Myeloid KLF4 deficiency augments atherogenesis in ApoE-/- mice (original) (raw)
. Author manuscript; available in PMC: 2013 Dec 1.
Published in final edited form as: Arterioscler Thromb Vasc Biol. 2012 Oct 11;32(12):2836–2838. doi: 10.1161/ATVBAHA.112.300471
Abstract
Objective
To investigate the role of Krüppel-like factor 4 (KLF4), an essential transcriptional regulator of macrophage polarization (M1/M2), in the pathogenesis of atherosclerosis.
Methods and Results
Despite the acknowledged importance of macrophages in atherosclerosis, the role of M1 (classically activated or pro-inflammatory) versus M2 (alternatively activated or anti-inflammatory) macrophages in this process remains incompletely understood. We recently identified KLF4 as a regulator of macrophage subset specification i.e. KLF4 promotes the M2 and inhibits the M1 phenotype. Here, we provide evidence that KLF4 deficient macrophages exhibit enhanced pro-inflammatory activation and foam cell formation in response to oxidized lipids. In vivo, myeloid KLF4 deficient mice (ApoE-/- background) develop significantly more vascular inflammation and atherosclerotic lesion formation.
Conclusion
Our findings identify myeloid KLF4 as an essential regulator of vascular inflammation and experimental atherogenesis.
Keywords: atherosclerosis, inflammation, KLF4, macrophage
Introduction
Macrophages are central to the development of vascular inflammation and atherogenesis1. Recent investigations highlight the importance of two subsets of macrophages termed M1 (classical) and M2 (alternative) in a broad array of biological processes2. These two subsets have also been identified in atherosclerotic lesions but their importance remains incompletely understood3-5.
KLF4, has been shown to regulate cell growth, development, differentiation, and function of diverse cell types6, 7. Recently, we showed that KLF4 control macrophage speciation by promoting the M2 phenotype and inhibiting the M1 phenotype8. However, the importance of myeloid KLF4 in atherogenesis remains unknown.
Results
Myeloid KLF4 regulates inflammatory targets in response to oxidized lipids
We first determined if pro-inflammatory cytokines and lipids modulate KLF4 expression. Peritoneal macrophages were treated with 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphatidylcholine (POV-PC), an OxLDL species9, IL-1β, and TNF-α. As shown in Fig 1A, KLF4 expression is significantly reduced by pro-inflammatory cytokines and modified lipids. Next, peritoneal macrophages from control (LysMCre/Cre:KLF4+/+ designated KLF4+/+) and myeloid KLF4 deficient mice (LysMCre/Cre:KLF4Flox/Flox; designated KLF4Δ/Δ) were treated with POV-PC and inflammatory targets were analyzed by qPCR (Fig 1B) and ELISA/enzymatic activity (supplementary Fig I). KLF4 deficient macrophages expressed higher levels of classical M1 markers such as iNOS, IL-6, and IL-1β. Next we assessed the role of KLF4 in modified lipid uptake. As shown in Fig 1C, the uptake of fluorescent labeled OxLDL (but not AcLDL, data not shown) was enhanced in the KLF4 deficient macrophages. Consistent with these observations, qPCR analysis revealed enhanced expression of CD36 (receptor for oxidized LDL) but not MSR1 (receptor for acetylated LDL) in KLF4Δ/Δ macrophages (Fig 1D).
Figure 1. Myeloid KLF4 regulates the response to oxidized lipids.
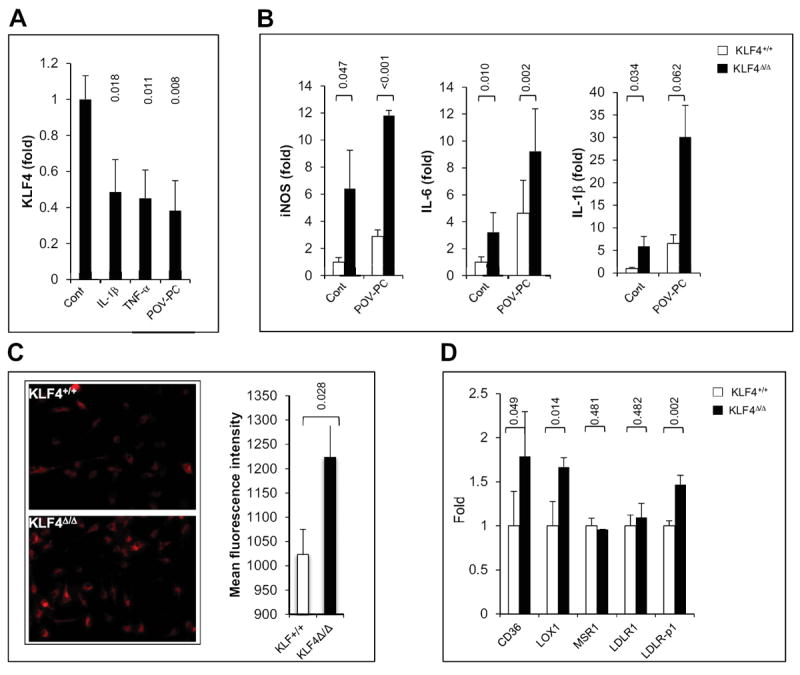
A) After 16hrs treatment of macrophage with IL-1β (20ng/ml), TNF-α (10ng/ml) or POV-PC (10μg/ml), KLF4 expression was analyzed by qPCR. (n=3) B) KLF4+/+ or KLF4Δ/Δ macrophages were treated with POV-PC and expression of pro-inflammatory markers iNOS, IL-6 and IL-1β was analyzed by qPCR. (n=3) C) KLF4+/+ or KLF4Δ/Δ macrophages were incubated with Dil-OxLDL (15μg/ml) for 4 hrs and uptake was analyzed by fluorescence-microscopy and flow cytometry. (n=4) D) Gene expression analysis of lipoprotein receptors in KLF4+/+ and KLF4Δ/Δ macrophages (n=4).
Myeloid KLF4 deficiency augments experimental atherosclerosis
To determine the role of myeloid KLF4 on atherogenesis, KLF4+/+ and KLF4Δ/Δ mice were crossed to ApoE-/- mice and fed a normal chow (n=9 per group) or HFD (n=18 per group). After 20 weeks of feeding, mice were euthanized and aortas harvested for assessment of atherosclerotic lesion area by Sudan IV staining. Intriguingly, as shown in Fig 2, enhanced lesion area was observed on both a normal chow (22.84±3.50 versus 9.15±2.85; P= < 0.001) as well as HFD (35.50±9.8 versus 24.38±10.42; P=0.002). q-PCR analysis of aortic tissues revealed increased expression of proinflammatory (M1) targets (supplementary Fig IIA&B). In addition, a reduction was observed in some M2 markers, particularly following HFD (supplementary Fig IIA&B). Mac-3 staining confirmed increased macrophage infiltration in aortic lesions (Supplementary Fig III). Finally, plasma lipid analyses revealed a modest increase in LDL, triglycerides, HDL and total cholesterol in KLF4Δ/Δ mice ApoE−/− following high fat diet but not chow diet (supplementary table).
Figure 2. Myeloid KLF4 deficiency augments atherosclerosis.
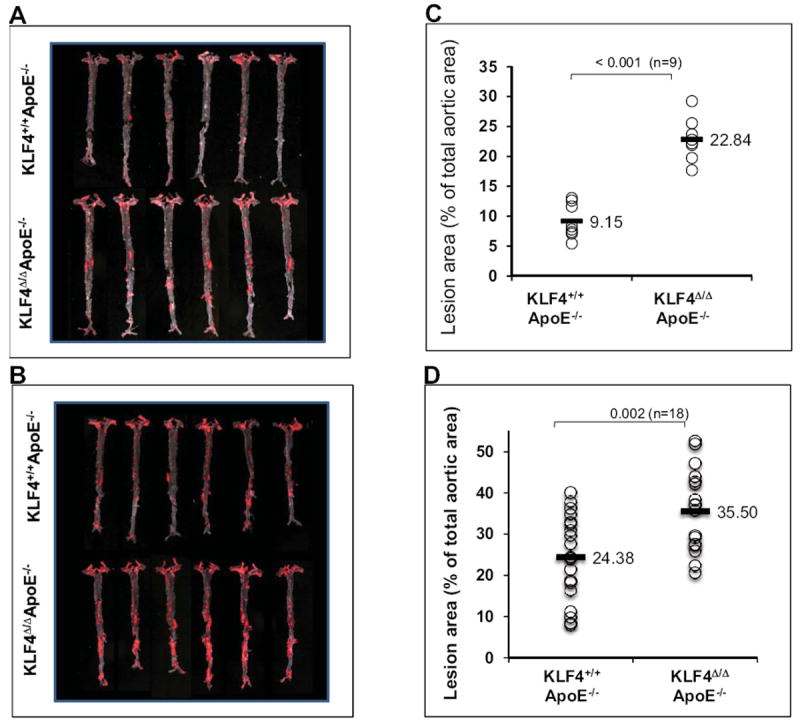
A&B) Representative Sudan IV staining indicating enhanced atherosclerotic lesions in KLF4Δ/ΔApoE−/− vs. KLF4Δ/ΔApoE+/+ mice on normal chow (A) and HFD (B). C&D) Quantitative analysis of lesion area on normal chow (C) and HFD (D) fed mice aortas.
Discussion
Macrophages are major cellular constituents of atherosclerotic lesions but how different subsets impact atherogenesis remains poorly understood3-5. We recently identified the transcription factor KLF4 as a key molecular determinant of macrophage subset specification8. Myeloid deficiency of KLF4 was found to enhance M1 gene expression and reduce M2 gene expression8. Furthermore, myeloid KLF4 deficient mice exhibited diet-induced obesity, insulin resistance, and glucose intolerance8. The availability of this model provided an opportunity to determine the effect of altering macrophage polarization on atherogenesis. Here we show that myeloid deficiency of KLF4 leads to enhanced atherosclerotic lesion formation and macrophage accumulation in the atherosclerotic lesion, supporting a critical role of myeloid KLF4 in vascular inflammation and atherogenesis following a HFD. Intriguingly, a significant increase in lesion formation and vascular inflammation (Fig 2 and supplementary Fig II) was also observed under normal chow conditions, observations that underscore the importance of KLF4 in disease pathogenesis. However, there are several caveats to our work. We note that, in response to HFD, myeloid KLF4 deficient mice exhibited altered lipid profiles that may contribute to the observed phenotype. Also, as there are differences in mouse versus human macrophage subsets, one must be cautious in directly extending these findings to the biology of human atherosclerosis.
Our study also expands our understanding of stimuli that regulate myeloid KLF4 expression. Previously, we showed that inflammatory cytokines reduce KLF4 expression in macrophages8. Here we extend these observations and show that oxidized lipids also reduce macrophage KLF4 expression. The importance of KLF4 is highlighted by the fact that its absence results in enhanced pro-inflammatory gene expression in response to POP-VC. As inflammatory cytokines and certain lipid species are well known to contribute to obesity and atherosclerosis, this down-regulation may be functionally quite important10. Indeed, consistent with this idea, we recently found that KLF4 expression was reduced in macrophages derived from obese subjects8.
In summary, our observations implicate myeloid KLF4 as a key regulator of experimental atherogenesis in mice. The fact that KLF4 has been shown to confer favorable anti-inflammatory effects in both the endothelium11 and myeloid compartments8 suggest that targeting this factor could be exploited for therapeutic gain.
Supplementary Material
1
Acknowledgments
This work is supported by NIH grants HL097593, HL086548, HL076754 HL084154, HL075427 (M.K.J), HL097023 (G.H.M), AHA grants 09POST2060203 and 11POST7200004 (N.S).
References
- 1.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol. 2009;20:370–378. doi: 10.1097/MOL.0b013e3283309848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. Ppargamma activation primes human monocytes into alternative m2 macrophages with anti-inflammatory properties. Cell metabolism. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The kruppel-like factor klf4 is a critical regulator of monocyte differentiation. Embo J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clement K, Jain MK. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor cd36. The Journal of biological chemistry. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 10.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 11.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1