Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner (original) (raw)
. Author manuscript; available in PMC: 2013 May 24.
SUMMARY
Microglia are the resident CNS immune cells and active surveyors of the extracellular environment. While past work has focused on the role of these cells during disease, recent imaging studies reveal dynamic interactions between microglia and synaptic elements in the healthy brain. Despite these intriguing observations, the precise function of microglia at remodeling synapses and the mechanisms that underlie microglia-synapse interactions remain elusive. In the current study, we demonstrate a role for microglia in activity-dependent synaptic pruning in the postnatal retinogeniculate system. We show that microglia engulf presynaptic inputs during peak retinogeniculate pruning and engulfment is dependent upon neural activity and the microglia-specific phagocytic signaling pathway, complement receptor 3(CR3)/C3. Furthermore, disrupting microglia-specific CR3/C3 signaling resulted in sustained deficits in synaptic connectivity. These results define a role for microglia during postnatal development and identify underlying mechanisms by which microglia engulf and remodel developing synapses.
INTRODUCTION
Early in development neurons make far more synaptic connections than are maintained in the mature brain. Synaptic pruning is an activity-dependent developmental program in which a large number of synapses that form in early development are eliminated while a subset of synapses are maintained and strengthened (Hua and Smith, 2004; Katz and Shatz, 1996; Sanes and Lichtman, 1999). While it is clear that neuronal activity plays a role, the precise cellular and molecular mechanisms underlying this developmental process remain to be elucidated.
Microglia are the resident CNS immune cells which have long been recognized as rapid responders to injury and disease, playing a role in a broad range of processes such as tissue inflammation and clearance of cellular debris (Hanisch and Kettenmann, 2007; Kreutzberg, 1996; Ransohoff and Perry, 2009). In contrast to disease pathology, the function of microglia in the normal, healthy brain is far less understood. However, recent studies suggest that microglia may play a role in synaptic remodeling and plasticity in the healthy brain (Davalos et al., 2005; Nimmerjahn et al., 2005; Paolicelli et al., 2011; Tremblay et al., 2010a; Wake et al., 2009; Schafer et al., 2012). For example, microglia within the juvenile visual cortex modify their association with dendritic spines in response to changes in visual sensory experience (Tremblay et al., 2010a). A more recent study provides evidence that disruptions in microglia function result in delayed maturation of hippocampal synaptic circuits (Paolicelli et al., 2011). Moreover, data from these studies suggest that microglia may be phagocytosing dendritic spines. These intriguing studies raise several interesting and important questions. The precise function of microglia at synaptic sites, the molecular mechanism(s) underlying microglia-mediated synaptic engulfment, and the long term consequence(s) of disrupting microglia function on synaptic circuits remain a mystery.
A candidate mechanism by which microglia could be interacting with developing synapses is the classical complement cascade. Complement cascade components C1q and C3 localize to immature synapses and are necessary for the developmental pruning of retinogeniculate synapses (Stevens et al., 2007; Stephan et al., 2012). While provocative, the mechanism by which complement mediates synaptic pruning has remained completely unknown. Complement components function in the immune system by binding and targeting unwanted cells and cellular debris for rapid elimination through several different pathways. Among the many mechanisms by which complement may mediate synaptic pruning is phagocytosis, which makes microglia, the resident CNS phagocyte, a candidate.
Given the questions that have now emerged regarding the role of microglia at CNS synapses, we sought to address precisely how microglia are interacting with developing synaptic circuits and determine the long-term consequences of disrupting microglia function on neural circuit development. In the current study, we demonstrate that microglia engulf presynaptic retinal inputs undergoing synaptic pruning in the postnatal brain and determine that this process is regulated by neuronal activity. Furthermore, we identify signaling through a phagocytic receptor, complement receptor 3 (CR3/CD11b-CD18/Mac-1), expressed on the surface of microglia and its ligand, complement component C3 localized to synaptically-enriched regions, as a key molecular mechanism underlying engulfment of developing synapses. Importantly, disruption of CR3/C3 signaling was specific to microglia in the CNS and resulted in sustained deficits in brain wiring. Taken together, these observations provide a role for microglia in the healthy, developing brain, and provide a cellular and molecular mechanism by which microglia are physically interacting with synaptic elements.
Results
Microglia engulf RGC inputs during a period of active synaptic pruning
To investigate the functional role of microglia in developmental synaptic remodeling, we used the mouse retinogeniculate system, a classic model for studying activity-dependent developmental synaptic pruning (Feller, 1999; Huberman et al., 2008; Shatz and Kirkwood, 1984). Early in development, retinal ganglion cells (RGCs) form exuberant synaptic connections with relay neurons throughout the dorsal lateral geniculate nucleus (dLGN) of the thalamus. During the pruning period, RGC synaptic inputs originating from the same eye as well as between eyes compete for territory throughout the dLGN (Chen and Regehr, 2000; Hooks and Chen, 2006; Jaubert-Miazza et al., 2005; Ziburkus and Guido, 2006). Spontaneous retinal activity plays critical role in this refinement process, however the underlying cellular and molecular mechanisms remain poorly understood. (Del Rio and Feller, 2006; Feller, 1999; Penn et al., 1998; Shatz, 1990; Torborg and Feller, 2005).
During this robust pruning period (P5 in mouse), we used high resolution confocal imaging to assess the interactions between microglia and synaptic inputs throughout the dLGN. Contralateral and ipsilateral presynaptic inputs from RGCs were visualized in the dLGN by intraocular injection of anterograde tracers, cholera toxin β subunit conjugated to Alexa 594 (CTB-594) and Alexa 647 (CTB-647), respectively (Figure 1A). Microglia were labeled using the CX3CR1+/GFP mouse line in which all microglia express EGFP under the control of fractalkine receptor, CX3CR1, expression (Figure 1, S1, and S5) (Cardona et al., 2006; Jung et al., 2000; Saederup et al., 2010).
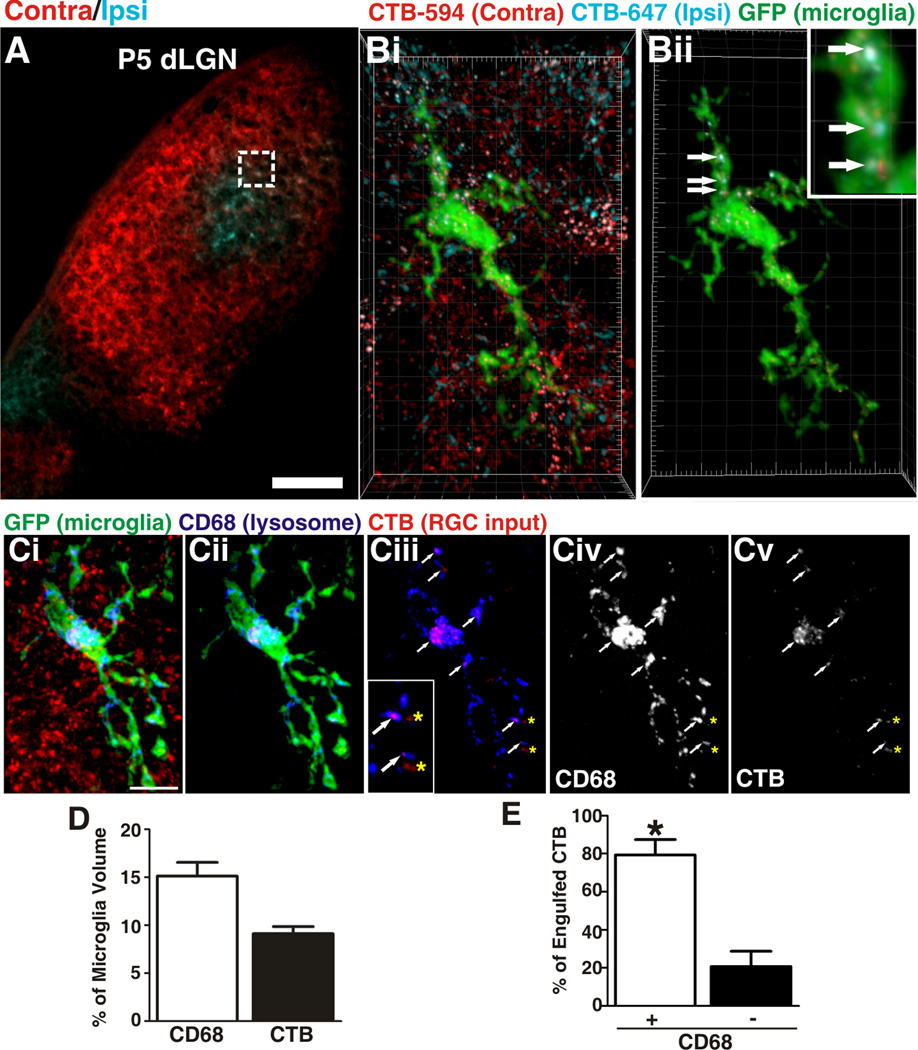
Figure 1. Microglia engulf RGC inputs undergoing active synaptic pruning in the dLGN.
A, A representative low magnification image of P5 dLGN. Ipsilateral inputs are labeled with CTB-647 (blue) and contralateral inputs are labeled with CTB-594 (red). Scale bar = 100 µm. Bi, A microglia (EGFP, green) sampled from the border region of ipsilateral (blue) and contralateral (red) projections (inset in A). Bii, All CTB fluorescence outside the microglial volume has been subtracted revealing RGC inputs (red and blue) that have been engulfed (arrows, enlarged in inset). Grid line increments = 5 µm. Ci, A representative microglia (green, EGFP) from P5 dLGN. RGC inputs from both eyes are labeled with CTB-594 (red) and lysosomes are labeled with anti-CD68 (blue). Cii, The same microglia in which all CTB fluorescence outside the microglia volume has been removed revealing lysosomes (blue) and engulfed RGC inputs (red). Ciii, The same cell in which only the lysosomes (blue) and RGC inputs (red) are visualized in which mostinputs (red) are localized within CD68-positive lysosomes (blue; white arrows). There are few instances in which CTB is not localized to lysosomes (yellow asterisks). Inset is enlarged region of Ciii. Civ-v, The CD68 (Civ) and CTB (Cv) channels alone. Scale bar = 10 µm. D, Quantification of % volume of microglia occupied by CD68-positive lysosomes (white bar) and RGC inputs (black bar), n=3 P5 mice. E, There are significantly more engulfed inputs localized to lysosomal compartments (white bars) versus non-lysosomal compartments (black bars). *P<0.001 by Student’s _t_-test, n=3 P5 mice. All error bars represent s.e.m. See also Figure S1 and Movie S1 and S2.
At an age consistent with robust synaptic pruning (P5), microglial processes were in close association with RGC presynaptic inputs (Figure 1B and S2A). Upon closer examination, we detected numerous fluorescently labeled RGC inputs within the processes and soma of microglia (Figure 1B; Movie S1 and S2). Internalization was further confirmed by assessing confocal z-stacks through individual microglia (Movie S2). This specific example is a microglia sampled from a region containing similar densities of overlapping ipsilateral (blue) and contralateral (red) RGC inputs (Figure 1A) which are undergoing active synaptic remodeling to establish non-overlapping eye specific territories (Figure 2A) (Godement et al., 1984; Guido, 2008; Huberman et al., 2008; Sretavan and Shatz, 1986; Ziburkus and Guido, 2006). Consistent with simultaneous pruning of inputs from both eyes, contralateral (red) and ipsilateral (blue) RGC inputs were engulfed and localized within the microglia (Figure 1B; Movie S1 and S2). In addition, consistent with widespread pruning of RGC inputs throughout the P5 dLGN, we observed engulfment of RGC inputs in all synaptic regions (monocular and binocular). These data suggest that microglia engulf RGC inputs undergoing active synaptic remodeling.
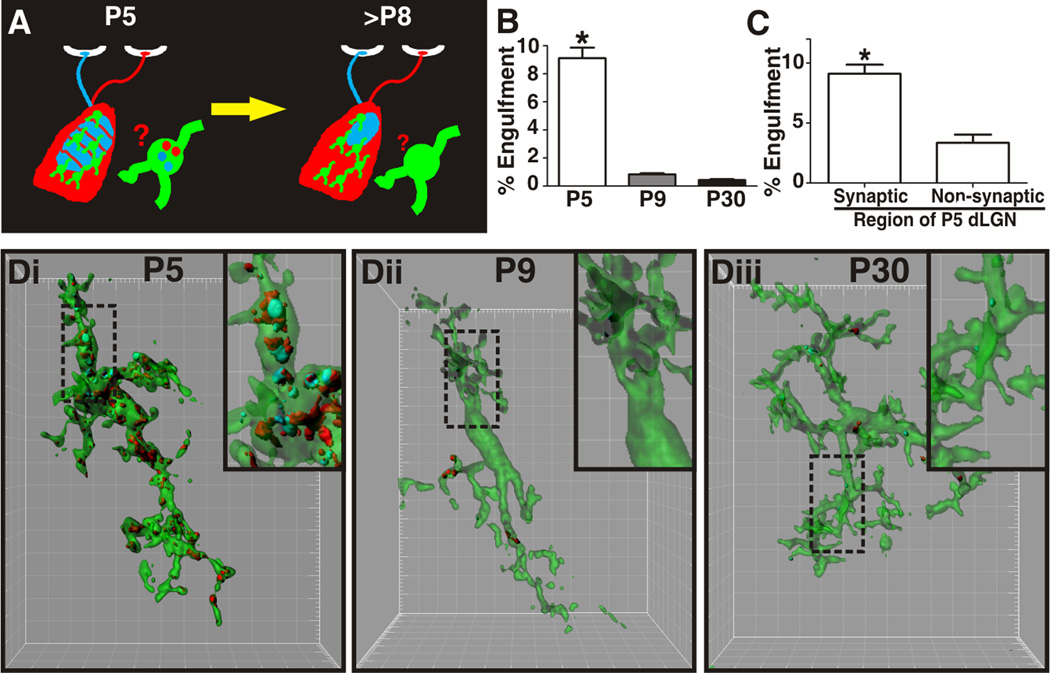
Figure 2. Microglia-mediated engulfment of RGC inputs is developmentally regulated.
A, Schematic of retinogeniculate pruning and strategy used for assessing engulfment. Contralateral (red) and ipsilateral (blue) inputs overlap at early postnatal ages (P5). Inputs from both eyes prune throughout the dLGN during the first postnatal week and is largely complete by P9/10. Engulfment was analyzed throughout the dLGN. B, Engulfment of RGC inputs is significantly increased during peak pruning in the dLGN (P5). *P<0.001 by one-way ANOVA, n=3 mice/age. C, Engulfment in P5 dLGN occurs most significantly in synapse-enriched (contralateral and ipsilateral dLGN) versus non-synaptic (optic tract) regions. *P<0.01 by Student’s _t_-test, n=3 P5 mice. All error bars represent s.e.m. D, Representative surface rendered microglia from P5 (fluorescent image is shown in 1B), P9, and P30 mouse dLGN. Engulfment of RGC inputs occurs during peak pruning (P5) versus older ages (P9 and P30). Enlarged insets denoted with a black dotted line. Grid line increments = 5 µm. See also Figure S2.
To confirm that inputs are phagocytosed by microglia, RGC inputs from both eyes were labeled with CTB-594 and colocalization with CD68, a marker of lysosomes specific to microglia, was assessed in P5 dLGN. As suggested by previous dye-labeling experiments, the majority of engulfed RGC inputs were completely colocalized within lysosomal compartments (Figure 1C–E). There were rare instances in which engulfed RGC inputs did not colocalize (Figure 1Ciii and E) and we suspect that these inputs are either in the process of being phagocytosed or are in phagosomal or endosomal compartments prior to lysosomal degradation. To further validate that microglia phagocytose RGC inputs, pHrodo-dextran, an anterograde tracer and pH-sensitive dye, was used to label RGC inputs (Figure S1A–B) (Deriy et al., 2009; Miksa et al., 2009). Because pHrodo only fluoresces once it enters acidic compartments of lysosomes, any pHrodo-positive fluorescence within a microglia confirms phagocytosis of RGC inputs. Similar to previous experiments, pHrodo-positive RGC inputs were localized within microglia (Figure S1A–B). Furthermore, in addition to anterograde tracing with CTB and pHrodo, RGC input engulfment was also assessed within the P5 dLGN using a genetic approach, double transgenic mice expressing tdTomato under the control of Chx10, a transcription factor expressed by RGCs (Chx10-cre/Rosa26-STOP-tdTomato) (Figure S1C–F). Similar to CTB experiments, we observed tdTomato-labeled RGC inputs within lysosomal compartments of microglia. Importantly, these experiments exclude the possibility that engulfment is due to injury secondary to ocular injections. Together, we demonstrate that microglia phagocytose RGC inputs during a peak period of synaptic pruning in the dLGN.
Microglia-mediated engulfment of RGC inputs is developmentally regulated
To begin to address whether microglia-mediated engulfment of RGC inputs contributes to the normal process of synaptic pruning, we assessed the developmental regulation of microglia phagocytic capacity. We first characterized microglia activation state through development and observed a unique class of microglia in the early postnatal dLGN as compared to older ages (P30) (Figure S2). Microglia within the early postnatal dLGN had characteristic features of more ‘activated’ cells traditionally associated with disease including increased phagocytic capacity (assessed by morphology and CD68 immunoreactivity; Figure S2C,D). Interestingly, early postnatal microglia also had processes, a morphological characteristic of ‘resting’ microglia which are resident in the healthy adult brain (Figure S2B) (Lynch, 2009; Ransohoff and Perry, 2009).
To address whether engulfment of RGC inputs was developmentally regulated, we developed an in vivo phagocytosis assay (Figure 2A). Using high resolution confocal microscopy followed by 3D reconstruction and surface rendering (Figure 2D), internalization of ipsilateral (CTB-647; blue) and contralateral (CTB-594; red) RGC inputs was quantified within the volume of each microglia (CX3CR1+/EGFP) throughout the dLGN. To control for variation in microglia volume, the following calculation was used: % Engulfment = Volume of internalized RGC inputs (µm3)/Volume of microglia (µm3). Consistent with microglial involvement in normal developmental synaptic pruning, engulfment of RGC inputs was developmentally regulated. During a developmental period of robust pruning (P5), engulfment was high (Figure 2B, Di). As few as 4 days later (P9), when much of the pruning is nearly complete, engulfment of RGC inputs was significantly reduced (Figure 2B,Dii). Thus, microglia-mediated engulfment of RGC inputs is temporally correlated with a period of robust synaptic pruning within the developing dLGN. Importantly, similar to P5 dLGN, microglia within the P9 dLGN still retained phagocytic capacity as assessed by morphology and CD68 expression (Figure S2C,D). These data suggest a more specific mechanism is driving engulfment specifically during the peak pruning period in the P5 dLGN.
Microglia-mediated engulfment of RGC inputs is regulated by neural activity
Synaptic pruning is thought to result from competition between neighboring axons for postsynaptic territory based on differences in patterns or levels of activity (Hua and Smith, 2004; Katz and Shatz, 1996; Sanes and Lichtman, 1999). In the dLGN, it is thought that RGC inputs compete for territory such that those inputs which are less active or ‘weaker’ are pruned and lose territory as compared to those inputs that are ‘stronger’ or more active, which elaborate and strengthen (Del Rio and Feller, 2006; Dhande et al., 2011; Huberman et al., 2008; Penn et al., 1998; Shatz, 1990; Torborg and Feller, 2005). This competition can occur between inputs from the same eye as well as between inputs from both eyes (Chen and Regehr, 2000; Hooks and Chen, 2006; Jaubert- Miazza et al., 2005; Ziburkus and Guido, 2006). To determine whether microglia-mediated engulfment of RGC inputs is regulated by neural activity, P4 CX3CR1+/EGFP mice were injected with TTX (0.5 µM) to block RGC activity or forskolin to increase activity (10 mM) (Cook et al., 1999; Dunn et al., 2006; Shatz and Stryker, 1988; Stellwagen and Shatz, 2002; Stellwagen et al., 1999) in the left eye and vehicle (saline or DMSO, respectively) in the right eye. In order to distinguish inputs from each eye, RGC inputs were anterogradely labeled with CTB-594 (TTX or forskolin inputs) and CTB 647 (vehicle inputs) following drug injection (Figure 3A,D). At P5, mice were sacrificed and engulfment was assessed in a region with a similar proportion of ipsilateral and contralateral eye inputs.
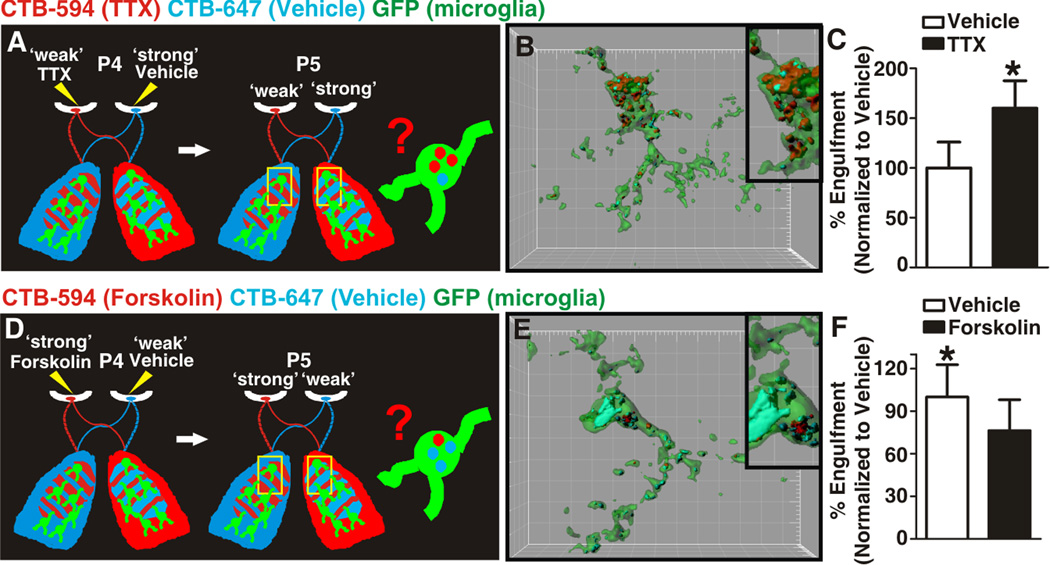
Figure 3. Microglia-mediated engulfment of RGC inputs is regulated by neural activity.
A, D Schematic of strategies used for assessing microglia engulfment following disruption of dLGN pruning by manipulation of neuronal activity. B. Representative P5 microglia (green) surface rendered from the border region of ipsilateral and contralateral projections in which left and right eyes were treated with TTX (red) and vehicle (blue), respectively. Inset is an enlarged region demonstrating the increase in engulfment of inputs from the ‘weaker’, TTX-treated eye (red) as compared to those inputs derived from the ‘stronger’ vehicle-treated eye (blue). Grid line increments = 5 µm. C, Significantly more TTX-treated inputs (black bar) are engulfed as compared to vehicle-treated inputs (white bar). *P<0.04 by Student’s _t_-test, n=4 mice/treatment. E. Representative P5 microglia (green) surface rendered from the border region of ipsilateral and contralateral projections in which left and right eyes were treated with forskolin (red) and vehicle (blue), respectively. Inset is an enlarged region demonstrating an increase in engulfment of inputs from the ‘weaker’, vehicle-treated eye (blue) as compared to those inputs derived from the ‘stronger’ forskolin-treated eye (red). Grid line increments = 5 µm. F, Significantly more vehicle-treated inputs (white bar) are engulfed as compared to forskolin-treated inputs (black bar) within the same dLGN. *P<0.04 by Student’s _t_-test, n= 5 mice/treatment. All error bars represent s.e.m. See also Figure S3.
When mice were injected with TTX and vehicle in the left and right eyes, respectively, microglia phagocytosed significantly more inputs from the less active TTX-treated eye (CTB-594, red) as compared to the vehicle-treated eye (CTB-647, blue) (Figure 3B,C). Likewise, mice injected with forskolin and vehicle engulfed significantly more inputs from the vehicle-treated eye (CTB-647, blue) as compared to the more active forskolin-treated eye (CTB-594, red) (Figure 3E,F). Importantly, this effect occurred in the absence of any significant increase in RGC death (Figure S3). Taken together, these data demonstrate that microglia-mediated engulfment of RGC inputs is regulated by activity such that microglia preferentially engulf inputs from the ‘weaker’ eye and suggest that microglia are active participants in synaptic pruning.
Microglia engulf presynaptic elements specific to RGCs
While it is clear that microglia engulf RGC inputs in a developmental and activity-dependent manner, it is unclear whether engulfed material is axonal and/or synaptic. Consistent with synaptic engulfment, significantly more RGC inputs were engulfed within synapse-enriched regions of the P5 dLGN compared to a non-synaptic region, the optic tract (Figure 2C). To better determine the identity of engulfed material, electron microscopy was performed.
Microglia were identified by EM using criteria previously described including a small, irregular-shaped nucleus containing substantial amounts of coarse chromatin and a cytoplasm rich in free ribosomes, vacuoles, and lysosomes (Mori and Leblond, 1969; Sturrock, 1981). Consistent with our confocal data, we observed several inclusions completely within the microglia cytoplasm including several double membrane-bound structures which contained 40 nm vesicles, data consistent with engulfment of presynaptic terminals (Figure 4A,B and S4). In a few instances, structures reminiscent of juxtaposed pre and postsynaptic structures were observed (Figure 4Aii).
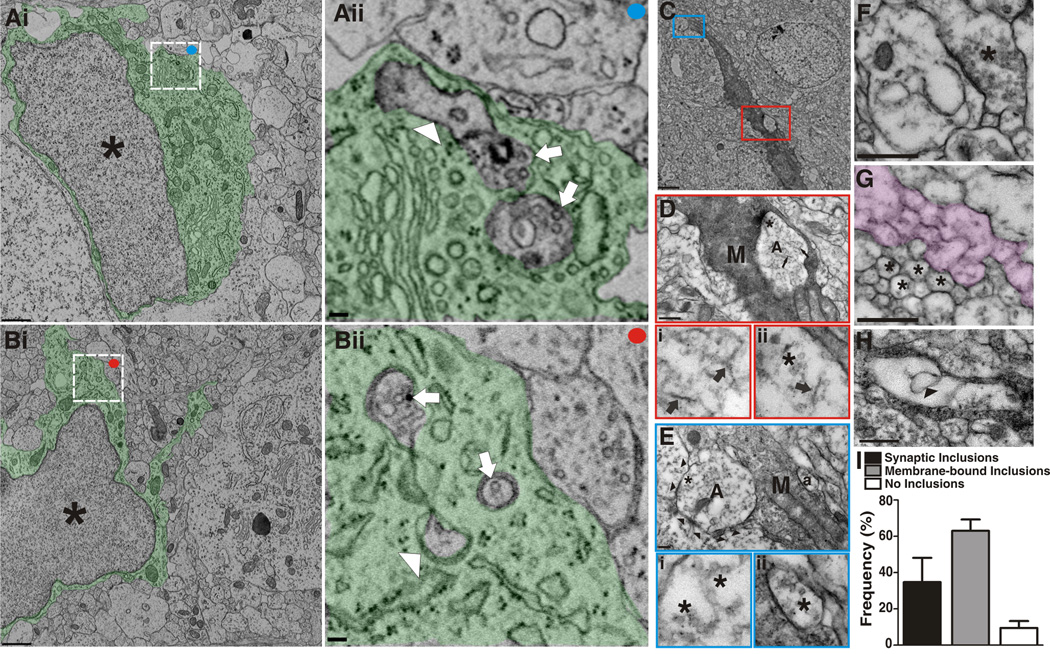
Figure 4. Microglia engulf preysnaptic elements undergoing active synaptic pruning.
Ai, Bi. Low magnification EM of microglia. Asterisks denote the nucleus and the cytoplasm is pseudocolored green. Scale bar = 1 µm. Aii, Bii. Magnified regions of Ai and Bi (white boxes) demonstrating membrane-bound elements engulfed by microglia. Arrows designate elements containing presynaptic machinery (40 nm vesicles). The arrowhead in Aii designates engulfed material resembling juxtaposed postsynaptic elements. Scale bar = 100 nm. C, Low magnification EM of a microglia immunolabeled for iba-1 in P5 dLGN (DAB-positive cell). Red and blue boxes indicate enlarged regions in D and E, respectively. Scale bar = 2 µm. D, RGC input (A) localized within the iba-1-positive microglia (M). Within the engulfed input, neurofilaments (arrows, enlarged in Di and Dii) and 40 nm vesicles (asterisks, enlarged in Dii) are indicative of presynaptic machinery. D, Scale bar = 500 nm. E, RGC input (A) enwrapped by a microglial process (M, arrowheads denote microglial process). 40 nm Vesicles are also visible (asterisks, enlarged region in Ei). Another presynaptic element (a) containing 40 nm vesicles is surrounded by microglia cytoplasm (enlarged region in Eii). Scale bars = 100 nm. F, An intact excitatory synapse in P5 dLGN in which the presynaptic terminal (asterisk) contains 40 nm vesicles. Scale bar = 500 nm. G, Cross (asterisks) or longitudinal sections (pseudocolor) through axons are relatively void of vesicles. Scale bars=500 nm. H, A membrane-bound structure (arrowhead) completely within a microglial (M) lysosome (L). Scale bar = 500 nm. I, The frequency at which engulfed material was observed in microglia from P5 dLGN, n=20 cells. Error bars represent s.e.m. See also Figure S4.
To further confirm microglia-mediated phagocytosis of synaptic elements, immunohistochemical electron microscopy (immunoEM) for the microglia marker iba-1 was performed and quantified in the P5 dLGN (Figure 4C) (Tremblay et al., 2010b). Consistent with EM data described above, we observed membrane-bound structures containing 40 nm presynaptic vesicles that were completely surrounded (Figure 4D) or enwrapped (Figure 4E) by DAB-positive microglial cytoplasm. To further support that microglia engulf material specific to presynaptic terminals, 40 nm vesicles were enriched in presynaptic terminals (Figure 4Bii,F) and very rarely visualized in cross or longitudinal sections of axons (Figure 4G). Indeed, presynaptic elements were observed within 35% of the microglia sampled (Figure 4I). Interestingly, several intact presynaptic terminals (Figure 4F) and all engulfed or enwrapped presynaptic inputs (Figure 4A,B,D,E) lacked mitochondria, a characteristic feature of presynaptic terminals. Previous work has suggested that sensory deprivation or pharmacological blockade of neuronal activity (i.e., TTX) results in reduced mitochondria in presynaptic terminals known to undergo subsequent elimination (Hevner and Wong-Riley, 1993; Tieman, 1984). Thus, we suspect that these terminals deficient in mitochondria may be those destined for elimination.
In addition to presynaptic element engulfment, 63% of the sampled cells contained structurally unidentifiable membrane-bound inclusions within microglial lysosomal compartments (Figure 4H). We suspect that this membraneous cellular material is synaptic material rapidly degraded in lysosomal compartments thereby rendering it undistinguishable by ultrastructure. Unlike presynaptic elements, engulfed material resembling postsynaptic elements was very rarely observed (Figure 4Aii). However, rapid degradation of structural elements may preclude visualization of the postsynaptic density. Importantly, there were rare instances in which no engulfed material was observed within microglia (Figure 4I, no inclusions, 10% of sampled cells).
To directly address whether microglia are engulfing RGC presynaptic terminals, immunohistochemistry in P5 dLGN for presynaptic machinery specific to RGCs (i.e., VGlut2) followed by high resolution imaging was performed. 3D structural illumination microscopy (3D-SIM), a technique enabling 2X the resolution of light microscopy (Gustafsson, 2000), was used to assess the P5 dLGN of CX3CR1+/EGFP mice immunolabeled for VGlut2. 3D-SIM data revealed VGlut2 immunoreactivity within the EGFP-positive cytoplasm of microglial cells (Figure 5A–D). Consistent with previous confocal and ultrastructural data (Figures 1–4), these data suggest that microglia are engulfing RGC presynaptic terminals.
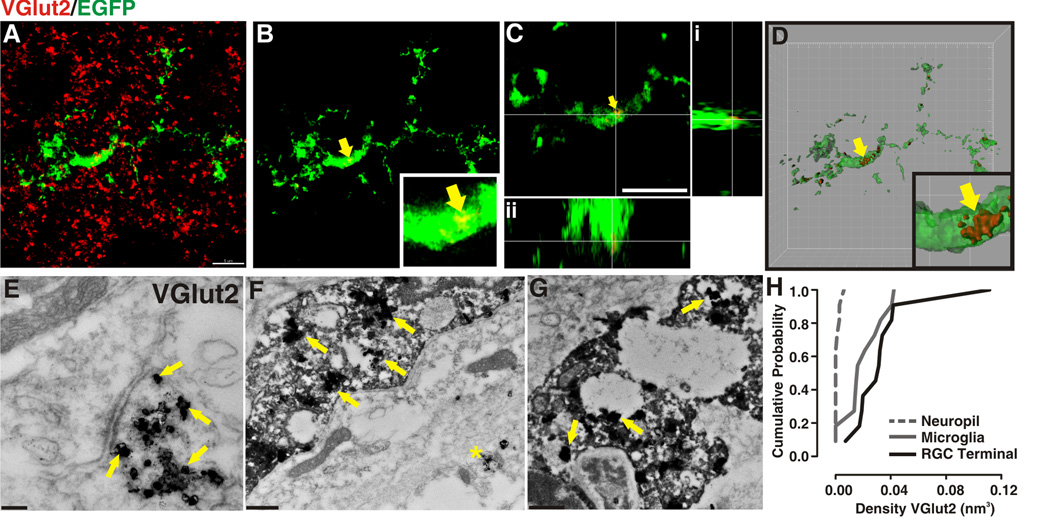
Figure 5. Microglia engulf presynaptic terminals specific to RGCs.
A–D, 3D-SIM in P5 CX3CR1+/EGFP dLGN in which microglia are labeled with EGFP (green) and RGC presynaptic terminals are immunolabeled with anti-VGlut2 (red). A, Maximum intensity projection (MIP) of microglia and VGlut2 immunostaining in P5 dLGN. B, MIP in which all VGlut2 fluorescence (red) that is not within the microglia (green) has been subtracted. Yellow arrow designates examples of engulfed VGlut2-positive elements, enlarged in inset. C,D Orthogonal views (C) and surface rendering (D) of region in B (yellow arrow and inset). A–D, Scale bar = 5 µm. D, grid line increments = 2 µm. E–G, Double immunoEM in P5 dLGN for iba-1 (DAB) and VGlut2 (immunogold). E, RGC presynaptic terminals are enriched with VGlut2 immunoreactivity (immunogold, yellow arrows). F,G, Similar to RGC terminals (E), microglial cytoplasm (DAB) and lysosomes contain VGlut2 immunogold labeling (yellow arrows). Asterisk in F denotes a VGlut2-positive presynaptic terminal within the same field of view as the microglia. Scale bars = 100nm. H, Cumulative probability demonstrates that there is increased probability of VGlut2 localization to a RGC terminal (black solid line) or microglia (grey solid line) versus random occurrence throughout the neuropil (grey dotted line). For each structure, n=10.
To further confirm that microglia were engulfing RGC presynaptic terminals, double immunoEM in P5 dLGN for iba-1 (DAB) and a presynaptic marker specific to RGC terminals, VGlut2 (immunogold; Figure 5E–G) was performed. Consistent with 3D-SIM data previously described, we observed immunogold labeling for VGlut2 within the microglia cytoplasm and lysosomes (Figure 5F,G). Because immunogold was overexposed in order to gain contrast against the DAB reactivity, vesicle membranes surrounding the VGlut2 labeling were not observed within intact presynaptic terminals (Figure 5E) or microglia (Figure 5F,G). In addition, cumulative probability calculations demonstrated an increased probability of VGlut2 localized to an RGC terminal or microglia as compared to random immunoreactivity throughout the neuropil (Figure 5H). Similar to results from confocal microscopy experiments (Figures 1–3), these ultrastructural data reveal that microglia engulf presynaptic terminals specific to RGCs.
Deletion of the CR3/C3-dependent phagocytic signaling decreases the capacity of microglia to engulf RGC inputs
What molecular mechanism(s) underlies microglia-mediated engulfment of synaptic inputs? In the peripheral immune system, phagocytic cells can interact with several different immune-related signaling pathways to mediate clearance of cellular material. Included among these pathways are proteins belonging to the classical complement cascade, which bind surface receptors expressed by phagocytic cells. Given previous work demonstrating that complement component C3 is enriched at synapses and is necessary for pruning of retinogeniculate synapses (Stevens et al., 2007), we hypothesized that C3 ligand-receptor signaling may be one molecular mechanism by which microglia interact with and engulf RGC synaptic inputs.
Consistent with this hypothesis, CR3, a high affinity receptor for activated C3 (Akiyama and McGeer, 1990; Perry et al., 1985), was specifically upregulated in microglia in the P5 dLGN and downregulated at later developmental time points (Figure 6A). Importantly, other cell types known to express the surface receptor CR3 and/or have phagocytic capacity (i.e., infiltrating monocytes, macrophages, etc.) were completely absent from the P5 dLGN and surrounding brain tissue (Figure S5) (Akiyama and McGeer, 1990; Perry et al., 1985). As a result, in the context of the P5 brain, genetic manipulation of CR3 is specific to microglial cells. Similar to CR3 and consistent with our previous work, immunohistochemistry for CR3 ligand, C3 was enriched in synaptic regions of P5 dLGN and downregulated by P9, an age when pruning is largely complete (Figure 6B) (Stevens et al., 2007). These data demonstrate that CR3 and its ligand, C3, are expressed at an appropriate age and location to mediate RGC input engulfment.
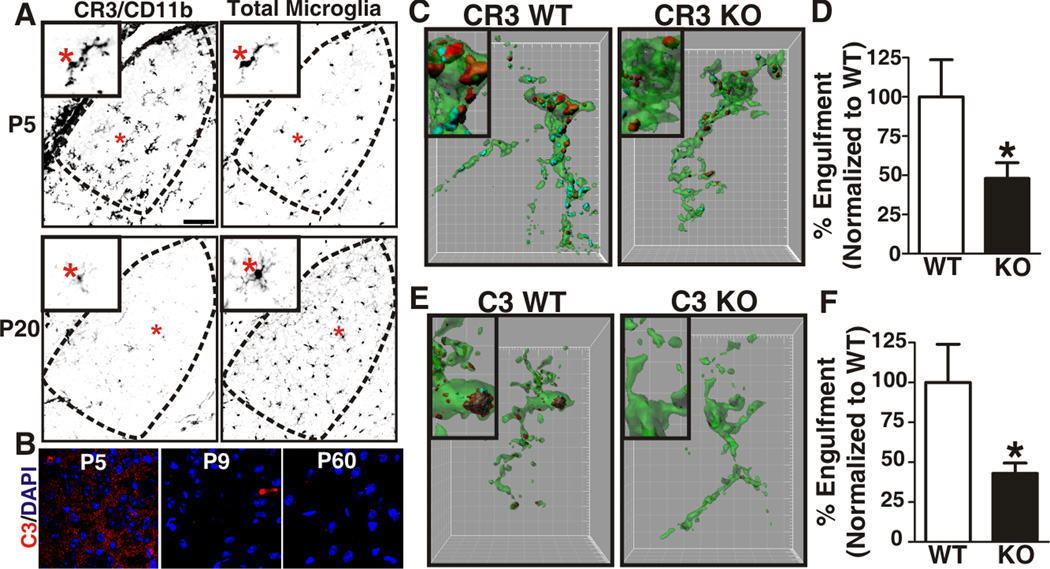
Figure 6. CR3/C3-dependent signaling regulates engulfment of synaptic inputs by microglia.
A, Immunohistochemistry for the alpha subunit of CR3 (CD11b) reveals that microglia express high levels of CR3/CD11b (left column) in the P5 dLGN (top panels) versus older ages (P20, bottom panels). Total microglia are visualized with GFP (CX3CR1+/EGFP, right column). Insets are magnified regions (red asterisks). Scale bar = 100 µm. B, Immunohistochemistry in the developing dLGN for C3 (red). A single plane confocal image reveals that C3 levels are increased in the P5 dLGN versus older ages (P9, P60). Scale bar = 10 µm. C, E, Representative surface rendered microglia (green) from P5 dLGN of WT (left) or KO (right) littermates in which RGC inputs were labeled with CTB-594 (red, contralateral) and CTB-647 (blue, ipsilateral). Insets are enlarged regions demonstrating reduced RGC input engulfment (red and blue) in CR3 (C) and C3 (E) KO mice. Grid line increments = 5 µm. D,F, P5 CR3 KO (D) and C3 KO (F) mice (black bars) engulf significantly fewer RGC inputs as compared to WT littermates (white bars). All data are normalized to WT control values. D, *P<0.04 by Student’s _t_-test, n=3 mice/genotype. E, *P<0.01 Student’s _t_-test, n=4 mice/genotype. All error bars represent s.e.m. See also Figure S5.
Using the in vivo phagocytosis assay previously described (Figure 2), engulfment was assessed in P5 mice lacking functional CR3 (CR3 KO) due to a genetic deletion of the alpha subunit, CD11b (Figure S5B) (Coxon et al., 1996) or mice deficient in CR3 ligand, C3 (C3 KO) (Figure S5A). Microglia sampled from P5 CR3 or C3 KO mice had a statistically significant decrease in capacity to engulf RGC inputs as compared to WT littermate controls (Figure 6C–F). Taken together, these data demonstrate that phagocytic signaling through CR3 and its ligand C3 is one molecular mechanism by which microglia engulf RGC inputs.
Disruption of CR3 signaling in microglia results in sustained deficits in structural remodeling of RGC inputs
During the first postnatal week, overlapping inputs from both eyes segregate into eye specific territories (i.e., eye-specific segregation) resulting in the termination of ipsilateral and contralateral inputs in distinct non-overlapping domains in the mature dLGN (see Figure 2A) (Godement et al., 1984; Guido, 2008; Huberman et al., 2008; Jaubert-Miazza et al., 2005; Sretavan and Shatz, 1986; Ziburkus and Guido, 2006). Consistent with our hypothesis that microglia play a role in synaptic pruning, C3 KO mice have previously been shown to have deficits in eye-specific segregation (Stevens et al., 2007). To determine whether microglia are mediators of C3-dependent synaptic refinement in the CNS, we quantified eye-specific segregation in CR3 KO mice. Ipsilateral and contralateral RGC inputs were labeled by intraocular injection of CTB-594 (red) and CTB-488 (green), respectively. Animals were subsequently sacrificed within 24 hrs of the initial dye injection and overlap (yellow) between contralateral and ipsilateral RGC projection territories was quantified. In this experimental paradigm, an increase in the % overlap between the ipsilateral and contralateral projections within the dLGN is indicative of a deficit in synaptic pruning (Bjartmar et al., 2006; Huh et al., 2000; Pham et al., 2001; Ravary et al., 2003; Stevens et al., 2007).
Consistent with the hypothesis that microglia mediate complement-dependent synaptic pruning, a statistically significant increase in ipsilateral and contralateral input overlap was observed in P10 and P30 CR3 KOs as compared to WT littermate controls (Figure 7 A–C). This increase in overlap was attributed to a significantly broader ipsilateral projection territory (Figure 7D) and a small, but not significant, increase in the contralateral projection territory (Figure 7E). Furthermore, at higher magnification we detected aberrant ipsilateral and contralateral RGC inputs within the inappropriate monocular region (contralateral and ipsilateral, respectively) in mature CR3 KO dLGN (P30; Figure 7F,G). In addition to genetic manipulation of CR3, microglia involvement in eye-specific segregation was further validated by manipulating microglia function pharmacologically using minocycline, an established inhibitor of microglial ‘activation’ (Buller et al., 2009) (Figure S6A–E). Similar to CR3 KO data, minocycline (P4–P8; 75 mg/kg) treatment during the peak of the pruning period resulted in reduced microglial phagocytic function (i.e., reduced RGC input engulfment) at P5 and a statistically significant deficit in eye-specific segregation at P10 (Figure S6C–E). Importantly, prior to any analyses we confirmed that any phenotype in KO or drug-treated mice was not due to differences in total RGC number within the retina and/or density of microglia within the dLGN (Figure S6F–K).
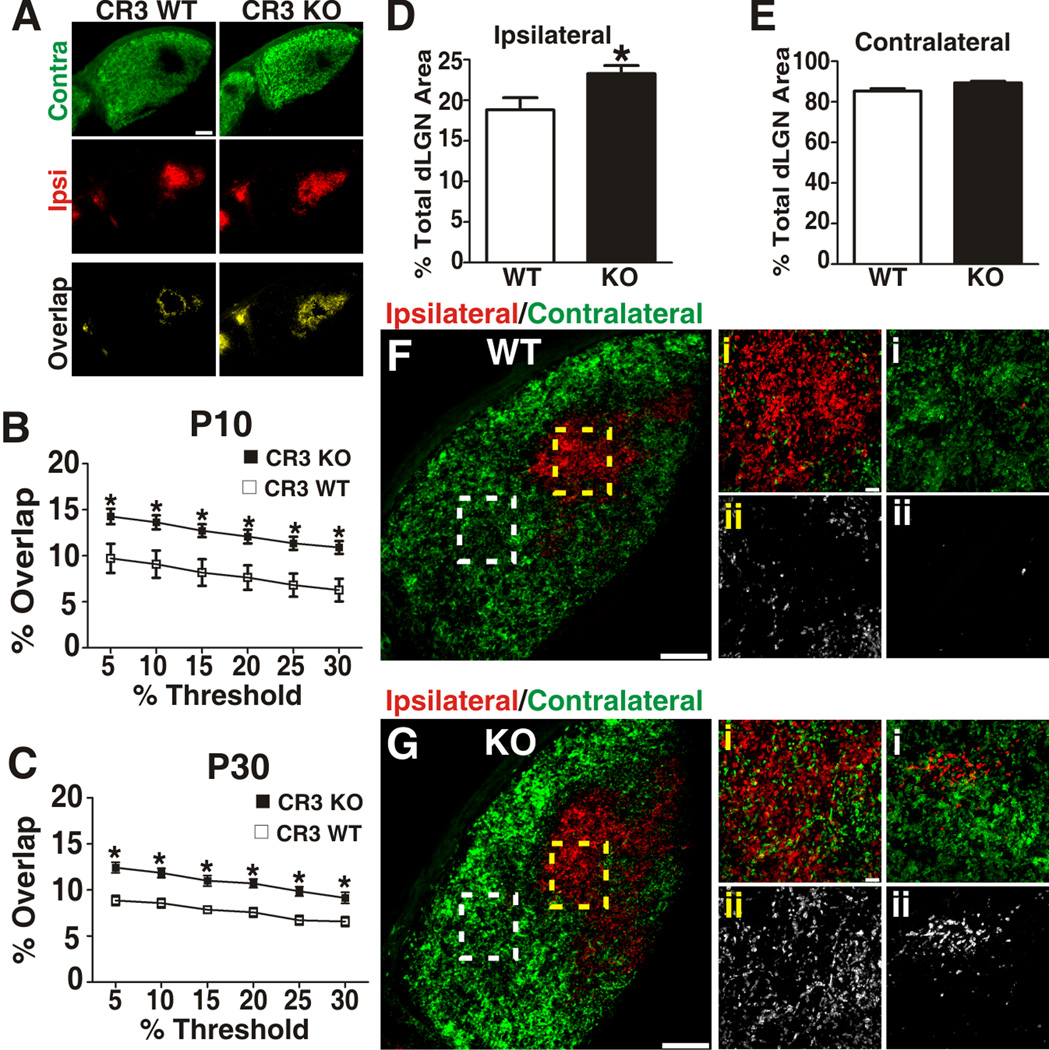
Figure 7. CR3 KO mice have sustained deficits in eye-specific segregation.
A, Representative image of a P30 WT (left) demonstrates minimal overlap (yellow) between ipsilateral (red) and contralateral (green) RGC inputs. Indicative of a synaptic pruning deficit, CR3 KO mice (right) had increased overlap (yellow) of ipsilateral (red) and contralateral (green) RGC inputs. Scale bar = 100 µm. B–C, P10 (B) and P30 (C) CR3 KO mice had statistically significant, threshold-independent deficits in retinogeniculate pruning. D, The percentage of ipsilateral territory is significantly increased in P30 CR3 KO mice as compared to WT littermate controls. E, Although trending towards an increase, there is no statistically significant difference in percentage of contralateral territory. F, G dLGN from P30 CR3 WT (F) or KO (G) mice, dotted line boxes in lower magnification image (left panels) correspond to ipsilateral region magnified in middle panels (yellow i–ii) or contralateral region magnified in far right panels (white i–ii). Bottom panels in F,G (ii) are contralateral (CTB-488, green, left panel) channel or ipislateral (CTB-594, red, right panel) alone. G, There were increased aberrant contralateral projections (middle panel; i, green and ii) within the ipsilateral territory in P30 CR3 KO mice as compared to WT littermates (F, middle panel). Similarly, there were aberrant ipsilateral projections (right panel; i, red and ii) within contralateral regions of the dLGN in CR3 KO mice as compared to WT littermates (F, right panel). Left panels, scale bar = 100 µm. Middle and right panels, scale bar = 10 µm. B–C, *P<0.0001 by Student’s _t_-test, n=6 (P10) or 4 (P30) mice/genotype. D, *P<0.03 by Student’s _t_-test, n=4 mice/genotype. All error bars represent s.e.m. See also Figure S6.
Taken together, disruption in microglia function by pharmacological (minocycline) or more specific genetic strategies (CR3 or C3 KOs) results in sustained deficits in eye-specific segregation within the dLGN. Furthermore, given that microglia are the only CNS cell that express CR3 in the postnatal dLGN, these data suggest that microglia are mediators of synaptic remodeling in the retinogeniculate system and represent a key cellular mechanism underlying complement-dependent synaptic pruning (Stevens et al., 2007).
Disruption in CR3/C3-dependent signaling in microglia results in sustained deficits in synaptic connectivity
If CR3/C3-dependent signaling in microglia is a mechanism underlying developmental synaptic pruning, then a sustained increase in synapse density would be expected in the absence of these molecules. To test this possibility, retinogeniculate synapse density was quantified in adult CR3 KOs (P32-35) using array tomography (AT), a powerful tool for high resolution imaging and quantification of synaptic density in vivo (Greer et al., 2010; Margolis et al., 2010; Micheva and Smith, 2007; Ross et al., 2010). RGC presynaptic terminals within the dLGN were labeled with an antibody directed against VGlut2 and postsynaptic excitatory sites were labeled with anti-GluR1. As suggested by the eye-specific segregation assay, there was a statistically significant increase (1.3-fold increase) in RGC synapse density (i.e., juxtaposed GluR1 and VGlut2 puncta) in adult CR3 KOs as compared to WT littermates (Figure 8 A,B). Consistent with our previously published work (Stevens et al., 2007), adult C3 KO mice had an identical 1.3-fold increase in VGlut2-containing synapses as compared to WT littermate controls (Figure S7). Interestingly, there was also a significant increase in the density of total (both synapse associated and non-associated) VGlut2-positive puncta in CR3 KOs (1.8-fold increase) as compared to WT littermates (Figure 8D). We hypothesize that these excess VGlut2-positive puncta represent residual immature synapses as well as retracted or unopposed immature presynaptic terminals that were not eliminated by phagocytic microglia. Taken together, these data implicate CR3/C3 signaling as a mechanism regulating synaptic connectivity.
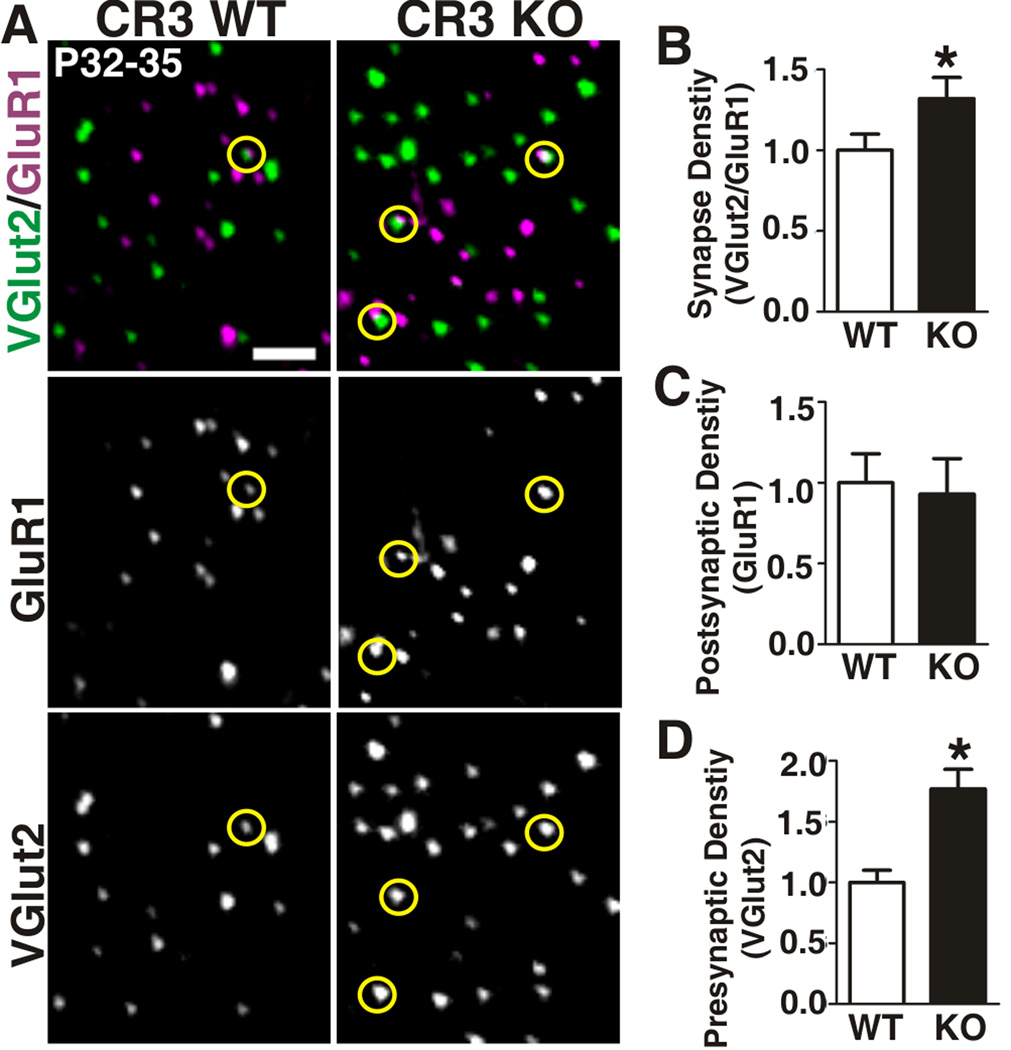
Figure 8. CR3 KO mice have a sustained increase in synapse density.
A, Single plane array tomography images for VGlut2 (green) to label RGC terminals and GluR1 (purple) to label postsynaptic excitatory sites in P32-35 dLGN of CR3 KO (right) and WT littermate controls (left). Yellow circles indicate synapses defined by VGlut2 and GluR1 immunoreactivity. Scale bar = 2 µm. B-D, Quantification of retinogeniculate synapse (B, VGlut2/GluR1-positive), postsynaptic (C, GluR1), and presynaptic/RGC terminal (D, VGlut2) density indicates that there is a statistically significant increase in retinogeniculate synapse density and total RGC terminal density in CR3 KOs as compared to WT littermates. *P<0.03 Man-Whitney U test, n=3 mice/genotype. Error bars represent s.e.m. See also Figure S7.
Because microglia are the only cell type within the P5 dLGN and surrounding brain tissue to express CR3 (Figure 6; Figure S5) (Akiyama and McGeer, 1990), our data directly implicate microglia as mediators of anatomical pruning and identify CR3/C3-dependent signaling as an underlying molecular mechanism.
DISCUSSION
In this study, we demonstrate that microglia are mediators of synaptic pruning in the normal, developing brain and identify neural activity and CR3/C3-dependent signaling as underlying mechanisms. Specifically, we demonstrate that: 1) Microglia in the postnatal dLGN engulf RGC presynaptic terminals during active synaptic remodeling. 2) Engulfment of RGC inputs is regulated by neuronal activity. 3) Engulfment of RGC inputs is regulated by CR3/C3-dependent phagocytic signaling specific to microglia. 4) Genetic (CR3 and C3 KO) or pharmacological perturbations that disrupt microglia function result in deficits in structural remodeling of synapses. 5) Defects in synaptic circuitry are sustained into adulthood in CR3 and C3 KO mice. We propose a model in which neural activity and complement work cooperatively to mediate engulfment of RGC inputs, a process that may underlie synaptic pruning in the developing CNS (Figure S7).
Microglia engulf RGC presynaptic inputs during peak synaptic pruning
One question arising is whether engulfment of RGC inputs by microglia is an active process. Particularly during CNS disease, microglia are known scavengers that phagocytose cellular debris (Hanisch and Kettenmann, 2007; Napoli and Neumann, 2009; Ransohoff and Perry, 2009). Furthermore, glia are known to engulf axonal material during large-scale developmental pruning of axons in Drosophila and synaptic pruning at the mammalian neuromuscular junction (Bishop et al., 2004; Freeman, 2006; Rochefort et al., 2002). While our results do not rule out the possibility that axonal material may also be engulfed, our data suggest that microglia play an active role in the removal of transient, intact presynaptic elements. Indeed, in comparison to large scale developmental axonal pruning, there is no evidence that local CNS synaptic pruning, such as in the case of the retinogeniculate system, involves classic axonal or synaptic degeneration (Dhande et al., 2011; Hahm et al., 1999; Snider et al., 1999; Sretavan and Shatz, 1984). Earlier EM work in the developing mammalian dLGN demonstrated that RGCs transiently synapse within the inappropriate region of the dLGN (Campbell and Shatz, 1992; Campbell et al., 1984). These transient synapses contained presynaptic machinery including a high density of vesicles, but were subsequently eliminated by an undetermined mechanism. Given our high resolution light microscopy and ultrastructure data, we suggest that microglia are actively pruning these transient synaptic connections via a phagocytic mechanism (Figure S7).
We provide several lines of evidence implicating microglia in the local pruning of transient, intact retinogeniculate synapses in the absence of axon debris or degeneration. First, in experiments involving anterograde tracing of RGCs (engulfment and eye-segregation assays), intraocular injections of dye occur less than 24 hrs prior to tissue harvesting and fixation. If neurons or axons were degenerating, we would not expect effective dye uptake and tracing of the entire RGC projection. Furthermore, previous work has demonstrated that RGC normal programmed cell death is essentially complete by P4/P5 (Farah and Easter, 2005). Taken together, any CTB labeling observed within the dLGN is, more likely, originating from a healthy RGC cell body and axon. Second, previous work using dye tracing or fluorescent protein to label small subsets of RGC afferents in the dLGN demonstrate that RGC axons and arbors within the dLGN undergoing active pruning remain intact and unfragmented (Dhande et al., 2011; Hahm et al., 1999; Snider et al., 1999; Sretavan and Shatz, 1984). Consistent with these data, our EM experiments demonstrated that engulfed material as well as surrounding dLGN neuropil did not appear to have classic signs of axonal or synaptic degeneration such as multilamellar bodies, electron-dense cytoplasm, lack of synaptic vesicles within presynaptic terminals, etc. (Hoopfer et al., 2006; Perry and O'Connor, 2010). Lastly, we observed sustained increases in the number of intact, structural synapses by eye specific segregation and array tomography analyses in mice with disrupted microglia function (C3 KO, CR3 KO, and minocycline-treated mice). If synapses degenerated prior to engulfment, we would not expect to observe increased numbers of healthy, intact synapses in KO mice. Taken together, our data suggest that engulfed presynaptic elements were healthy, intact, and specifically engulfed by microglia.
Engulfment of RGC inputs by microglia is an activity-dependent process
Previous work has demonstrated that microglia have the capacity to interact with synaptic elements in response to neurotransmitter release and/or sensory experience (Biber et al., 2007; Fontainhas et al., 2011; Nimmerjahn et al., 2005; Ransohoff and Perry, 2009; Tremblay et al., 2010a; Wake et al., 2009). Furthermore, microglia can contribute to synaptic plasticity in the adult CNS and, more recently, in the context of the normal developing hippocampus (Paolicelli et al., 2011; Pascual et al., 2011; Roumier et al., 2008). Our data provide insight into mechanisms by which microglia may interact with synapses and contribute to activity-dependent synaptic plasticity. When competition between inputs from the two eyes was enhanced by pharmacological manipulation (i.e., TTX or forskolin), we found that microglia preferentially engulfed inputs from the eye in which neuronal activity was decreased or ‘weaker’. Although it is not yet known whether or how microglia target specific ‘weaker’ synapses, these data are consistent with previous work demonstrating that such a competition results in decreased territory of the ‘weaker’ inputs and increased territory of ‘stronger’ inputs within the dLGN (Del Rio and Feller, 2006; Huberman et al., 2008; McLaughlin et al., 2003; Penn et al., 1998; Shatz, 1990; Shatz and Stryker, 1988; Stellwagen and Shatz, 2002; Stellwagen et al., 1999; Torborg and Feller, 2005).
In the retina, spontaneous, correlated neuronal activity from both eyes (i.e., retinal waves) drives the elimination of synapses and segregation of inputs into eye-specific territories in the dLGN (Del Rio and Feller, 2006; Feller, 1999; Huberman et al., 2008; McLaughlin et al., 2003; Penn et al., 1998; Stellwagen and Shatz, 2002; Torborg and Feller, 2005). Interestingly, complement and complement receptor-deficient mice have similar pruning deficits to mice in which this correlated firing has been disrupted (e.g., cAMP-analog injection, β_2nAChR_−/− mice, etc.) (Stevens et al., 2007), suggesting the intriguing possibility that complement cascade activation and function is regulated by neural activity. Neural activity could also directly regulate microglia function (i.e., activation, recruitment, phagocytic capacity) through complement-independent mechanisms. Alternatively, neural activity may drive the elimination of synapses by other mechanisms which ultimately lead to complement activation and/or microglia-mediated engulfment. Future studies will aim to address how neural activity, complement, and microglia may interact to contribute to developmental synaptic pruning (Figure S7).
CR3/C3-dependent signaling: A molecular pathway underlying microglia-mediated synaptic pruning
Synaptic pruning likely involves several mechanisms that cooperatively interact to establish precise synaptic circuits. We suggest that microglia may be a common link and identify CR3/C3 signaling as one pathway underlying microglia-synapse interactions and microglia-dependent pruning in the developing CNS. One of the major questions raised by these findings is precisely how secreted complement proteins mediate the selective elimination of synapses by microglia. In the immune system, C3 is cleaved into an activated form, iC3b, which covalently binds to the surface of cells or debris and targets them for elimination by macrophages via specific phagocytic receptor signaling (e.g., CR3) (Lambris and Tsokos, 1986; van Lookeren Campagne et al., 2007). Similar to the immune system, we propose that activated C3 (iC3b/C3b) could selectively ‘tag’ weak synapses (Figure S7). Consistent with C3 “tagging” subsets of RGC terminals, previous confocal analysis revealed colocalization of C3 with pre and postsynaptic markers in the developing dLGN (Stevens et al., 2007). Furthermore, mice deficient in CR3, C3, and C1q, the initiating protein of the classical complement cascade, exhibit strikingly similar defects in developmental synaptic pruning (Figure 7,8 and S7). Alternatively, other complement-dependent and/or independent mechanisms may be involved. For example, C3 could bind all synapses and only those synapses that are ‘stronger’ or more active are selectively protected by membrane-bound complement regulatory molecules (Kim and Song, 2006; Song, 2006). In contrast, selective, activity-dependent elimination of synapses could be driven by a complement-independent mechanism which subsequently results in complement binding and/or microglia-mediated engulfment. For example, MHC class I molecules, another class of immune molecules demonstrated to play a role in retinogeniculate pruning, have been shown to be activity-dependent, localized to synapses, and co-localized with C1q leaving the possibility that MHC class I molecules may play an upstream role in microglia-mediated pruning of synapses (Corriveau et al., 1998; Datwani et al., 2009; Goddard et al., 2007; Huh et al., 2000).
While our data demonstrate that CR3/C3 signaling specific to microglia is involved in the pruning of developing circuits and suggest that engulfment is the underlying mechanism, CR3 and C3 may be acting through other pathways independent of phagocytosis or may be downstream of other signaling pathways to mediate pruning. In addition, engulfment deficits in CR3 and C3 KO mice were reduced to approximately 50% of WT littermate control values suggesting that other complement receptor-dependent (e.g., CR4, CRig, etc.) and independent phagocytic mechanisms may also be involved. Future studies will aim to address whether and how specific synapses are eliminated by complement and other microglia-dependent mechanisms and how neural activity plays a role in this process.
Complement-dependent engulfment of synaptic inputs: A more global mechanism underlying remodeling of neural circuits in the healthy and diseased CNS?
Our data raise the question as to whether complement and/or microglia-dependent engulfment of synaptic inputs represents a more global mechanism underlying CNS neural circuit plasticity. While in at least one other developing system local axonal retraction and synapse elimination appear to occur independent of microglia (Cheng et al., 2010), recent work describes a role for microglia at developing hippocampal synapses (Paolicelli et al., 2011). In addition, in vivo imaging studies in the cortex revealed that microglia dynamics and interactions with neuronal compartments change in response to neural activity and experience (Davalos et al., 2005; Nimmerjahn et al., 2005; Tremblay et al., 2010a; Wake et al., 2009). While these studies describe microglia dynamics at synapses, a precise function and molecular mechanism(s) underlying microglia-synapse interactions in these brain regions was unknown. Our study provides mechanistic insight on the dynamic between microglia and developing synapses and provides complement-dependent signaling as a potential mechanism in other brain regions. Consistent with this idea, deficits in complement component C1q results in an increase in the number of presynaptic boutons and exuberant excitatory connectivity in the cortex (Chu et al., 2010). Future studies will aim to test the role of complement in microglia-synapse interactions in other CNS regions known to undergo activity-dependent synaptic remodeling.
In addition to relevance in global remodeling of circuits in the healthy brain, our findings have important implications for understanding mechanisms underlying synapse elimination in the diseased brain. Consistent with this idea, abnormal microglia function and complement cascade activation have been associated with neurodegeneration of the CNS (Alexander et al., 2008; Beggs and Salter, 2010; Rosen and Stevens, 2010; Schafer and Stevens, 2010; Stephan et al. 2012). Indeed, in a mouse model of glaucoma, a neurodegenerative disease associated with RGC loss and gliosis, C1q and C3 are highly upregulated and deposited on retinal synapses and C1q deficiency or microglial ‘inactivation’ with minocycline provide significant neuroprotection (Howell et al., 2011; Steele et al., 2006; Stevens et al., 2007). In addition to diseases associated with neurodegeneration, recent data from genome-wide association studies and analyses of postmortem human brain tissue have suggested that microglia and/or the complement cascade may also be involved in the development and pathogenesis of neurodevelopmental and psychiatric disorders (e.g., autism, obsessive compulsive disorder, schizophrenia, etc.) (Chen et al., 2010; Havik et al., 2011; Monji et al., 2009; Pardo et al., 2005; Vargas et al., 2005). Thus, an intriguing possibility remains that microglia and/or complement dysfunction may be directly involved in diseases associated with synapse loss, dysfunction, and/or development.
Together, our data offer insight into mechanisms underlying activity-dependent synaptic pruning in the developing CNS, provide a role for microglia in the healthy brain, and provide important mechanistic insight into microglia-synapse interactions in the healthy and diseased CNS.
EXPERIMENTAL PROCEDURES
Engulfment analysis
Mice, except tdTomato-expressing mice (CHX10-cre::tdTomato), received intraocular injections of anterograde tracers at P4. All mice were sacrificed at P5 and brains were 4% PFA fixed overnight (4°C). Only those brains with sufficient dye fills were analyzed (see Supplementary Methods for details).
Intraocular injection of TTX or forskolin
P4 CX3CR1::EGFP heterozygotes were anesthetized with isoflurane and given an intraocular injection of drug (0.5 µM TTX or 10mM forskolin) and vehicle (saline or DMSO) into the left and right eyes, respectively. Injection volume was approximately 200 nL. 4–5 hrs after first injection, mice received a second intraocular injection of CTB 594 and 647 into the left and right eyes, respectively. Mice were sacrificed at P5 for analysis.
Electron Microscopy
EM was performed in collaboration with J. Lichtman laboratory. Tissue was prepared and imaged as previously described with minor modifications (Hayworth et al., 2006). For immunoEM, dLGN from postnatal mice were prepared and immunostained with rabbit anti-Iba-1 (Wako) as previously described (Tremblay et al., 2010b). See Supplementary Methods for details.
Eye segregation analysis
Mice received intraocular injection of cholera toxin-β subunit (CTB) and were sacrificed the following day. Tissue was processed and analyzed as previously described (Jaubert-Miazza et al., 2005; Stevens et al., 2007). All analyses were performed blind with littermate controls.
Array Tomography
Array Tomography was performed as previously described with minor modifications (Greer et al., 2010; Margolis et al., 2010; Micheva and Smith, 2007; Ross et al., 2010; Stevens et al., 2007). See Supplemental Methods for details.
Supplementary Material
01
02
03
updated supplement _121312
Acknowledgements
We thank C. Chen, B. Sabatini, Z. He, L. Benowitz, A. Huberman, M. Tessier-Lavigne, G. Corfas and X. He for their helpful discussions and reading of this manuscript; J. Sanes for the advice regarding CHX10-tdTomato experiments; J. Lichtman and R. Schalek for the technical expertise regarding EM experiments; E. Polk for performing preliminary engulfment analysis experiments; the imaging core at Children’s Hospital Boston including T. Hill and L. Bu for their technical support; the electron microscopy core at Harvard Medical School including L. Benecchi and M. Ericsson for their technical support; C. Heller for assistance in quantification of data. Work was supported by grants from the Smith Family Foundation (BS), Dana Foundation (BS), John Merck Scholars Program (BS), NINDS (RO1NS07100801-A1; BS), NRSA (F32NS066698-01;D.P.S.), NIDA (RO1-DA-15043; BAB), NIH (NIH-P30-HD-18655; MRDDRC Imaging Core), NIH (RO1-NS-045500; M.E.G.), NIH (RO1-NS-32151; R.M.R.), National MS Society (RG4550; R.M.R)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation--neuro-protection and -degeneration. J Neurochem. 2008;107:1169–1187. doi: 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Salter MW. Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol. 2010;20:474–480. doi: 10.1016/j.conb.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller KM, Carty ML, Reinebrant HE, Wixey JA. Minocycline: a neuroprotective agent for hypoxic-ischemic brain injury in the neonate? J Neurosci Res. 2009;87:599–608. doi: 10.1002/jnr.21890. [DOI] [PubMed] [Google Scholar]
- Campbell G, Shatz CJ. Synapses formed by identified retinogeniculate axons during the segregation of eye input. J Neurosci. 1992;12:1847–1858. doi: 10.1523/JNEUROSCI.12-05-01847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G, So KF, Lieberman AR. Normal postnatal development of retinogeniculate axons and terminals and identification of inappropriately-located transient synapses: electron microscope studies of horseradish peroxidase-labelled retinal axons in the hamster. Neuroscience. 1984;13:743–759. doi: 10.1016/0306-4522(84)90093-9. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, Capecchi MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TW, Liu XB, Faulkner RL, Stephan AH, Barres BA, Huberman AD, Cheng HJ. Emergence of lamina-specific retinal ganglion cell connectivity by axon arbor retraction and synapse elimination. J Neurosci. 2010;30:16376–16382. doi: 10.1523/JNEUROSCI.3455-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PM, Prusky G, Ramoa AS. The role of spontaneous retinal activity before eye opening in the maturation of form and function in the retinogeniculate pathway of the ferret. Vis Neurosci. 1999;16:491–501. doi: 10.1017/s0952523899163107. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA, Mayadas TN. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Del Rio T, Feller MB. Early retinal activity and visual circuit development. Neuron. 2006;52:221–222. doi: 10.1016/j.neuron.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Deriy LV, Gomez EA, Zhang G, Beacham DW, Hopson JA, Gallan AJ, Shevchenko PD, Bindokas VP, Nelson DJ. Disease-causing mutations in the cystic fibrosis transmembrane conductance regulator determine the functional responses of alveolar macrophages. J Biol Chem. 2009;284:35926–35938. doi: 10.1074/jbc.M109.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer ES, Feller MB, Crair MC. Development of single retinofugal axon arbors in normal and beta2 knock-out mice. J Neurosci. 2011;31:3384–3399. doi: 10.1523/JNEUROSCI.4899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TA, Wang CT, Colicos MA, Zaccolo M, DiPilato LM, Zhang J, Tsien RY, Feller MB. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J Neurosci. 2006;26:12807–12815. doi: 10.1523/JNEUROSCI.3238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MH, Easter SS., Jr Cell birth and death in the mouse retinal ganglion cell layer. J Comp Neurol. 2005;489:120–134. doi: 10.1002/cne.20615. [DOI] [PubMed] [Google Scholar]
- Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron. 1999;22:653–656. doi: 10.1016/s0896-6273(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6:e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR. Sculpting the nervous system: glial control of neuronal development. Curr Opin Neurobiol. 2006;16:119–125. doi: 10.1016/j.conb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaun J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol. 1984;230:552–575. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guido W. Refinement of the retinogeniculate pathway. J Physiol. 2008;586:4357–4362. doi: 10.1113/jphysiol.2008.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MG. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J Microsc. 2000;198:82–87. doi: 10.1046/j.1365-2818.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- Hahm JO, Cramer KS, Sur M. Pattern formation by retinal afferents in the ferret lateral geniculate nucleus: developmental segregation and the role of N-methyl-D-aspartate receptors. J Comp Neurol. 1999;411:327–345. doi: 10.1002/(sici)1096-9861(19990823)411:2<327::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Havik B, Le Hellard S, Rietschel M, Lybaek H, Djurovic S, Mattheisen M, Muhleisen TW, Degenhardt F, Priebe L, Maier W, et al. The Complement Control-Related Genes CSMD1 and CSMD2 Associate to Schizophrenia. Biol Psychiatry. 2011;70:35–42. doi: 10.1016/j.biopsych.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Hayworth KJ, Kasthuri N, Schalek R, Lichtman JW. Automating the collection of ultrathin serial sections for large volume TEM reconstructions. Microsc Microanal. 2006;12:86–87. [Google Scholar]
- Hevner RF, Wong-Riley MT. Mitochondrial and nuclear gene expression for cytochrome oxidase subunits are disproportionately regulated by functional activity in neurons. J Neurosci. 1993;13:1805–1819. doi: 10.1523/JNEUROSCI.13-05-01805.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O'Leary DD, Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Howell GR, Macalinao DG, Sousa GL, Walden M, Soto I, Kneeland SC, Barbay JM, King BL, Marchant JK, Hibbs M, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121:1429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci. 2005;22:661–676. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lambris JD, Tsokos GC. The biology and pathophysiology of complement receptors. Anticancer Res. 1986;6:515–523. [PubMed] [Google Scholar]
- Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40:139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, Lipton DM, Mandel-Brehm C, Wills ZP, Mardinly AR, Hu L, Greer PL, Bikoff JB, Ho HY, et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143:442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O'Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksa M, Komura H, Wu R, Shah KG, Wang P. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J Immunol Methods. 2009;342:71–77. doi: 10.1016/j.jim.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Leblond CP. Identification of microglia in light and electron microscopy. J Comp Neurol. 1969;135:57–80. doi: 10.1002/cne.901350104. [DOI] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011 doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2 doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TA, Rubenstein JL, Silva AJ, Storm DR, Stryker MP. The CRE/CREB pathway is transiently expressed in thalamic circuit development and contributes to refinement of retinogeniculate axons. Neuron. 2001;31:409–420. doi: 10.1016/s0896-6273(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Ravary A, Muzerelle A, Herve D, Pascoli V, Ba-Charvet KN, Girault JA, Welker E, Gaspar P. Adenylate cyclase 1 as a key actor in the refinement of retinal projection maps. J Neurosci. 2003;23:2228–2238. doi: 10.1523/JNEUROSCI.23-06-02228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort N, Quenech'du N, Watroba L, Mallat M, Giaume C, Milleret C. Microglia and astrocytes may participate in the shaping of visual callosal projections during postnatal development. J Physiol Paris. 2002;96:183–192. doi: 10.1016/s0928-4257(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Rosen AM, Stevens B. The role of the classical complement cascade in synapse loss during development and glaucoma. Adv Exp Med Biol. 2010;703:75–93. doi: 10.1007/978-1-4419-5635-4_6. [DOI] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier A, Pascual O, Bechade C, Wakselman S, Poncer JC, Real E, Triller A, Bessis A. Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS One. 2008;3:e2595. doi: 10.1371/journal.pone.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Stevens B. The "quad-partite" synapse: Microglia-synapse interactions in the developing and mature CNS. Glia. 2012 doi: 10.1002/glia.22389. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans. 2010;38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Competitive interactions between retinal ganglion cells during prenatal development. J Neurobiol. 1990;21:197–211. doi: 10.1002/neu.480210113. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Kirkwood PA. Prenatal development of functional connections in the cat's retinogeniculate pathway. J Neurosci. 1984;4:1378–1397. doi: 10.1523/JNEUROSCI.04-05-01378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988;242:87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Snider CJ, Dehay C, Berland M, Kennedy H, Chalupa LM. Prenatal development of retinogeniculate axons in the macaque monkey during segregation of binocular inputs. J Neurosci. 1999;19:220–228. doi: 10.1523/JNEUROSCI.19-01-00220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WC. Complement regulatory proteins and autoimmunity. Autoimmunity. 2006;39:403–410. doi: 10.1080/08916930600739647. [DOI] [PubMed] [Google Scholar]
- Sretavan D, Shatz CJ. Prenatal development of individual retinogeniculate axons during the period of segregation. Nature. 1984;308:845–848. doi: 10.1038/308845a0. [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ. Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat's lateral geniculate nucleus. J Neurosci. 1986;6:234–251. doi: 10.1523/JNEUROSCI.06-01-00234.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller MB. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 1999;24:673–685. doi: 10.1016/s0896-6273(00)81121-6. [DOI] [PubMed] [Google Scholar]
- Stephan A, Barres BA, Stevens B. The complement system: An unexpected role in synaptic pruning during development and disease. Ann. Rev. Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. Microglia in the prenatal mouse neostriatum and spinal cord. J Anat. 1981;133:499–512. [PMC free article] [PubMed] [Google Scholar]
- Tieman SB. Effects of monocular deprivation on geniculocortical synapses in the cat. J Comp Neurol. 1984;222:166–176. doi: 10.1002/cne.902220203. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010a;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Riad M, Majewska A. Preparation of mouse brain tissue for immunoelectron microscopy. J Vis Exp. 2010b doi: 10.3791/2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 2007;9:2095–2102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol. 2006;96:2775–2784. doi: 10.1152/jn.01321.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
updated supplement _121312