Gemcitabine Plus nab-Paclitaxel Is an Active Regimen in Patients With Advanced Pancreatic Cancer: A Phase I/II Trial (original) (raw)
Abstract
Purpose
The trial objectives were to identify the maximum-tolerated dose (MTD) of first-line gemcitabine plus _nab_-paclitaxel in metastatic pancreatic adenocarcinoma and to provide efficacy and safety data. Additional objectives were to evaluate positron emission tomography (PET) scan response, secreted protein acidic and rich in cysteine (SPARC), and CA19-9 levels in relation to efficacy. Subsequent preclinical studies investigated the changes involving the pancreatic stroma and drug uptake.
Patients and Methods
Patients with previously untreated advanced pancreatic cancer were treated with 100, 125, or 150 mg/m2 _nab_-paclitaxel followed by gemcitabine 1,000 mg/m2 on days 1, 8, and 15 every 28 days. In the preclinical study, mice were implanted with human pancreatic cancers and treated with study agents.
Results
A total of 20, 44, and three patients received _nab_-paclitaxel at 100, 125, and 150 mg/m2, respectively. The MTD was 1,000 mg/m2 of gemcitabine plus 125 mg/m2 of _nab_-paclitaxel once a week for 3 weeks, every 28 days. Dose-limiting toxicities were sepsis and neutropenia. At the MTD, the response rate was 48%, with 12.2 median months of overall survival (OS) and 48% 1-year survival. Improved OS was observed in patients who had a complete metabolic response on [18F]fluorodeoxyglucose PET. Decreases in CA19-9 levels were correlated with increased response rate, progression-free survival, and OS. SPARC in the stroma, but not in the tumor, was correlated with improved survival. In mice with human pancreatic cancer xenografts, _nab_-paclitaxel alone and in combination with gemcitabine depleted the desmoplastic stroma. The intratumoral concentration of gemcitabine was increased by 2.8-fold in mice receiving _nab_-paclitaxel plus gemcitabine versus those receiving gemcitabine alone.
Conclusion
The regimen of _nab_-paclitaxel plus gemcitabine has tolerable adverse effects with substantial antitumor activity, warranting phase III evaluation.
INTRODUCTION
Metastatic pancreatic ductal adenocarcinoma (PDA) is a lethal disease with approximately 6 months of median survival.1,2 Gemcitabine is the only approved single agent, with a median survival of 5.7 months and 20% 1-year survival.3 Except for erlotinib, all phase III trials exploring gemcitabine-based combinations have failed to improve overall survival (OS).4 Nevertheless, a recent meta-analysis of randomized trials revealed a general survival benefit for gemcitabine-based chemotherapies for patients with good performance status.5 Because of the moderate activity of the current standard gemcitabine and gemcitabine-based regimens,3–7 improved therapeutic options are greatly needed.
The selection of _nab_-paclitaxel, a 130-nm albumin-bound formulation of paclitaxel particles (Celgene, Summit, NJ), in combination with the standard gemcitabine was based on a molecular profiling of PDA tumor samples,8 in which secreted protein acidic and rich in cysteine (SPARC), an albumin-binding protein, was noted to be overexpressed. _nab_-Paclitaxel has shown antitumor activity in various advanced cancer types that overexpress SPARC,9–11 including breast,12–14 lung,15,16 and melanoma.17
The objectives of this trial were to identify the maximum-tolerated dose (MTD) of gemcitabine plus _nab_-paclitaxel as first-line therapy in patients with metastatic PDA and to provide efficacy and safety data to permit the planning of a possible pivotal phase III trial. Additional exploratory objectives were to evaluate SPARC and CA19-9 levels and positron emission tomography (PET) scan response in relation to efficacy. Subsequent preclinical studies in human pancreatic cancer xenografts investigated the underlying biology of the substantial clinical activity seen in this phase I/II study.
PATIENTS AND METHODS
Phase I/II Clinical Study
The study was conducted at four centers in the United States in accordance with the Declaration of Helsinki and Good Clinical Practice, Guidelines of the International Conference on Harmonization. Written informed consent was obtained from all patients before entering the study. Eligibility criteria included age ≥ 18 years and histologically or cytologically confirmed metastatic PDA with measurable disease by computed tomography scan as defined by the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.0 guidelines.18 Patients had no previous treatment for metastatic disease. Prior adjuvant treatment with fluorouracil or gemcitabine administered as a radiation sensitizer during and up to 4 weeks after radiation therapy was allowed. If a patient received adjuvant therapy, tumor recurrence must have occurred ≥ 6 months after the last treatment. Patients had an Eastern Cooperative Oncology Group performance status of 0 or 1 and had adequate hematologic, hepatic, and renal function.
Study Design
This was an open-label phase I/II study. In the phase I portion, the primary end point was to identify the MTD and dose-limiting toxicities (DLTs) of gemcitabine (1,000 mg/m2) followed by _nab_-paclitaxel (100, 125, or 150 mg/m2), administered intravenously (IV) on days 1, 8, and 15, every 28 days, using the standard 3 + 3 phase I dose-escalation design.19 Per protocol, DLTs were treatment-related toxicities during cycle 1 per National Cancer Institute Common Terminology Criteria of Adverse Events version 3.0, including any grade 4 hematologic toxicity; grade 3 thrombocytopenia with hemorrhage; grade ≥ 3 nausea, vomiting, or diarrhea despite prophylaxis; or any grade ≥ 3 treatment-related nonhematologic toxicity, excluding alopecia and fatigue. Dose escalation was stopped when ≥ one of three patients had DLTs, and the dose below was declared the MTD. Patients continued treatment until disease progression or unacceptable toxicity. In the phase II portion, accrual continued at the MTD to ≥ 42 patients to evaluate the efficacy and safety of the combination. This clinical study also evaluated PET scan response, CA19-9, and SPARC levels in relation to antitumor activity.
Assessments
All patients who received at least one dose of study drugs were evaluated for efficacy and safety. Response was assessed by computed tomography scans at baseline and every 4 weeks on day 1 of each cycle (per RECIST v1.0); an initial response (complete [CR] or partial response [PR]) had to be confirmed at least 4 weeks later. Metabolic activity was assessed by [18F]fluorodeoxyglucose (FDG) PET scans at baseline and at 6 and 12 weeks on the basis of the European Organisation for Research and Treatment of Cancer criteria by an independent investigator.20 Safety was assessed by the incidence of treatment-related adverse events (AEs), according to the National Cancer Institute Common Terminology Criteria of Adverse Events version 3.0, and incidence of patients experiencing dose modifications, dose interruptions, and/or premature discontinuation of study drug. CA19-9 levels were monitored by investigators at every cycle. Archived tumor blocks, if available, were collected for SPARC analysis.
Statistical Methods for Efficacy End Points and Biomarkers
With a total of 44 patients treated at the MTD, there was ≥ 95% power of observing a serious AE that had an incidence of ≥ 7%. The percentage of patients (with 95% CI) who achieved an objective CR or PR using RECIST criteria were summarized using descriptive statistics. Disease control rate was defined as the percentage of patients with CR, PR, and stable disease (SD) ≥ 16 weeks. Progression-free survival (PFS) was defined as the time from first dose of study drug to the start of disease progression or patient death, whichever occurred first. OS was defined as the time from first dose of study drug to patient death. PFS and OS were analyzed using Kaplan-Meier methods.
To assess possible relationships between CA19-9 and efficacy outcomes, the correlation of maximum decrease from baseline in CA19-9 with survival was analyzed. SPARC immunohistochemistry was performed using a monoclonal and a polyclonal antibody and proprietary methodology. Seven tissue components including tumor cells and stromal components such as fibroblast and inflammatory cells were evaluated. For each tissue component and each antibody, three measures were recorded by two board-certified pathologists at a Clinical Laboratory Improvement Amendments laboratory: maximum intensity, percentage of cells at the maximum intensity, and overall score, providing 42 variables. All variables were standardized across patients via z-score transformation and averaged between the two pathologists. For each patient, an average z-score was calculated across variables. On the basis of the average z-scores ≥ or less than 0, patients were classified into a high- or low-SPARC group, respectively. The difference in OS between the low- and high-SPARC groups was assessed by the log-rank test, and a multivariate Cox regression model was used to assess the independent predictive power of SPARC levels. All statistical analyses for SPARC were carried out in R version 2.12.0.21
Preclinical Study Methods
The objectives of these preclinical studies were to evaluate tumor progression, potential changes in the pancreatic stroma, and intratumoral drug penetration.
Xenograft Establishment and Treatment
Fresh pancreatic cancer tissues obtained from 11 chemotherapy-naive patients who underwent surgery at the Johns Hopkins (JH) Hospital were propagated as subcutaneous tumors in 6-week-old female athymic nude mice as a live PancXenoBank.22 Mice with tumor size of ∼200 mm3 were randomly assigned to four treatment groups (seven to 10 tumors/group): (1) control, (2) gemcitabine 100 mg/kg intraperitoneally (IP) on days 1 and 5 weekly for 4 weeks, (3) _nab_-paclitaxel 30 mg/kg/d IV for 5 consecutive days, and (4) gemcitabine plus _nab_-paclitaxel in the preceding regimens for 4 weeks. A response was defined as a more than 50% regression in tumor size. Animals were killed on day 28. The experimental protocol was approved by the Animal Care and Use Committee at JH University.
Immunohistochemistry
Tumors obtained at euthanasia were immediately flash frozen, and a portion of each tumor was kept in 10% formalin for paraffin embedding. The extent of stromal desmoplasia was determined by an immunohistochemistry assay for collagen 1 (1:500; Abcam, Cambridge, MA).23 Stromal vascularity was assessed using an anti-CD31 antibody (1:200; Santa Cruz Biotechnologies, Santa Cruz, CA).
Quantitative Real-Time PCR
Endothelial cell content was quantified by real-time polymerase chain reaction (qRT-PCR) for murine-specific nestin (mNestin) transcripts.24 For qRT-PCR, total RNA was isolated (RNeasy Mini Kit, Qiagen, Santa Clarita, CA), followed by cDNA production (SuperScript III First Strand synthesis kit, Invitrogen, Carlsbad, CA). Relative fold expression of mNestin was calculated using the 2-ΔΔCt method.25
Gemcitabine Uptake in Tumors
Mice harboring PANC265 xenograft were treated with gemcitabine at 100 mg/kg IP on day 5 or gemcitabine 100 mg/kg on day 5 plus _nab_-paclitaxel 30 mg/kg/d IV for 5 consecutive days. Animals were killed and tumors were harvested 1 hour after the last gemcitabine dose. Gemcitabine concentrations in tumors were measured in the JH Analytic Pharmacology Core. Briefly, tumor tissue homogenates were prepared. After liquid extraction and evaporation of homogenates, the sample was dissolved in 100 μL of methanol/water (10:90, volume/volume). The analytes were separated on a YMC Jsphr M80TM C18 column (Waters, Millford, MA), and gemcitabine and dFdU (a gemcitabine metabolite) were monitored by tandem mass spectrometry.
RESULTS
Patients
A total of 67 patients were enrolled and evaluated (Table 1). All patients have discontinued therapy either because of progressive disease (48%), unacceptable toxicity without progressive disease (18%), patient discretion (17%), investigator discretion (8%), AE (8%), or other (2%). The most common treatment-related AEs that led to treatment discontinuation were neuropathy and fatigue.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | _nab_-Paclitaxel mg/m2 | |||||
|---|---|---|---|---|---|---|
| 100 (n = 20) | 125 (n = 44) | 150 (n = 3) | ||||
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Median | 62 | 61 | 69 | |||
| Range | 30–86 | 28–78 | 53–72 | |||
| Female sex | 9 | 45 | 25 | 57 | 1 | 33 |
| ECOG | ||||||
| 0 | 9 | 45 | 22 | 50 | 2 | 67 |
| 1 | 11 | 55 | 22 | 50 | 1 | 33 |
| Site of metastatic disease | ||||||
| Abdomen/peritoneal* | 16 | 80 | 38 | 86 | 2 | 67 |
| Liver | 11 | 55 | 34 | 77 | 2 | 67 |
| Liver only | 1 | 5 | 2 | 5 | 1 | 33 |
| Lung | 5 | 25 | 18 | 41 | 1 | 33 |
| Lung only | 1 | 5 | 5 | 11 | 1 | 33 |
| Other | 10 | 50 | 12 | 27 | 1 | 33 |
| No. of metastatic sites | ||||||
| 1 | 6 | 30 | 8 | 18 | 1 | 33 |
| 2 | 8 | 40 | 18 | 41 | 2 | 67 |
| ≥ 3 | 6 | 30 | 18 | 41 | 0 | |
| CA19-9 baseline levels, n | 15 | 37 | 2 | |||
| Normal† | 2 | 13 | 6 | 16 | 1 | 50 |
| Elevated | 13 | 87 | 31 | 84 | 1 | 50 |
| CA19-9 baseline, μ/mL | ||||||
| Median | 1,148 | 881 | 181 | |||
| Range | 14–180,062 | 1–96,990 | 23–339 | |||
| Previous treatment | ||||||
| Prior chemotherapy‡ | 3 | 15 | 10 | 23 | 1 | 33 |
| Prior adjuvant therapy | 3 | 15 | 10 | 23 | 1 | 33 |
| With gemcitabine | 1 | 5 | 5 | 11 | 0 | |
| With capecitabine | 1 | 5 | 4 | 9 | 0 | |
| With FU | 2 | 10 | 1 | 2 | 0 | |
| With docetaxel | 0 | 2 | 5 | 0 | ||
| With erlotinib | 0 | 0 | 1 | 33 | ||
| Time since adjuvant therapy,§ months | 5 | |||||
| Median | 64 | 12 | ||||
| Range | 9–81 | 1–23 |
MTD and DLTs
Of the first six patients treated at dose level 1 (100 mg/m2 _nab_-paclitaxel cohort), two patients had their day 8 treatment held: one patient with a possible history of ethanol abuse had asymptomatic neutropenia (absolute neutrophil count 0.85 × 109 cells/L), and a 79-year-old patient had asymptomatic thrombocytopenia (platelet count 60 × 109 cells/L). In three of those first six patients, radiologic responses were observed. Because of the confounding factors in two patients with dose delays, the potentially promising level of antitumor activity with this regimen, and the excellent tolerability in the remaining patients, the protocol was modified to allow for a total of 20 patients at dose level 1 rather than considering this dose level as having exceeded the MTD. Subsequently, dose escalation proceeded to dose level 2 and then 3. Of the three patients at dose level 3 (150 mg/m2 of _nab_-paclitaxel), one patient died as a result of treatment-related systemic infection (neutropenia in the presence of a biliary stent) during cycle 1, and the MTD was established at dose level 2 (125 mg/m2 of _nab_-paclitaxel). The other two patients at dose level 3 had grade 3 AEs that were resolved (leukopenia, fatigue, and neutropenia). A total of 44 patients were enrolled at dose level 2.
Efficacy Results
Survival.
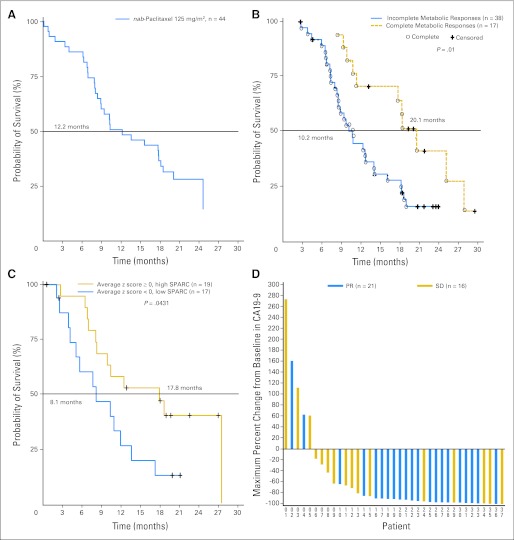
In patients treated at the MTD of 125 mg/m2 of _nab_-paclitaxel (n = 44), the median PFS was 7.9 months (95% CI, 5.8 to 11.0 months), median OS was 12.2 months (95% CI, 8.9 to 17.9 months; Fig 1A), and the 1-year survival was 48%. For all 67 patients, median PFS was 7.1 months (95% CI, 5.7 to 8.0 months), with median OS of 10.3 months (95% CI, 8.4 to 13.6).
Fig 1.
(A) Median overall survival in patients receiving 125 mg/m2 of albumin-bound (nab) paclitaxel followed by 1,000 mg/m2 of gemcitabine. (B) Median overall survival correlated with a complete metabolic response compared with baseline, defined according to the European Organisation for Research and Treatment of Cancer criteria by the absence of [18F]fluorodeoxyglucose uptake (cohorts 1 and 2). (C) Median survival correlated with secreted protein acidic and rich in cysteine (SPARC; all cohorts). (D) Maximum percentage change in CA19-9 levels in patients receiving 125 mg/m2 of _nab_-paclitaxel followed by 1,000 mg/m2 of gemcitabine. PR, partial response; SD, stable disease.
Response rate.
The overall response rate (ORR) was 46% for all patients (N = 67). In the 100 (n = 20) and 125 (n = 44) mg/m2 _nab_-paclitaxel cohorts, the response rates were 45% and 48%, respectively (Table 2). The overall disease control rate was 60% and 68%, respectively.
Table 2.
Response Rates, Disease Progression, and Disease Control Rates for All Patients and in the 125 mg/m2 _nab_-Paclitaxel Cohort
| Response Result | Dose Level 2 (n = 44) | All Dose Levels(n = 67) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Complete response | 0 | 3 | 4 | |
| Partial response | 21 | 48 | 28 | 42 |
| Stable disease* | 9 | 20 | 12 | 18 |
| Progressive disease | 7 | 16 | 15 | 22 |
| Disease control rate† | 30 | 68 | 43 | 64 |
PET scan analysis.
FDG PET scans were available for 55 patients. The median decrease in metabolic activity was 79% for all three cohorts together at 12 weeks. In the 125 mg/m2 _nab_-paclitaxel cohort (n = 38), the reduction in FDG uptake was greater compared with the 100 mg/m2 cohort (n = 14; 68% v 53%; P = .044) at 6 weeks, but not at 12 weeks (74% v 76%; P = .13, respectively). When PET analyses from all three cohorts were combined, patients with a complete metabolic response, defined according to the European Organisation for Research and Treatment of Cancer criteria by the absence of FDG uptake, had a significantly improved OS compared with patients without a complete metabolic response (median 20.1 v 10.3 months, respectively; P = .01; Fig 1B).
Treatment Exposure
Across all _nab_-paclitaxel doses, patients received 81% of the planned dose and 85% of the planned gemcitabine dose. The median number of cycles administered was 6.0 (range, 1 to 24) for all patients. Twenty-five percent of patients had a _nab_-paclitaxel dose reduction, with 20% in the 125 mg/m2 cohort. Thirty-one percent of patients had a gemcitabine dose reduction, with 43% in the 125 mg/m2 cohort. For all patients and in the 125 mg/m2 cohort, 72% and 70% of patients had a _nab_-paclitaxel dose delayed, respectively, mainly due to AEs. For all patients, 73% patients had a dose delay of gemcitabine sometime in their treatment, mainly because of AEs.
Safety Results
The DLTs were sepsis and neutropenia. The most common treatment-related AEs of any grade were anemia (98%), leukopenia (91%), neutropenia (89%), thrombocytopenia (83%), fatigue (76%), alopecia (76%), sensory neuropathy (63%), and nausea (48%). Most of these treatment-related AEs were grade 1 and 2 (Table 3). Specifically, the most common grade ≥ 3 _nab_-paclitaxel–related nonhematologic AEs were fatigue (21%) and sensory neuropathy (15%). Of the grade ≥ 3 treatment-related hematologic AEs, neutropenia (67%), leukopenia (44%), and thrombocytopenia (23%) were the most common.
Table 3.
Selected Treatment-Related Adverse Events
| Adverse Events | Dose Level 1(n = 20) | Dose Level 2(n = 44) | Dose Level 3(n = 3) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Nonhematologic events | ||||||
| Diarrhea | ||||||
| Grade 1 | 1 | 5 | 7 | 16 | 1 | 33 |
| Grade 2 | 1 | 5 | 6 | 14 | 0 | |
| Grade 3 | 3 | 15 | 1 | 2 | 0 | |
| Grade 4 | 0 | 0 | 0 | |||
| Fatigue | ||||||
| Grade 1 | 4 | 20 | 10 | 23 | 0 | |
| Grade 2 | 9 | 45 | 13 | 30 | 1 | 33 |
| Grade 3 | 1 | 5 | 12 | 27 | 1 | 33 |
| Grade 4 | 0 | 0 | 0 | |||
| Nausea | ||||||
| Grade 1 | 7 | 35 | 11 | 25 | 1 | 33 |
| Grade 2 | 2 | 10 | 9 | 20 | 1 | 33 |
| Grade 3 | 0 | 1 | 2 | 0 | ||
| Grade 4 | 0 | 0 | 0 | |||
| Sensory neuropathy | ||||||
| Grade 1 | 5 | 25 | 15 | 34 | 0 | |
| Grade 2 | 1 | 5 | 9 | 20 | 2 | 67 |
| Grade 3 | 1 | 5 | 9 | 20 | 0 | |
| Grade 4 | 0 | 0 | 0 | |||
| Vomiting | ||||||
| Grade 1 | 1 | 5 | 10 | 23 | 1 | 33 |
| Grade 2 | 2 | 10 | 3 | 7 | 1 | 33 |
| Grade 3 | 0 | 3 | 7 | 0 | ||
| Grade 4 | 0 | 0 | 0 | |||
| Hematologic events | ||||||
| Anemia | ||||||
| Grade 1 | 7 | 35 | 10 | 23 | 2 | 67 |
| Grade 2 | 11 | 55 | 27 | 63 | 1 | 33 |
| Grade 3 | 1 | 5 | 6 | 14 | 0 | |
| Grade 4 | 0 | 0 | 0 | |||
| Leukopenia | ||||||
| Grade 1 | 2 | 10 | 6 | 14 | 1 | 33 |
| Grade 2 | 12 | 60 | 9 | 21 | 1 | 33 |
| Grade 3 | 4 | 20 | 16 | 37 | 1 | 33 |
| Grade 4 | 0 | 8 | 19 | 0 | ||
| Neutropenia | ||||||
| Grade 1 | 4 | 20 | 6 | 14 | 0 | |
| Grade 2 | 3 | 15 | 1 | 2 | 1 | 33 |
| Grade 3 | 8 | 40 | 11 | 26 | 2 | 67 |
| Grade 4 | 2 | 10 | 21 | 49 | 0 | |
| Febrile neutropenia | ||||||
| Grade 1 | 0 | 0 | 0 | |||
| Grade 2 | 0 | 0 | 0 | |||
| Grade 3 | 1 | 5 | 1 | 2 | 0 | |
| Grade 4 | 1 | 5 | 0 | 0 | ||
| Thrombocytopenia | ||||||
| Grade 1 | 5 | 25 | 18 | 42 | 2 | 67 |
| Grade 2 | 5 | 25 | 9 | 21 | 1 | 33 |
| Grade 3 | 2 | 10 | 8 | 19 | 0 | |
| Grade 4 | 1 | 5 | 4 | 9 | 0 |
Biomarkers
SPARC.
SPARC status was evaluated in 36 patients. Applying the average z-score algorithm to all 42 variables, patients were classified into high-SPARC (average z-scores ≥ 0, n = 19) and low-SPARC groups (average z-scores < 0, n = 17). A significant increase in OS was observed for patients in the high-SPARC group compared with patients in the low-SPARC group (median OS, 17.8 v 8.1 months, respectively; P = .0431; Fig 1C). Furthermore, SPARC level remained a significant predictor for the OS in a multivariate Cox regression model after adjusting for clinical covariates, including sex, race, age, treatment, and baseline CA19-9 level (P = .041). Additionally, stromal SPARC was significantly correlated with OS (P = .013), but not SPARC in tumor cells (P = .15).
CA19-9 levels.
Rapid decreases in CA19-9 levels were observed, with the median time to maximum decrease of 89 days. In the 125 mg/m2 cohort, 92% evaluable patients (34 of 37) had a ≥ 20% decrease in CA19-9, 78% (29 of 37) had a ≥ 50% decrease, and 70% (26 of 37) had a ≥ 70% decrease in CA19-9. The median maximum percentage change in CA19-9 level was 91% for all patients and also for patients in the 125 mg/m2 cohort (Fig 1D). CA19-9 levels were correlated with increased survival. Patients with ≥ 50% decrease in CA19-9 levels had a 62% ORR and 8.0 and 13.6 median months of PFS and OS, respectively, whereas those with less than 50% decrease in CA19-9 level had a 33% ORR and 3.6 and 6.5 months of PFS and OS, respectively (P = .105, < .001, and .004 for ORR, PFS, and OS, respectively).
Preclinical Study Results
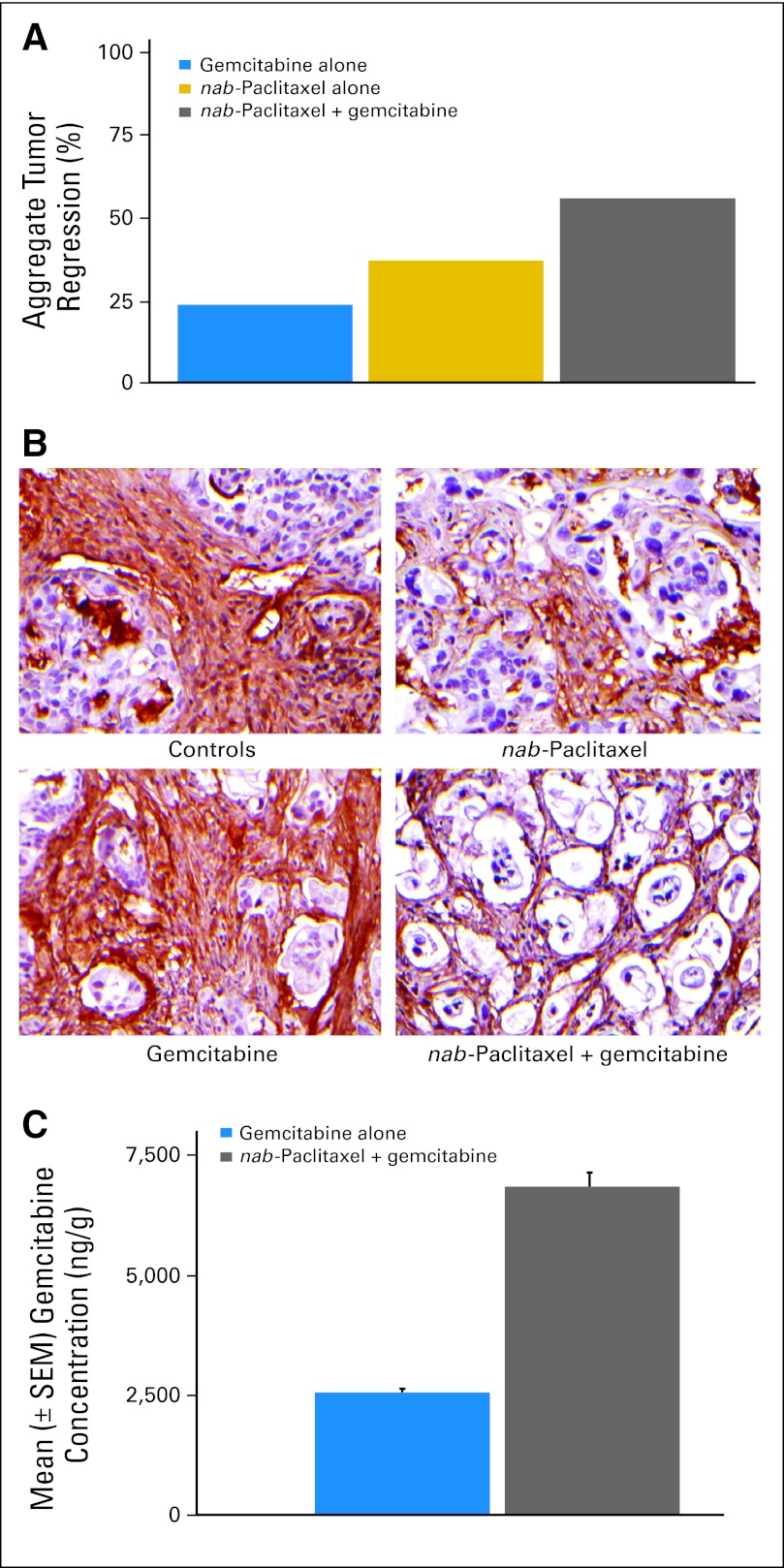
Gemcitabine and _nab_-paclitaxel alone resulted in tumor regressions in two (18%) and four (36%) of 11 patient-derived xenografts, respectively. However, gemcitabine plus _nab_-paclitaxel chemotherapy resulted in tumor regressions in seven (64%) of 11 cases. The aggregate tumor regression response in individual xenografts derived from the 11 parental cases were 22 (24%) of 90, 34 (36%) of 95, and 53 (55%) of 96 for gemcitabine, _nab_-paclitaxel, and gemcitabine plus _nab_-paclitaxel, respectively (Fig 2A).
Fig 2.
(A) Percentage incidence of aggregate tumor regression in response to gemcitabine, albumin-bound (nab) paclitaxel, and gemcitabine plus _nab_-paclitaxel in individual xenografts derived from the 11 parental cases. (B) Immunohistochemical assay for collagen type 1 fibers in a gemcitabine-resistant human pancreatic cancer xenograft treated with _nab_-paclitaxel, gemcitabine, or gemcitabine plus _nab_-paclitaxel. (C) Intratumor concentration of gemcitabine in human pancreatic cancer xenografts.
We analyzed the stromal content of two gemcitabine-resistant tumors in each of the treatment groups. Mice treated with vehicle or gemcitabine exhibited a profuse desmoplastic stroma, as demonstrated by the collagen type1 fibers (Fig 2B). In contrast, _nab_-paclitaxel treatment depleted the desmoplastic stroma as evidenced by compact “back-to-back” arrangement of neoplastic glands separated by “wisps” of collagen. The reduction in stromal content was accompanied by dilated blood vessels in the tumor milieu, which were particularly prominent in the combination therapy cohort. An approximately three-fold increase in mNestin, marker of endothelial cells, was observed in xenografts receiving combination therapy as compared with control tumors, consistent with increased stromal endothelial cell content. The reduction in tumor stroma and the accompanied increase in vascularization facilitated the delivery of gemcitabine to these tumors.
The intratumor concentration of gemcitabine increased by 2.8-fold in the gemcitabine plus _nab_-paclitaxel treated tumors compared with gemcitabine-alone treated mice (Fig 2C).
DISCUSSION
The MTD (the recommended dose for phase III) was 1,000 mg/m2 of gemcitabine plus 125 mg/m2 of _nab_-paclitaxel administered weekly for 3 weeks, repeated every 4 weeks. The 48% ORR, 12.2 months of OS, and 1-year survival of 48% at the MTD is among the highest reported for a phase II study in patients with PDA, including the fluorouracil, leucovorin, irinotecan, and oxaliplatin regimen,26 which in a recent randomized phase III trial produced significantly improved survival compared with gemcitabine alone.27 Additionally, this current study is among the first to formally assess PET scan responses in pancreatic cancer. Results showed that a complete loss of FDG metabolic activity was associated with favorable survival. In accordance with published results showing that CA19-9 is a prognostic marker for both PFS and OS,28 decrease from baseline CA19-9 in the present study was an independent prognostic factor for OS. Overall, SPARC expression was not correlated with baseline CA19-9 levels, indicating that SPARC is a predictive marker independent of CA19-9 levels. Although an increase in SPARC level was correlated with improved OS, the significant increase was specific to elevated stromal SPARC and not SPARC in tumor cells. This is particularly important because historically, SPARC expression in the stroma, but not in the tumor, has been associated with poor survival,29,30 suggesting that a unique mechanism of action of the present regimen may play a role in this reverse outcome. Together these observations indicate that stromal SPARC expression may be an important marker of early activity of gemcitabine plus _nab_-paclitaxel combination regimens in advanced pancreatic cancer.
The preclinical studies were subsequently initiated on the basis of the encouraging responses seen in the clinical trial. In the present preclinical study, _nab_-paclitaxel alone and in combination with gemcitabine depleted the peritumoral desmoplastic stroma, and intratumoral concentration of gemcitabine increased in mice treated with _nab_-paclitaxel versus those receiving gemcitabine alone. We speculate that reducing the dense tumor stroma, a histologic hallmark of PDA, may allow the chemotherapeutics to reach the tumor tissue more efficiently. Although these preclinical results were compelling in the athymic mouse, it has been noted that the mouse stromal cells may be transformed in the presence of human xenograft.31,32 Other existing models of PDA (eg, Kras mutations that harbor similar precancerous lesions as humans) may be needed to confirm the stromal depletion seen in this model. The stromal depletion and the increased survival with SPARC expression observed in this study indicate that, in addition to intrinsic antitumor effects against the cancer cell, _nab_-paclitaxel may target stromal SPARC and facilitate delivery of chemotherapy. These data are consistent with a recent preclinical study targeting the hedgehog pathway in pancreatic cancer23 and suggest that stroma-directed treatments may be a new treatment strategy. In particular, the antitumor activity of gemcitabine plus _nab_-paclitaxel combination therapy may, in part, be explained by the use of the albumin receptor(gp60)–caveolin-1–caveolae–SPARC pathway to increase intratumoral drug concentrations.33
Although the results of this clinical phase I/II study are promising, as with any nonrandomized study, patient selection may have influenced the outcome, and validation by a larger randomized trial is necessary. Given the favorable safety profile and the encouraging antitumor activity of the _nab_-paclitaxel plus gemcitabine regimen, a phase III study comparing gemcitabine plus _nab_-paclitaxel and gemcitabine alone has been initiated.
Acknowledgment
We thank the Pancreatic Cancer Research Team at TGen and the Pancreas Cancer Research Dream Team, a Stand Up To Cancer program, for the support with the clinical and preclinical study, respectively. We thank Niki A. Ottenhof, MD, Johns Hopkins University, for performing the immunohistochemistry and nestin assays and Michelle A. Rudek, PhD, Johns Hopkins University, for help in mass spectrometry. We also thank Amanda Johnson, Paul Bhar, and Pankaj Patel of Celgene for biostatistical and data management assistance. Additionally, we thank Preeti Pramanik and Kouros Motamed, PhD, Tina Treece, PhD, Larn Huang, PhD, and Xiaoyue Zhao, PhD, of Celgene for SPARC assays and analysis. Expert medical writing assistance was provided by Anita N. Schmid, PhD, Celgene.
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL; the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL; the 46th Annual Meeting of the American Society of Clinical Oncology, June 4-8, 2010, Chicago, IL; American Association for Cancer Research 2008 Annual Meeting, April 12-16, 2008, San Diego, CA; the American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics, November 15–19, 2009, Boston, MA; and European Society of Medical Oncology Congress, October 8–12, 2010, Milan, Italy.
Footnotes
Sponsored by Abraxis BioScience, Los Angeles, CA, a wholly owned subsidiary of Celgene, Summit, NJ. The preclinical studies were supported in part by National Institutes of Health Grants No. CA116554and CA129963 (M.H.) and CA113669 and CA134767 (A.M.) and by a Stand Up To Cancer-American Association for Cancer Research Dream Team Translational Cancer Research Grant No. SU2C-AACR-DT0509.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00398086.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Neil Desai, Celgene (C); Vuong Trieu, Celgene (C); Jose L. Iglesias, Celgene (C); Hui Zhang, Celgene (C); Patrick Soon-Shiong, Abraxis BioScience (C); Tao Shi, Celgene (C) Consultant or Advisory Role: None Stock Ownership: Neil Desai, Celgene; Vuong Trieu, Celgene; Jose L. Iglesias, Celgene; Patrick Soon-Shiong, Celgene; Tao Shi, Celgene Honoraria: Ramesh K. Ramanathan, Abraxis BioScience Research Funding: Daniel D. Von Hoff, Celgene; Ramesh K. Ramanathan, Abraxis BioScience; Mitesh J. Borad, Abraxis Oncology; Daniel A. Laheru, Celgene; Lon S. Smith, Abraxis BioScience; Ronald L. Korn, Scottsdale Medical Imaging Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Daniel D. Von Hoff, Ramesh K. Ramanathan, Ronald L. Korn, Neil Desai, Vuong Trieu, Jose L. Iglesias, Patrick Soon-Shiong, N.V. Rajeshkumar, Anirban Maitra, Manuel Hidalgo
Provision of study materials or patients: Daniel D. Von Hoff, Ramesh K. Ramanathan, Mitesh J. Borad, Daniel A. Laheru, Lon S. Smith, Tina A. Wood, Manuel Hidalgo
Collection and assembly of data: Daniel D. Von Hoff, Ramesh K. Ramanathan, Mitesh J. Borad, Daniel A. Laheru, Lon S. Smith, Tina E. Wood, Ronald L. Korn, N.V. Rajeshkumar, Anirban Maitra, Manuel Hidalgo
Data analysis and interpretation: Daniel D. Von Hoff, Ramesh K. Ramanathan, Mitesh J. Borad, Daniel A. Laheru, Lon S. Smith, Tina E. Wood, Ronald L. Korn, Neil Desai, Vuong Trieu, Jose L. Iglesias, Hui Zhang, Tao Shi, N.V. Rajeshkumar, Anirban Maitra, Manuel Hidalgo
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Cancer facts and figures 2010. Atlanta, GA: American Cancer Society; 2010. p. 65. [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Eli Lilly. Gemzar prescribing information. Indianapolis, IN: 1996. pp. 1–23. [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: Evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer. 2008;8:82. doi: 10.1186/1471-2407-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Pancreatic adenocarcinoma. Fort Washington, PA: National Comprehensive Cancer Network; 2011. pp. 1–67. [Google Scholar]
- 8.Von Hoff DD, Penny R, Shack S, et al. Frequency of potential therapeutic targets identified by immunohistochemistry (IHC) and DNA microarray (DMA) in tumors from patients who have progressed on multiple therapeutic agents. J Clin Oncol. 2006;24(suppl):138s. abstr 3071. [Google Scholar]
- 9.Watkins G, Douglas-Jones A, Bryce R, et al. Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids. 2005;72:267–272. doi: 10.1016/j.plefa.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Koukourakis MI, Giatromanolaki A, Brekken RA, et al. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 11.Massi D, Franchi A, Borgognoni L, et al. Osteonectin expression correlates with clinical outcome in thin cutaneous malignant melanomas. Hum Pathol. 1999;30:339–344. doi: 10.1016/s0046-8177(99)90014-x. [DOI] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 13.Lobo C, Lopes G, Silva O, et al. Paclitaxel albumin-bound particles (abraxane(TM)) in combination with bevacizumab with or without gemcitabine: Early experience at the University of Miami/Braman Family Breast Cancer Institute. Biomed Pharmacother. 2007;61:531–533. doi: 10.1016/j.biopha.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Yardley DA, Daniel BR, Inhorn RC, et al. SPARC microenvironment signature (SMS) analysis of a phase II trial of neoadjuvant gemcitabine (G), epirubicin (E), and nab-paclitaxel (nab-P) in locally advanced breast cancer (LABC) J Clin Oncol. 2010;28(suppl):741s. abstr 10574. [Google Scholar]
- 15.Socinski MA, Manikhas GM, Stroyakovsky DL, et al. A dose finding study of weekly and every-3-week nab-Paclitaxel followed by carboplatin as first-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:852–861. doi: 10.1097/jto.0b013e3181d5e39e. [DOI] [PubMed] [Google Scholar]
- 16.Socinski MA, Bondarenko I, Karaeva N, et al. Survival results of a randomized, phase 3 trial of nab-paclitaxel and carboplatin compared with cremophor-based paclitaxel and carboplatin as first-line therapy in advanced non-small cell lung cancer. J Clin Oncol. 2011;29(suppl):488s. abstr 7551. [Google Scholar]
- 17.Hersh E, O'Day S, Ribas A, et al. A phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naive patients with metastatic melanoma. Cancer. 2010;116:155–163. doi: 10.1002/cncr.24720. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, O'Dwyer PJ, Christian M, et al. Phase I clinical trial design in cancer drug development. J Clin Oncol. 2000;18:684–692. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 20.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 21.The R project for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 22.Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 23.Olive K, Jacobetz M, Davidson C, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Ychou M, Desseigne F, Guimbaud R, et al. Randomized phase II trial comparing FOLFIRINOX (5FU/leucovorin [LV], irinotecan [I] and oxaliplatin [O]) vs gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA). First results of the ACCORD 11 trial. J Clin Oncol. 2007;25(suppl):201s. abstr 4516. [Google Scholar]
- 27.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 28.Maisey R, Norman A, Hill A, et al. CA19-9 as a prognostic factor in inoperable pancreatic cancer: The implication for clinical trials. Br J Cancer. 2005;93:740–743. doi: 10.1038/sj.bjc.6602760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 30.Guweidhi A, Kleeff J, Adwan H, et al. Osteonectin influences growth and invasion of pancreatic cancer cells. Ann Surg. 2005;242:224–234. doi: 10.1097/01.sla.0000171866.45848.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparrow A, Jones M, Billington S, et al. The in vivo malignant transformation of mouse fibroblasts in the presence of human tumour xenografts. Br J Cancer. 1986;53:793–797. doi: 10.1038/bjc.1986.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neesse A, Michl P, Frese K, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 33.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of Cremophor-free, albumin-bound paclitaxel, ABI-007, compared with Cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]