Dietary intake of berries and flavonoids in relation to cognitive decline (original) (raw)
. Author manuscript; available in PMC: 2013 Jul 1.
Published in final edited form as: Ann Neurol. 2012 Apr 26;72(1):135–143. doi: 10.1002/ana.23594
Abstract
Objective
Berries are high in flavonoids, especially anthocyanidins, and improve cognition in experimental studies. We prospectively evaluated whether greater long-term intakes of berries and flavonoids are associated with slower rates of cognitive decline in older women.
Methods
Beginning in 1980, a semi-quantitative food frequency questionnaire was administered every four years to Nurses’ Health Study participants. In 1995–2001, we began measuring cognitive function in 16,010 participants, aged ≥70 years; follow-up assessments were conducted twice, at two-year intervals. To ascertain long-term diet, we averaged dietary variables from 1980 through the initial cognitive interview. Using multivariable-adjusted, mixed linear regression, we estimated mean differences in slopes of cognitive decline by long-term berry and flavonoid intakes.
Results
Greater intakes of blueberries and strawberries were associated with slower rates of cognitive decline (e.g., for a global score averaging all six cognitive tests, for blueberries: p-trend=0.014 and mean difference=0.04 [95% CI=0.01, 0.07] comparing extreme categories of intake; for strawberries: p-trend= 0.022 and mean difference=0.03 [95% CI=0.00, 0.06] comparing extreme categories of intake), after adjusting for multiple potential confounders. These effect estimates were equivalent to those we find for approximately 1.5 to 2.5 years of age in our cohort, indicating that berry intake appears to delay cognitive aging by up to 2.5 years. Additionally, in further supporting evidence, greater intakes of anthocyanidins and total flavonoids were associated with slower rates of cognitive decline (p-trends= 0.015 and 0.053, respectively, for the global score).
Interpretation
Higher intake of flavonoids, particularly from berries, appears to reduce rates of cognitive decline in older adults.
INTRODUCTION
Substantial experimental data have established that berry supplementation enhances neuronal function and survival and ameliorates age-related cognitive impairment in rodents 1–7. Very small trials in older persons (≤15 participants) have also observed improved short-term cognitive performance with flavonoid-rich fruit juices, including blueberry juice 8, 9. Supporting these findings, berries are particularly high in a subclass of flavonoids called anthocyanidins, which can cross the blood-brain barrier and localize in areas of learning and memory (e.g., hippocampus) 10. Moreover, flavonoids more generally have powerful antioxidant and anti-inflammatory properties, and both oxidative stress and inflammation are thought to be important contributors to cognitive impairment; thus, increased flavonoid consumption could be a potential strategy for reducing cognitive decline in older adults 11–13.
However, no epidemiologic studies have specifically examined berry or anthocyanidin intake and cognition, and few large-scale epidemiologic studies have explored dietary flavonoids in relation to cognitive decline in older adults. Because easily-utilized databases of diverse flavonoids recently became available, we evaluated the associations of long-term dietary intake of berries and flavonoids, including anthocyanidins, with cognitive decline in a large, prospective cohort of older women in the Nurses’ Health Study. Our a priori hypothesis was that greater intake of berries and flavonoids would be associated with slower rates of cognitive decline.
METHODS
The Nurses’ Health Study began in 1976, when 121,700 female, registered nurses, aged 30–55 years, completed a mailed questionnaire on their health and lifestyle. Follow-up questionnaires are sent biennially; follow up is >90%. A food frequency questionnaire was added in 1980 and updated every four years. During 1995–2001, women who were ≥70 years old and free of stroke were invited to participate in a telephone-based study of cognitive function. For the first interview, 93% percent of eligible women participated (n=19,415) and 7% refused; follow up was >90% in the second and third interviews. Median time between the first and second interviews was 1.8 years, and between the first and third interviews was 4.2 years. The Institutional Review Board of Brigham and Women’s Hospital approved this study, and informed consent was implied by return of the mailed questionnaire and completion of the telephone cognitive interview.
Population for analysis
Of the 19,415 women who completed the initial cognitive interview, we excluded those who did not provide dietary data (n=3,405); thus, our analytic sample consisted of 16,010 women (see Supplementary Figure 1). Women included in our analysis were similar to those who were excluded (e.g., mean age=74.2 vs. 74.4 years; mean body-mass index=26.0 vs. 26.3 kg/m2; 77 vs. 80% with an associate’s degree, 23 vs. 20% with a bachelor’s degree or higher, respectively).
Dietary assessment
We used a 61-item, Willett semi-quantitative food frequency questionnaire to ascertain dietary habits in 1980, and an expanded 130-item version in 1984, 1986, and every four years thereafter 14. Foods were specified in a common unit or portion size (e.g., ½ cup of blueberries), and participants reported how often, on average, they consumed each food over the previous year (nine response categories ranged from “almost never” to “≥6 times per day”). For each participant, the consumption frequency of each food was multiplied by its flavonoid content, and values were summed across all foods. We also calculated intakes for 31 individual flavonoids representing six major flavonoid subclasses (anthocyanidins, flavonols, flavones, flavanones, flavan-3-ols, and polymeric flavonoids) that are commonly found in US diets. Flavonoid information was obtained using the US Department of Agriculture database, an extensive European database (EuroFIR eBASIS, http://www.eurofir.org), and consultation with nutritional experts. Individual flavonoid intakes were summed to estimate total flavonoid intake.
In a validation study, intakes of major flavonoid-containing foods were found to correlate highly when measurements from the food frequency questionnaire were compared to four, one-week dietary records collected over one year (e.g. correlations were 0.93 for tea, 0.80 for apples, 0.84 for orange juice, and 0.74 for oranges) 15.
Cognitive assessment
We administered six cognitive tests: Telephone Interview of Cognitive Status, a telephone adaptation of the Mini-Mental State Examination; East Boston Memory Test – immediate and delayed recalls; category fluency; delayed recall of the Telephone Interview of Cognitive Status 10-word list; and digit span backward 16–18. In a validation study, our telephone-based cognitive battery performed well compared to detailed, in-person interviews among 61 highly-educated women aged ≥70 years old (ρ=0.81 comparing the two modes of assessment). Inter-interviewer reliability was also high across ten interviewers each scoring the same cognitive interview (ρ>0.95 for interviewers’ scoring of each cognitive test). For analysis, we evaluated three primary cognitive outcomes selected a priori: two measures of overall cognition (a global composite score averaging all tests, and the Telephone Interview of Cognitive Status) and a verbal memory composite score, averaging four tests of episodic memory (which is particularly affected in early Alzheimer’s disease) 19–21. Because our six cognitive tests were each scaled differently, we used z-scores to create the two composite measures; specifically, we calculated the difference between each participant’s individual score on a given test measure and the overall mean score for our population, and then divided by the population standard deviation. To create the global score, we averaged together z-scores for all six cognitive tests; for the verbal score, we averaged the immediate and delayed recalls of both the East Boston Memory Test and Telephone Interview of Cognitive Status 10-word list.
Statistical analysis
Because cognitive decline develops over many years, long-term dietary habits are likely most relevant to brain health; thus, to obtain stable measures reflecting long-term diet, we averaged berry and flavonoid intakes from each food frequency questionnaire beginning in 1980, through each participant’s last dietary report prior to initial cognitive assessment 22. Based on our a priori hypothesis, we evaluated the association of berry and anthocyanidin intake with cognitive decline as our primary analysis. Because flavonoids might have cognitive benefits more generally, we also examined other flavonoid subclasses as well as total flavonoid intake. To complement these analyses, we evaluated intakes of other flavonoid-rich foods (tea, apples, oranges, and onions) because dietary recommendations are easier to make based on foods rather than nutrients.
Multivariable-adjusted, mixed linear regression was utilized to estimate mean differences in rates of cognitive decline over approximately four years, across categories of berry and other flavonoid-rich food consumption (sensible categories were determined based on the distribution of intake for each food) and quintiles of flavonoid intakes. Flavonoid variables were energy adjusted using the residual method 23. We used repeated-measures modeling with random intercepts and slopes, which permits description of individual cognitive trajectories over time and provides explicit tests regarding the relation of exposures to rates of cognitive decline. To construct our mixed models, we included terms for the exposure, covariates, and continuous time to account for associations with initial cognitive scores, and added interaction terms for the exposure and covariates with continuous time to estimate relations with cognitive decline. We calculated 95% confidence intervals and performed linear tests of trend using continuous variables composed of the median values of exposure categories.
We considered multiple potential confounders: age, (continuous), education (associate’s degree, bachelor’s degree, graduate degree), antidepressant use (yes, no), smoking (never, past, current), physical activity (metabolic-equivalent-hours/week in quintiles, missing), body-mass index (<22, 22–24, 25–30, 30+ kg/m2), history of high blood pressure (yes, no), history of high cholesterol (yes, no), history of type 2 diabetes (yes, no), history of myocardial infarction (yes, no), total caloric intake (kcal/day in quintiles), and dietary factors including fish intake (≤3 servings/month, 1 serving/week, 2–4 servings/week, ≥5 servings/week, missing), alcohol intake (none, 0–14 g/day, 15+ g/day, missing), and overall dietary scores (the Alternative Healthy Eating Index and Alternative Mediterranean Diet, in quintiles). We were particularly concerned that socioeconomic status might confound associations with berries, as they tend to be relatively high-priced fruits; thus, we considered adjustments for marital status (yes, no), husband’s highest level of educational attainment (high school degree or less, college degree, graduate school, missing), mother’s occupation at age 16 (upper white-collar, lower white-collar and skilled manual, unskilled manual, farming, other/missing), father’s occupation at age 16 (upper white-collar, lower white-collar and skilled manual, unskilled manual, farming, other/missing), and several variables based on the census tract of a participant’s residence, geocoded to the 2000 US Census, including median annual household income (quintiles) and median home value (quintiles). Covariate status was determined at the time of initial cognitive interview.
In our model building, we evaluated initial models that were adjusted for age and education only, and then carefully considered adjustment for numerous other potential confounding factors listed above. In particular, we built several sets of models adjusting for age and education plus the following covariates in successive models: 1) antidepressant use; 2) lifestyle factors (smoking, physical activity, body-mass index, alcohol intake); 3) cardiovascular factors (history of high blood pressure, high cholesterol, type 2 diabetes, and myocardial infarction); 4) dietary factors (total energy intake, fish intake, and dietary patterns); and 5) socioeconomic status (martial status, husband’s highest level of educational attainment, mother’s occupation when nurse was age 16, father’s occupation when nurse was age 16, annual household income, and median home value). Effect estimates were very similar in all of these models, and we therefore chose to develop a parsimonious model including: age, education, alcohol intake (except in flavonoid analyses where red wine contributed to the exposure), physical activity, body-mass index, total caloric intake, and annual household income. These covariates were selected because they were related to berry intake (see Table 1) and were either significantly associated with cognitive decline in our participants, or were key a priori risk factors for cognitive decline based on the literature.
Table 1.
Baseline characteristics of women in the Nurses’ Health Study cognitive study by categories of berry intake 1
| Blueberry intake | Strawberry intake | |||||
|---|---|---|---|---|---|---|
| <1 serving/mo(35.2%) | 1–3 servings/mo(44.8%) | ≥1 serving/wk(20.0%) | <1 serving/wk(44.5%) | 1 serving/wk(33.9%) | ≥2 servings/wk(21.6%) | |
| Mean age (SD), y | 74.4 | 74.2 | 74.2 | 74.3 | 74.2 | 74.3 |
| Education, % | ||||||
| Associate’s degree | 80.6 | 76.9 | 73.1 | 79.4 | 76.5 | 75.0 |
| Bachelor’s degree | 14.1 | 16.8 | 18.8 | 15.1 | 16.8 | 17.6 |
| Graduate degree | 5.3 | 6.3 | 8.1 | 5.5 | 6.7 | 7.4 |
| Antidepressant use, % | 6.3 | 5.6 | 5.7 | 5.8 | 5.7 | 6.3 |
| Smoking, % | ||||||
| Never | 44.9 | 47.1 | 49.5 | 43.9 | 49.2 | 49.1 |
| Past | 45.1 | 45.8 | 45.3 | 46.6 | 43.9 | 45.5 |
| Current | 10.0 | 7.1 | 5.2 | 9.5 | 6.9 | 5.4 |
| Median physical activity (IQR), metabolic-equivalent-hours/week 2 | 7.9 | 10.2 | 11.6 | 8.4 | 10.5 | 11.0 |
| Body mass index, kg/m2, % | ||||||
| <22 | 23.2 | 19.5 | 20.0 | 22.9 | 19.6 | 18.7 |
| 22–24 | 26.4 | 27.6 | 25.7 | 27.2 | 26.4 | 26.7 |
| 25–30 | 32.5 | 35.8 | 37.1 | 33.3 | 36.1 | 36.2 |
| 30+ | 17.9 | 17.1 | 17.2 | 16.6 | 17.9 | 18.4 |
| High blood pressure, % 3 | 55.7 | 55.1 | 54.1 | 55.8 | 53.8 | 55.7 |
| High cholesterol, % 3 | 65.8 | 65.8 | 63.3 | 66.4 | 64.0 | 65.0 |
| Type 2 diabetes, % 3 | 9.4 | 10.0 | 9.9 | 9.0 | 10.2 | 10.7 |
| Myocardial infarction, % 3 | 5.8 | 6.0 | 5.8 | 6.4 | 5.1 | 6.0 |
| Mean caloric intake, kcal/d | 1619.9 | 1721.5 | 1816.1 | 1636.7 | 1731.2 | 1803.5 |
| Fish intake, % | ||||||
| ≤3 servings/month | 12.4 | 5.8 | 3.8 | 9.7 | 6.6 | 5.4 |
| 1 serving/week | 24.0 | 16.9 | 12.7 | 22.5 | 16.6 | 13.3 |
| 2–4 servings/week | 55.3 | 64.1 | 60.8 | 58.6 | 62.9 | 60.1 |
| ≥5 servings/week | 8.3 | 13.2 | 22.7 | 9.2 | 13.9 | 21.2 |
| Alcohol intake, g/d, % | ||||||
| None | 54.5 | 47.7 | 44.0 | 51.4 | 47.3 | 48.1 |
| 0–14 | 36.4 | 43.7 | 47.8 | 38.9 | 44.1 | 44.9 |
| 15+ | 9.1 | 8.6 | 8.2 | 9.7 | 8.6 | 7.0 |
| Mean score on the Alternative Healthy Eating Index, points | 39.3 | 43.0 | 46.6 | 40.1 | 43.3 | 45.8 |
| Husband’s education, % | ||||||
| High school degree or less | 58.0 | 50.1 | 45.5 | 54.1 | 50.8 | 49.3 |
| College degree | 25.5 | 28.5 | 29.7 | 26.6 | 28.5 | 28.6 |
| Graduate school | 16.5 | 21.4 | 24.8 | 19.3 | 20.7 | 22.1 |
| Father’s occupation when nurse was age 16, % | ||||||
| Upper white-collar | 23.9 | 25.7 | 26.1 | 24.3 | 25.4 | 26.4 |
| Lower white-collar and skilled manual | 59.4 | 58.2 | 59.2 | 59.7 | 58.4 | 57.7 |
| Unskilled manual | 3.4 | 3.4 | 3.3 | 3.6 | 3.4 | 3.0 |
| Farming | 13.3 | 12.7 | 11.4 | 12.4 | 12.8 | 12.9 |
| Median annual household income, in US dollars | 53,607.0∣53,607.0 | 53,607.0∣56,484.0 | 59,635.0∣59,635.0 | 59,635.0∣55,645.5 | 55,785.0∣55,785.0 | 55,785.0∣57,912.5 |
| Mean baseline cognitive scores 4 | ||||||
| Global score, standard units | −0.03 | 0.02 | −0.01 | −0.02 | 0.02 | −0.03 |
| Verbal memory score, standard units | −0.03 | 0.01 | −0.01 | −0.03 | 0.01 | −0.02 |
| Telephone Interview of Cognitive Status, points | 33.7 | 33.9 | 33.8 | 33.7 | 33.9 | 33.7 |
In secondary analyses, we evaluated effect modification by smoking status because smoking greatly increases oxidative stress, and berries and flavonoids could affect cognition through antioxidant mechanisms. We also repeated our analyses while excluding women in the bottom 10% of the score distribution on our initial cognitive tests, as these women may have been experiencing early stages of dementia that also caused them to reduce their intake of healthy foods, such as blueberries and strawberries.
All statistical analyses were performed in SAS version 9 (SAS Institute Incorporated, Cary, North Carolina).
RESULTS
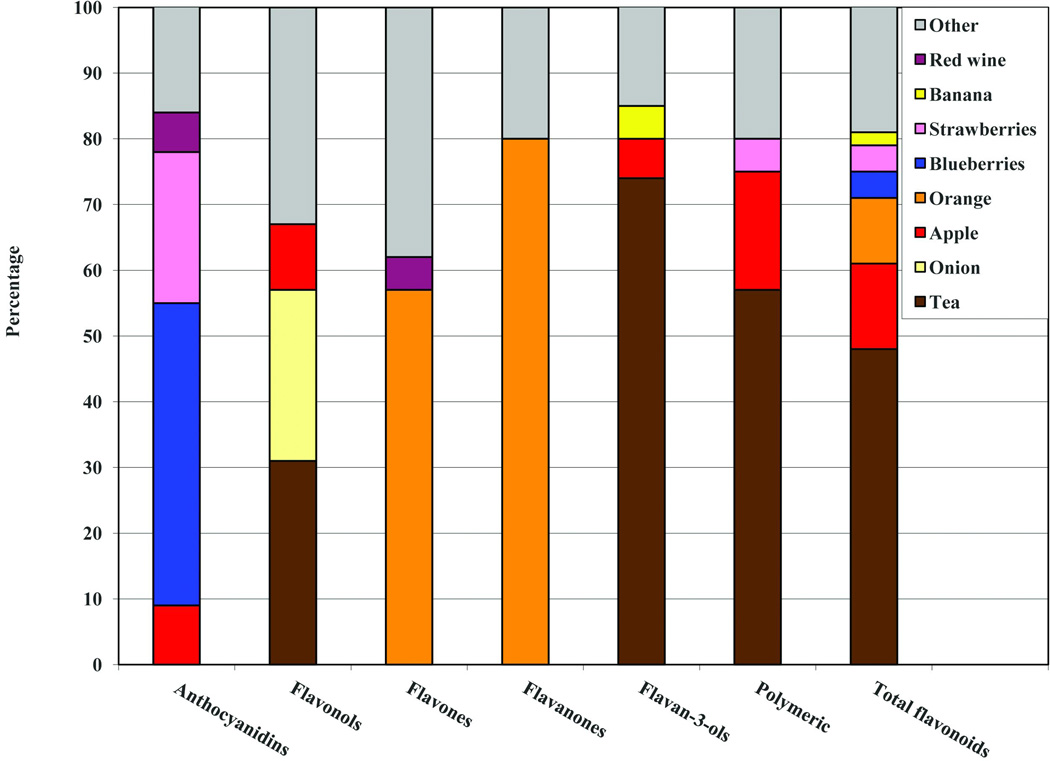
Blueberries and strawberries were the primary foods contributing to anthocyanidin intake in our cohort, whereas tea, apples, and oranges were the top contributors to other flavonoid subclasses and total flavonoid intake (Figure 1). In total, tea, apples, oranges, berries, and onions accounted for >80% of between-person variation in total flavonoid consumption in this cohort. We observed few meaningful differences in participants’ characteristics at the initial cognitive interview across categories of blueberry and strawberry intake (Table 1). However, women with the highest blueberry or strawberry intakes had somewhat higher physical activity levels and annual household income compared to those with the lowest berry intakes; as expected, total caloric intake and fish intake were both greater across increasing quintiles of berry intake. Similarly, participant characteristics did not differ markedly across categories of total flavonoid intake either (data not shown in table).
Figure 1.
Top food contributors to six flavonoid subclasses (anthocyanidins, flavonols, flavones, flavanones, flavan-3-ols, and polymeric flavonoids) and total flavonoid intake in the Nurses’ Health Study.
After adjustment for age and education, greater consumption of blueberries was highly associated with slower decline in the global score (p-trend=0.010), the verbal score (p-trend=0.016), and the Telephone Interview of Cognitive Status (p-trend=0.027) (Table 2). The mean difference in rate of global decline was 0.04 standard units over the follow-up period (95% CI=0.01, 0.07) comparing women who ate ≥1 serving of blueberries per week to those who ate <1 serving per month. Similarly, greater intake of strawberries was related to slower decline in the global and verbal scores when we adjusted for age and education (p-trends=0.021 and 0.014, respectively); for example, women who ate ≥2 servings of strawberries per week had an average decline in the global score that was 0.04 standard units less over follow up compared to women who ate <1 serving per week (95% CI=0.01, 0.08). To help interpret the mean differences, we find that one year of age in our population is associated with a mean decline of 0.02 standard units on the global score over the follow-up period; thus, the mean differences that we observed comparing extreme categories of blueberry and strawberry intakes were equivalent to approximately 1.5 to 2.5 years of cognitive aging. In other words, women with higher berry intake appeared to have delayed cognitive aging by up to 2.5 years. These trends all remained similar upon adjustment for multiple potential confounders, including annual household income.
Table 2.
Mean differences in rates of cognitive decline over four years of follow up, across categories of berry intake 1,2
| Blueberries | ||||
|---|---|---|---|---|
| <1 serving/mo(35.2%) | 1−3 servings/mo(44.8%) | ≥1 serving/wk(20.0%) | p-trend | |
| Global score | ||||
| Model 1 3 | 0.00 | 0.02−0.01, 0.04 | 0.040.01, 0.07 | 0.010 |
| Model 2 4 | 0.00 | 0.02−0.01, 0.04 | 0.040.01, 0.07 | 0.014 |
| Verbal memory score | ||||
| Model 1 3 | 0.00 | 0.02−0.01, 0.05 | 0.050.01, 0.09 | 0.016 |
| Model 2 4 | 0.00 | 0.02−0.01, 0.06 | 0.050.01, 0.09 | 0.022 |
| Telephone Interview of Cognitive Status | ||||
| Model 1 3 | 0.00 | 0.11−0.01, 0.22 | 0.160.02, 0.31 | 0.027 |
| Model 2 4 | 0.00 | 0.11−0.01, 0.23 | 0.170.03, 0.32 | 0.022 |
| Strawberries | ||||
| <1 serving/wk(44.5%) | 1 serving/wk(33.9%) | ≥2 servings/wk(21.6%) | p-trend | |
| Global score | ||||
| Model 1 3 | 0.00 | 0.00−0.03, 0.02 | 0.030.00, 0.06 | 0.021 |
| Model 2 4 | 0.00 | 0.00−0.03, 0.02 | 0.030.00, 0.06 | 0.022 |
| Verbal memory score | ||||
| Model 1 3 | 0.00 | 0.00−0.03, 0.03 | 0.040.01, 0.08 | 0.014 |
| Model 2 4 | 0.00 | 0.00−0.04, 0.03 | 0.040.01, 0.08 | 0.015 |
| Telephone Interview of Cognitive Status | ||||
| Model 1 3 | 0.00 | 0.01−0.10, 0.13 | 0.08−0.05, 0.21 | 0.24 |
| Model 2 4 | 0.00 | 0.01−0.11, 0.13 | 0.09−0.05, 0.22 | 0.22 |
We also found that greater intake of anthocyanidins was related to cognitive decline (e.g. p-trend=0.015 for the global score), after adjusting for multiple potential confounders (results not shown in tables). For example, the mean difference in global score decline over the follow-up period was 0.03 standard units (95% CI=−0.01, 0.06) comparing extreme quintiles of anthocyanidin intake. We observed a modest association for flavonols as well (e.g. for the global score, p-trend=0.052 and mean difference=0.03 [95% CI=0.00, 0.07] comparing extreme quintiles of flavonol intake). Overall, qualitatively, we found suggestions of reduced cognitive decline with higher flavonoid intake across most flavonoid subclasses.
Consistent with these results, higher total flavonoid intake was associated with slower rates of cognitive decline for all three of our primary outcome measures in age- and education- adjusted models (e.g. p-trend=0.029 for the global score; Table 3). For example, women in the highest quintile of total flavonoid intake had an average decline in the global score that was 0.04 standard units less over the follow-up period (95% CI=0.00, 0.07) compared to women in the lowest quintile. These results were very similar when we additionally adjusted for multiple potential confounders, and mean differences in decline were equivalent to 1.5–2.5 years of cognitive aging. When we examined other important food contributors to flavonoid intake, consumption of tea, apples, onions, and oranges was not related to cognitive decline (e.g., for the global score, for tea: p-trend=0.23 and mean difference=0.02 [95% CI=−0.02, 0.06] comparing extreme categories of intake; for apples: p-trend=0.84 and mean difference=0.01 [95% CI=− 0.03, 0.04] comparing extreme categories of intake; for onions: p-trend=0.095 and mean difference=0.02 [95% CI=−0.02, 0.06] comparing extreme categories of intake; and for oranges: p-trend=0.69 and mean difference=0.01 [95% CI=−0.02, 0.04] comparing extreme categories of intake; results not shown in tables).
Table 3.
Mean differences in rates of cognitive decline over four years of follow up, across quintiles of total flavonoid intake 1,2
| Quintiles of total flavonoid intake | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p-trend | |
| Median flavonoid intake, milligrams/d | 145.4 | 220.5 | 297.7 | 412.0 | 684.1 | |
| Global composite score | ||||||
| Model 1 3 | 0.00 | 0.01−0.02, 0.05 | 0.01−0.02, 0.05 | 0.02−0.01, 0.05 | 0.040.00, 0.07 | 0.029 |
| Model 2 4 | 0.00 | 0.01−0.02, 0.04 | 0.01−0.02 0.04 | 0.02−0.02, 0.05 | 0.030.00, 0.07 | 0.053 |
| Verbal memory composite score | ||||||
| Model 1 3 | 0.00 | 0.03−0.02, 0.07 | 0.04−0.01, 0.08 | 0.040.00, 0.09 | 0.050.01, 0.09 | 0.028 |
| Model 2 4 | 0.00 | 0.02−0.02, 0.07 | 0.03−0.01, 0.08 | 0.040.00, 0.08 | 0.050.00, 0.09 | 0.040 |
| Telephone Interview of Cognitive Status | ||||||
| Model 1 3 | 0.00 | 0.08−0.08, 0.24 | 0.02−0.14, 0.18 | 0.04−0.11, 0.20 | 0.180.02, 0.33 | 0.041 |
| Model 2 4 | 0.00 | 0.06−0.09, 0.22 | 0.00−0.16, 0.16 | 0.03−0.13, 0.19 | 0.15−0.01, 0.31 | 0.072 |
Finally, in secondary analyses, smoking status did not modify the associations of berries, flavonoids, and cognitive decline, and results were nearly identical when we excluded women with the lowest cognitive scores at our initial cognitive interview (results not shown in tables).
DISCUSSION
Higher, long-term consumption of berries, anthocyanidins, and total dietary flavonoids were related to significantly slower rates of cognitive decline in this cohort of older women, even after careful consideration of confounding by socioeconomic status. We report the first epidemiologic evidence that greater intakes of blueberries and strawberries (top food contributors to anthocyanidin intake) were highly associated with slower rates of cognitive decline – consistent with a large body of experimental data supporting cognitive benefits of berries. The magnitude of associations that we identified for berries and flavonoids were equivalent to the cognitive differences that we observe in women up to 2.5 years apart in age – that is, women with higher intake of berries and total flavonoids appeared to have delayed cognitive aging by as much as 2.5 years.
Very small trials have indicated that berry supplementation can enhance cognitive function over twelve weeks in older adults with early cognitive impairments 8, 9. In a study of older men (n=342), total flavonoid consumption was related to a lower risk of cognitive impairment, although findings were not statistically significant (comparing the top versus bottom tertiles: odds ratio=0.86, 95% confidence interval=0.39, 1.89) 24. However, this study had limited power to detect associations between flavonoids and cognition due to its modest size. A larger study in France (n=1,640) identified a relation between greater flavonoid intake and slower cognitive decline over a ten-year period (p-trend=0.05 in models adjusted for age, sex, and education) 25. These results were attenuated and became non-significant upon adjustment for smoking, body-mass index, and fruit and vegetable intake; however, the inclusion of fruit and vegetable intake probably represents over adjustment because these foods contain flavonoids, and therefore the adjusted results are difficult to interpret. In all of these studies, no specific information on long-term dietary habits was available; as previously noted, long-term diet is likely to be most relevant for cognitive decline. In our data, correlations were modest across repeated measures of berry intake (ρ=0.2–0.3), which probably reflects true within-person changes in berry consumption over time (e.g., due to changes in berry availability by year and region26, 27) and also reflects some amount of random measurement error. By averaging reported intakes over multiple dietary assessments, we account for within-person changes in intake and also reduce random measurement error (as has been previously documented by our group28). Thus, for biologic and statistical reasons, existing epidemiologic data probably underestimate any relation between flavonoids and cognition.
Substantial biologic evidence supports our finding that berry and flavonoid intake may be related to cognition. Berry-derived anthocyanidins are uniquely and specifically capable of both crossing the blood-brain barrier and localizing in brain regions involved in learning and memory (e.g., the hippocampus).10 In multiple studies of rats, blueberry or strawberry supplementation significantly reduced age-related declines in neuronal signaling and cognitive behavior, and supplementation at older ages reversed neuronal and cognitive decline 29. More generally, several flavonoids have been shown to inhibit the c-jun N-terminal kinases, apoptosis signal-regulating kinase-1, and p38 pathways, which can lead to reductions in neuroinflammation and enhanced neuronal viability. In addition, flavonoid-rich foods can activate extracellular receptor kinase and protein kinase B/Akt pathways, which are thought to enhance memory and cognition. Overall, although flavonoids are traditionally regarded for their antioxidant properties, the discovery of these various molecular targets suggests that flavonoids may act through multiple mechanisms; this might explain why, other than a specific effect of anthocyanidins, we largely observed that greater overall flavonoid intake appeared more important than any single flavonoid subclass or flavonoid-rich food.
Our study has limitations. First, this is an observational study, and we cannot rule out the possibility that residual confounding explains observed associations. However, we adjusted for a large variety of health and lifestyle factors, as well as socioeconomic and dietary variables, and observed very little change in our effect estimates before and after adjustment, which suggests that residual confounding is unlikely to further influence our findings meaningfully. Second, the self-reported dietary information contains some random misclassification; however, this would tend to bias our results toward the null and therefore cause an underestimate of the relation between flavonoids and cognition. Finally, we identified associations between higher berry and flavonoid intake and slower cognitive decline in an all-female cohort, and therefore our results could be limited in their generalizability to men. Yet, few existing studies have reported substantial sex differences in dietary risk factors for cognitive decline and dementia, suggesting that limited generalizability is probably not a large concern in our study. Still, future studies should consider men in analyses of berries, flavonoids, and cognitive decline.
In conclusion, we found that higher consumption of berries and anthocyanidins, as well as total flavonoids, is associated with a slower progression of cognitive decline in older women. These findings potentially have substantial public health implications, as increasing berry intake represents a fairly simple dietary modification to test in older adults for maintaining cognition.
Supplementary Material
Supp Fig S1. Supplementary Figure 1.
Study flow design.
* Women who were excluded because of missing dietary data were similar to those who were included in our analyses (e.g., mean age=74.4 vs. 74.2 years; mean body-mass index=26.3 vs. 26.0 kg/m2, respectively).
ACKNOWLEDGEMENTS
This study was funded by grants from the National Institutes of Health (P01 CA87969) and the California Strawberry Commission. Dr. Devore received a postdoctoral fellowship from the National Institutes of Health (F32 AG031633) to carry out this work.
REFERENCES
- 1.Spencer JP. Flavonoids: modulators of brain function? Br J Nutr. 2008 May;99(E Suppl 1):ES60–ES77. doi: 10.1017/S0007114508965776. [DOI] [PubMed] [Google Scholar]
- 2.Patil CS, Singh VP, Satyanarayan PS, Jain NK, Singh A, Kulkarni SK. Protective effect of flavonoids against aging- and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology. 2003 Oct;69(2):59–67. doi: 10.1159/000072357. [DOI] [PubMed] [Google Scholar]
- 3.Unno K, Takabayashi F, Yoshida H, et al. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology. 2007 Apr;8(2):89–95. doi: 10.1007/s10522-006-9036-8. [DOI] [PubMed] [Google Scholar]
- 4.He M, Zhao L, Wei MJ, Yao WF, Zhao HS, Chen FJ. Neuroprotective effects of (−)-epigallocatechin-3-gallate on aging mice induced by D-galactose. Biol Pharm Bull. 2009 Jan;32(1):55–60. doi: 10.1248/bpb.32.55. [DOI] [PubMed] [Google Scholar]
- 5.Willis LM, Shukitt-Hale B, Joseph JA. Modulation of cognition and behavior in aged animals: role for antioxidant- and essential fatty acid-rich plant foods. Am J Clin Nutr. 2009 May;89(5):1602S–1606S. doi: 10.3945/ajcn.2009.26736J. [DOI] [PubMed] [Google Scholar]
- 6.Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006 Mar;22(3):295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Shukitt-Hale B, Cheng V, Joseph JA. Effects of blackberries on motor and cognitive function in aged rats. Nutr Neurosci. 2009 Jun;12(3):135–140. doi: 10.1179/147683009X423292. [DOI] [PubMed] [Google Scholar]
- 8.Krikorian R, Shidler MD, Nash TA, et al. Blueberry supplementation improves memory in older adults. J Agric Food Chem. 2010 Apr 14;58(7):3996–4000. doi: 10.1021/jf9029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krikorian R, Nash TA, Shidler MD, Shukitt-Hale B, Joseph JA. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br J Nutr. 2010 Mar;103(5):730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- 10.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005 Apr;8(2):111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt-Schillig S, Schaffer S, Weber CC, Eckert GP, Muller WE. Flavonoids and the aging brain. J Physiol Pharmacol. 2005 Mar;56(Suppl 1):23–36. [PubMed] [Google Scholar]
- 12.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004 Aug 5;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behl C. Amyloid beta-protein toxicity and oxidative stress in Alzheimer's disease. Cell Tissue Res. 1997 Dec;290(3):471–480. doi: 10.1007/s004410050955. [DOI] [PubMed] [Google Scholar]
- 14.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985 Jul;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 15.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989 Dec;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 16.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991 Apr;57(3–4):167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 17.Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer's disease. A longitudinal study. Brain. 1991 Dec;114(Pt 6):2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- 18.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992 Dec;49(12):1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 19.Locascio JJ, Growdon JH, Corkin S. Cognitive test performance in detecting, staging, and tracking Alzheimer's disease. Arch Neurol. 1995 Nov;52(11):1087–1099. doi: 10.1001/archneur.1995.00540350081020. [DOI] [PubMed] [Google Scholar]
- 20.Small BJ, Fratiglioni L, Backman L. Canaries in a coal mine: cognitive markers of preclinical Alzheimer disease. Arch Gen Psychiatry. 2001 Sep;58(9):859–860. doi: 10.1001/archpsyc.58.9.859. [DOI] [PubMed] [Google Scholar]
- 21.Welsh K, Butters N, Hughes J, Mohs R, Heyman A. Detection of abnormal memory decline in mild cases of Alzheimer's disease using CERAD neuropsychological measures. Arch Neurol. 1991 Mar;48(3):278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- 22.Launer LJ. The epidemiologic study of dementia: a life-long quest? Neurobiol Aging. 2005 Mar;26(3):335–340. doi: 10.1016/j.neurobiolaging.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997 Apr;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 24.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997 Jan 1;145(1):33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 25.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007 Jun 15;165(12):1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- 26.Economics, Statistics, and Market Information System: U.S. Strawberry Industry [database on the Internet] United States Department of Agriculture; [cited March 7, 2012] [Google Scholar]
- 27.Economics, Statistics, and Market Information System: U.S. Blueberry Industry [database on the Internet] United States Department of Agriculture; [cited March 7, 2012] [Google Scholar]
- 28.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. American journal of epidemiology. 1999 Mar 15;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 29.Joseph JA, Shukitt-Hale B, Willis LM. Grape juice, berries, and walnuts affect brain aging and behavior. J Nutr. 2009 Sep;139(9):1813S–1817S. doi: 10.3945/jn.109.108266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Fig S1. Supplementary Figure 1.
Study flow design.
* Women who were excluded because of missing dietary data were similar to those who were included in our analyses (e.g., mean age=74.4 vs. 74.2 years; mean body-mass index=26.3 vs. 26.0 kg/m2, respectively).