subjects. Several studies have shown that early-onset diabetes case subjects are more obese (25,26), and some have suggested an inverse association between age at onset and BMI at the time of diabetes diagnosis (27–29). Similarly, fasting insulin also explained most of the slope differences between early- and late-onset diabetes case subjects, although the direction of the relationship between adiponectin and insulin levels is not yet characterized. Thus, it is not known whether insulin sensitivity is a confounding factor or a mediator.
The finding that adiponectin trajectories preceding diabetes had different slopes in males and females although they were parallel in male and female control subjects suggests that adiponectin may have different regulation in high-risk male and female subjects. This is unlikely to be related to the adiponectin gene (ADIPOQ) that explains ∼7% of the phenotypic variation because the gene is unlikely to cause sex differences in adiponectin levels (30). A potential explanation could involve sex hormone levels. Adiponectin and sex hormone–binding globulin (SHBG) levels have similar bidirectional associations with insulin sensitivity, and both are independent predictors of type 2 diabetes. SHBG is involved in sexual dimorphism (31), and some (32) but not all (33,34) studies have suggested that SHBG is more strongly related to diabetes development in women.
Our results confirm previously observed associations between adiponectin and sex (35–37), obesity (6–8,35–39), and diabetes (13). Because of the low number of nonwhite participants, we were unable to explore the previously described ethnic variation in adiponectin levels (36–38), but the potential ethnic differences were controlled for in our analysis. Several cross-sectional studies report age to be positively associated with adiponectin levels in unadjusted analysis (7,37,40) and even after adjustment for blood glucose or obesity measures (35). Longitudinal studies using repeat measures of adiponectin, however, show no consistent association between age and adiponectin levels: one study suggests increasing levels with age and weight loss (22), some report no significant changes in adiponectin levels over time (10,19), and one study among males reports that adiponectin levels decrease with age (18). The contradictory findings in cross-sectional and longitudinal studies suggest that healthy selection may bias cross-sectional findings. Our longitudinal results are also consistent with the above findings and also suggest that changes in obesity over time could also effect the direction of the age-adiponectin association.
The current study has several limitations. First, the retrospective nature of our analysis does not allow us to draw conclusions about the pathophysiological role of adiponectin in diabetes development. Rather, our estimated trajectories help to describe the natural history of diabetes development. Second, some reports suggest that high–molecular weight adiponectin might be more strongly related to the development of type 2 diabetes (12,13,35), but we were unable to measure adiponectin isoforms in our cohort. Third, it is possible that different adipose tissue compartments are more strongly related to adiponectin levels than BMI or waist circumference (7,39). Thus, controlling for additional time-varying measures of adiposity might have attenuated the sex differences even further, but we believe that our converging results using two measures of obesity validate our findings. Fourth, Whitehall II is an occupational cohort, so our findings may not be generalizable to the general population. Furthermore, the participants of this case-cohort study were somewhat healthier compared with the original Whitehall sample; however, this is unlikely to bias the differences between subgroups.
Our study benefits from the use of a well-characterized cohort, the use of a widely accepted method of diabetes diagnosis, and the up to three times repeated measures of risk factors preceding diabetes diagnosis (4,23). We applied a sophisticated method to data analysis that accounts for the interrelationship between within-individual repeated measures. The fact that we were unable to prove any nonlinearity of adiponectin development, although we reported nonlinear trajectories of glycemic measures and interleukin-1 receptor antagonist in this dataset, suggests that adiponectin indeed decreases linearly before diabetes diagnosis (5,16). Similar findings in the main and sensitivity analyses further confirm that the estimated trajectories are likely to describe real changes in adiponectin levels in the investigated population.
In conclusion, we described adiponectin trajectories preceding the diagnosis of type 2 diabetes and compared them with the corresponding trajectories in control participants in a middle-aged cohort of British civil servants. We found significantly lower adiponectin levels in males than females and in participants who developed diabetes compared with those who did not. Adiponectin levels showed a faster decline prior to diabetes in participants with early-onset diabetes and in female case subjects. While the first was explained by a faster increase in adiposity, the latter may be related to sex-specific mechanisms that relate to both diabetes risk and adiponectin levels.
Supplementary Material
Supplementary Data
Acknowledgments
The Whitehall II Study was funded by the Medical Research Council (London, U.K.); the Economic and Social Research Council (Swindon, U.K.); the British Heart Foundation (London, U.K.); the Health and Safety Executive (Merseyside, U.K.); the Department of Health (London, U.K.); the National Institutes of Health National Heart, Lung, and Blood Institute (HL-36310); the National Institutes of Health National Institute on Aging (AG-13196); the Agency for Health Care Policy Research (HS-06516); and the John and Catherine MacArthur Foundation. This case-cohort study was funded by Medical Research Council New Investigator Grant G0501184, the Federal Ministry of Health (Berlin, Germany), and the Ministry of Innovation, Science, Research, and Technology of the state North Rhine-Westphalia (Düsseldorf, Germany). M.K. is supported by the Bupa Foundation Specialist Research Grant (London, U.K.) and the Academy of Finland (Helsinki, Finland). M.J.S. is supported by a grant from the British Heart Foundation (London, U.K.). The funders had no role in study design, data collection and analysis, the decision to publish the manuscript, or the preparation of the manuscript.
No potential conflicts of interest relevant to this article were reported.
A.G.T. researched data, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. M.C., D.R.W., E.J.B., and M.J.S. researched data, contributed to discussion, and reviewed and edited the manuscript. M.J. and M.R. contributed to discussion and reviewed and edited the manuscript. M.K. and C.H. researched data, contributed to discussion, and reviewed and edited the manuscript. A.G.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 45th Annual Meeting of the European Association for the Study of Diabetes, Vienna, Austria, 29 September 2009–2 October 2009.
The authors thank Ulrike Poschen and Karin Röhrig (both from the German Diabetes Center, Leibniz Center for Diabetes Research at Heinrich Heine University Düsseldorf) and Enas El-Safa (Whitehall Study, Department of Epidemiology and Public Health, University College London) for their excellent technical assistance.
Footnotes
References
- 1.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 2002;13:84–89 [DOI] [PubMed] [Google Scholar]
- 2.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995;270:26746–26749 [DOI] [PubMed] [Google Scholar]
- 3.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr 2010;91:258S–261S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 5.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet 2009;373:2215–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fumeron F, Aubert R, Siddiq A, et al. Epidemiologic Data on the Insulin Resistance Syndrome (DESIR) Study Group Adiponectin gene polymorphisms and adiponectin levels are independently associated with the development of hyperglycemia during a 3-year period: the epidemiologic data on the insulin resistance syndrome prospective study. Diabetes 2004;53:1150–1157 [DOI] [PubMed] [Google Scholar]
- 7.Daimon M, Oizumi T, Saitoh T, et al. Funagata study Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese Population: the Funagata study. Diabetes Care 2003;26:2015–2020 [DOI] [PubMed] [Google Scholar]
- 8.Snijder MB, Heine RJ, Seidell JC, et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in older men and women: the hoorn study. Diabetes Care 2006;29:2498–2503 [DOI] [PubMed] [Google Scholar]
- 9.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002;360:57–58 [DOI] [PubMed] [Google Scholar]
- 10.Mather KJ, Funahashi T, Matsuzawa Y, et al. Diabetes Prevention Program Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes 2008;57:980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spranger J, Kroke A, Möhlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003;361:226–228 [DOI] [PubMed] [Google Scholar]
- 12.Heidemann C, Sun Q, van Dam RM, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med 2008;149:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2009;302:179–188 [DOI] [PubMed] [Google Scholar]
- 14.Schwarz PE, Towers GW, Fischer S, et al. Hypoadiponectinemia is associated with progression toward type 2 diabetes and genetic variation in the ADIPOQ gene promoter. Diabetes Care 2006;29:1645–1650 [DOI] [PubMed] [Google Scholar]
- 15.Tabák AG, Brunner EJ, Miller MA, et al. Low serum adiponectin predicts 10-year risk of type 2 diabetes and HbA1c independently of obesity, lipids, and inflammation: Whitehall II study. Horm Metab Res 2009;41:626–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carstensen M, Herder C, Kivimäki M, et al. Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study. Diabetes 2010;59:1222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289:1799–1804 [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Hirose H, Saito I, Nishikai K, Saruta T. Adiponectin, an adipocyte-derived protein, predicts future insulin resistance: two-year follow-up study in Japanese population. J Clin Endocrinol Metab 2004;89:87–90 [DOI] [PubMed] [Google Scholar]
- 19.Langenberg C, Bergstrom J, Laughlin GA, Barrett-Connor E. Ghrelin, adiponectin, and leptin do not predict long-term changes in weight and body mass index in older adults: longitudinal analysis of the Rancho Bernardo cohort. Am J Epidemiol 2005;162:1189–1197 [DOI] [PubMed] [Google Scholar]
- 20.Okauchi Y, Kishida K, Funahashi T, et al. Changes in serum adiponectin concentrations correlate with changes in BMI, waist circumference, and estimated visceral fat area in middle-aged general population. Diabetes Care 2009;32:e122. [DOI] [PubMed] [Google Scholar]
- 21.Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 2001;50:1126–1133 [DOI] [PubMed] [Google Scholar]
- 22.Arawaka N, Daimon M, Oizumi T, et al. Correlation between change in body weight rather than current body weight and change in serum adiponectin levels in a Japanese population — the Funagata study. Metabolism 2006;55:324–330 [DOI] [PubMed] [Google Scholar]
- 23.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int J Epidemiol 2005;34:251–256 [DOI] [PubMed] [Google Scholar]
- 24.Wannamethee SG, Papacosta O, Lawlor DA, et al. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012;55:80–87 [DOI] [PubMed] [Google Scholar]
- 25.Ng MCY, Lee SC, Ko GTC, et al. Familial early-onset type 2 diabetes in Chinese patients: obesity and genetics have more significant roles than autoimmunity. Diabetes Care 2001;24:663–671 [DOI] [PubMed] [Google Scholar]
- 26.Aguilar-Salinas CA, Rojas R, Gómez-Pérez FJ, et al. Prevalence and characteristics of early-onset type 2 diabetes in Mexico. Am J Med 2002;113:569–574 [DOI] [PubMed] [Google Scholar]
- 27.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care 2001;24:1522–1527 [DOI] [PubMed] [Google Scholar]
- 28.Logue J, Walker JJ, Colhoun HM, et al. Scottish Diabetes Research Network Epidemiology Group Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 2011;54:3003–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2006;84:427–433 [DOI] [PubMed] [Google Scholar]
- 30.Heid IM, Henneman P, Hicks A, et al. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis 2010;208:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook JR, Semple RK. Hypoadiponectinemia—cause or consequence of human “insulin resistance”? J Clin Endocrinol Metab 2010;95:1544–1554 [DOI] [PubMed] [Google Scholar]
- 32.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288–1299 [DOI] [PubMed] [Google Scholar]
- 33.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009;361:1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry JR, Weedon MN, Langenberg C, et al. MAGIC Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet 2010;19:535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Retnakaran R, Hanley A, Tungtrongchitr R, Shaw C, Sweeney G. Total and high molecular weight but not trimeric or hexameric forms of adiponectin correlate with markers of the metabolic syndrome and liver injury in Thai subjects. J Clin Endocrinol Metab 2007;92:4313–4318 [DOI] [PubMed] [Google Scholar]
- 36.Duncan BB, Schmidt MI, Pankow JS, et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2004;53:2473–2478 [DOI] [PubMed] [Google Scholar]
- 37.Koenig W, Khuseyinova N, Baumert J, Meisinger C, Löwel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. J Am Coll Cardiol 2006;48:1369–1377 [DOI] [PubMed] [Google Scholar]
- 38.Mente A, Razak F, Blankenberg S, et al. Study of the Health Assessment And Risk Evaluation. Study of the Health Assessment And Risk Evaluation in Aboriginal Peoples Investigators Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care 2010;33:1629–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain SH, Massaro JM, Hoffmann U, et al. Cross-sectional associations between abdominal and thoracic adipose tissue compartments and adiponectin and resistin in the Framingham Heart Study. Diabetes Care 2009;32:903–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herder C, Hauner H, Haastert B, et al. Hypoadiponectinemia and proinflammatory state: two sides of the same coin?: results from the Cooperative Health Research in the Region of Augsburg Survey 4 (KORA S4). Diabetes Care 2006;29:1626–1631 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data