Promiscuous Interactions of gp78 E3 ligase CUE domain with polyubiquitin chains (original) (raw)
. Author manuscript; available in PMC: 2013 Dec 5.
Published in final edited form as: Structure. 2012 Nov 1;20(12):2138–2150. doi: 10.1016/j.str.2012.09.020
Abstract
Recognition of ubiquitin and polyubiquitin chains by ubiquitin-binding domains (UBDs) is vital for ubiquitin-mediated signaling pathways. The endoplasmic reticulum resident RING finger ubiquitin ligase (E3) gp78 regulates critical proteins via the ubiquitin-proteasome system to maintain cellular homeostasis and includes a UBD known as the CUE domain, which is essential for function. A probable role of this domain is to recognize ubiquitin modified substrates, enabling gp78 to assemble polyubiquitin chains on these substrates and mark them for degradation. Here, we report the molecular details of the interaction of gp78CUE domain with ubiquitin and diubiquitin. The gp78CUE domain exhibits a well-defined set of interactions with ubiquitin and a dynamic, promiscuous interaction with diubiquitin chains. This leads to a model where the CUE domain functions to both facilitate substrate binding and enables switching between adjacent ubiquitin molecules of a growing chain to facilitate processivity in ubiquitination.
Introduction
The covalent attachment of ubiquitin (Ub) to cellular proteins, most often to primary amines (ε-amino groups of Lys and to the N-termini of proteins) targets proteins for proteasomal degradation and also plays critical roles in mediating a variety of non-proteasomal functions (Komander and Rape 2012). This post-translational modification is the result of a multi-step process involving three classes of proteins, known as ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2s) and substrate-specific ubiquitin protein ligases (E3) (Komander and Rape 2012). Common to most ubiquitination is the sequential formation of high energy thioester bonds between the active site Cys of E1 and E2 and the C-terminus of ubiquitin (E1~Ub, E2~Ub). Thereafter, there is a divergence of mechanism. The homologous to E6-AP carboxyl terminus (HECT) E3s form a thioester intermediate with ubiquitin (E3~Ub) analogous to E1 and E2 before nucleophillic attack by E3-bound substrate. The large majority of ubiquitin ligases are characterized by RING fingers or the related PHD/LAP fingers and U-box domains. These E3s bind both the E2~Ub and substrates and mediate the transfer of ubiquitin from one to the other. Substrates may be ubiquitinated on a single or several lysines resulting in monoubiquitination. However, critical to the combinatorial complexity of signaling through ubiquitin is the capacity of lysines on the substrate-conjugated ubiquitin to act as acceptors during sequential rounds of ubiquitination, thereby assembling polyubiquitin chains on substrate. Depending on which of the seven lysines of ubiquitin act as the acceptor, the ubiquitin chains will have different types of linkages with diverse conformations and create a range of molecular signals in the cell (Komander and Rape 2012). For example, K48-linked polyubiquitin chains generally target proteins for proteasomal degradation (Thrower, Hoffman et al. 2000), a function now known to be shared with K11-linked chains, whereas K63-linked chains can regulate kinase activation, DNA repair, signal transduction, and endocytosis (Passmore and Barford 2004; Chen and Sun 2009).
A primary determinant of the outcome of ubiquitination is the recognition of ubiquitin or specific ubiquitin chains by ubiquitin binding domains. To date, about 20 types of UBDs have been characterized, including ubiquitin associated domains (UBAs) and the related coupling of ubiquitin conjugation to ER degradation (CUE) domains (Hurley, Lee et al. 2006; Ikeda and Dikic 2008; Wu, Lo et al. 2010; Winget and Mayor 2011); ubiquitin-interacting motifs (UIMs) and zinc-fingers (ZFs). Recent structural studies indicate that these diverse classes of UBDs have distinct specificities and modes of interaction (Hurley, Lee et al. 2006; Wu, Lo et al. 2010; Winget and Mayor 2011). Even among UBA domains, one sub-group can preferentially bind to Lys48-linked chains, another to Lys63-linked chains and a third group binds to both without any preference (Raasi, Varadan et al. 2005). Moreover, the diverse modes of interaction do not segregate with their functional roles. For example, hHR23A shuttles K48-linked substrates to the proteasome and its UBA domain binds to a closed and ‘sandwich-like’ conformation of di-ubiquitin (Varadan, Assfalg et al. 2005). Alternatively, the proteasome receptors (S5a and Rpn13) recognize the same K48-linked substrates, but binds to an open conformation of di-ubiquitin (Husnjak, Elsasser et al. 2008; Zhang, Wang et al. 2009). E2’s like Ubc1 and E2-25K assemble K48-linked chains but their UBA domains bind to K63-linked chains with higher affinity (Raasi, Varadan et al. 2005). This underscores the need for further structural investigations of various UBD/polyubiquitin interactions in order to gain an understanding of their mechanism of action.
CUE domains were identified in a database search based on similarity to a region of the yeast Cue1 protein, which is implicated in yeast Endoplasmic Reticulum Associated Degradation (ERAD) (Ponting 2000). The mechanistic role of CUE domains in ERAD remains unclear. The yeast protein Vps9 includes a CUE domain and is implicated in the yeast endocytotic pathway. An extensive study of ubiquitin recognition by Vps9 has suggested that the CUE domain binds to the ubiquitin conjugated to HECT E3 Rsp5 and promotes self monoubiquitination of Vps9 (Prag, Misra et al. 2003; Shih, Prag et al. 2003). The generality of this mechanism to other HECT E3s or RING E3s is not known.
A prominent human protein containing a CUE domain is the mammalian ERAD E3 gp78 (also known as the human tumor autocrine motility factor receptor or RNF45). This E3 has a variety of targets implicated in diverse physiological and pathophysiological processes including lipid metabolism, metastasis, cystic fibrosis and neurodegeneration (Song, Sever et al. 2005; Tsai, Mendoza et al. 2007; Morito, Hirao et al. 2008). gp78 has a complex domain structure that includes a polytopic N-terminal trans-membrane region and a C-terminal cytosolic domain (gp78C, aa: 313–643), which contains a RING finger domain, a specific binding site for its cognate E2 (Ube2g2) known as the G2BR, and a CUE domain that has the capacity to bind ubiquitin (Figure 1a). The G2BR and RING domains interact with Ube2g2, and a recent study revealed that G2BR allosterically enhances E2:RING binding and consequently ubiquitination (Das, Mariano et al. 2009). The position of the CUE domain (gp78CUE:453–504) is approximately central to the region between the RING and G2BR, where it is separated from each of them by structurally dynamic regions of ~50aa and ~70aa, respectively (Das and Byrd, unpublished data). All three of these regions within gp78C are required for the cellular function of gp78 including its self regulation. However, the functional role(s) of the gp78CUE domain have remained enigmatic (Chen, Mariano et al. 2006).
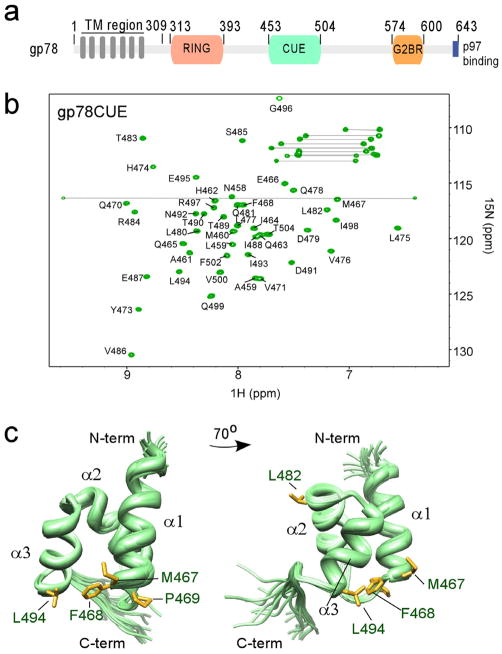
Figure 1.
Structure of gp78CUE domain. (a) Domain structure of gp78. (b) 15N-1H HSQC spectrum of isolated gp78CUE domain at 298 K. About 95% of the backbone (labeled) and sidechain atoms are assigned. Sidechain amide resonances of glutamine and asparagine residues are connected by lines. (c) Ensemble of twenty lowest energy gp78CUE structures shown in two different orientations. The three helices α1, α2 and α3 are marked. The two conserved leucines (L482 and L494) and the MFP sequence are shown in the gp78CUE domain.
Two recent studies have provided a clue to the function of gp78CUE domain. In vitro, gp78 can ubiquitinate an otherwise non-substrate protein fused to ubiquitin, presumably via the gp78CUE/ubiquitin interaction (Morito, Hirao et al. 2008; Das, Mariano et al. 2009). In vivo, gp78 can catalyze polyubiquitin chain formation on the pathogenic CFTRΔ508 after initial ubiquitination by another ERAD E3 RMA1/RNF5, with the gp78CUE domain being critical for this polyubiquitination (Morito, Hirao et al. 2008). These data point to a model where one E3 can initiate substrate ubiquitination, targeting it to gp78 through the latter’s CUE domain for polyubiquitination. This mechanism of chain elongation, where gp78 functions in an E4-like manner is reminiscent of several other examples where one E2:E3 pair monoubiquitinates the target protein and another pair subsequently assembles polyubiquitin chains (Ulrich and Jentsch 2000; Christensen, Brzovic et al. 2007; Rodrigo-Brenni and Morgan 2007). These studies however, do not rule out the possibility of recruiting substrates directly via other regions in the cytosolic or the transmembrane region of gp78.
With each cycle of ubiquitination a new ubiquitin molecule is added to the end of the growing polyubiquitin chain. For this processivity to occur the spatial relationship of the last ubiquitin in the chain to the catalytic center, the RING-bound E2~Ub, must be maintained. Hence, there must be mechanisms to reposition the growing substrate-bound ubiquitin chain. In this report, we present the structures of gp78CUE domain, its complex with ubiquitin, and examine the interaction between gp78CUE and di-ubiquitin using the two conformationally and functionally diverse ubiquitin chains; K48-linked di-ubiquitin (K48-Ub2) and K63-linked di-ubiquitin (K63-Ub2). To our knowledge, these findings provide the first detailed insight into the dynamic structural conformations of a CUE domain intrinsic to an E3 interacting with di-ubiquitin chains, and mechanistic clues for the recruitment and processive elongation of ubiquitin chains on gp78 substrates.
Results
gp78CUE domain forms a compact three helix bundle
The gp78CUE domain (453–504) was expressed in E. coli, isolated and purified (details in Experimental Procedures). Chemical shifts of approximately 95% of the backbone and side-chain 13C, 1H and 15N atoms of the domain were assigned by standard three-dimensional NMR experiments (Sattler, Schleucher et al. 1999)(Figure 1b). The three-dimensional solution structure of gp78CUE was calculated using 1189 distance restraints determined from 13C- and 15N-edited NOESY-HSQC experiments (averaging > 14 medium or long-range restraints per residue) and 97 dihedral angle restraints (Figure 1c, structural statistics in Table 1). The high-resolution structure reveals a three helix bundle comprising of helices α1 (S455-M467), α2 (Y473-L482) and α3 (V486-E495). A total of 64 explicit inter-helical contacts between these three helices hold them together in a compact manner. The structure superposes well with another NMR structure of the CUE domain in gp78 (452–502, pdb id: 2ejs, RMSD=0.69).
Table 1.
Structural Statistics of gp78CUE structure
| gp78CUE | |
|---|---|
| NOE Restraints | |
| Intra-residue (|i-j | =0) |
| Sequential (|i-j | =1) |
| Mediun-range (|i-j | <5) |
| Long-range (|i-j | ≥5) |
| Dihedral Angles (φ, ϕ) | 97 |
| RMS deviation | |
| Bond Angles | 0.7° |
| Bond lengths | 0.007Å |
| RMSD (Å)* | |
| All Backbone | 0.64 ±0.17 |
| All Heavy Atoms | 1.14 ±0.17 |
| Molprobity** | |
| Clashscore | 13 |
| Score | 1.6 |
| PROCHECK* | |
| Most favored region | 94.0% |
| Allowed region | 6.0% |
| Generously allowed region | 0.0% |
| Disallowed region | 0.0% |
gp78CUE recognizes the L8-I44-V70 hydrophobic patch on ubiquitin
The interaction between gp78CUE domain and ubiquitin was probed by Isothermal Titration Calorimetry (ITC). gp78CUE was titrated into ubiquitin, and the resulting exothermic curve was fit to a “One set of sites” binding model yielding a Kd of 12.8(±0.7) μM (Figure 2a). A variety of NMR experiments were then used to study the gp78CUE/ubiquitin interaction. First, unlabeled ubiquitin was titrated into 15N-labeled gp78CUE and observed by 1H, 15N-HSQC (supplementary Figure 1). The difference of chemical shifts between the free and the ubiquitin-bound states of gp78CUE is plotted as the Chemical Shift Perturbation (CSP) in Figure 2b, which indicates the binding interface of gp78CUE. Peaks of some gp78CUE residues like M467, F468, Q470, E487 and L494 had significant shifts. These peaks showed a signature of intermediate exchange, which is in agreement with the low Kd determined by ITC. The same NMR titration was repeated from the ubiquitin side and plotted in Figure 2c and in supplementary Figure 2. A fit of the peak positions against ligand:protein ratio provide the dissociation constant Kd=13.6(±12) μM by NMR (Table 2, Supplementary Figure 3).
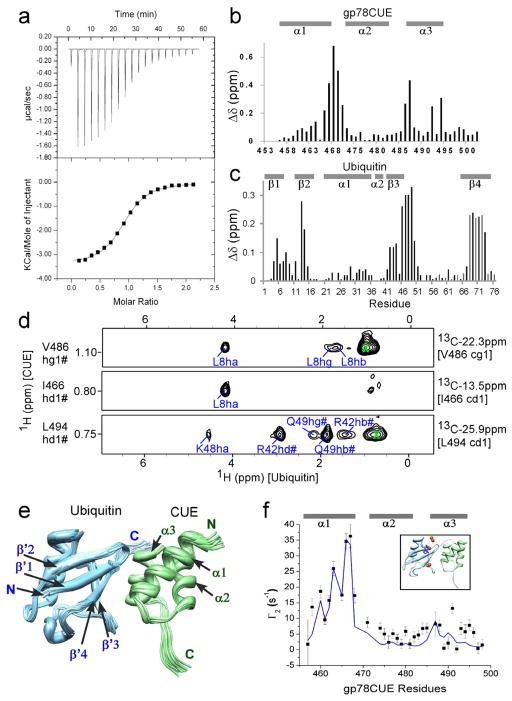
Figure 2.
Interaction between gp78CUE and ubiquitin. (a) ITC evaluation of the interaction between gp78CUE and ubiquitin (D77). The titration curve indicates an exothermic reaction with the dissociation constant Kd= 12.8(±0.7) μM, stoichiometry = 0.91:1, enthalpy ΔH = −14.3(±0.1) kJ mol−1 and entropy ΔS = 46.2 J K−1 mol−1T−1. Amide CSP mapping of the interaction at the saturation for (b) gp78CUE and (c) ubiquitin. (d) Selected strips from the 13C-filtered, 13C-edited NOESY spectra depicting intermolecular NOEs between 13C bound protons of CUE domain and ubiquitin. 13C and 1H assignment of CUE atoms are given on the right and left of the strips respectively. The protons of ubiquitin that show NOEs to CUE are assigned in blue. (e) Solution structure of the gp78CUE/ubiquitin complex. Twenty lowest energy structures are shown, where gp78CUE is colored in green and ubiquitin is colored in light blue. The N-terminal, C-terminal and various secondary structural regions are marked for the two proteins. (f) The calculated (blue line) PREs and observed (black squares, errors: 1 standard deviation) PREs of gp78CUE domain amide protons from the nitroxide atom of MTSL tagged at T12C of ubiquitin in the gp78CUE/ubiquitin complex correlate well (Q-factor =0.33). The lowest energy structure of (e) was used to back-calculate the PREs and is shown as an inset Five red spheres represent the conformational freedom of the spin label and the MTSL sidechain of one conformation is shown in blue.
Table 2.
Dissociation constants between gp78CUE and mono/di-ubiquitin
| Sample (Cell) | Titrant (syringe) | Kd (μM) | Method |
|---|---|---|---|
| ubiquitin | gp78CUE | 12.8 (±0.7) | ITC |
| ubiquitin | gp78CUE | 13.6 (±12.0) | NMR |
| K48-Ub2 | gp78CUE | 12.7 (±0.7) | ITC |
| K48-Ub2 (15N-distal) | gp78CUE | 13.4 (±8.2) | NMR |
| K48-Ub2 (15N-proximal) | gp78CUE | 22.6 (±16.4) | NMR |
| K63-Ub2 | gp78CUE | 14.5 (±1.0) | ITC |
| K63-Ub2 (15N-distal) | gp78CUE | 11.5 (±2.4) | NMR |
| K63-Ub2 (15N-proximal) | gp78CUE | 24.0 (±14.1) | NMR |
Contacts at the gp78CUE/ubiquitin interface were identified by detecting intermolecular NOEs between isotopically 2H, 13C, 15N, ILV-labeled gp78CUE and unlabeled ubiquitin. In the 2H, 13C, 15N, ILV-gp78CUE, all the non-exchangeable protons in gp78CUE domain were substituted with deuterium except for the methyls of Isoleucine, Leucine and Valine residues (ILV). In addition, all the backbone and side-chain amides were found to be protonated via exchange with water. A 13C/15N-filtered, 13C/15N-edited NOESY-HSQC (Zwahlen, Legault et al. 1997)(Figure 2d) detected 42 intermolecular NOEs between gp78CUE and ubiquitin. Using the data of CSP and intermolecular NOEs, a solution structure of gp78CUE/ubiquitin was calculated using HADDOCK (de Vries, van Dijk et al. 2007)(Figure 2e). All 200 structures after the final step of calculation were found in a single cluster with overall RMSD of 0.7 Å. The buried surface area at the interface is 1326(±60) Å2. The structural statistics are provided in Table 3 and the non-covalent contacts at the interface are depicted in Figure 3. The structure reveals that α1 and α3 helices of gp78CUE recognize the L8-I44-V70 hydrophobic patch on the β-sheet of ubiquitin, which is known to be the hot-spot of interaction between ubiquitin and its co-factors (Winget and Mayor 2011). We have also verified the heterodimeric structure of gp78CUE/ubiquitin complex using paramagnetic relaxation effects (PREs). A 2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-yl methyl methanesulfonothioate (MTSL) molecule was tagged at C12 in a T12C ubiquitin mutant. When the MTSL-ubiquitin binds to gp78CUE, the chemical shifts of gp78CUE remain identical to those of gp78CUE when it binds free ubiquitin, indicating that the MTSL has not disrupted the interaction between the molecules. However, some peaks in gp78CUE broaden due to PRE effects as expected. The observed PREs correlate well with predicted PREs from the HADDOCK calculated structure (Q-factor=0.33, Figure 2f), thereby validating the fidelity of the gp78CUE/ubiquitin structure.
Table 3.
Structural Statistics of gp78CUE/ubiquitin complex
| gp78CUE/Ub complex | |
|---|---|
| Intermolecular Restraints | |
| Ambiguous (CSP) | |
| Active | 11 |
| Passive | 10 |
| Unambiguous | |
| NOE | 42 |
| Haddock Parameters | |
| Haddock Score | −82(±12) |
| AIR viols | 1.29(±0.9) |
| Surface Area | 1326(±60) |
| RMS deviation | |
| Bond Angles | 0.6° |
| Bond lengths | 0.004Å |
| RMSD (Å)* | |
| All Backbone | 0.50 ±0.08 |
| All Heavy Atoms | 0.73 ±0.07 |
| Molprobity** | |
| Clashscore | 4.84 |
| Score | 2.07 |
| PROCHECK* | |
| Most favored region | 91.4% |
| Allowed region | 8.6% |
| Generously allowed region | 0.0% |
| Disallowed region | 0.0% |
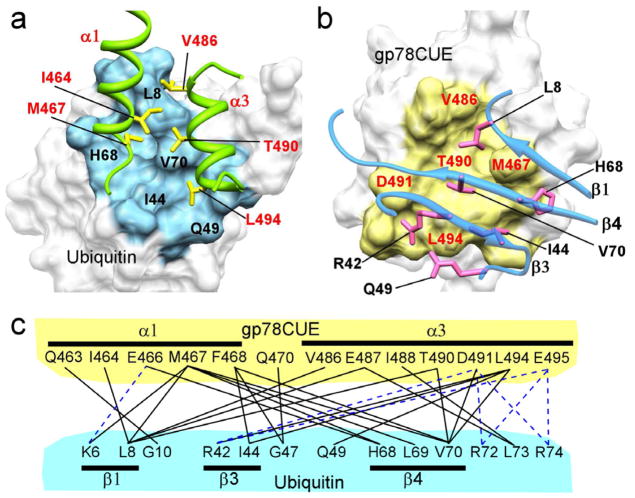
Figure 3.
Noncovalent interactions at the gp78CUE/ubiquitin interface. (a) A view of the contact interface of ubiquitin colored in blue and the critical residues are marked in black. The CUE interacting sidechains are shown in yellow and labeled in red. The α1 and α3 helices of CUE are colored in green while the helix α2 is hidden for clarity. (b) A view of the interface on gp78CUE is shown in yellow and residues labeled in red. The beta-sheet structure of ubiquitin is rendered semi-transparent, while the interacting side-chain is shown in pink and labeled in black. (c) Contacts at the interface between gp78CUE domain and ubiquitin. The salt-bridges are shown as blue broken lines and the other contacts are shown black solid lines.
gp78CUE exhibits similar interactions with both proximal and distal units of K48-Ub2
The interaction of K48-Ub2 with gp78CUE domain was studied using ITC and NMR spectroscopy. In the ITC experiment, gp78CUE was titrated into K48-Ub2 and the resulting exothermic curve could be fit to yield the Kd of 12.4(±0.7) μM (Figure 4a). The stoichiometry of the interaction was found to be 1.9, suggesting that, when gp78CUE is added in excess, two gp78CUE molecules can bind to a single K48-Ub2 molecule. CSP data from NMR studies indicated that the interface of gp78CUE when bound to K48-Ub2 was similar to that of gp78CUE when bound to ubiquitin (compare 2b with 4b). The interactions of gp78CUE domain with proximal and distal ubiquitin were studied individually by designing K48-Ub2 molecules where either the proximal or the distal units are 15N isotope labeled. These molecules were then titrated individually with unlabeled gp78CUE. The CSP data indicated that both the proximal and the distal ubiquitin present the same L8-I44-V70 interface to gp78CUE domain (compare Figure 2c with Figure 4c and Figure 4d). The Kd of gp78CUE was estimated to be 13.4(±8.2) μM and 22.6(±16.4) μM for distal and proximal units, respectively (Supplementary Figure 4).
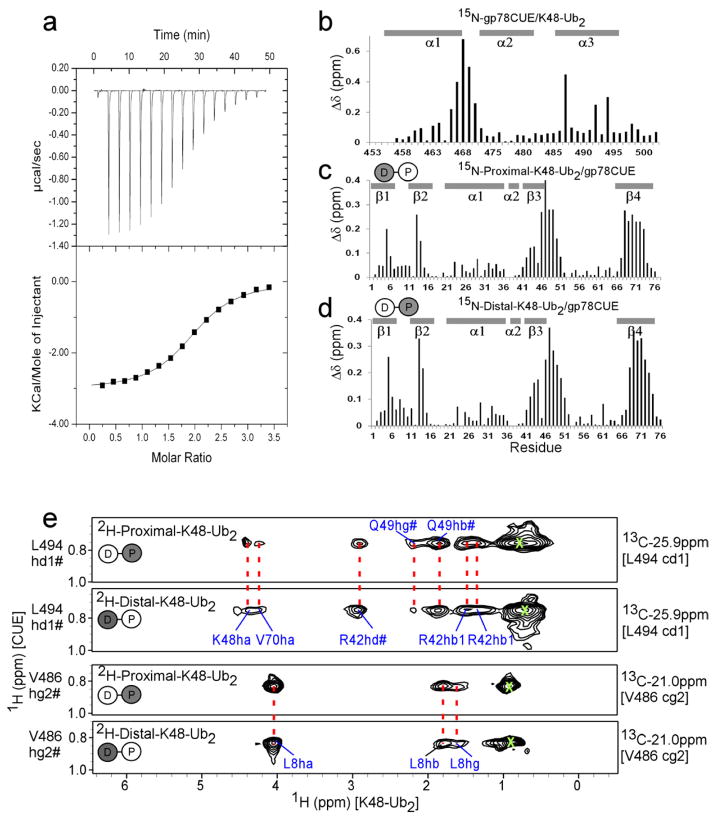
Figure 4.
Interaction between gp78CUE and K48-Ub2. (a) ITC evaluation of the interaction between gp78CUE and K48-Ub2. The titration curve indicates an exothermic reaction with the dissociation constant Kd= 12.4(±0.7) μM, stoichiometry = 1.94:1, enthalpy ΔH = −12.8(±0.09) kJ mol−1 and entropy ΔS = 51.7 J K−1 mol−1T−1. Amide CSP mapping of the interaction at saturation in the titration for (b) gp78CUE, (c) 15N-labeled proximal unit of K48-Ub2 and (d) 15N-labeled distal unit of K48-Ub2. The di-ubiquitin Ub2 is modeled as two circles connected with a line and the 15N-labeled labeled unit is colored gray. (e) Selected strips from the 13C-filtered, 13C-edited NOESY spectra depicting intermolecular NOESY between 13C bound protons of CUE domain to specifically either proximal or distal unit of K48-Ub2. The di-ubiquitin Ub2 is modeled as two circles connected with a line. Here, the 2H-labeled labeled unit is colored gray and does not contribute to the NOESY spectrum. The 13C and 1H assignment of CUE atoms are given on the right and left of the strips respectively. The protons of the ubiquitin (either proximal or distal) unit that show NOEs to CUE are assigned in blue. Identical NOEs from CUE to distal and proximal units of ubiquitin are connected by red broken lines. Diagonal peaks arising due to leaked magnetization are marked as green X.
To map the contacts between gp78CUE and K48-linked Ub2, intermolecular NOEs between gp78CUE and K48-linked Ub2 were measured. The same 2H, 13C, 15N, ILV-gp78CUE as above was used along with fully protonated di-ubiquitin (K48-Ub2), where both distal and proximal ubiquitin molecules are protonated. Intermolecular NOEs observed between gp78CUE and K48-Ub2 were similar to those observed between gp78CUE and ubiquitin (Supplementary Figure 5). To detect explicit contacts from individual ubiquitin molecules in K48-Ub2 and gp78CUE, two different samples of K48-linked Ub2 were used: (i) proximal was deuterated but distal was protonated (2H-Proximal-K48-Ub2), and (ii) distal was deuterated but proximal was protonated (2H-Distal-K48-Ub2). Since the intermolecular NOESY experiment detects NOEs between protons exclusively, the spectra of the gp78CUE/2H-proximal-K48-Ub2 complex was blind to NOEs between the proximal ubiquitin and gp78CUE, but exclusively detected NOEs between the distal ubiquitin and gp78CUE. The inverse was true for gp78CUE/2H-Distal-K48-Ub2 spectra. However, the intermolecular NOESY spectra from both samples were largely identical (Figure 4e). The NOEs from distal and proximal were also similar to the NOEs from fully protonated K48-Ub2 (data not shown), which should be a mere addition of the spectra from samples (i) and (ii). In addition, these NOEs were similar to that of gp78CUE/ubiquitin (compare Figure 2c and Figure 4d), and, identified the same interface between the hydrophobic patch of ubiquitin and the helices α1 and α3 of gp78CUE. Although α2 did not participate at the major interface, one exclusive NOE was observed between α2 of gp78CUE and the proximal ubiquitin. This NOE was attributed to a contact formed between T66 of proximal ubiquitin and L482 of a gp78CUE molecule whose α1 and α3 were bound to the distal ubiquitin (Supplementary Figure 6). The intermolecular NOEs observed from protonated K48-Ub2 and 2H-Distal-K48-Ub2 were normalized against the intensity of the L482-T66 NOE, and compared to yield the ratio of distal- and proximal-bound population of gp78CUE. The ratio was found to be distal:proximal~1.6:1 indicating a slightly higher population of distal bound gp78CUE. It is interesting to note that the ratio of Kds between gp78CUE and the distal/proximal ubiquitin in K48-Ub2 is Kd15N-proximal:Kd15N-distal ~ 1.6 (Table 2), which is consistent with the inference from intermolecular NOE comparison. Overall, the similarity of NOEs between gp78CUE and the two ubiquitin units of di-ubiquitin indicates that the gp78CUE binds to each of them in an identical mode.
Multiple conformations of K48-Ub2 in the presence of gp78CUE
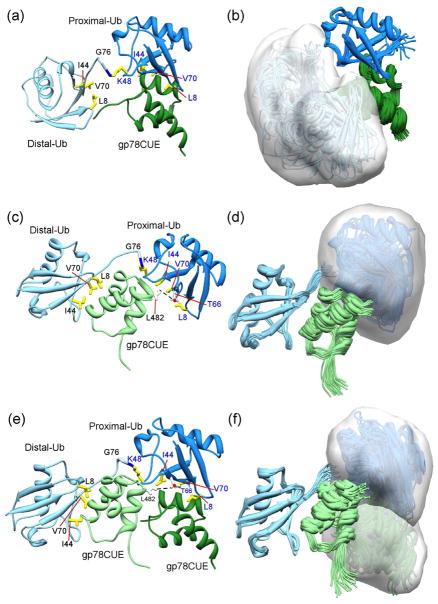
To assess the range of conformational dynamics of K48-Ub2 in the presence of gp78CUE, we used HADDOCK to calculate structures of; (i) gp78CUE bound to the proximal ubiquitin in K48-Ub2 (Figures 5a and 5b) and (ii) gp78CUE bound to the distal ubiquitin in K48-Ub2 (Figure 5c and 5d). The NMR data used in these calculations include the CSP and intermolecular NOEs observed between gp78CUE and distal/proximal ubiquitin (Supplementary Table 1). The pairwise RMSD between these structures and the gp78CUE/Ub complex are modest (RMSD ≤1, Supplementary Table 2) indicating that interface is essentially equivalent when gp78CUE binds to either proximal or distal ubiquitin. In these structures the ubiquitin that is not bound to gp78CUE can experience a range of conformations, which could be specified with the volume that subsumes the entire ensemble of structures. This ensemble volume is 42,000 Å3 for the proximal ubiquitin when gp78CUE is bound to the distal ubiquitin (Figure 5d). The ensemble volume of the distal ubiquitin, when gp78CUE is bound to the proximal ubiquitin, is 62,000 Å3 (Figure 5b).
Figure 5.
Multiple conformations of K48-Ub2 in the presence of gp78CUE. HADDOCK calculation of the complex between gp78CUE and proximal ubiquitin in K48-Ub2 was carried out and the highest scored structure is shown in (a) while an ensemble of 20 structures representing all the clusters is shown in (b). The structures are superposed with reference to proximal ubiquitin. A partially transparent surface in white is drawn to indicate the conformational space accessed by the flexible distal ubiquitin. gp78CUE is colored dark green, proximal ubiquitin is colored dark blue and the distal ubiquitin is colored light blue. The same calculation of the complex between gp78CUE and the distal ubiquitin yielded (c) highest scored structure, and, (d) ensemble of 20 best-scored structures superposed with reference to distal ubiquitin. A surface is drawn to indicate the conformational space accessed by the proximal ubiquitin. The gp78CUE molecule is colored light green. Another consistently observed unique interaction between distal-bound gp78CUE and the proximal ubiquitin (apart from the L482-T66) in Figure 5c,d is a hydrogen bond between the sidechain of R484 (gp78CUE) and the sidechain of S65 or backbone of N60 (proximal ubiquitin, not shown for clarity). The HADDOCK calculation is repeated between two gp78CUE molecules and K48-Ub2; (e) shows the highest scored structure and (f) shows the ensemble of 20 best-scored structures superposed with reference to distal ubiquitin. Partially transparent surfaces are drawn to indicate the conformational space accessed by the proximal ubiquitin and the gp78CUE molecule bound to it. The critical residues L8, I44 and V70 of ubiquitin are shown in yellow. The unique L482-T66 contact residues are shown in white and marked in Figure 5c and e.
The ITC experiments between gp78CUE and K48-Ub2 indicated that, when K48-Ub2 is saturated with excess gp78CUE, the stoichiometry can be 2. To verify the possibility of 2:1 (gp78CUE:K48-Ub2) complex, size-exclusion chromatography was carried out on gp78CUE/K48-Ub2 complexes formed in the approximate ratio of 2:1 and 1:1 (Supplementary Figure 7). The profile of the complex at 2:1 ratio had a peak at the size of 28 KDa indicating that two gp78CUE molecules were bound to one K48-Ub2 molecule. In contrast, the complex formed in a ratio of 1.3:1 peaked at the molecular size of 22KDa, indicating that at equivalent concentrations one gp78CUE molecule binds to one K48-Ub2 molecule. To further study the structural implications of the 2:1 complex, we modeled the complex where two gp78CUE molecules are bound to K48-Ub2, one each to the proximal and distal ubiquitin (Figure 5e and 5f). This modeling was performed in HADDOCK by using the restraints observed individually from the proximal and distal ubiquitins in the 1:1 complex. No major AIR violations, clashes or major alterations in pairwise RMSD were observed in the structures (Supplementary tables 1 and 2), indicating that K48-Ub2 can bind to two gp78CUE molecules simultaneously without any steric occlusion. These structure calculations and the sizing measurements suggest that K48-Ub2 can adopt multiple conformations in the presence of gp78CUE, including open conformations.
Interactions of gp78CUE with K48-Ub2 and K63-Ub2 are similar
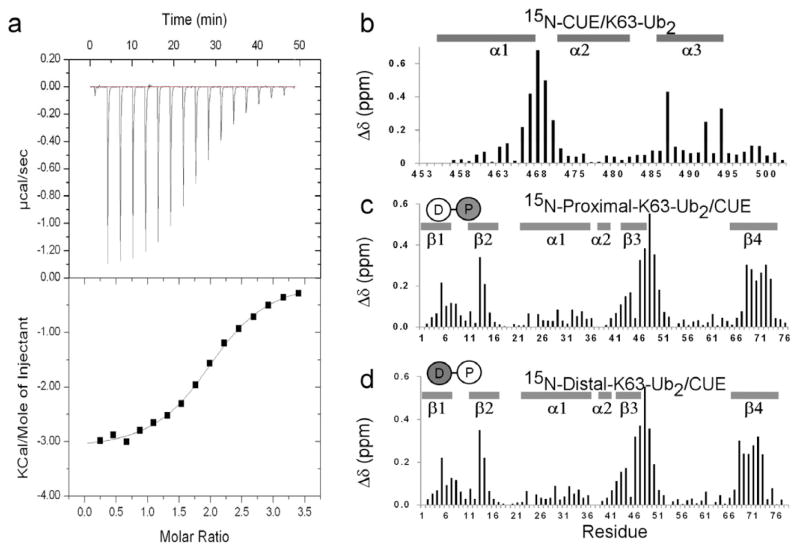
To compare the interaction of gp78CUE with K48-Ub2 and K63-Ub2, the interaction of gp78CUE with K63-Ub2 was studied using ITC and NMR. In an ITC study, gp78CUE was titrated into K63-Ub2, and the resulting exothermic curve was fit to yield the Kd of 14.5(±1.0) μM (Figure 6a). CSP data from NMR studies indicated that the interface of gp78CUE with K63-Ub2 was similar to that with mono-ubiquitin and K48-Ub2 (compare Figure 6b with Figures 2b and 4b). The interaction of gp78CUE domain with proximal and distal ubiquitin was studied individually by designing K63-Ub2 molecules where either the proximal or the distal units were 15N isotope labeled. These molecules were then titrated individually with unlabeled gp78CUE. The CSP data indicated that both the proximal and the distal ubiquitin presented the L8-I44-V70 interface to gp78CUE domain just as mono-ubiquitin and K48-Ub2. The Kd of gp78CUE was estimated to be 11.5(±2.4) μM and 24(±14.1) μM for distal and proximal units respectively. All of the above data indicates that gp78CUE interacts in a similar fashion to both K48-Ub2 and K63-Ub2.
Figure 6.
Interaction between gp78CUE and K63-Ub2. (a) ITC analysis of the binding between gp78CUE and K63-Ub2. The titration curve indicates an exothermic reaction with the dissociation constant Kd= 14.5(±1) μM, stoichiometry = 2.02:1, enthalpy ΔH = −13.4(±0.16) kJ mol−1 and entropy ΔS = 47.9 J K−1 mol−1T−1. Amide CSP mapping of the interaction at saturation in the titration for (b) gp78CUE, (c) 15N-labeled proximal unit of K63-Ub2 and (d) 15N-labeled distal unit of K63-Ub2. The di-ubiquitin Ub2 is modeled as two circles connected with a line and the 15N-labeled labeled unit is colored gray.
Discussion
Our structural studies reveal gp78CUE to be a compact three helical bundle, comprised of helices α1, α2 and α3, which is characteristic among CUE domains (Ponting 2000; Kang, Daniels et al. 2003; Prag, Misra et al. 2003; Shih, Prag et al. 2003). A sequence comparison of CUE domains across species reveals three highly conserved regions; (i) a region where a proline residue is preceded by two hydrophobic residues, (ii) a leucine residue in the middle of the domain, and, (iii) another leucine residue towards the end of the domain (Ponting 2000) (also in Supplementary Figure 8). In gp78CUE, the conserved proline residue P469 occurs in the loop between α1 and α2 and stabilizes the turn to align α2 relative to α1. Among the two preceding hydrophobic residues M467 and F468, residue F468 maintains several crucial intra-molecular contacts, especially with α3, and a F468A mutant disrupts the fold of the protein (Das, Li and Byrd, unpublished work). Additionally, F468 is at the binding interface in the gp78CUE/ubiquitin complex and makes several inter-molecular contacts at this interface (Figure 3). The M467 side-chain is solvent exposed and a M467A mutant is folded (Das, Li and Byrd, unpublished work), indicating that this residue is not important for the intrinsic fold of the protein but could be crucial for interaction with co-factors. Indeed, the M467 side-chain packs tightly between the β1 and β4 strands of ubiquitin and forms several critical contacts at the interface of the gp78CUE/ubiquitin complex (Figure 3). In a previous study, it was found that the Kd between yeast CUE domains and ubiquitin varies from 20 μM (Vps9-CUE, which contains the MFP sequence) to 160 μM (Cue1-CUE domain, which contains an LAP sequence) (Shih, Prag et al. 2003). In gp78CUE, the low Kd ~13 μM observed between ubiquitin and gp78CUE, is consistent with the MFP sequence and the role of M467 and F468. The two other highly conserved leucine residues in CUE domains, L494 and L482, play important roles in gp78CUE/ubiquitin recognition. Residue L494 is exposed at the C-terminal end of α3, is not crucial for the fold of gp78CUE domain as determined by a L494A mutant (Das, Li and Byrd, unpublished work), and makes contacts with R42, V70 and Q49 of ubiquitin at the interface of the complex. Residue L482 is exposed at the N-terminal end of α2, but does not participate in the gp78CUE/ubiquitin complex. Instead, L482 forms a contact with the proximal ubiquitin of K48-linked di-ubiquitin when the α1 and α3 helices interact with the distal ubiquitin. These conserved residues either stabilize the fold of gp78CUE or form critical contacts for recognition of mono- or di-ubiquitin via the hydrophobic patch on the β-sheet structure of ubiquitin (Figure 3), indicating structural and binding homology among the CUE family. The ubiquitin hydrophobic patch includes the conserved residues L8, I44 and V70, and has been identified as the hot-spot of interaction between ubiquitin and its binding partners (Winget and Mayor 2011). In the gp78CUE complex, L8 is docked in between the hydrophobic residues M467 and V486 of gp78CUE. Residue I44 also forms hydrophobic interactions with the conserved residues F468 and L494, and V70 is positioned central to the interface exhibiting an extensive set of van der Waals contacts with gp78CUE. In addition, we find several salt-bridges formed between basic residues in ubiquitin (R42, R72 and R74) and acidic residues of gp78CUE (E466, D491 and E495). The large set of interactions between the molecules (Figure 3) provides specificity to the gp78CUE/ubiquitin complex and is consistent with the binding affinity.
While it is known that the gp78CUE is essential for biological function (Tsai, Mendoza et al. 2007), the mechanistic role is as yet unknown. A putative role for the gp78CUE domain may be to recruit substrates that already bear one or more ubiquitin moieties (or a ubiquitin-like domain, UBL). It has been shown previously that proteins can be made into a substrate for ubiquitination by gp78 (the E3) acting with Ube2g2 (the E2) if the target protein bears a ubiquitin moiety, formed as a fusion (Tsai, Mendoza et al. 2007; Morito, Hirao et al. 2008; Das, Mariano et al. 2009). We postulate that this is due to recruitment and possible positioning of the fusion target by the gp78CUE domain. Similarly, some substrates of gp78, e.g. the in vitro substrate Herp (Li, Tu et al. 2009), include an UBL that is highly similar to ubiquitin in sequence and structure. The Herp UBL presents residues equivalent to ubiquitin I44 and V70, as well as R74, suggesting that the contacts observed in the gp78CUE/ubiquitin structure are supportive of gp78 recruiting UBL-containing substrates. Subsequent to the first rounds of ubiquitination, the substrate is modified with mono-ubiquitin or a chain of two or more ubiquitin moieties, and the gp78CUE domain will presumably recruit the substrate by binding to the mono- or polyubiquitin chain. It is of interest to consider how gp78CUE interacts with polyubiquitin chains and successfully positions the last ubiquitin of the chain for extension. We demonstrated that neither the affinity nor the mode of interaction with each ubiquitin moiety in K48-Ub2 differed significantly from that determined for mono-ubiquitin (Table 2, Figure 4). NMR experiments confirm that the gp78CUE uses the same α1–α3 interface for binding to the identical L8-I44-V70 interface in both the proximal and distal ubiquitin molecules, which is also synonymous to the interface of mono-ubiquitin (Figure 4), In support of our findings, a previous study has found that, at the resolution of GST-based pulldown assays, yeast CUE domains which interact with ubiquitin weakly also bind polyubiquitin weakly and the CUE domains which interacted with ubiquitin strongly bind polyubiquitin equally strong (Shih, Prag et al. 2003). Thus, the gp78CUE domain appears to act as a general recruitment partner for ubiquitin and UBL-containing or ubiquitin-modified substrates. The distal ubiquitin consistently shows a slightly higher affinity for gp78CUE (Kdproximal/Kddistal ~1.6), which results in a higher population of distal-bound gp78CUE species. Since the distal ubiquitin acts as the acceptor in the next round of ubiquitin transfer, this state may be most favorable for gp78CUE mediated positioning. The modest, and chain-length independent, affinity supports a broad recruitment mechanism and the tendency to shift equilibrium towards the distal bound state supports a possible positioning mechanism for gp78CUE domain in the ubiquitination process.
It is known that gp78 assembles primarily K48-linked polyubiquitin chains on its substrates (Li, Tu et al. 2007). However, the data presented here shows that gp78CUE is a promiscuous binding partner for ubiquitin chains, as it binds to both K48- and K63-linked chains with similar affinities and interfaces (Table 2, Figure 6). Hence, a role of gp78CUE in linkage type regulation, if any, arises more likely from a spatial context influenced largely by the RING finger:E2~Ub complex. Since gp78CUE can equally recognize K63-modified substrates, there exists a possibility that gp78 coordinates with other E3s to assemble K63 and K48 mixed polyubiquitin chains on a substrate. For example, two kinase adaptors were found that require K63-linked chains initially to initiate NF-κB signaling, while at later times K48-linked polyubiquitin modification targets them for proteasomal degradation (Newton, Matsumoto et al. 2008). However, it is not yet known if extension by gp78/Ube2g2 occurs on a K63-linked substrate to create such a mixed chain.
Recent studies have described free ubiquitin chains as dynamic ensembles of multiple conformations (Hirano, Serve et al.; Datta, Hura et al. 2009; Lai, Zhang et al. 2012). The conformation of ubiquitin chains in the presence of co-factors, however, is varied (Varadan, Assfalg et al. 2005; Zhang, Raasi et al. 2008; Wu, Lo et al. 2010; Winget and Mayor 2011). While K48-Ub2 forms a closed, single conformation bound to the hHR23A-UBA domain (Varadan, Assfalg et al. 2005), it has an open conformation bound to the ubiquilin-UBA domain (Zhang, Raasi et al. 2008). The NMR-driven structural modeling presented here demonstrated that K48-Ub2 can have multiple conformations in the presence of gp78CUE domain. When gp78CUE is bound to the proximal ubiquitin, the distal ubiquitin can adopt a variety of three-dimensional conformations spanning a volume of 62,000 Å3 (Figure 5b). When gp78CUE binds the distal ubiquitin, the flexibility of proximal ubiquitin is reduced to volume of 42,000 Å3 due to additional contacts with gp78CUE (Supplementary Table 1), but it still exchanges among multiple conformations including open conformations that can bind another co-factor (Figure 5d). In fact, our structure calculations, ITC, and size-estimation experiments indicate that in excess concentration of gp78CUE, both the proximal and distal ubiquitins can simultaneously recruit a gp78CUE molecule each, substantiating that the K48-Ub2 can adopt open conformations in the presence of gp78CUE.
It is not clear whether the gp78CUE:Ub2 (2:1) complex observed in vitro is relevant to the function of gp78. In the E3 Cbl-b, dimerization of a UBA domain was found to be critical for binding polyubiquitin chains and activity (Peschard, Kozlov et al. 2007). The gp78CUE:Ub2 (2:1) complex observed in vitro suggests that a diubiquitin chain could further assist dimerization of two gp78 molecules induced by close proximity. CUE domains have to date been reported as monomers in solution, and the isolated gp78CUE domain (453–504) did not form dimers up to concentrations of 1mM. Moreover, the net affinity of the 2:1 complex is roughly equivalent to the 1:1 complex, indicating that there is no apparent driving force by gp78CUE alone to form a gp78 dimer. However, an oligomerization domain does exist in gp78 at residues N-terminal (419–448) to the CUE domain, which is supportive of a model where multiple gp78 molecules work with Ube2g2 in a coordinated fashion (Li, Tu et al. 2009). The self-association of the oligomerization domain combined with spatially proximate gp78CUE domains binding polyubiquitin could act synergistically to localize multiple gp78 molecules in the ER membrane. The contribution of such complexes to the overall functional mechanism in ERAD is an area of further research.
In conclusion, we have defined the structure of the gp78CUE domain, its binding complexes with ubiquitin and K48-Ub2, and demonstrated that there is no affinity preference for mono- versus di-ubiquitin or K48-Ub2 versus K63-Ub2. It is known that gp78CUE occurs in the middle of an unstructured and dynamic part of gp78C between the RING and G2BR domains, thus providing spatial flexibility for the gp78CUE domain relative to Ube2g2. We have also shown that K48-linked and K63-linked polyubiquitin chains have a flexible interaction with gp78CUE, where chains can adopt multiple conformations in its presence. These data lead to two important mechanistic postulations for the role of gp78CUE. First, gp78CUE could recruit a ubiquitin chain (or UBL domain) on the target substrate by binding to any ubiquitin molecule on the chain. Secondly, the combined spatial flexibility of gp78CUE and the conformational flexibility of the polyubiquitin chain would allow gp78CUE to shuffle between adjacent ubiquitin molecules and correctly position the last ubiquitin of the chain for attack on the thioester-linked ubiquitin at the active site of Ube2g2. Since gp78CUE has no preference for K48-Ub2 versus K63-Ub2 in recruitment, the factors determining the linkage formation with the conjugated ubiquitin at the active site likely involve interactions with, or conformational restriction by, other components of the entire gp78:Ube2g2~Ub complex and require further structural examination. Multiple E3s like Cbls, Nedd4, Huwe1, Rad18, etc, include ubiquitin binding regions and their contribution in the polyubiquitination of substrates is an exciting subject of future research.
Experimental Procedures
gp78CUE expression and purification
The CUE (aa: 453–504) of gp78 was subcloned into pET3a vector between Ndel and BamHi restriction sites from the template of full-length gp78 clone (gp78FL/pGEX). gp78CUE/PET3a gene construct was transformed to BL21 Star E. coli (Invitrogen). Cells were grown at 37°C to an OD600nm of 0.8 in M9 medium and protein expression was induced by 1.0 mM IPTG, followed by another 4hr growth and lysis. After lysis the pellet was dissolved in 4M Urea in 50 mM Tris, pH 7.2. The supernatant was refolded in 4M Urea to 0M Urea in 50 mM Tris, pH 7.2 and loaded on to Q-Sepharose HP column (GE Healthcare) followed by superdex 75 (GE Healthcare) on AKTA-FPLC for purification. For 15N-labeled gp78CUE, recombinant E. Coli cells were grown in M9 media with 15NH4Cl as the only nitrogen source. For expression of DCN–ILV (2H,13C,15N-1H (Ileδ1, Leu, Val) gp78CUE, regular M9/deuterium media (1L) containing 2 g/L 13C,2H-glucose and 1 g/L 15N NH4Cl was supplemented with 100 mg/L 2-keto-3-(methyl-d3)-butyric acid-1,2,3,4-13C4, 50 mg/L 2-Ketobutyric acid-13C4, 3, 3-d2 (Isotech). Cell growth and protein purification were performed as unlabeled gp78CUE.
Expression, purification of ubiquitin mutants and synthesis of K48-Ub2 and K63-Ub2
Plasmids of wild type ubiquitin, K48A-, D77- and D77/T12C-ubiquitins (encoded by pET/pRSET-based plasmids) were transformed into BL21 Star E. coli, respectively. Expression and purification of the above Ub mutants were as described (Haldeman, Xia et al. 1997). For 15N-labeled Ub mutants, E. Coli cells were grown in M9 media with 15NH4Cl as the only nitrogen source. Deuterated Ub mutants were expressed in deuterated M9 medium, using 2H,12C-glucose and D2O. K48-Ub2 and K63-Ub2 were synthesized as previously described (Piotrowski, Beal et al. 1997; Hofmann and Pickart 2001).
ITC and SEC studies
The binding affinity and stoichiometry of CUE with monoUb and Ub2 was determined using iTC200 (MicroCal, LLC) at 25°C. gp78CUE and ubiquitin were dialyazed together against the same 50mM Tris buffer, pH 7.2, 100 mM NaCl. Nineteen aliquots of a 2.6 mM gp78CUE in syringe (ligand) were injected into a 0.27 mM UbD77 (cell) stirred at 1000 rpm, 25°C. The integrated interaction heat values were normalized as a function of ligand concentration, and the data were fit using MicroCal Origin software. The same procedure was performed on gp78CUE interacting with K48-Ub2 or K63-Ub2. For SEC experiments, samples were injected directly into the sample loop and onto an analytical Superdex 75 (10/300, GE Healthcare) column at a flow rate of 0.5 mL/minute in the 50mM Tris, 150mM NaCl, pH 7.2 buffer. The protein peaks were detected by an UV monitor at a wavelength of 280nm.
Site-directed spin labeling of monoUb and PRE 1H-T2 measurements
Residue T12 of Ub(D77) was mutated to a Cysteine in order to enable covalent tagging with a paramagnetic spin label, methanethiosulfonate (MTSL, Toronto Research Chemicals). Lyophilized Ub(D77/T12C) was dissolved in 50 mM Tris buffer, pH7.2, with 0.2 mM DTT, followed by addition of MTSL (20X) and incubation for 3 hrs. Dialysis of the protein against 50 mM Tris, pH 7.2, eliminated excess MTSL. The extent of tagging was confirmed to be 100% based on mass spectrometry. The concentration of MTSL tagged Ub(D77/T12C) was determined by Bradford protein assay. gp78CUE was mixed with MTSL tagged Ub(D77/T12C) at 1:1.5 and the final concentration of MTSL tagged Ub(D77/T12C) was 0.368mM. The reduced form of MTSL tagged protein was obtained by addition of 10-fold excess of ascorbic acid. pH of the sample was adjusted back to pH 7.2 using 1M Trizma (Sigma). The PRE 1H-T2 effect was measured and calculated as described previously (Iwahara, Schwieters et al. 2004). Recognizing the conformational flexibility of the MTSL tag, 1HN-Γ2 rates were back-calculated in Xplor-NIH using a five-conformer ensemble for the spin-label together with the Solomon-Bloembergen Model Free (SBMF) representation, optimizing the coordinate positions in torsion angle space by simulated annealing to minimize the difference between observed and calculated 1HN-Γ2 PRE rates, as described previously (Iwahara, Schwieters et al. 2004).
NMR Spectroscopy
NMR samples were prepared in 50 mM Tris, pH 7.0, 50mM NaCl, and all experiments were performed at 25 °C. NMR spectra were acquired on 500, 600 and 800 MHz Varian INOVA spectrometers, or 600 and 700 MHz Bruker Avance spectrometers equipped with triple-resonance gradient cryoprobes. All NMR data were processed with NMRPipe (Delaglio, Grzesiek et al. 1995). The 1H, 15N and 13C backbone and side-chain resonances were assigned using standard 3D triple resonance NMR experiments (Sattler, Schleucher et al. 1999) and peaks were analyzed by Sparky (Goddard). Distance information were obtained from 3D 15N/13C-edited NOESY-HSQC (τm=100 ms) (Sattler, Schleucher et al. 1999). Intermolecular distance restraints in the gp78CUE:ubiquitin (1mM:1.2mM) complex and the gp78CUE:K48-Ub2 (0.8mM:0.96mM) complex were determined via a 13C/15N-filtered, 13C/15N-edited NOESY experiment (τm=150 ms) (Zwahlen, Legault et al. 1997). The experiments were repeated with smaller mixing time (τm=75 ms) and produced similar spectra with lower peak intensities, ruling out artifacts due to spin-diffusion. These experiments were also repeated with non-decoupling during t1-increments to rule out peaks that appear due to leaked magnetization during the purging pulses.
Titration of Ub or Ub2 with CUE was monitored by 2D 1H-15N HSQC spectra of 15N Ub(D777) or Ub2 with one unit 15N-labeled, respectively. The 2D HSQC spectra of 1mM 15N Ub(D77) were acquired with increased amount of unlabeled gp78CUE at Ub/gp78CUE molar ratio of 0, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 2.0 and 3.0. The same procedure was applied in the Ub2/gp78CUE titration. The chemical shifts (δH and δN) observed in ubiquitin, K48-Ub2 and K63-Ub2 upon CUE titration was extracted from the spectra using NMRViewJ and calculated as Δδ=((δHsat−δHfree)2+((δNsat−δNfree)/5)2)1/2 (Johnson 2004). The Kd of gp78CUE/monoubiquitin interaction was determined with the equation for one-binding-site mode in a matlab program (Varadan, Assfalg et al. 2005). To obtain the Kd between proximal/distal Ub of K48-Ub2 and gp78CUE domain, the 15N-proximal/distal labeled K48-Ub2 (0.2mM) was titrated with gp78CUE upto molar ratio of 4 (0.8mM, final concentration). The same experiment for K63-Ub2 and gp78CUE domain, the 15N-proximal/distal labeled K63-Ub2 (0.24mM) was titrated with gp78CUE upto molar ratio of 4 (0.96mM, final concentration). The Kd values of gp78CUE/K48-Ub2 and gp78CUE/K63-Ub2 were determined using a matlab macro written for two independent binding sites (Varadan, Assfalg et al. 2005).
Structural restraints defining the position of the gp78CUE relative to the ubiquitin were determined from measurements of chemical shift perturbations (CSP) and intermolecular NOEs. Ambiguous interaction restraints (AIR) were defined for amide protons of gp78CUE residues that have Δδ bigger than 0.2 ppm to amide protons of the ubiquitin residues with Δδ bigger than 0.1 ppm. The solution structure was calculated in HADDOCK (Dominguez, Boelens et al. 2003) using the lowest energy NMR structure of gp78CUE (this work) and ubiquitin (pdb id:1d3z). All AIR and inter-molecular NOE-derived restraints were used during the docking steps. The interface of gp78CUE and ubiquitin were kept semi-flexible during simulated annealing and the water refinement. The C-terminal end of gp78CUE (aa:500–504) and ubiquitin (aa:74–76) were kept flexible throughout the structure calculation. The structures of gp78CUE with K48-Ub2 were calculated similarly, except a distance restraint was imposed between the C-terminal end of distal ubiquitin and the K48 sidechain of proximal ubiquitin.
Supplementary Material
01
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Data depositions: The coordinates have been deposited in the PDB as entries 2lvn (gp78CUE), 2lvo (gp78CUE/ubiquitin), 2lvp and 2lvq (gp78CUE/K48-Ub2 proximal and distal) and data has been deposited in the BioMagResBank as entry 18581–18584.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Chen B, Mariano J, et al. The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site. Proc Natl Acad Sci U S A. 2006;103(2):341–346. doi: 10.1073/pnas.0506618103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33(3):275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Christensen DE, Brzovic PS, et al. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat Struct Mol Biol. 2007;14(10):941–948. doi: 10.1038/nsmb1295. [DOI] [PubMed] [Google Scholar]
- Das R, Mariano J, et al. Allosteric activation of E2-RING finger-mediated ubiquitylation by a structurally defined specific E2-binding region of gp78. Mol Cell. 2009;34(6):674–685. doi: 10.1016/j.molcel.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta AB, Hura GL, et al. The structure and conformation of Lys63-linked tetraubiquitin. J Mol Biol. 2009;392(5):1117–1124. doi: 10.1016/j.jmb.2009.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries SJ, van Dijk AD, et al. HADDOCK versus HADDOCK: new features and performance of HADDOCK2.0 on the CAPRI targets. Proteins. 2007;69(4):726–733. doi: 10.1002/prot.21723. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6(3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Dominguez C, Boelens R, et al. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125(7):1731–1737. doi: 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- Goddard TD, Kneller DG. SPARKY. p. 3. [Google Scholar]
- Haldeman MT, Xia G, et al. Structure and function of ubiquitin conjugating enzyme E2-25K: the tail is a core-dependent activity element. Biochemistry. 1997;36(34):10526–10537. doi: 10.1021/bi970750u. [DOI] [PubMed] [Google Scholar]
- Hirano T, Serve O, et al. Conformational dynamics of wild-type Lys-48-linked diubiquitin in solution. J Biol Chem. 286(43):37496–37502. doi: 10.1074/jbc.M111.256354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann RM, Pickart CM. In vitro assembly and recognition of Lys-63 polyubiquitin chains. J Biol Chem. 2001;276(30):27936–27943. doi: 10.1074/jbc.M103378200. [DOI] [PubMed] [Google Scholar]
- Hurley JH, Lee S, et al. Ubiquitin-binding domains. Biochem J. 2006;399(3):361–372. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husnjak K, Elsasser S, et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453(7194):481–488. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Dikic I. Atypical ubiquitin chains: new molecular signals. ‘Protein Modifications: Beyond the Usual Suspects’ review series. EMBO Rep. 2008;9(6):536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahara J, Schwieters CD, et al. Ensemble approach for NMR structure refinement against (1)H paramagnetic relaxation enhancement data arising from a flexible paramagnetic group attached to a macromolecule. J Am Chem Soc. 2004;126(18):5879–5896. doi: 10.1021/ja031580d. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol Biol. 2004;278:313–352. doi: 10.1385/1-59259-809-9:313. [DOI] [PubMed] [Google Scholar]
- Kang RS, Daniels CM, et al. Solution structure of a CUE-ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell. 2003;113(5):621–630. doi: 10.1016/s0092-8674(03)00362-3. [DOI] [PubMed] [Google Scholar]
- Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- Lai MY, Zhang D, et al. Structural and biochemical studies of the open state of Lys48-linked diubiquitin. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbamcr.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Tu D, et al. A ubiquitin ligase transfers preformed polyubiquitin chains from a conjugating enzyme to a substrate. Nature. 2007;446(7133):333–337. doi: 10.1038/nature05542. [DOI] [PubMed] [Google Scholar]
- Li W, Tu D, et al. Mechanistic insights into active site-associated polyubiquitination by the ubiquitin-conjugating enzyme Ube2g2. Proc Natl Acad Sci U S A. 2009;106(10):3722–3727. doi: 10.1073/pnas.0808564106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morito D, Hirao K, et al. Gp78 cooperates with RMA1 in endoplasmic reticulum-associated degradation of CFTRDeltaF508. Mol Biol Cell. 2008;19(4):1328–1336. doi: 10.1091/mbc.E07-06-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Matsumoto ML, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134(4):668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Passmore LA, Barford D. Getting into position: the catalytic mechanisms of protein ubiquitylation. Biochem J. 2004;379(Pt 3):513–525. doi: 10.1042/BJ20040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschard P, Kozlov G, et al. Structural basis for ubiquitin-mediated dimerization and activation of the ubiquitin protein ligase Cbl-b. Mol Cell. 2007;27(3):474–485. doi: 10.1016/j.molcel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Piotrowski J, Beal R, et al. Inhibition of the 26 S proteasome by polyubiquitin chains synthesized to have defined lengths. J Biol Chem. 1997;272(38):23712–23721. doi: 10.1074/jbc.272.38.23712. [DOI] [PubMed] [Google Scholar]
- Ponting CP. Proteins of the endoplasmic-reticulum-associated degradation pathway: domain detection and function prediction. Biochem J. 2000;351(Pt 2):527–535. [PMC free article] [PubMed] [Google Scholar]
- Prag G, Misra S, et al. Mechanism of ubiquitin recognition by the CUE domain of Vps9p. Cell. 2003;113(5):609–620. doi: 10.1016/s0092-8674(03)00364-7. [DOI] [PubMed] [Google Scholar]
- Raasi S, Varadan R, et al. Diverse polyubiquitin interaction properties of ubiquitin-associated domains. Nat Struct Mol Biol. 2005;12(8):708–714. doi: 10.1038/nsmb962. [DOI] [PubMed] [Google Scholar]
- Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130(1):127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Sattler M, Jr, Schleucher, et al. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Progress in Nuclear Magnetic Resonance Spectroscopy. 1999;34(2):93–158. [Google Scholar]
- Shih SC, Prag G, et al. A ubiquitin-binding motif required for intramolecular monoubiquitylation, the CUE domain. Embo J. 2003;22(6):1273–1281. doi: 10.1093/emboj/cdg140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BL, Sever N, et al. Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol Cell. 2005;19(6):829–840. doi: 10.1016/j.molcel.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Thrower JS, Hoffman L, et al. Recognition of the polyubiquitin proteolytic signal. Embo J. 2000;19(1):94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Mendoza A, et al. The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat Med. 2007;13(12):1504–1509. doi: 10.1038/nm1686. [DOI] [PubMed] [Google Scholar]
- Ulrich HD, Jentsch S. Two RING finger proteins mediate cooperation between ubiquitin-conjugating enzymes in DNA repair. Embo J. 2000;19(13):3388–3397. doi: 10.1093/emboj/19.13.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadan R, Assfalg M, et al. Structural determinants for selective recognition of a Lys48-linked polyubiquitin chain by a UBA domain. Mol Cell. 2005;18(6):687–698. doi: 10.1016/j.molcel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Winget JM, Mayor T. The diversity of ubiquitin recognition: hot spots and varied specificity. Mol Cell. 2011;38(5):627–635. doi: 10.1016/j.molcel.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Wu H, Lo YC, et al. Recent advances in polyubiquitin chain recognition. F1000 Biol Rep. 2010;2:1–5. doi: 10.3410/B2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raasi S, et al. Affinity makes the difference: nonselective interaction of the UBA domain of Ubiquilin-1 with monomeric ubiquitin and polyubiquitin chains. J Mol Biol. 2008;377(1):162–180. doi: 10.1016/j.jmb.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wang Q, et al. Structure of the s5a:k48-linked diubiquitin complex and its interactions with rpn13. Mol Cell. 2009;35(3):280–290. doi: 10.1016/j.molcel.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwahlen C, Legault P, et al. Methods for Measurement of Intermolecular NOEs by Multinuclear NMR Spectroscopy: Application to a Bacteriophage λ N-Peptide/boxB RNA Complex. Journal of the American Chemical Society. 1997;119(29):6711–6721. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01