IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium (original) (raw)
Abstract
Acute injury to the intestinal mucosa is a major dose-limiting complication of abdominal radiation therapy. We studied the role of the transcription factor NF-κB in protection against radiation-induced apoptosis in the intestinal epithelium in vivo. We use mice in which NF-κB signaling through IκB-kinase (IKK)-β is selectively ablated in intestinal epithelial cells to show that failure to activate epithelial cell NF-κB in vivo results in a significant increase in radiation-induced epithelial cell apoptosis. Furthermore, bacterial lipopolysaccharide, which is normally a radioprotective agent, is radiosensitizing in IKKβ-deficient intestinal epithelial cells. Increased apoptosis in IKKβ-deficient intestinal epithelial cells was accompanied by increased expression and activation of the tumor suppressor p53 and decreased expression of antiapoptotic Bcl-2 family proteins. These results demonstrate the physiological importance of the NF-κB system in protection against radiation-induced death in the intestinal epithelium in vivo and identify IKKβ as a key molecular target for radioprotection in the intestine. Selective preactivation of NF-κB through IKKβ in intestinal epithelial cells could provide a therapeutic modality that allows higher doses of radiation to be tolerated during cancer radiotherapy.
Ionizing radiation (IR) is used to treat many malignant intraabdominal neoplasms (1). Rapidly proliferating cells, like tumor cells, undergo apoptosis in response to IR-induced DNA double strand breaks, whereas nondividing and slowly dividing cells rarely die after IR. This distinction provides a rationale for the use of IR in cancer therapy. Epithelial cells located in the small intestinal crypts also divide rapidly and are among the most susceptible cells in the body to IR-induced death. Consequently, intestinal tract injury is a major adverse effect of cancer radiotherapy. Higher doses of radiation produce superior antitumor effects than lower doses, but the ability of patients to tolerate acute radiation side effects caused by intestinal epithelial injury and the accompanying mucositis limits the dose that can be administered (2, 3). Therefore, radiation oncologists have sought radioprotective agents for the intestine that would limit epithelial cell death, but lack of knowledge of molecular and physiologically relevant antiapoptotic mechanisms operating in the intestinal epithelium has hampered those efforts.
The molecular mechanisms of IR-induced apoptosis involve several pathways. Importantly, the tumor suppressor p53 is required for acute IR-induced killing of intestinal epithelial cells (4). In contrast, comparatively little is known about protective mechanisms that limit radiation-induced cell death. In this regard, bacterial lipopolysaccharide (LPS) (5), prostaglandin E2 (6, 7), keratinocyte and other growth factors (8–10), lysophosphatidic acid (11), IL-1, and tumor necrosis factor α (TNFα) (12) were all shown to decrease the sensitivity of cells to IR-induced apoptosis, although the molecular basis of radioprotection by these agents is not known.
DNA damage induced by IR has been reported to activate the transcription factor nuclear factor κB (NF-κB) in HeLa, lymphoblastoid, and colon cancer cell lines (13–15) and in murine liver and kidney (13, 14). NF-κB constitutes a family of dimeric transcription factors that regulate gene transcription by binding to κB elements in the promoter regions of specific target genes (16). Signaling cascades initiated by LPS, IL-1, TNFα, or DNA double strand breaks culminate in activation of the IκB-kinase (IKK) complex, which phosphorylates IκB proteins leading to their degradation (17). This enables NF-κB to enter the nucleus and induce target gene transcription. Mice genetically engineered to lack key components of the NF-κB system, including RelA/p65 (18), IKKβ (19), or IKKγ (20), are highly sensitive to TNFα-induced hepatocyte apoptosis during embryonic development and, in some cancer cells, constitutive NF-κB activation appears to inhibit the cytotoxicity of antineoplastic drugs (21) and radiation (15). These observations indicate an important cell-survival role for NF-κB and suggest that this transcription factor might protect normal cells against DNA damage-induced apoptosis. Therefore, we tested the hypothesis that NF-κB signals an antiapoptotic response in the irradiated intestinal epithelium in vivo by using mice genetically engineered to lack the canonical NF-κB-activation kinase, IKKβ, in intestinal epithelial cells.
Methods
Mice. LoxP sites were introduced into the _Ikk_β locus of embryonic stem cells, and gene-targeted mice containing the _Ikk_βF floxed allele were obtained from these cells as described (22). A Cre recombinase cDNA (gift of Steve O'Gorman, The Salk Institute for Biological Studies) was subcloned downstream of a 9-kb fragment of the murine Vil gene promoter (23). This construct was microinjected into the pronucleus of single-cell mouse embryos, and founders were generated. Transgenic Vil-Cre mice were crossed with Gtrosa26 Cre/loxP recombination reporter mice (24) (The Jackson Laboratory) and various tissues were isolated from the offspring. Fixed tissues were stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) to identify β-galactosidase expression indicative of loxP site recombination. To ablate IKKβ, transgenic Vil-Cre mice were crossed with _Ikk_βF mice. Genotypes were determined by PCR (see supporting information, which is published on the PNAS web site).
Analysis of the Ikkβ Locus. To assess recombination of the _Ikk_βF locus by Southern blot analysis, genomic DNA digested with _Hin_dIII was probed with a 1.4-kb fragment of intronic DNA from inside the _Hin_dIII sites and outside the loxP sites (see Fig. 2_C_).
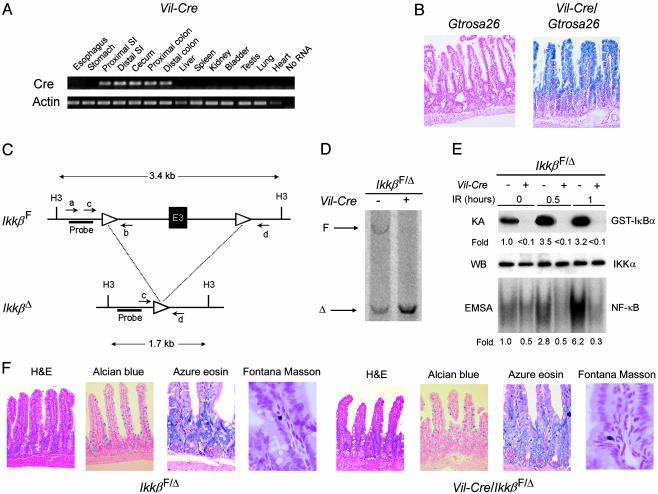
Fig. 2.
Conditional intestinal epithelial cell-specific IKKβ knockout mice. (A) Intestine-specific expression of Cre recombinase mRNA in Vil-Cre mice. mRNA expression of Cre and β-actin was assessed by RT-PCR of RNA extracted from the indicated tissues of Vil-Cre mice. SI, small intestine. (B) Intestinal epithelial cell-specific recombination of loxP sites in Vil-Cre mice. β-Galactosidase activity was assessed in the small intestine of Cre/loxP recombination reporter mice (Gtrosa26) and Gtrosa26/Vil-Cre mice by staining with X-Gal and nuclear fast red (counterstain). Blue staining indicates loxP site recombination in Gtrosa26/Vil-Cre epithelial cells of crypts and villi. (C) Schematic representation of _Ikk_βF and _Ikk_βΔ alleles. H3, _Hin_dIII site; a, b, c, and d, annealing sites of primers used for genotyping (see supporting information); E3, exon 3; triangles, loxP sites; Probe, annealing site for Southern blot probe. (D) Southern blot analysis of the _Ikk_β locus using _Hin_dIII-digested DNA from isolated intestinal epithelial cells of _Ikk_βF/Δ and Vil-Cre/_Ikk_βF/Δ mice. The positions of the _Ikk_βF and _Ikk_βΔ restriction fragments are indicated. (E) Defective IR-induced IKK and NF-κB activation in IKKβ-deficient intestinal epithelial cells. Intestinal epithelial cells were isolated from _Ikk_βF/Δ and Vil-Cre/_Ikk_βF/Δ mice at the indicated times after 8-Gy IR. IKK activity was determined by an in vitro kinase assay (KA), and IKKα levels (loading control) were assessed by Western blotting (WB). NF-κB binding activity was determined by electrophoretic mobility-shift assay (EMSA). Fold induction by IR of IKK and NF-κB binding activities relative to epithelial cells from nonirradiated control mice is shown under each lane. (F) Normal intestinal epithelial lineage development in the absence of IKKβ. Sections of the small intestine from 6-week-old _Ikk_βF/Δ and Vil-Cre/_Ikk_βF/Δ mice were prepared and stained as indicated from left to right to detect enterocytes, goblet cells, Paneth cells, and enteroendocrine cells. H&E, hematoxylin and eosin.
In Vivo Irradiation or LPS Stimulation. Mice were exposed to 8 Gy γ-radiation from a 137Cesium source, at an exposure rate of 7.94 Gy/min. Mice were injected i.p. with 5 mg/kg LPS from Escherichia coli O111:B4.
Analysis of Apoptosis. A total of 4.5 h after irradiation or 8.5 h after LPS injection, the mid-small intestine was removed, fixed in 10% neutral buffered formalin, sectioned, and stained with hematoxylin and eosin. Two blinded observers quantified intestinal epithelial cell apoptosis. The numbers of cells in at least 10 well oriented crypts that displayed characteristic features of apoptosis were counted (25). Activation of caspase 3 in tissue sections was assessed by immunofluorescence using rabbit anti-cleaved caspase-3 antibody (Cell Signaling Technologies, Beverly, MA) and Cy3-conjugated goat anti-rabbit IgG antibody (Jackson ImmunoResearch).
Kinase Assay, Electrophoretic Mobility-Shift Assay (EMSA), Western Blotting, and Real-Time RT-PCR. Methods and reagents are provided in the supporting information.
Statistical Analysis. Differences between means were compared by t tests and by ANOVA with post hoc Tukey tests. P values <0.05 were considered significant.
Results
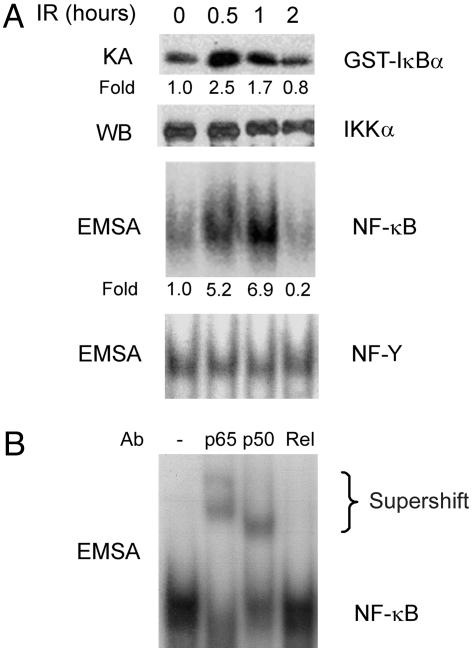
IR Activates Intestinal Epithelial Cell NF-κB in Vivo. IR induces nuclear translocation of NF-κB proteins through a pathway that involves ATM, the product of the ataxia telangiectasia gene (14) and the IKK complex (13). ATM deficiency results in defective NF-κB activation and increased sensitivity to IR (14), suggesting that NF-κB activation mediates, at least in part, the radioprotective activity of ATM. To determine whether IR activates IKK and NF-κB in intestinal epithelial cells in vivo, we exposed normal mice to whole-body γ-radiation and assayed IKK kinase activity and NF-κB DNA-binding activity. We found a marked activation of IKK and increased NF-κB DNA binding activity, consisting of p65/p50 heterodimers, in small intestinal epithelial cells of irradiated mice (Fig. 1). IR-induced NF-κB activation was paralleled by a 2.5-fold increase in mRNA levels of the NF-κB target gene IκBα after 4 h. These results suggested a physiological role for NF-κB in the intestinal radiation response.
Fig. 1.
IR activates NF-κB in intestinal epithelial cells. (A) Mice were exposed to 8-Gy IR and, after the indicated times, epithelial cells were isolated from the mid-small intestine. IKK activity was determined by an in vitro kinase assay (KA), and IKKα levels were assessed as a loading control by Western blotting (WB). NF-κB binding activity was determined by electrophoretic mobility-shift assay (EMSA) with NF-Y binding activity used as a loading control. The fold induction of IKK and NF-κB activities in irradiated relative to nonirradiated mice are shown under each lane. (B) The components of the IR-induced NF-κB complex were investigated by supershift assay, using antibodies (Ab) against the indicated NF-κB proteins.
Generation and Characterization of Conditional Intestinal Epithelial Cell IKKβ Knockout Mice. To address the functional role of radiation-induced IKK and NF-κB activation in vivo, signaling through the canonical NF-κB activation pathway was ablated by disrupting the gene encoding IKKβ (Ikk_β) selectively in intestinal epithelial cells of mice using the Cre/loxP system of somatic DNA recombination (26). An intestinal epithelial cell-specific Cre recombinase-expressing transgenic mouse line (Vil-Cre) was established (Fig. 2_A_). LoxP_ sites at a reporter gene locus (Gtrosa26) were efficiently and specifically recombined in intestinal epithelial cells throughout the crypt/villus units of Vil-Cre mice (Fig. 2_B_). Exon 3 of the murine _Ikk_β gene was flanked with loxP sites by using gene targeting to generate _Ikk_β+/F mice that transmit the floxed _Ikk_βF allele to their progeny (Fig. 2_C_) (22). In Vil-Cre mice predicted to be homozygous for the _Ikk_βF allele, complete recombination of this locus (producing the _Ikk_βΔ allele) occurred in small intestinal epithelial cells (Fig. 2_D_, right lane) and in colon epithelial cells. Quantification of residual _Ikk_βF using real-time PCR of genomic DNA, revealed that <5% nonrecombined locus remained in isolated intestinal epithelial cells of the small intestine. Approximately 50% of the _Ikk_β locus of mice predicted to be homozygous for _Ikk_βF but negative for Cre was recombined in intestinal epithelial cells (Fig. 2_D_, left lane) and other cell types (data not shown). Further analysis of _Ikk_β in various tissues of Vil-Cre/_Ikk_β+/F mice using a quantitative PCR designed to amplify preferentially the recombined _Ikk_βΔ allele (Fig. 2_C_, primers c and d) revealed the presence of a small amount of _Ikk_βΔ DNA in the testis (see supporting information). Negligible amounts of _Ikk_βΔ DNA were identified in other tissues. Thus, it appears that the recombined _Ikk_βΔ allele, reflecting recombination in the gonads, is transmitted through the germ line, producing an _Ikk_βF/Δ genotype. Like _Ikk_β+/F mice (22) and _Ikk_β+/– mice (19), _Ikk_βF/Δ mice were normal and were used as controls in further experiments.
To determine whether radiation-induced IKK and NF-κB activation depended on IKKβ in intestinal epithelial cells, _Ikk_βF/Δ and Vil-Cre/_Ikk_βF/Δ mice were irradiated. We found a marked defect in IKK and NF-κB activation in intestinal epithelial cells in Vil-Cre/_Ikk_βF/Δ mice (Fig. 2_E_). Most Vil-Cre/_Ikk_βF/Δ mice appeared normal, gained weight at the same rate as _Ikk_βF/Δ mice, and were fertile, although occasional Vil-Cre/_Ikk_βF/Δ mice exhibited growth retardation and skin inflammation of unclear cause. This phenotype may represent extraintestinal Cre expression in some mice causing a cutaneous IKKβ knockout, as recently described by others (27). Enterocyte lineage development was not different in Vil-Cre/_Ikk_βF/Δ mice and _Ikk_βF/Δ mice (Fig. 2_F_).
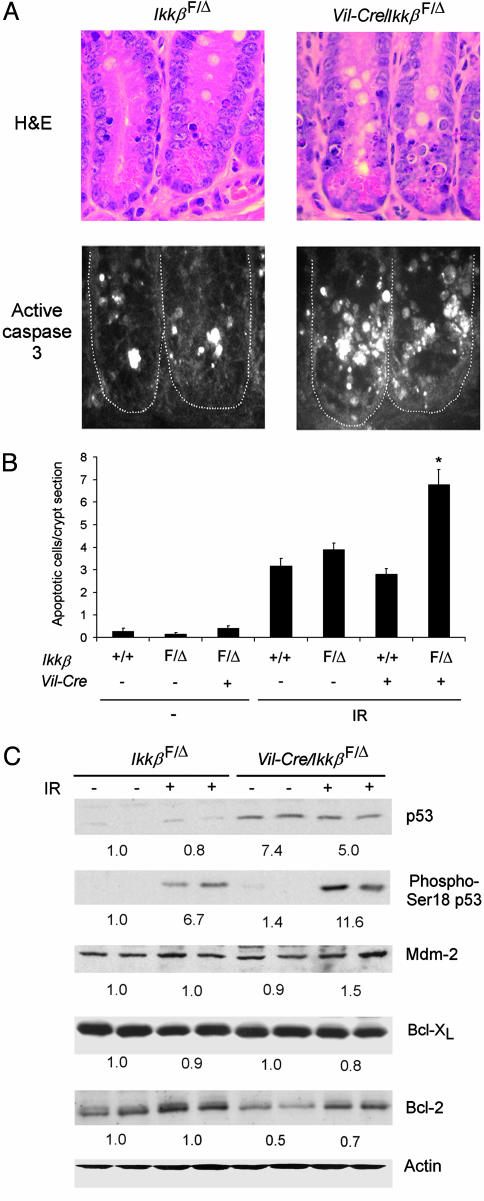
IKKβ-Deficient Small Intestinal Epithelial Cells Are More Sensitive to IR. To test the hypothesis that NF-κB signals an antiapoptotic response in intestinal epithelial cells of irradiated mice, apoptosis was quantified in small intestinal crypts of wild-type, _Ikk_βF/Δ, Vil-Cre, and Vil-Cre/_Ikk_βF/Δ mice (Fig. 3 A and B). Apoptotic small intestinal crypt epithelial cells were infrequent in nonirradiated Vil-Cre/_Ikk_βF/Δ and control mice. Consistent with prior reports (25), exposure to 8-Gy γ-radiation greatly increased apoptosis of small intestinal crypt epithelial cells, as identified morphologically and by caspase 3 activation. Notably, the number of apoptotic cells was ≈2-fold greater in irradiated Vil-Cre/_Ikk_βF/Δ than in irradiated control mice. Thus, IKKβ-dependent NF-κB activation is an important component of the intestinal epithelial cell response to IR in vivo, and serves to protect small intestinal crypt epithelial cells from apoptotic death.
Fig. 3.
Increased apoptosis in small intestinal crypt epithelial cells of intestinal epithelial cell-specific IKKβ knockout mice. (A) Crypt epithelial cell morphology and caspase 3 activation in _Ikk_βF/Δ and Vil-Cre/_Ikk_β F/Δ mice after irradiation. Mice of the indicated genotypes were irradiated (8 Gy), and, after 4.5 h, sections of small intestine were prepared and stained with hematoxylin and eosin (H&E) (Upper) or with anti-cleaved caspase-3 antibody (Lower). (B) Increased radiation-induced apoptosis in IKKβ-deficient intestinal epithelial cells. Mean (±SE) number of apoptotic cells per crypt section in the indicated nonirradiated mice (–, n = 4–8 mice per genotype) or in irradiated mice (IR, n = 9–16 mice per genotype). ANOVA, P < 0.0001; *, post hoc test, P < 0.01 compared to the three other genotypes exposed to IR. (C) Western blotting of protein extracts from small intestinal epithelial cells of _Ikk_βF/Δ or Vil-Cre/_Ikk_βF/Δ mice for p53, phosphoserine-18 p53, Mdm-2, Bcl-XL, Bcl-2, and actin. + indicates mice exposed to IR 4.5 h before cell isolation. Average relative protein abundance normalized to actin is shown beneath each pair of samples.
Increased p53 Levels in the Epithelium of Conditional IKKβ Knockout Mice. p53 is an essential mediator of the early phase of the intestinal epithelial cell apoptotic response to IR (4). Therefore, we assessed the expression of p53 in isolated intestinal epithelial cells from conditional IKKβ knockout and control mice. Whole-cell extracts of isolated small intestinal epithelial cells of Vil-Cre/_Ikk_βF/Δ mice contained higher levels of p53 than those of _Ikk_βF/Δ mice, both before and after irradiation (Fig. 3_C_). Irradiation resulted in a marked increase in serine-18 phosphorylation of p53 (corresponding to phosphorylation of human p53 at serine 15), which is important for p53 transactivation (28). A recent report using IKKα/β-null mouse embryonic fibroblasts suggested that IKKβ promotes p53 destabilization through Mdm-2 expression (29), but the levels of Mdm-2 in isolated intestinal epithelial cells of Vil-Cre/_Ikk_βF/Δ mice were similar to those observed in _Ikk_βF/Δ mice (Fig. 3_C_). These data suggest that increased p53 plays a role in the greater radiosensitivity of IKKβ-deficient intestinal epithelial cells, and that IKKβ regulates p53 protein levels in these cells by a mechanism independent of Mdm-2. p53 mRNA levels, determined by real-time PCR, were not different between Vil-Cre/_Ikk_βF/Δ and _Ikk_βF/Δ mice (data not shown), suggesting that IKKβ regulates p53 protein expression by a posttranslational mechanism. To determine whether additional mechanisms might mediate NF-κB-dependent antiapoptosis in irradiated intestinal epithelium, we evaluated expression of antiapoptotic NF-κB target genes (30). The mRNA levels of c-IAP1, c-IAP2, Gadd45β, Bcl-2, and Bcl-XL were not significantly different between control and IKKβ-deficient intestinal epithelial cells (data not shown), nor were the protein levels of Bcl-XL (Fig. 3_C_). Interestingly, baseline Bcl-2 protein levels were consistently lower in Vil-Cre/_Ikk_βF/Δ than in _Ikk_βF/Δ mice (Fig. 3_C_), although the functional significance of this difference is not known.
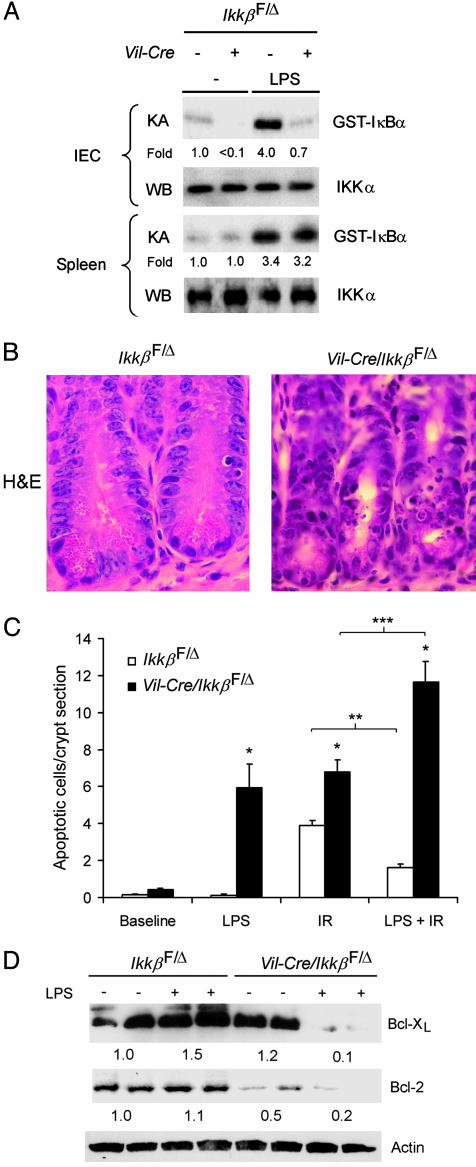
Inducible Radioprotection Depends on IKKβ. Because IR activated NF-κB in intestinal epithelial cells, which protected cells from IR-induced apoptosis, we reasoned that prior activation of NF-κB, for example, by systemic LPS injection, might induce greater resistance to subsequent irradiation. i.p. LPS injection resulted in marked IKK activation in the intestinal epithelial cells of _Ikk_βF/Δ mice after 1 h, but not in those of Vil-Cre/_Ikk_βF/Δ mice (Fig. 4_A_). LPS injection activated IKK similarly in the spleens of _Ikk_βF/Δ mice and Vil-Cre/_Ikk_βF/Δ mice, confirming specificity of the loss of IKK activity in intestinal epithelium. LPS injection before IR significantly decreased the number of apoptotic intestinal epithelial cells in _Ikk_βF/Δ (Fig. 4 B and C) and in wild-type mice (not shown) by ≈60%. In contrast, LPS injection before IR significantly increased the number of apoptotic intestinal epithelial cells in Vil-Cre/_Ikk_βF/Δ mice by ≈70% (Fig. 4_C_). LPS injection alone induced apoptosis in intestinal epithelial cells of Vil-Cre/_Ikk_βF/Δ mice, but not _Ikk_βF/Δ mice (Fig. 4_C_). Thus, IKKβ protects crypt epithelial cells from both LPS- and IR-induced apoptosis, and mediates the radioprotective effects of LPS. Together, these findings establish IKKβ as a critical survival factor for crypt epithelial cells of the small intestine.
Fig. 4.
Dependence of LPS-activated protection from radiation-induced apoptosis on epithelial cell IKKβ. (A) LPS-induced IKK activation in intestinal epithelial cells (IEC) and spleen cells. Mice of the indicated genotypes were injected with vehicle (–) or LPS 1 h before isolation of IEC or spleen cells as a source of cell extracts for the in vitro IKK kinase assay (KA) and IKKα Western blotting (WB). Fold induction of IKK activity by LPS is shown. (B) Crypt epithelial cell morphology of LPS pretreated (4 h) _Ikk_βF/Δ and Vil-Cre/_Ikk_βF/Δ mice 4.5 h after IR. Mice were injected with LPS before IR, the small intestine was removed, and sections were stained with hematoxylin and eosin (H&E). (C) Effects of LPS and IR on intestinal epithelial cell apoptosis. Mice of the indicated genotypes were left untreated (Baseline), injected with LPS (LPS), irradiated (IR), or injected with LPS and irradiated 4 h later (LPS + IR), and apoptosis was quantified. Mean (±SE) number of apoptotic cells per crypt section are shown (n = 4–10 mice per condition). *, t test, P < 0.05 compared to _Ikk_βF/Δ subjected to the same treatment; ** and ***, t test P < 0.05 for LPS-induced decrease or increase, respectively, in apoptotic cells. (D) Expression of Bcl-XL, Bcl-2, and actin were evaluated by Western blotting using protein extracts from isolated small intestinal epithelial cells of _Ikk_βF/Δ and Vil-Cre/_Ikk_βF/Δ mice 3 h after LPS (+) or vehicle (–) injection. Average relative protein abundance normalized to actin is shown beneath each pair of samples.
To identify molecular mechanisms by which IKKβ governs the apoptotic response of small intestinal epithelial cells to LPS treatment, we injected _Ikk_βF/Δ mice and Vil-Cre/_Ikk_βF/Δ mice with LPS and, after 3 h, analyzed expression levels of pro- and antiapoptotic proteins. p53 expression was not affected by LPS (data not shown). However, immunoblot analysis revealed a marked loss of expression of the antiapoptotic Bcl-XL and Bcl-2 proteins in IKKβ-deficient, but not in control, intestinal epithelial cells (Fig. 4_D_). Interestingly, decreased Bcl-XL expression was observed only after injection of Vil-Cre/_Ikk_βF/Δ mice with LPS, whereas, as noted in Fig. 3_C_, basal levels of Bcl-2 were already lower in Vil-Cre/_Ikk_βF/Δ mice before LPS injection. This finding suggests that LPS-stimulated radio-protection of intestinal epithelium might depend in part on NF-κB to maintain expression of antiapoptotic Bcl-2 family members, although the mechanism by which IKKβ prevents the LPS-induced decrease of Bcl-2 and Bcl-XL expression is not known.
Discussion
Our results show that IKKβ-dependent NF-κB activation is a key signaling event required for protection against radiation-induced apoptosis in the intestinal epithelium in vivo. IKKβ and NF-κB are likely to be activated by IR in a cell-autonomous manner, because prior studies had shown that ATM was required for the activation of NF-κB and p53 phosphorylation by IR in cell lines and gene targeted mice (14). Activation may also be secondary to apoptotic damage to intestinal endothelium (31). Although that study used significantly higher doses of IR than used herein, it is possible that IR-stimulated NF-κB activation in intestinal epithelial cells depends, in part, on endothelial injury. Irrespective of the mechanism by which IR induces NF-κB activation in intestinal epithelial cells in vivo, the defective antiapoptotic response of these cells in the absence of NF-κB activation shows the potent survival effect of this signaling cascade. Furthermore, the high sensitivity of IKKβ-deficient intestinal epithelial cells of LPS-pretreated mice to radiation-induced apoptosis reveals IKKβ and NF-κB as molecular mediators of the radioprotective effects of LPS. A prior study showing that LPS injection increased crypt survival in wild-type mice after IR further supports our conclusion (5). Together, these findings solidly establish the NF-κB system as a candidate target for mediating radioprotection in the irradiated intestine.
Our data suggest that increased levels of activated p53 contribute to the increased apoptosis of IKKβ-deficient epithelium following IR. Apoptosis in response to radiation requires the activation of p53 (4). We found that IKKβ-deficient intestinal epithelial cells from irradiated mice contained markedly increased levels of serine-18 phosphorylated p53, which is important for p53-dependent gene expression (28, 32). Although the molecular mechanism responsible for increased constitutive expression of p53 in IKKβ-deficient intestinal epithelium is not known, it possibly reflects the absence of competition with NF-κB for limiting amounts of the transcriptional coactivator p300 (33), resulting in greater availability of p300 for p53 stabilization (34). A recent report using IKKα/β-null mouse embryonic fibroblasts suggested that IKKβ promotes p53 destabilization through Mdm-2 expression (29). However, this is not the case in radiation-induced DNA damage of intestinal epithelium because Mdm-2 levels were not lower in IKKβ-deficient than in control intestinal epithelial cells, indicating a potential cell type-specific role for IKKβ in regulating Mdm-2 expression. Our results suggest that regulation of a proapoptotic mechanism (i.e., p53 activation) is a key determinant of NF-κB-dependent antiapoptosis in the irradiated intestinal epithelium.
Members of the Bcl-2 family, a subset of which are regulated by NF-κB, inhibit cell death in response to a variety of apoptosis-inducing agents (30, 35, 36). Prior studies had shown that the antiapoptotic Bcl-2 family member Bcl-XL, which is encoded by a NF-κB target gene, can restore apoptosis resistance in NF-κB-defective cells (36). Systemic injection of LPS profoundly lowered Bcl-XL protein in intestinal epithelial cells of conditional IKKβ-null mice. Expression of Bcl-2 was also lower in IKKβ-deficient intestinal epithelial cells, suggesting a dependence on NF-κB for its expression. Although the mechanism by which NF-κB preserves Bcl-XL and Bcl-2 expression after LPS injection is not known, lower expression of these antiapoptotic proteins in IKKβ-deficient intestinal epithelial cells was accompanied by a marked increase in apoptosis after irradiation. This finding suggests that LPS-mediated radioprotection, and perhaps that mediated by other NF-κB-activating factors (e.g., TNFα, IL-1 and lysophosphatidic acid), depends, at least in part, on the regulated expression of antiapoptotic Bcl-2 family members.
The results of this study reveal prevention of radiation-induced intestinal damage as a previously unrecognized physiologic function for NF-κB in vivo. Moreover, our results, taken together with prior reports of NF-κB-mediated resistance to chemotherapy (21, 29, 37) and radiation (15) in cancer cell lines, further highlight the crucial functions of NF-κB in governing the outcomes of cancer treatment. Whereas most efforts have been directed at abrogating the activation of IKKβ/NF-κB to inhibit inflammatory responses, the potent cell survival effect of IKKβ-dependent NF-κB activation points to the therapeutic potential of activating this signaling system. Thus, selective preactivation of NF-κB through IKKβ in intestinal epithelium, but not in the targeted cancer, could provide a therapeutic modality to limit acute radiation-induced damage to the intestine and allow higher doses of radiation to be tolerated by patients.
Supplementary Material
Supporting Information
Acknowledgments
This work was supported by National Institutes of Health Grants DK35108 and DK58960 (to M.F.K.), CA76188 and AI043477 (to M.K.), DK60792 (to L.J.E.), and AI56075 (to L.E.), grants from the Cystic Fibrosis Foundation (to M.F.K.) and the Superfund Basic Research Program (to M.K.), and a fellowship of the Cancer Research and Prevention Foundation (to F.R.G.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: IKK, IκB kinase; IR, ionizing radiation; LPS, lipopolysaccharide.
References
- 1.Lichter, A. S. (2000) in Clinical Oncology, ed. Abeloff, M. D. (Churchill Livingstone, New York), pp. 423–464.
- 2.Dubois, A. & Walker, R. I. (1988) Gastroenterology 95**,** 500–507. [DOI] [PubMed] [Google Scholar]
- 3.Cohn, S. & Bickston, S. J. (2003) in Textbook of Gastroenterology, ed. Yamada, T. (Lippincott Williams & Wilkins, Philadelphia), Vol. 2, pp. 2760–2771. [Google Scholar]
- 4.Merritt, A. J., Potten, C. S., Kemp, C. J., Hickman, J. A., Balmain, A., Lane, D. P. & Hall, P. A. (1994) Cancer Res. 54**,** 614–617. [PubMed] [Google Scholar]
- 5.Riehl, T., Cohn, S., Tessner, T., Schloemann, S. & Stenson, W. F. (2000) Gastroenterology 118**,** 1106–1116. [DOI] [PubMed] [Google Scholar]
- 6.Houchen, C. W., Sturmoski, M. A., Anant, S., Breyer, R. M. & Stenson, W. F. (2003) Am. J. Physiol. 284**,** G490–G498. [DOI] [PubMed] [Google Scholar]
- 7.Cohn, S. M., Schloemann, S., Tessner, T., Seibert, K. & Stenson, W. F. (1997) J. Clin. Invest. 99**,** 1367–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell, C. L., Bready, J. V., Rex, K. L., Chen, J. N., DiPalma, C. R., Whitcomb, K. L., Yin, S., Hill, D. C., Wiemann, B., Starnes, C. O., et al. (1998) Cancer Res. 58**,** 933–939. [PubMed] [Google Scholar]
- 9.Houchen, C. W., George, R. J., Sturmoski, M. A. & Cohn, S. M. (1999) Am. J. Physiol. 276**,** G249–G258. [DOI] [PubMed] [Google Scholar]
- 10.Okunieff, P., Mester, M., Wang, J., Maddox, T., Gong, X., Tang, D., Coffee, M. & Ding, I. (1998) Radiat. Res. 150**,** 204–211. [PubMed] [Google Scholar]
- 11.Deng, W., Balazs, L., Wang, D. A., Van Middlesworth, L., Tigyi, G. & Johnson, L. R. (2002) Gastroenterology 123**,** 206–216. [DOI] [PubMed] [Google Scholar]
- 12.Neta, R. (1997) Stem Cells 15**,** 87–94. [DOI] [PubMed] [Google Scholar]
- 13.Li, N. & Karin, M. (1998) Proc. Natl. Acad. Sci. USA 95**,** 13012–13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, N., Banin, S., Ouyang, H., Li, G. C., Courtois, G., Shiloh, Y., Karin, M. & Rotman, G. (2001) J. Biol. Chem. 276**,** 8898–8903. [DOI] [PubMed] [Google Scholar]
- 15.Russo, S. M., Tepper, J. E., Baldwin, A. S., Jr., Liu, R., Adams, J., Elliott, P. & Cusack, J. C., Jr. (2001) Int. J. Radiat. Oncol. Biol. Phys. 50**,** 183–193. [DOI] [PubMed] [Google Scholar]
- 16.Rothwarf, D. M. & Karin, M. (1999) Sci. STKE 1999**,** RE1. [DOI] [PubMed] [Google Scholar]
- 17.Karin, M. & Ben-Neriah, Y. (2000) Annu. Rev. Immunol. 18**,** 621–663. [DOI] [PubMed] [Google Scholar]
- 18.Beg, A. A. & Baltimore, D. (1996) Science 274**,** 782–784. [DOI] [PubMed] [Google Scholar]
- 19.Li, Z. W., Chu, W., Hu, Y., Delhase, M., Deerinck, T., Ellisman, M., Johnson, R. & Karin, M. (1999) J. Exp. Med. 189**,** 1839–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makris, C., Godfrey, V. L., Krahn-Senftleben, G., Takahashi, T., Roberts, J. L., Schwarz, T., Feng, L., Johnson, R. S. & Karin, M. (2000) Mol. Cell 5**,** 969–979. [DOI] [PubMed] [Google Scholar]
- 21.Wang, C. Y., Cusack, J. C., Jr., Liu, R. & Baldwin, A. S., Jr. (1999) Nat. Med. 5**,** 412–417. [DOI] [PubMed] [Google Scholar]
- 22.Li, Z. W., Omori, S. A., Labuda, T., Karin, M. & Rickert, R. C. (2003) J. Immunol. 170**,** 4630–4637. [DOI] [PubMed] [Google Scholar]
- 23.Pinto, D., Robine, S., Jaisser, F., El Marjou, F. E. & Louvard, D. (1999) J. Biol. Chem. 274**,** 6476–6482. [DOI] [PubMed] [Google Scholar]
- 24.Mao, X., Fujiwara, Y. & Orkin, S. H. (1999) Proc. Natl. Acad. Sci. USA 96**,** 5037–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potten, C. S. (1990) Int. J. Radiat. Biol. 58**,** 925–973. [DOI] [PubMed] [Google Scholar]
- 26.Nagy, A. (2000) Genesis 26**,** 99–109. [PubMed] [Google Scholar]
- 27.Pasparakis, M., Courtois, G., Hafner, M., Schmidt-Supprian, M., Nenci, A., Toksoy, A., Krampert, M., Goebeler, M., Gillitzer, R., Israel, A., et al. (2002) Nature 417**,** 861–866. [DOI] [PubMed] [Google Scholar]
- 28.Chao, C., Saito, S., Anderson, C. W., Appella, E. & Xu, Y. (2000) Proc. Natl. Acad. Sci. USA 97**,** 11936–11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tergaonkar, V., Pando, M., Vafa, O., Wahl, G. & Verma, I. (2002) Cancer Cell 1**,** 493–503. [DOI] [PubMed] [Google Scholar]
- 30.Karin, M. & Lin, A. (2002) Nat. Immunol. 3**,** 221–227. [DOI] [PubMed] [Google Scholar]
- 31.Paris, F., Fuks, Z., Kang, A., Capodieci, P., Juan, G., Ehleiter, D., Haimovitz-Friedman, A., Cordon-Cardo, C. & Kolesnick, R. (2001) Science 293**,** 293–297. [DOI] [PubMed] [Google Scholar]
- 32.Siliciano, J. D., Canman, C. E., Taya, Y., Sakaguchi, K., Appella, E. & Kastan, M. B. (1997) Genes Dev. 11**,** 3471–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong, H., Voll, R. E. & Ghosh, S. (1998) Mol. Cell 1**,** 661–671. [DOI] [PubMed] [Google Scholar]
- 34.Yuan, Z. M., Huang, Y., Ishiko, T., Nakada, S., Utsugisawa, T., Shioya, H., Utsugisawa, Y., Yokoyama, K., Weichselbaum, R., Shi, Y. & Kufe, D. (1999) J. Biol. Chem. 274**,** 1883–1886. [DOI] [PubMed] [Google Scholar]
- 35.Chen, C., Edelstein, L. C. & Gelinas, C. (2000) Mol. Cell. Biol. 20**,** 2687–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rayet, B., Fan, Y. & Gelinas, C. (2003) Mol. Cell. Biol. 23**,** 1520–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldwin, A. S. (2001) J. Clin. Invest. 107**,** 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information