Chromosomal Microarray versus Karyotyping for Prenatal Diagnosis (original) (raw)
. Author manuscript; available in PMC: 2013 Jun 6.
Published in final edited form as: N Engl J Med. 2012 Dec 6;367(23):2175–2184. doi: 10.1056/NEJMoa1203382
Abstract
Background
Chromosomal microarray analysis has emerged as a primary diagnostic tool for the evaluation of developmental delay and structural malformations in children. We aimed to evaluate the accuracy, efficacy, and incremental yield of chromosomal microarray analysis as compared with karyotyping for routine prenatal diagnosis.
Methods
Samples from women undergoing prenatal diagnosis at 29 centers were sent to a central karyotyping laboratory. Each sample was split in two; standard karyotyping was performed on one portion and the other was sent to one of four laboratories for chromosomal microarray.
Results
We enrolled a total of 4406 women. Indications for prenatal diagnosis were advanced maternal age (46.6%), abnormal result on Down’s syndrome screening (18.8%), structural anomalies on ultrasonography (25.2%), and other indications (9.4%). In 4340 (98.8%) of the fetal samples, microarray analysis was successful; 87.9% of samples could be used without tissue culture. Microarray analysis of the 4282 nonmosaic samples identified all the aneuploidies and unbalanced rearrangements identified on karyotyping but did not identify balanced translocations and fetal triploidy. In samples with a normal karyotype, microarray analysis revealed clinically relevant deletions or duplications in 6.0% with a structural anomaly and in 1.7% of those whose indications were advanced maternal age or positive screening results.
Conclusions
In the context of prenatal diagnostic testing, chromosomal microarray analysis identified additional, clinically significant cytogenetic information as compared with karyotyping and was equally efficacious in identifying aneuploidies and unbalanced rearrangements but did not identify balanced translocations and triploidies. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and others; ClinicalTrials.gov number, NCT01279733.)
The development of array-based molecular cytogenetic techniques has improved the detection of small genomic deletions and duplications (called copy-number variants) that are not routinely seen on karyotyping, the standard cytogenetic analysis performed. Copy-number variants result in a variation from the expected number of copies of a segment of DNA (i.e., the number in a normal genome). Copy-number variants can be either benign or pathogenic, depending on their location and genetic content. They are identified with the use of chromosomal microarray analysis in which a test sample of DNA from the patient is compared directly or indirectly with a reference (normal) genome.
Identification of copy-number variants has been particularly helpful in the evaluation of children with congenital structural anomalies or altered neurocognitive development, including those diagnosed with autism spectrum disorders.1-5 Chromosomal microarray analysis identifies a genetic cause in an additional 12 to 15% of affected children, as compared with the current standard of karyotyping, leading to recommendations that microarray analysis become the first-tier test for such children.6,7
In this issue of the Journal, Reddy and colleagues demonstrate the incremental value of chromosomal microarray in the analysis of stillborn pregnancies.8 As compared with karyotyping, microarray analysis had a higher likelihood of obtaining a result and identified an increased incidence of genomic abnormalities among stillborn infants.
Microarray analysis for prenatal diagnosis has been evaluated in small studies involving women whose fetus had a high probability of having chromosome abnormalities, such as those resulting in structural anomalies.9-13 These studies have shown the technical feasibility of microarray analysis. It remains uncertain, however, whether microarray analysis reliably detects all chromosome abnormalities diagnosed on standard karyotyping, and how often abnormal microarray results are not detected on karyotyping. We conducted a large, prospective study of prenatal diagnostic samples to assess, in blinded fashion, the ability of microarray analysis to diagnose common chromosome abnormalities and to gauge the extent of additional information provided by microarray analysis as compared with standard karyotyping.
Methods
Study Conduct
The study was approved by the institutional review boards of all participating sites. The authors vouch for the accuracy of the data and the fidelity of the study to the protocol, which is available with the full text of this article at NEJM.org. All the authors were involved in the design and conduct of the study and made the decision to submit the manuscript for publication, and all approved the content of the article. The academic authors performed the data analysis. Agilent Technologies and Affymetrix donated all microarray kits and reagents and provided training without reimbursement but were not otherwise involved in either the conduct of the study or the preparation of the manuscript. Integrated Genetics received study funding to cover staff costs for handling study-specific sample and conventional cytogenetic data but was not involved in any aspect of the microarray analysis or results or in manuscript preparation. Integrated Genetics approved the content of the manuscript without changes.
Participant Recruitment and Sample Collection
Women presenting with a singleton gestation to 1 of 29 prenatal diagnostic centers for either chorionic-villus sampling or amniocentesis for indications including advanced maternal age, a positive aneuploidy screening result, and structural anomalies detected on ultrasonography were offered participation in our study. Those choosing to participate provided written informed consent after discussion of the potential advantages and risks of chromosomal microarray testing, including the possibility of findings of uncertain clinical significance and the identification of genetic variants in the fetus or a parent that cause adult-onset disorders. Chorionic-villus sampling was performed in the usual fashion. For women undergoing amniocentesis, an additional 10 ml of amniotic fluid was retrieved.
All samples were submitted to a single cytogenetics laboratory (Integrated Genetics) for karyotype analysis. The laboratory established cultures required for cytogenetic analysis, from which karyotype results were obtained and reported to the referring physician according to standard clinical practice. A sample of 7 to 10 ml of amniotic fluid, or of at least 2 mg of chorionic-villus tissue, was deidentified and sent together with peripheral-blood samples from each parent, to one of four laboratories (at Baylor College of Medicine, Columbia University, Emory University, or Signature Genomic Laboratories), where microarray analysis was performed.
Study Outcomes
For purposes of the primary analysis, each microarray result was assessed as being true positive, true negative, false positive, or false negative relative to the karyotype finding. Karyotyping was considered the standard against which the performance of chromosomal microarray in identifying common autosomal and sex-chromosome aneuploidies was measured. Per protocol, participants for whom mosaicism was determined by means of karyotyping were excluded from the primary analysis. Secondary outcomes included the overall occurrence and classification of copy-number variants identified with the use of chromosomal microarray in the presence of a normal karyotype, the success (or failure) of microarray analysis, and the ability of chromosomal microarray to identify uncommon cytogenetic abnormalities seen on karyotyping (e.g., marker chromosomes, rearrangements, or polyploidy).
Microarray Laboratory Procedures
DNA extraction was performed according to local protocols. We tested the purified DNA for contamination with maternal DNA (with the use of the Identifiler kit, Applied Biosystems) and excluded samples with more than 10% contamination.
Microarray assays were performed according to the manufacturer’s protocol and training provided by the industry donors of the microarray kits and reagents (Agilent Technologies and Affy metrix). The study began in 2007, and the choice of microarray platform and design reflects the state of technology available at that time; the choice was not updated during the course of the study. Two array platforms were used. One was an Agilent 4-plex array designed by the investigators. Each array on the fourplex consisted of 44,000 oligonucleotide probes covering targeted regions of known disease association (each covered by a minimum of 9 probes), 43 pericentromeric and 41 subtelomeric regions, and a genomic backbone with spacing of approximately 1 probe per 75 kb.14 (Coverage is listed in Table S2 in the Supplementary Appendix, available at NEJM.org.) The second platform was the Affymetrix Genome-Wide Human SNP Array 6.0, containing 1.8 million oligonucleotide probes (approximately 906,600 single-nucleotide polymorphisms [SNPs] and approximately 946,000 unique sequence probes) on a single slide. Data were masked by the analysis software to emulate the same resolution and coverage as the Agilent platform; thus, review of the SNPs was not performed. All four laboratories used the Agilent array; three of the four also used the Affymetrix array. Overall, 71% of the microarray assays were performed on the Agilent array.
We initially carried out array analysis on DNA extracted from paired uncultured and cultured samples for the first 259 participants with chorionic-villus samples and 275 participants with amniotic-fluid samples. We observed acceptable yields of interpretable results regardless of culturing and subsequently used uncultured samples whenever available. An independent tissue culture was retained at Integrated Genetics in the event that an uncultured sample was unavailable, uncultured array analysis failed, or metaphase fluorescence in situ hybridization (FISH) was required for confirmation. All results in this report are from uncultured samples, unless such samples were unavailable or failed, in which case the results from the backup culture are reported.
Confirmation of Array Results
Microarray analysis of DNA from maternal and paternal blood samples was used to determine whether copy-number variants detected in the fetal samples were inherited. We confirmed all de novo array findings seen in samples with a normal karyotype by a second method, preferentially FISH. For rare cases in which metaphase FISH was not possible because of the size of the variant or was impractical to perform within a clinically relevant time frame, we sought confirmation using a different array platform or quantitative polymerase-chain-reaction assay.
Classification and Reporting of Array Results
Karyotype and array results from each array laboratory were submitted separately to an independent data coordinating center (George Washington University Biostatistics Center). A positive microarray result was defined as a copy-number variant identified within or overlapping a targeted region (a region of the genome associated with a well-defined, clinically relevant phenotype and represented with dense probe coverage on the array) regardless of size (Table S2 in the Supplementary Appendix), a copy-number variant of 1 Mb or greater in size in the pericentromeric or subtelomeric regions or in the genomic backbone, or a copy-number variant of less than 1 Mb in size in a nontargeted region but including a gene or portion of a gene implicated in a known chromosomal syndrome, an autosomal dominant Mendelian disorder, or an X-linked disorder.
Deletions and duplications identified exclusively by means of microarray analysis were classified as “pathogenic” when they encompassed a region implicated in a well-described abnormal phenotype (Table S2 in the Supplementary Appendix). We reported these directly to the participant. Prior to the start of the study, we listed frequently observed benign copy-number variants present in our own databases of copy-number variants detected in the course of postnatal analysis, in peer-reviewed publications, and in curated databases of apparently unaffected persons (Table S3 in the Supplementary Appendix). We did not further evaluate these benign copy-number variants when we observed them in this study, nor did we report them to the participant. All other deletions and duplications were categorized as being of “uncertain clinical significance.” These were then reviewed by the study’s clinical geneticist with the laboratory directors, who determined whether copy-number variants should be classified as “likely benign” on the basis of small size, absence of notable gene content, unremarkable family history, and normal result on ultrasonography. We submitted all copy-number variants not judged as “likely benign” to an independent clinical advisory group composed of clinical geneticists, cytogeneticists, and a genetic counselor, who reviewed the clinical findings, literature, available databases, and gene content and size to determine whether there was sufficient information on which to base the prediction of phenotype and, if so, whether the phenotype was of sufficient clinical relevance to be reported. The variants determined not to be of clinical importance were classified as “likely benign” and were not reported to the participant. The rest were determined to be of potential clinical significance and were reported to the participant.
Statistical Analysis
An original sample size of 4000 women was chosen to achieve the desired precision of the estimate of sensitivity. In 2009, after karyotype results from the first 755 women showed that 8.3% of participants had an abnormal karyotype, the sample size was increased to 4400. This number of participants was sufficient to yield an exact lower 95% confidence interval of at least 99% for an observed sensitivity of 100%, assuming that 367 participants would have an abnormal karyotype.
SAS software (SAS Institute) was used for analysis. Where proportions were estimated (including sensitivity and specificity), we calculated exact confidence intervals.
Results
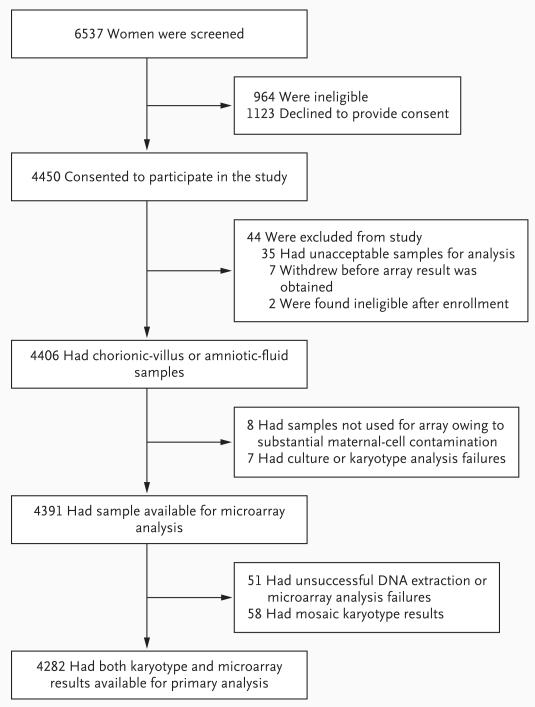
We screened 6537 women from October 2008 through July 2011. Of these, 4450 were eligible and consented to participate in the study; we obtained adequate samples from 4406 (with 2275 undergoing chorionic-villus sampling and 2131 undergoing amniocentesis) (Fig. 1). Characteristics of the study population, including indications for prenatal testing, are provided in Table 1.
Figure 1. Screening and Enrollment of the Study Participants.
Table 1. Baseline Characteristics and Primary Indication for Prenatal Testing and Characteristics of the 4406 Study Participants with Adequate Samples for Analysis.*.
| Characteristic | Indication for Invasive Sampling | ||||
|---|---|---|---|---|---|
| Anomaly onUltrasonography(N = 1109) | MaternalAdvanced Age(N = 2054) | Positive Result onDown’s SyndromeScreening (N = 827) | Other (N = 416) | All (N = 4406) | |
| Maternal age — yr | 32.2±5.8 | 38.5±2.5 | 34.0±5.2 | 33.1±4.5 | 35.6±5.1 |
| Gestational age at procedure — wk | |||||
| Chorionic-villus sampling | 12.5±1.6 | 11.8±0.8 | 12.8±0.8 | 11.9±0.8 | 12.1±1.1 |
| Amniocentesis | 21.1±4.0 | 17.4±1.3 | 18.3±1.9 | 17.8±2.1 | 18.8±3.1 |
| Race or ethnic group — no. (%)† | |||||
| Black | 114 (10.3) | 80 (3.9) | 65 (7.9) | 27 (6.5) | 286 (6.5) |
| Hispanic | 164 (14.8) | 163 (7.9) | 110 (13.3) | 46 (11.1) | 483 (11.0) |
| Other | 831 (74.9) | 1811 (88.2) | 652 (78.8) | 343 (82.5) | 3637 (82.5) |
We obtained an adequate sample for microarray analysis for 4391 (99.7%) of these 4406 participants. Overall, microarray was successful in 98.8% of cases (4340 of 4391). The microarray analysis was performed on uncultured samples for 3860 (87.9%) of the 4391 participants. We successfully obtained study results in 3408 (88.3%) of these 3860 uncultured samples: 1781 (93.2%) of the 1910 chorionic-villus samples and 1627 (83.4%) of the 1950 amniotic-fluid samples. The study result was derived from the cultured, rather than uncultured, sample in the remaining 932 (21.5%) of the 4340 cases of successful microarray.
Fifty-eight samples showed mosaicism on karyotyping and were excluded from this study. The remaining 4282 samples were included in the primary analysis (Table 2). Of these, 317 (7.4%) common autosomal and 57 (1.3%) sex-chromosome aneuploidies were identified by means of standard karyotyping. Microarray analysis identified all of these aneuploidies. Eight of these cases, all from uncultured chorionic-villus samples, were mosaic on the microarray and could represent mosaicism not detected on karyotyping. None of these cases had maternal-cell contamination. All 22 unbalanced rearrangements also were identified by microarray (1 as a mosaic).
Table 2. Results of Karyotype and Microarray Analysis in 4282 Samples with a Nonmosaic Karyotype, According to Cytogenetic Abnormality.
| Abnormality | Detected onKaryotyping | Detected on Microarray* | ||
|---|---|---|---|---|
| Total | FullComplement | MosaicComplement | ||
| no. (%) | no. (%) | no. | no. | |
| Any autosomal or sex-chromosome abnormality | 374 (8.7) | 374 (100) | 366 | 8 |
| Any common autosomal trisomy | 317 (7.4) | 317 (100) | 312 | 5 |
| Trisomy 21 | 188 | 188 (100) | 185 | 3 |
| Trisomy 18 | 93 | 93 (100) | 91 | 2 |
| Trisomy 13 | 36 | 36 (100) | 36 | 0 |
| Other autosomal trisomy | 4 (0.1) | 4 (100) | 4 | 0 |
| Any sex-chromosome aneuploidy | 57 (1.3) | 57 (100) | 54 | 3 |
| 45,X | 39 | 39 (100) | 36 | 3 |
| 47,XXX; 47,XXY; 47,XYY | 18 | 18 (100) | 18 | 0 |
| Structural rearrangement | 65 (1.5) | |||
| Balanced | 40 | 0 | 0 | 0 |
| Unbalanced | 22 | 22 (100) | 21 | 1 |
| Marker | 3 | 2 (66.7) | 2† | 0 |
| Triploidy | 17 (0.4) | 0‡ | 0 | 0 |
As expected, none of the apparently balanced rearrangements identified on karyotyping were identified with the use of microarray analysis, suggesting that these rearrangements were truly balanced. Of the three marker chromosomes detected on karyotyping, we detected two on microarray. Results obtained on FISH suggested that the third marker chromosome contained no euchromatin and thus was unlikely to contain genes, be detected by means of microarray, or have clinical significance. Seventeen triploid samples (0.4%) were present in our series; none were identified on microarray; in 15 (88.2%), the test for maternal-cell contamination revealed aberrant findings suggestive of triploidy. One other was recorded as mosaic 47,XXY on microarray. Three of the 17 triploid fetuses had normal ultrasonography images at the time of chorionic-villus sampling.
Microarray analysis revealed clinically significant, segmental aneuploidies not detected on karyotyping (Table 3, and Table S1 in the Supplementary Appendix). On microarray, 1399 samples were identified as having copy-number variants; of these, 1234 (88.2%) were classified as common benign, including 26 in which the fetus was a carrier (3 were deletions of the steroid sulfatase [microsomal], isozyme S gene [_STS_] on chromosome Xp22.31 in females, 22 were deletions of the juvenile nephronophthisis 1 gene [_NPHP1_] on chromosome 2q13, and 1 involved a deletion of gamma sarcoglycan and sacsin genes [SGCG and SACS, respectively] on chromosome 13q12.12). Thirty-five copy-number variants, occurring in 35 of the 3822 fetuses (0.9%), were on the predetermined list of pathogenic copy-number variants. Of the remaining 130 samples, 36 (27.7%) were considered likely to be benign by the study’s clinical geneticist, and 94 maintained a classification of uncertain significance and were adjudicated by the Clinical Advisory Committee. The committee felt that 61 of these 94 (64.9%) had sufficient clinical relevance to be reported to the participant. Overall, a total of 96 of the 3822 fetal samples with normal karyotypes (2.5%; 95% confidence interval [CI], 2.1 to 3.1) had a microdeletion or duplication of clinical significance.
Table 3. Frequency and Clinical Interpretation of Microdeletions and Duplications on Chromosomal Microarray in the 3822 Samples with a Normal Karyotype, According to Indication for Prenatal Testing.
| Indication for Prenatal Diagnosis | NormalKaryotype | CommonBenign | Pathogenic | Uncertain ClinicalSignificance (N = 130) | Total Known Pathogenicand Potential for ClinicalSignificance* | |
|---|---|---|---|---|---|---|
| Likely to BeBenign | Potentialfor ClinicalSignificance | |||||
| no. | no. (%) | no. (%) [95% CI]* | ||||
| Any | 3822 | 1234 (32.3) | 35 (0.9) | 69 (1.8)‡ | 61 (1.6) | 96 (2.5) [2.1–3.1] |
| Advanced maternal age | 1966 | 628 (31.9) | 9 (0.5) | 37 (1.9) | 25 (1.3) | 34 (1.7) [1.2–2.4] |
| Positive on Down’s syndrome screening | 729 | 247 (33.9) | 3 (0.4) | 13 (1.8) | 9 (1.2) | 12 (1.6) [0.9–2.9] |
| Anomaly on ultrasonography | 755 | 247 (32.7) | 21 (2.8) | 16 (2.1) | 24 (3.2) | 45 (6.0) [4.5–7.9] |
| Other§ | 372 | 112 (30.1) | 2 (0.5) | 3 (0.8) | 3 (0.8) | 5 (1.3) [0.6–3.1] |
We examined the results from microarray analysis in subgroups of women with normal karyotypes (Table 3). In samples from fetuses with suspected growth or structural anomalies, 45 of the 755 (6.0%; 95% CI, 4.5 to 7.9) had clinically relevant findings on microarray that were not found on karyotyping. A total of 34 of the 1966 women without ultrasonography-identified anomalies who were tested because of advanced maternal age had a normal karyotype and a clinically relevant finding on microarray (1.7%; 95% CI, 1.2 to 2.4), as did 12 of the 729 women who tested positive on Down’s syndrome screening (1.6%; 95% CI, 0.9 to 2.9). Recurrent copy-number variants associated with autism and neurocognitive alterations were relatively prevalent in our study series (Table S1 in the Supplementary Appendix)5; we detected these copy-number variants in 1.3% (51 of 3822) of karyotypically normal pregnancies: 3.6% (27 of 755) with and 0.8% (24 of 3067) without structural anomalies.
Discussion
We have shown that microarray analysis is equivalent to standard karyotype analysis for the prenatal diagnosis of common aneuploidies. Microarray analysis provided additional clinically relevant information in 1.7% of pregnancies with standard indications for prenatal diagnosis (such as advanced maternal age and positive aneuploid screening result) and in 6.0% of cases with an anomaly on ultrasonography. These data indicate a benefit to chromosomal microarray analysis as a standard part of prenatal testing, bearing in mind that, as with karyotyping, the detection of variants of uncertain clinical significance present a challenge for counseling and cause anxiety.15
We used an array design that maximized the detection of well-characterized microdeletions and duplications but also included oligonucleotides representing regions distributed throughout the genome to identify additional chromosomal imbalances. Uncertain findings occurred in 3.4% (130 of 3822) of all karyotypically normal cases analyzed with the use of microarray. Of these 130 cases, 94 (72.3%) had findings that were not easily dismissed as likely to be benign and therefore required expert adjudication for clinical relevance. Since the start of our study 5 years ago, the literature and databases of array results and associated phenotypes have expanded, providing additional information with which to predict the phenotype.1,3,4 The laboratory directors therefore reinterpreted their initial categorization on the basis of the current literature. Were data available in 2012 used for the ascertainment, only 56 of the original 94 uncertain results requiring evaluation by the clinical advisory committee would remain in that category; 30 are now clearly pathogenic and 8 are now likely to be benign (Table S1 in the Supplementary Appendix). With this additional information, the pathogenicity of only 1.5% of copy-number variants detected on microarray in karyotypically normal samples remains uncertain, and this number should continue to fall as additional experience is acquired. The interpretation of uncertain results will continue to require a close working relationship among laboratory directors, clinical geneticists, counselors, and practitioners.
We chose to obtain results preferentially from uncultured samples so as to avoid the additional time needed for, and the artifacts of, cell and tissue culture. However, experience with traditional cytogenetic analysis and confined placental mosaicism in chorionic-villus samples has occasionally revealed discrepant results between direct (uncultured) analysis that evaluates predominantly the cytotrophoblast and cultured samples that typically derive from the mesenchymal core of the villi.16 Microarray analysis of uncultured samples captures the genomic content of both cell lineages. Although our initial comparison of microarray results from paired cultured and uncultured samples was reassuring, the limited sample size makes further evaluation necessary.
After approximately 12 weeks’ gestation, most triploid pregnancies show abnormalities on ultrasonography, which could alert the physician to request further evaluation by means of karyotyping. Ultrasonographic images obtained earlier than 12 weeks may miss these abnormalities. Arrays including SNP probes can identify triploidy with the use of genotype data,17 but this information was not included in our study design. Our array analysis did not use the genotype data derived from the SNP probes on the Affymetrix array because we initiated the study before their development for clinical use. However, a post hoc review determined that had the SNP data been analyzed, the triploid cases would have been detected. We therefore suggest that arrays used for prenatal testing should contain SNP probes that can reliably identify triploidy.
Balanced chromosomal translocations and inversions occur in approximately 0.08 to 0.09% of prenatal diagnostic samples18 and are not detectable with the use of an array because there is no gain or loss of genetic material. An inherited balanced rearrangement will have no consequences for the current pregnancy but is relevant to future reproductive counseling. A de novo, apparently balanced rearrangement identified by means of standard karyotyping is associated with a 6.7% risk of congenital abnormalities,19 many of which may be caused by a genomic gain or loss at the breakpoints and may be discoverable with the use of an array.20 Further investigation is necessary to quantify the residual risk of a balanced rearrangement when a microarray analysis is normal and to determine when and whether additional genomic analysis is necessary.
One in 60 pregnancies that underwent genetic testing because of advanced maternal age or positive aneuploidy screening had a clinically relevant copy-number variant in our study. However, many of these copy-number variants are typically smaller microdeletions or microduplications than those identified with the use of chromosome banding or FISH and have much greater variability in their associated phenotypes.5 Copy-number variants associated with substantial phenotypic variability are listed in Table S1 in the Supplementary Appendix. When encountered in the prenatal setting, this increased range of phenotypic features can make genetic counseling challenging; many of these copy-number variants do not always result in severe impairments. Because of their smaller size and milder phenotypic effects and the possibility that these copy-number variants exert a phenotypic effect only in the presence of other genetic variants,21-23 these copy-number variants may be inherited from a parent with minimal or no recognizable features. Although data from symptomatic infants evaluated postnatally gives some guidance for prenatal counseling, this group almost certainly represents a biased, more severe, and incomplete characterization of the phenotype. To address this bias, we are following the children with copy-number variants ascertained in this prenatal study, as well as others discovered in utero, to understand the associated phenotypic variability more comprehensively and to gauge the relative contribution of copy-number variants to the 13 to 14% of children who receive a diagnosis of developmental delay.24
The comparatively high rate of discovery, with the use of microarray, of clinically relevant genomic disorders may result in more requests for invasive prenatal diagnostic testing. At present, on the basis of the relative balance between the genetic risk and the risk of procedure-induced miscarriage, a risk of aneuploidy of 1:270 or higher is the generally accepted threshold for offering invasive testing.25 However, the decision to have an amniocentesis or chorionic-villus sampling is based on many factors, including the risk that the fetus will have an abnormality, the risk of pregnancy loss from an invasive procedure, and the consequences of having an affected child. Potential parents weigh these potential outcomes in different ways.26-28 If the observed 1.7% (1:60) frequency of clinically relevant microdeletions and microduplications in pregnancies sampled for indications other than fetal structural anomalies is confirmed by others, offering invasive testing and microarray analysis to all pregnant women would seem to be appropriate. This is consistent with the recommendations of the American Congress of Obstetricians and Gynecologists, who suggest that all women, regardless of risk, should be offered the option of invasive testing.25 Counseling should include a discussion of the risk of invasive testing, the frequency and severity of clinically relevant microarray findings, and the more limited identification of common aneuploidies currently achievable with the use of noninvasive screening.29
We are still in the process of gauging the extent of incremental information that should be sought in the context of prenatal testing and how that information should be introduced into care. Lessons learned from microarray analysis will be helpful when whole-genome sequencing of the fetus, perhaps with the use of maternal blood samples, becomes clinically available30-32 and should help to ensure the sensible application of new technology.
Supplementary Material
Supplement1
Acknowledgments
Supported by grants from the National Institute of Child Health and Human Development (R01HD05565101, R01HD055651-03S1, and RC2HD064525). Agilent Technologies and Affymetrix donated all microarrays and reagents used in the study.
We thank Deborah A. Driscoll, M.D., Sherman Elias, M.D., Deborah L. Eunpu, M.S., Kurt Hirschhorn, M.D., Charles Lee, Ph.D., Eugene Pergament, M.D., and Dorothy Warburton, Ph.D., for their work as the Clinical Advisory Committee; Maternal Fetal Specialists, Atlanta (Daniel Eller, M.D.), Baylor College of Medicine (Anthony Johnson, D.O.), NJ Perinatal Associates/St. Barnabas Medical Center (Richard Miller, M.D.), Main Line Health and Women’s Health Care Group of PA (Alan Donnenfeld, M.D.), Phoenix Perinatal Associates (Rodney Edwards, M.D.), Maternal Fetal Medicine of Central PA (Terry Tressler, D.O.), Thomas Jefferson University (Stuart Weiner, M.D.), Fetal Diagnostic Center (Greggory DeVore, M.D.), Virtua Health System (Ronald Librizzi, D.O.), Valley Health System (Andrei Rebarber, M.D.), Christiana Care Health System (Anthony Sciscione, D.O.), San Francisco Perinatal Associates (James Goldberg, M.D.), University of Medicine and Dentistry, New Jersey-Robert Wood Johnson (Todd Rosen, M.D.), University of California, Irvine (Deborah Wing, M.D.), Vanderbilt Center for Women’s Health (William Walsh, M.D.), Children’s Hospital of Philadelphia (Mark Johnson, M.D.), Ohio State University Maternal Fetal Medicine (Richard O’Shaughnessy, M.D.), St. Vincent Women’s Hospital (Maurice Eggleston, M.D., and James Sumners, M.D.), Englewood Hospital (Ying Chan, M.D.), University of Utah (Robert Silver, M.D.), and Magella Medical Group (Melissa Bush, M.D.) for their role as participating recruitment sites; Nicolasa H. Chavez, Jessica Feinberg, and Andrea M. Murad for their coordinating and recruitment efforts; Lindsay Doherty and Mark McNellis from the George Washington University Data Coordinating Center; the laboratory technologists including Emily Carron from Columbia University, Vanessa Jump from Emory University, Caron Glotzbach from Signature Genomic Laboratories, PerkinElmer, and Patricia Hixson at Baylor College of Medicine; Eliza Sanchez for her work at Integrated Genetics; and the participants and the North American Fetal Therapy Network for their support of the study.
Footnotes
Dr. Aggarwal reports receiving lecture fees from Agilent Technologies and consulting fees from Affymetrix through her institution. Drs. Ballif, Lamb, and Shaffer report being employees of and holding stock in PerkinElmer (Signature Genomics). Dr. Eng reports receiving lecture fees from Genzyme and Shire. Dr. Jackson reports serving as an expert witness in malpractice cases regarding genetic concerns. Dr. Klugman reports serving as an expert witness in a case regarding counseling for a family with a fetus with a rare genetic disorder. Dr. Ledbetter reports receiving consulting fees from and holding stock in CombiMatrix and receiving consulting fees from Roche NimbleGen and Celula. Dr. Levy reports receiving consulting fees from and holding stock in Natera and receiving consulting fees from RMA Genetics and Celula and lecture fees and travel support from Affymetrix and Agilent Technologies. Dr. Scholl reports being an employee of LabCorp (Integrated Genetics). Dr. Simpson reports receiving consulting fees from Bio Dx, Bayer, and Novartis. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org
References
- 1.Sagoo GS, Butterworth AS, Sanderson S, Shaw-Smith C, Higgins JP, Burton H. Array CGH in patients with learning disability (mental retardation) and congenital anomalies: updated systematic review and meta-analysis of 19 studies and 13,926 subjects. Genet Med. 2009;11:139–46. doi: 10.1097/GIM.0b013e318194ee8f. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer LG, Kashork CD, Saleki R, et al. Targeted genomic microarray analysis for identification of chromosome abnormalities in 1500 consecutive clinical cases. J Pediatr. 2006;149:98–102. doi: 10.1016/j.jpeds.2006.02.006. Erratum, J Pediatr 2006;149:585. [DOI] [PubMed] [Google Scholar]
- 3.Cooper GM, Coe BP, Girirajan S, et al. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43:838–46. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaminsky EB, Kaul V, Paschall J, et al. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet Med. 2011;13:777–84. doi: 10.1097/GIM.0b013e31822c79f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mefford HC, Batshaw ML, Hoffman EP. Genomics, intellectual disability, and autism. N Engl J Med. 2012;366:733–43. doi: 10.1056/NEJMra1114194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller DT, Adam MP, Aradhya S, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–64. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manning M, Hudgins L. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med. 2010;12:742–5. doi: 10.1097/GIM.0b013e3181f8baad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy UM, Page GP, Saade GR, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med. 2012;367:2185–93. doi: 10.1056/NEJMoa1201569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillman SC, Pretlove S, Coomarasamy A, et al. Additional information from array comparative genomic hybridization technology over conventional karyotyping in prenatal diagnosis: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:6–14. doi: 10.1002/uog.7754. [DOI] [PubMed] [Google Scholar]
- 10.Maya I, Davidov B, Gershovitz L, et al. Diagnostic utility of array-based comparative genomic hybridization (aCGH) in a prenatal setting. Prenat Diagn. 2010;30:1131–7. doi: 10.1002/pd.2626. [DOI] [PubMed] [Google Scholar]
- 11.Srebniak M, Boter M, Oudesluijs G, et al. Application of SNP array for rapid prenatal diagnosis: implementation, genetic counselling and diagnostic flow. Eur J Hum Genet. 2011;19:1230–7. doi: 10.1038/ejhg.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH, Woo JH, Shim SH, et al. Application of a target array comparative genomic hybridization to prenatal diagnosis. BMC Med Genet. 2010;11:102. doi: 10.1186/1471-2350-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faas BH, van der Burgt I, Kooper AJ, et al. Identification of clinically significant, submicroscopic chromosome alterations and UPD in fetuses with ultrasound anomalies using genome-wide 250k SNP array analysis. J Med Genet. 2010;47:586–94. doi: 10.1136/jmg.2009.075853. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin EL, Lee JY, Blake DM, et al. Enhanced detection of clinically relevant genomic imbalances using a targeted plus whole genome oligonucleotide microarray. Genet Med. 2008;10:415–29. doi: 10.1097/GIM.0b013e318177015c. [DOI] [PubMed] [Google Scholar]
- 15.McGillivray G, Rosenfeld JA, McKinlay Gardner RJ, Gillam LH. Genetic counselling and ethical issues with chromosome microarray analysis in prenatal testing. Prenat Diagn. 2012;32:389–95. doi: 10.1002/pd.3849. [DOI] [PubMed] [Google Scholar]
- 16.Wolstenholme J. Confined placental mosaicism for trisomies 2, 3, 7, 8, 9, 16, and 22: their incidence, likely origins, and mechanisms for cell lineage compartmentalization. Prenat Diagn. 1996;16:511–24. doi: 10.1002/(SICI)1097-0223(199606)16:6<511::AID-PD904>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Tyreman M, Abbott KM, Willatt LR, et al. High resolution array analysis: diagnosing pregnancies with abnormal ultrasound findings. J Med Genet. 2009;46:531–41. doi: 10.1136/jmg.2008.065482. [DOI] [PubMed] [Google Scholar]
- 18.Giardino D, Corti C, Ballarati L, et al. De novo balanced chromosome rearrangements in prenatal diagnosis. Prenat Diagn. 2009;29:257–65. doi: 10.1002/pd.2215. [DOI] [PubMed] [Google Scholar]
- 19.Warburton D. De novo balanced chromosome rearrangements and extra marker chromosomes identified at prenatal diagnosis: clinical significance and distribution of breakpoints. Am J Hum Genet. 1991;49:995–1013. [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins AW, Alkuraya FS, Bosco AF, et al. Characterization of apparently balanced chromosomal rearrangements from the Developmental Genome Anatomy Project. Am J Hum Genet. 2008;82:712–22. doi: 10.1016/j.ajhg.2008.01.011. Erratum, Am J Hum Genet 2008;83: 425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gau SS, Liao HM, Hong CC, Chien WH, Chen CH. Identification of two inherited copy number variants in a male with autism supports two-hit and compound heterozygosity models of autism. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:710–7. doi: 10.1002/ajmg.b.32074. [DOI] [PubMed] [Google Scholar]
- 22.Girirajan S, Rosenfeld JA, Cooper GM, et al. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010;42:203–9. doi: 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girirajan S, Rosenfeld JA, Bradley PC, et al. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N Engl J Med. 2012;367:1321–31. doi: 10.1056/NEJMoa1200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg SA, Zhang D, Robinson CC. Prevalence of developmental delays and participation in early intervention services for young children. Pediatrics. 2008;121(6):e1503–e1509. doi: 10.1542/peds.2007-1680. [DOI] [PubMed] [Google Scholar]
- 25.ACOG practice bulletin no. 77: screening for fetal chromosomal abnormalities. Obstet Gynecol. 2007;109:217–27. doi: 10.1097/00006250-200701000-00054. [DOI] [PubMed] [Google Scholar]
- 26.Grobman WA, Dooley SL, Welshman EE, Pergament E, Calhoun EA. Preference assessment of prenatal diagnosis for Down syndrome: is 35 years a rational cutoff? Prenat Diagn. 2002;22:1195–200. doi: 10.1002/pd.494. [DOI] [PubMed] [Google Scholar]
- 27.Kuppermann M, Nease RF, Learman LA, Gates E, Blumberg B, Washington AE. Procedure-related miscarriages and Down syndrome-affected births: implications for prenatal testing based on women’s preferences. Obstet Gynecol. 2000;96:511–6. doi: 10.1016/s0029-7844(00)00969-8. [DOI] [PubMed] [Google Scholar]
- 28.Caughey AB, Washington AE, Kuppermann M. Perceived risk of prenatal diagnostic procedure-related miscarriage and Down syndrome among pregnant women. Am J Obstet Gynecol. 2008;198(3):333.e1–333.e8. doi: 10.1016/j.ajog.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 29.Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genet Med. 2011;13:913–20. doi: 10.1097/GIM.0b013e3182368a0e. [DOI] [PubMed] [Google Scholar]
- 30.Bodurtha J, Strauss JF., III Genomics and perinatal care. N Engl J Med. 2012;366:64–73. doi: 10.1056/NEJMra1105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitzman JO, Snyder MW, Ventura M, et al. Noninvasive whole-genome sequencing of a human fetus. Sci Transl Med. 2012;4:137–76. doi: 10.1126/scitranslmed.3004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan HC, Gu W, Wang J, Blumenfeld YJ, El-Sayed YY, Quake SR. Non-invasive prenatal measurement of the fetal genome. Nature. 2012;487:320–4. doi: 10.1038/nature11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1