Tumor Microsatellite-Instability Status as a Predictor of Benefit from Fluorouracil-Based Adjuvant Chemotherapy for Colon Cancer (original) (raw)
. Author manuscript; available in PMC: 2013 Feb 28.
Published in final edited form as: N Engl J Med. 2003 Jul 17;349(3):247–257. doi: 10.1056/NEJMoa022289
Abstract
BACKGROUND
Colon cancers with high-frequency microsatellite instability have clinical and pathological features that distinguish them from microsatellite-stable tumors. We investigated the usefulness of microsatellite-instability status as a predictor of the benefit of adjuvant chemotherapy with fluorouracil in stage II and stage III colon cancer.
METHODS
Tumor specimens were collected from patients with colon cancer who were enrolled in randomized trials of fluorouracil-based adjuvant chemotherapy. Microsatellite instability was assessed with the use of mononucleotide and dinucleotide markers.
RESULTS
Of 570 tissue specimens, 95 (16.7 percent) exhibited high-frequency microsatellite instability. Among 287 patients who did not receive adjuvant therapy, those with tumors displaying high-frequency microsatellite instability had a better five-year rate of overall survival than patients with tumors exhibiting microsatellite stability or low-frequency instability (hazard ratio for death, 0.31 [95 percent confidence interval, 0.14 to 0.72]; P=0.004). Among patients who received adjuvant chemotherapy, high-frequency microsatellite instability was not correlated with increased overall survival (hazard ratio for death, 1.07 [95 percent confidence interval, 0.62 to 1.86]; P=0.80). The benefit of treatment differed significantly according to the microsatellite-instability status (P=0.01). Adjuvant chemotherapy improved overall survival among patients with microsatellite-stable tumors or tumors exhibiting low-frequency microsatellite instability, according to a multivariate analysis adjusted for stage and grade (hazard ratio for death, 0.72 [95 percent confidence interval, 0.53 to 0.99]; P=0.04). By contrast, there was no benefit of adjuvant chemotherapy in the group with high-frequency microsatellite instability.
CONCLUSIONS
Fluorouracil-based adjuvant chemotherapy benefited patients with stage II or stage III colon cancer with microsatellite-stable tumors or tumors exhibiting low-frequency microsatellite instability but not those with tumors exhibiting high-frequency microsatellite instability.
Colorectal cancer is the fourth most common type of cancer in Western society and the second leading cause of cancer-related death in North America.1 Although surgical resection alone is potentially curative, local or distant recurrences develop in many patients, and those with the highest risk of recurrence are advised to receive fluorouracil-based systemic adjuvant chemotherapy, which has been shown to be beneficial in a number of cooperative-group trials and analyses.2–12
Traditional pathological staging systems have been useful in predicting the outcome of colorectal cancer, but it is now evident that these cancers are heterogeneous. The natural history of colorectal cancer correlates strongly with genetic alterations that occur during the progression from adenoma to carcinoma to metastatic disease.13,14 The most common genetic alterations, occurring in approximately 85 percent of colorectal cancers, are allelic losses or loss of heterozygosity, chromosomal amplifications, and translocations.15–19 These alterations are characteristic of the chromosomal-instability pathway, also known as the microsatellite-stability pathway. The remaining 15 percent of colorectal cancers display frame-shift mutations and base-pair substitutions that are commonly found in short, tandemly repeated nucleotide sequences known as microsatellites.16,20–27 This form of genetic destabilization is most commonly caused by the loss of the DNA mismatch-repair function and is referred to as the microsatellite-instability pathway. The phenotype of tumors with this defect is termed the high-frequency–microsatellite-instability phenotype.16,20,22,28–32 The chromosomal-instability phenotype and the high-frequency–microsatellite-instability phenotype do not represent alterations of single genes, but rather discrete molecular pathways involving multiple somatic genetic targets.20
Recently, distinct clinical and pathological features of colorectal tumors arising from these two separate mutational pathways have been identified. High-frequency microsatellite instability is observed more frequently in colorectal cancers that occur proximal to the splenic flexure. These tumors also exhibit poor differentiation, mucinous cell type, and peritumoral lymphocytic infiltration; they are usually diploid, unlike microsatellite-stable tumors, which are commonly aneuploid.26,27,33–35 Colorectal cancers exhibiting high-frequency microsatellite instability have also been associated with a larger size of the primary tumor but a more favorable stage distribution.22,33 Patients with colorectal cancers that exhibit high-frequency microsatellite instability have longer survival than stage-matched patients with cancers exhibiting microsatellite stability.16,22,23,26,36–38
Few studies have examined the effect of adjuvant treatment in colorectal cancers with high-frequency microsatellite instability.23,24,36,39,40 Furthermore, studies that have controlled for the effects of adjuvant therapy have had small or nonrandomized study populations with potential selection biases.24,36,39,40 We used specimens from patients with resected stage II or stage III colon cancer who were previously enrolled in prospective, randomized trials of fluorouracil-based chemotherapy. The pooled data base for these trials includes phase 3 studies with groups that received no treatment, thus permitting an analysis of the true survival advantage for patients whose tumors exhibited high-frequency microsatellite instability and who had not received adjuvant chemotherapy. In addition, studying this population of patients allowed us to analyze whether the phenotype of high-frequency microsatellite instability could be an independent predictor of a benefit from fluorouracil-based adjuvant chemotherapy.
METHODS
PATIENTS AND SPECIMENS
We studied specimens from 570 patients with colon cancer who had previously been enrolled in five phase 3 trials of adjuvant chemotherapy (Table 1). The primary objective of each of the trials was to determine whether fluorouracil-based adjuvant chemotherapy improved disease-free survival, overall survival, or both among patients who had undergone curative resection of stage II or stage III colon cancer. Three studies randomly assigned patients to fluorouracil plus leucovorin or no treatment, and two studies randomly assigned patients to fluorouracil plus levamisole or no treatment. The median duration of follow-up for all patients was 7.4 years. The current analysis was in accordance with the original informed consent signed by all patients.
Table 1.
Characteristics of the Trials.*
| Characteristic | Clinical Trials Group, National Cancer Institute of Canada | North Central Cancer Treatment Group | Gastrointestinal Intergroup, National Cancer Institute | Fondation Française de Cancérologie Digestive | |
|---|---|---|---|---|---|
| Protocol 784852 | Protocol 874651 | ||||
| Details of trial | |||||
| Date of first randomization | May1987 | May1978 | February1988 | January1985 | October1982 |
| Total no. of patients randomized | 370 | 267 | 111 | 936 | 268 |
| Total no. of samples collected and analyzed for microsatellite-instability status | 292 | 66 | 34 | 143 | 35 |
| Eligibility criteria | |||||
| Age limit | None | None | None | None | ≤75 yr |
| Minimum no. of days between surgery and beginning of chemotherapy | 56 | 35 | 35 | 35 | 35 |
| Adjuvant chemotherapy | |||||
| Dose of fluorouracil (mg/m2)† | 370 | 450 | 425 | 450 | 400 |
| Dose of leucovorin (mg/m2)† | 200 | — | 20 | — | 200 |
| Dose of levamisole (mg/m2)† | — | 50 | — | 50 | — |
| Duration of therapy (mo) | 6 | 12 | 6 | 12 | 6 |
| Median follow-up (yr) | 6.2 | 11.6 | 7.8 | 8.8 | 4.9 |
Blocks of formalin-fixed, paraffin-embedded specimens were requested from the relevant pathology departments. In total, 570 specimens were included in the analysis of microsatellite instability. Collected specimens that were excluded from the analysis had low tumor cellularity (<60 percent) or could not be amplified by polymerase chain reaction (PCR).
MICROSATELLITE-INSTABILITY TESTING AND ANALYSIS
Extracted DNA was amplified by PCR with the use of 2 to 11 microsatellite loci. Specifically, specimens from the North Central Cancer Treatment Group (protocols 784852 and 874651) and Gastrointestinal Intergroup trial 0035 of the National Cancer Institute were screened with 4 to 11 dinucleotide markers, as described previously.23,41 Nearly all specimens collected from the C.03 trial of the National Cancer Institute of Canada were amplified with 5 to 10 microsatellite loci derived from the panel of microsatellite loci defined by the National Cancer Institute, as described previously.22,34,42,43 Specimens obtained from the trial conducted by the Fondation Française de Cancérologie Digestive, as well as five specimens from the C.03 trial of the National Cancer Institute of Canada, were screened only with mononucleotide markers BAT25 and BAT26, since non-neoplastic control tissue was not available.44
The presence of additional bands observed in the PCR products from tumor DNA that were not observed in DNA from normal tissue from the same patient was scored as instability at that particular locus. In accordance with consensus definitions of the National Cancer Institute, tumor samples were classified as displaying high-frequency microsatellite instability (instability at 30 percent or more of the loci screened), low-frequency microsatellite instability (instability at less than 30 percent of the loci screened), or microsatellite stability (stability at all the loci tested).34,42 Since extensive data indicate that tumors with low-frequency microsatellite instability are not biologically distinct from those exhibiting microsatellite stability, these two molecular phenotypes were grouped together in all analyses.23,45
The microsatellite-instability status of tumors from patients without available corresponding normal tissue was analyzed with the use of the BAT25 and BAT26 markers, without the need for amplified normal DNA, as described previously.40,46 Specifically, samples with instability at both markers were scored as exhibiting high-frequency microsatellite instability, whereas samples with no instability at these markers were scored as microsatellite-stable. No specimen exhibited instability at only one of the two mononucleotide markers.
CLINICAL DATA BASE
A common clinical data base had previously been established and verified by investigators from centers in the International Multicentre Pooled Analysis of Colon Cancer Trials. This data base was maintained by the Clinical Trials Group of the National Cancer Institute of Canada and was recently merged with the clinical data bases of Gastrointestinal Intergroup trial 0035 of the National Cancer Institute and protocols 784852 and 874651 of the North Central Cancer Treatment Group for combined analysis. All data bases were prepared and managed by persons with no knowledge of the molecular data.
STATISTICAL ANALYSIS
For the outcome analysis, patients were classified according to the presence of high-frequency microsatellite instability, low-frequency microsatellite instability, or microsatellite stability in the tumor specimens. The primary outcomes were overall survival and disease-free survival. Overall survival was defined as the time from study entry to death. Disease-free survival was defined as the time from study entry to the first confirmed relapse or death, whichever occurred first. Data on overall and disease-free survival were censored at eight years from the date of randomization. Survival curves were generated according to the method of Kaplan and Meier, and univariate survival distributions were compared with the use of the log-rank test.47 Hazard ratios and 95 percent confidence intervals for univariate and multivariate models were computed with the use of Cox proportional-hazards regression.48 P values for tests of interaction were computed with the use of the likelihood-ratio statistic in comparisons between a model including main effects but no interaction and the same model with the inclusion of the term for interaction.
Differences in base-line prognostic factors according to the microsatellite-instability status of the patients’ tumors were tested for statistical significance with the use of a chi-square test for categorical variables or an unpaired Student’s t-test for continuous variables. The use of randomized clinical trials comparing fluorouracil-based adjuvant treatment with no adjuvant treatment permitted us to test directly for an effect of chemotherapy. In addition, a test for interaction between microsatellite-instability status and treatment effect was performed with the use of Cox proportional-hazards regression. The probability that chemotherapy is associated with a 5 percent or greater increase in the rate of five-year survival among patients with colon cancers exhibiting high-frequency microsatellite instability was analyzed with the use of both a simulation based on a Weibull survival model and a standard bootstrap technique.
All time-to-event analyses were stratified according to the type of treatment protocol (levamisole or leucovorin in addition to fluorouracil-based adjuvant chemotherapy). Specifically, the C.03 trial of the National Cancer Institute of Canada, the trial of the Fondation Française de Cancérologie Digestive, and protocol 874651 of the North Central Cancer Treatment Group were treated as one stratum, and protocol 784852 of the North Central Cancer Treatment Group and Gastrointestinal Intergroup trial 0035 of the National Cancer Institute were treated as a separate stratum for univariate analyses. Additional analyses were further stratified according to the stage of disease, as indicated. All reported P values are two-sided, and P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
CHARACTERISTICS OF THE PATIENTS AND MICROSATELLITE-INSTABILITY STATUS
Of 570 tumor samples tested for microsatellite instability, 95 (16.7 percent) demonstrated high-frequency microsatellite instability, 60 (10.5 percent) demonstrated low-frequency microsatellite instability, and 415 (72.8 percent) were microsatellite-stable. High-frequency microsatellite instability was associated with localization of the tumor to a site proximal to the splenic flexure (P<0.001) and a high histologic tumor grade (P<0.001) (Table 2). In other respects, the patients with tumors exhibiting high-frequency microsatellite instability were similar to the patients with tumors exhibiting microsatellite stability or low-frequency microsatellite instability (Table 2).
Table 2.
Characteristics of the 570 Patients with Colon Cancer.*
| Characteristic | All Eligible Patients (N=570) | Patients with Tumors Exhibiting High-Frequency Microsatellite Instability (N=95) | Patients with Tumors Exhibiting Microsatellite Stability or Low-Frequency Microsatellite Instability (N=475) | P Value |
|---|---|---|---|---|
| Treatment — no. (%) | 0.19 | |||
| Adjuvant chemotherapy | 283 (50) | 53 (56) | 230 (48) | |
| No adjuvant chemotherapy | 287 (50) | 42 (44) | 245 (52) | |
| Age — yr | 59.8±11.2 | 60.7±13.0 | 59.7±10.8 | 0.13 |
| Sex — no. (%) | 0.45 | |||
| Male | 326 (57) | 51 (54) | 275 (58) | |
| Female | 244 (43) | 44 (46) | 200 (42) | |
| Stage of disease — no. (%) | 0.18 | |||
| II | 312 (55) | 58 (61) | 254 (53) | |
| III | 258 (45) | 37 (39) | 221 (47) | |
| Site of tumor — no. (%)† | <0.001 | |||
| Proximal | 257 (45) | 84 (89) | 173 (36) | |
| Distal | 305 (54) | 9 (10) | 296 (62) | |
| Multiple | 6 (1) | 1 (1) | 5 (1) | |
| Tumor grade — no. (%)† | <0.001 | |||
| Well differentiated (G1) | 97 (17) | 8 (9) | 89 (19) | |
| Moderately differentiated (G2) | 376 (66) | 49 (53) | 327 (69) | |
| Poorly differentiated (G3) | 65 (11) | 24 (26) | 41 (9) | |
| Undifferentiated (G4) | 28 (5) | 12 (13) | 16 (3) | |
| No. of positive nodes — no. (%) | 0.21 | |||
| 0 | 312 (55) | 58 (61) | 254 (53) | |
| 1–4 | 128 (22) | 15 (16) | 113 (24) | |
| >4 | 130 (23) | 22 (23) | 108 (23) | |
| Vital status at 8 yr — no. (%) | 0.03 | |||
| Alive | 385 (68) | 73 (77) | 312 (66) | |
| Dead | 185 (32) | 22 (23) | 163 (34) |
RELATION BETWEEN MICROSATELLITE-INSTABILITY STATUS AND SURVIVAL
In total, 185 of the 570 patients (32.5 percent) died during a median follow-up period of 7.4 years. In a pooled analysis that did not control for the use or nonuse of adjuvant chemotherapy, the rate of five-year disease-free survival among patients with tumors exhibiting high-frequency microsatellite instability (75.3 percent) was significantly greater than that among patients with tumors exhibiting low-frequency microsatellite instability or microsatellite stability (64.1 percent; P=0.04) (Table 3). In univariate analyses, there was no significant difference in five-year overall survival between these groups of patients (P=0.07) (Table 3). In multivariate models adjusted for the stage of disease and tumor grade, high-frequency microsatellite instability was significantly associated with overall survival (hazard ratio for death, 0.61 [95 percent confidence interval, 0.38 to 0.96]; P=0.03) (Table 4).
Table 3.
Univariate Analysis of Survival among Patients with Stage II or Stage III Colon Cancer According to Microsatellite-Instability Status or Adjuvant-Chemotherapy Status.*
| Analysis | No. of Patients | Patients Surviving at 5 Yr | P Value | Patients Surviving without Disease at 5 Yr | P Value |
|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | ||||
| According to microsatellite-instability status | |||||
| All patients | 570 | ||||
| Stability or low-frequency instability | 475 | 71.9 (67.9–76.1) | 0.07 | 64.1 (59.9–68.6) | 0.04 |
| High-frequency instability | 95 | 78.4 (70.3–87.3) | 75.3 (67.0–84.6) | ||
| Patients receiving no adjuvant chemotherapy | 287 | ||||
| Stability or low-frequency instability | 245 | 68.4 (62.7–74.6) | 0.004 | 58.7 (52.8–65.3) | 0.004 |
| High-frequency instability | 42 | 88.0 (78.7–98.4) | 82.9 (72.1–95.3) | ||
| Patients receiving adjuvant chemotherapy | 283 | ||||
| Stability or low-frequency instability | 230 | 75.5 (70.1–81.4) | 0.66 | 69.8 (64.1–76.0) | 0.85 |
| High-frequency instability | 53 | 70.7 (59.2–84.5) | 69.3 (57.8–83.1) | ||
| According to adjuvant-chemotherapy status | |||||
| All patients | 570 | ||||
| Adjuvant chemotherapy | 283 | 74.6 (69.6–80.0) | 0.12 | 69.7 (64.5–75.3) | 0.06 |
| No adjuvant chemotherapy | 287 | 71.2 (66.1–76.8) | 62.3 (56.9–68.2) | ||
| Stability or low-frequency instability | 475 | ||||
| Adjuvant chemotherapy | 230 | 75.5 (70.1–81.4) | 0.02 | 69.8 (64.1–76.0) | 0.01 |
| No adjuvant chemotherapy | 245 | 68.4 (62.7–74.6) | 58.7 (52.8–65.3) | ||
| High-frequency instability | 95 | ||||
| Adjuvant chemotherapy | 53 | 70.7 (59.2–84.5) | 0.07 | 69.3 (57.8–83.1) | 0.11 |
| No adjuvant chemotherapy | 42 | 88.0 (78.7–98.4) | 82.9 (72.1–95.3) |
Table 4.
Hazard Ratios for Death with Adjustment for Stage of Disease and Tumor Grade.*
| Analysis | Hazard Ratio for Death (95% CI) | P Value |
|---|---|---|
| According to microsatellite-instability status | ||
| All patients | 0.61 (0.38–0.96) | 0.03 |
| Patients receiving no adjuvant chemotherapy | 0.32 (0.14–0.75) | 0.008 |
| According to adjuvant-chemotherapy status | ||
| All patients | 0.81 (0.60–1.08) | 0.15 |
| Patients with tumors exhibiting microsatellite stability or low-frequency microsatellite instability | 0.72 (0.53–0.99) | 0.04 |
| Patients with tumors exhibiting high-frequency microsatellite instability | 2.14 (0.83–5.49) | 0.11 |
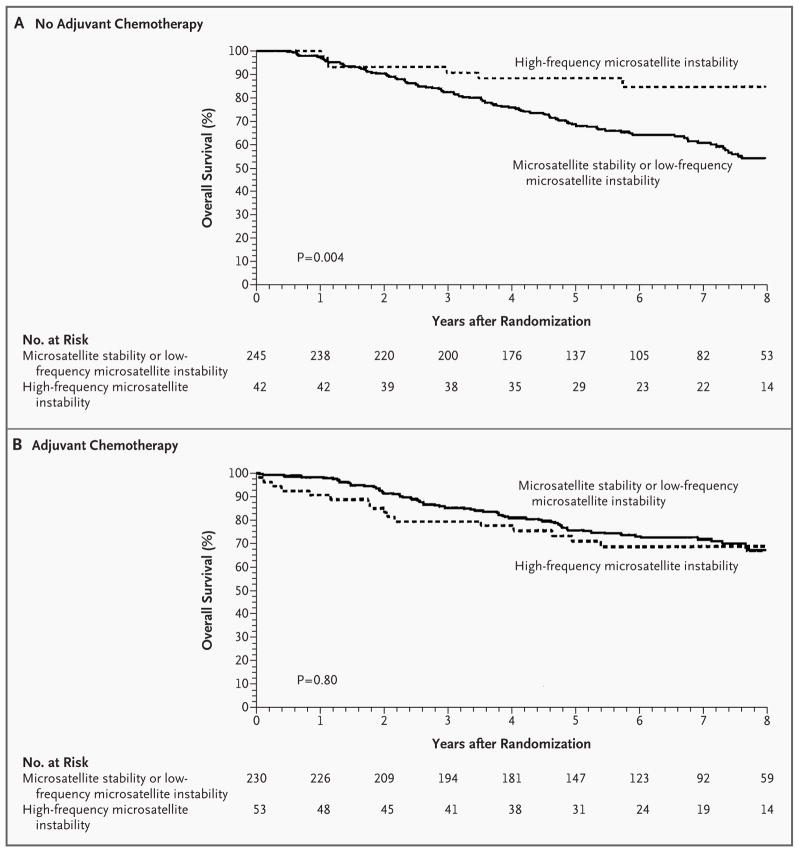
Among patients who had not received adjuvant chemotherapy, patients with tumors exhibiting high-frequency microsatellite instability had longer overall survival (Fig. 1A) and higher rates of five-year disease-free survival (Table 3) than patients with tumors exhibiting low-frequency microsatellite instability or microsatellite stability. Multivariate analysis controlled for the stage of disease and tumor grade also demonstrated that high-frequency microsatellite instability in patients not receiving fluorouracil-based adjuvant chemotherapy was significantly and independently associated with survival (hazard ratio for death, 0.32 [95 percent confidence interval, 0.14 to 0.75]; P=0.008) (Table 4). However, the analysis of patients who did receive adjuvant therapy failed to show significant differences in overall or disease-free survival according to microsatellite-instability status (Table 3 and Fig. 1B).
Figure 1. Kaplan–Meier Estimates of Overall Survival among Patients with Stage II or Stage III Colon Cancer According to the Microsatellite-Instability Status of the Tumor.
In the absence of adjuvant chemotherapy, the patients with tumors displaying high-frequency microsatellite instability had significantly longer overall survival than patients with tumors exhibiting microsatellite stability or low-frequency microsatellite instability (hazard ratio for death, 0.31 [95 percent confidence interval, 0.14 to 0.72]; P=0.004)(Panel A). When the analysis was limited to the group receiving adjuvant chemotherapy, patients with tumors exhibiting high-frequency microsatellite instability did not have a significant increase in overall survival as compared with patients with tumors exhibiting microsatellite stability or low-frequency microsatellite instability (hazard ratio for death, 1.07 [95 percent confidence interval, 0.62 to 1.86]; P=0.80)(Panel B). The analysis included data for eight years from the date of randomization.
RELATION BETWEEN MICROSATELLITE-INSTABILITY STATUS AND BENEFIT OF ADJUVANT CHEMOTHERAPY
Analyses were performed to determine whether the effect of treatment, microsatellite-instability status, or both differed according to the stage of disease. Neither interaction was found to be significant (P=0.48 and P=0.21, respectively), and therefore, patients were pooled regardless of stage for the analyses examining treatment effect and microsatellite-instability status. When the entire group of 570 patients was analyzed, we found no significant difference between those who were treated with fluorouracil-based adjuvant chemotherapy and those who were not in the rates of five-year overall survival (P=0.12) or five-year disease-free survival (P=0.06) (Table 3). However, a significant interaction was observed between microsatellite-instability status and the benefit of treatment (P=0.01). This interaction remained significant after stratification according to the stage of disease (P=0.02). Among patients with tumors exhibiting low-frequency microsatellite instability or microsatellite stability, adjuvant chemotherapy was associated with significant increases in the duration of overall survival (Fig. 2A) and the rates of five-year disease-free survival (Table 3). This survival benefit was also seen in a multivariate analysis controlled for stage and grade (Table 4). There was no evidence of a three-way interaction among treatment effect, microsatellite-instability status, and stage of disease (P=0.39).
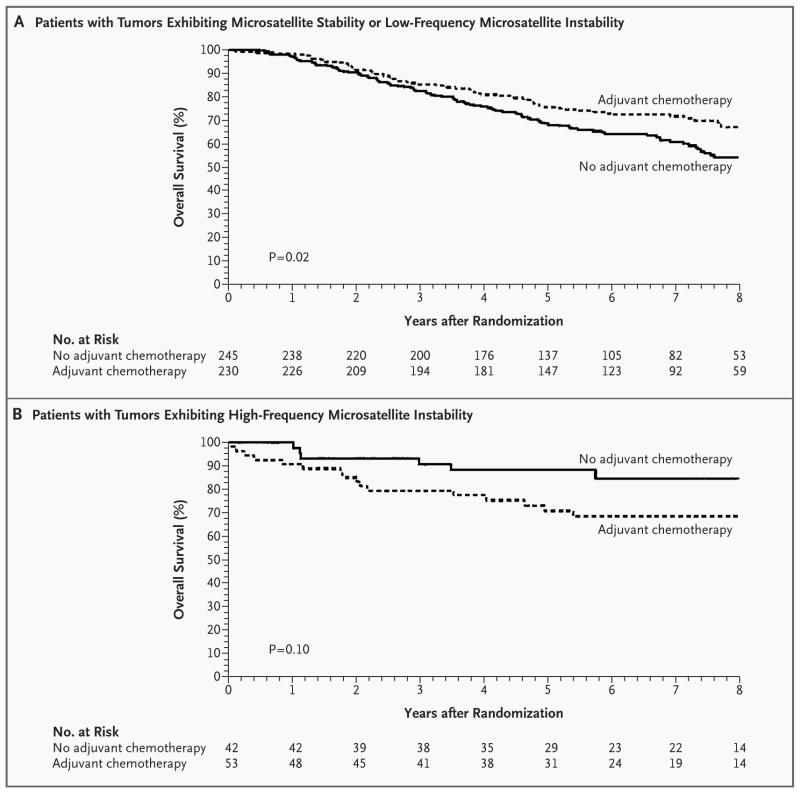
Figure 2. Kaplan–Meier Estimates of Overall Survival among Patients with Stage II or Stage III Colon Cancer According to Treatment Status.
Patients with tumors exhibiting microsatellite stability or low-frequency microsatellite instability who received adjuvant chemotherapy had a significant increase in overall survival as compared with patients who received no adjuvant chemotherapy (hazard ratio for death, 0.69 [95 percent confidence interval, 0.50 to 0.94]; P=0.02) (Panel A). Among patients with tumors exhibiting high-frequency microsatellite instability, there was no significant difference in the duration of overall survival between patients who received adjuvant chemotherapy and those who did not (hazard ratio for death, 2.17 [95 percent confidence interval, 0.84 to 5.55]; P=0.10) (Panel B). The analysis included data for eight years from the date of randomization.
Conversely, among patients with tumors exhibiting high-frequency microsatellite instability, fluorouracil-based chemotherapy did not improve the outcome as compared with no chemotherapy (Table 3 and Fig. 2B). The probability that fluorouracil-based chemotherapy was associated with an increase of at least 5 percent in the rate of five-year survival among patients with tumors exhibiting high-frequency microsatellite instability was less than 1 percent.
These trends were consistently maintained in analyses of subgroups defined according to the stage of disease. Treatment was associated with an improved outcome among patients with stage II or stage III cancers with low-frequency microsatellite instability or microsatellite stability (hazard ratio for death among treated patients as compared with untreated patients, 0.67 [95 percent confidence interval, 0.39 to 1.15] among patients with stage II cancer and 0.69 [95 percent confidence interval, 0.47 to 1.01] among patients with stage III cancer). In contrast, among patients with tumors exhibiting high-frequency microsatellite instability, treatment was associated with a worse outcome for both stage II and stage III cancer (hazard ratio for death, 3.28 [95 percent confidence interval, 0.86 to 12.48] among patients with stage II cancer and 1.42 [95 percent confidence interval, 0.36 to 5.56] among patients with stage III cancer).
DISCUSSION
Recent studies of colorectal cancer have identified two molecular pathways leading to the malignant phenotype — the pathway of high-frequency microsatellite instability and that of microsatellite stability — which respond differently to DNA damage. It is unlikely that tumors with these distinct pathways would respond similarly to chemotherapeutic agents that damage DNA. Since it may be unethical to withhold chemotherapy in a clinical trial for potentially curable advanced-stage colon cancer, we used samples from previous multicenter, prospective, randomized, controlled trials to determine whether microsatellite-instability status could serve as a predictor of a survival benefit with fluorouracil-based adjuvant chemotherapy.
Our results in patients with stage II or stage III colon cancer confirm previous reports of a survival benefit for patients with tumors exhibiting high-frequency microsatellite instability.22,23,26,33,35–38 In a univariate analysis that did not control for the possible effect of chemotherapy, high-frequency microsatellite instability was associated with improved five-year disease-free survival among patients with stage II or stage III colon cancer. We also found that patients with tumors exhibiting microsatellite stability or low-frequency microsatellite instability tended to benefit from fluorouracil-based adjuvant chemotherapy, whereas such chemotherapy did not benefit patients with high-frequency microsatellite instability and may in fact have led to worse outcomes among such patients. These results remained consistent in models that adjusted for the stage of disease and in models stratified according to stage, and they held true for both patients with stage II cancer and patients with stage III cancer.
In vitro studies have shown that colon-cancer cell lines displaying high-frequency microsatellite instability are less responsive than microsatellite-stable cell lines to fluorouracil.49–54 However, our findings contrast with those of a large, selected case series of patients with stage III colon cancer, which demonstrated a significant association between an increased duration of survival and high-frequency microsatellite instability among patients receiving adjuvant chemotherapy.40 But this nonrandomized case series has the potential for bias. For example, patients not receiving chemotherapy were, on average, 13 years older than those who received adjuvant fluorouracil therapy. Increasing age has been demonstrated to be significantly and independently associated with a poor outcome among patients with colorectal cancer, after adjustment for the microsatellite-instability status of the tumor.24 A significantly older mean age also makes it likely that the presence of coexisting disease was an important reason why some patients in this nonrandomized study were not offered adjuvant treatment.40
Although the results of our analysis and previous data from in vitro studies suggest that fluorouracil-based adjuvant chemotherapy is not beneficial in patients with colon cancer exhibiting high-frequency microsatellite instability, other drugs, such as the topoisomerase-I inhibitor camptothecin, have been shown to kill mismatch-repair–deficient cancer cells exhibiting high-frequency microsatellite instability.55 It would therefore seem important to conduct molecular analyses of specimens from recent clinical trials of non–fluorouracil-based chemotherapies and to ensure that future trials include analyses of molecular pathways.56
In our retrospective analysis, the finding that fluorouracil-based adjuvant chemotherapy does not significantly increase, and may potentially decrease, overall and disease-free survival among patients with tumors exhibiting high-frequency microsatellite instability raises several provocative issues regarding postoperative management of stage II and stage III colon cancer. However, we would urge caution and not advocate altering clinical decision making on the basis of our findings. If confirmed by other analyses of previous, well-designed clinical trials or by future prospective, randomized, controlled studies, however, our findings would indicate that microsatellite-instability testing should be conducted routinely and the results used to direct rational adjuvant chemotherapy in colon cancer.
Acknowledgments
Supported in part by grants from the National Cancer Institute of Canada (012200) and the National Cancer Institute, National Institutes of Health (CA25224, CA60100, CA21115, CA62924, CA23318), and fellowship funding from the American Society of Colon and Rectal Surgeons.
References
- 1.Miller BA, Kolonel LN, Bernstein L, et al. Racial/ethnic patterns of cancer in the United States 1988–1992. Bethesda, Md: National Cancer Institute; 1996. (NIH publication no. 96-4104.) [Google Scholar]
- 2.Makela JT, Laitinen ST, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer: results of a prospective randomized trial. Arch Surg. 1995;130:1062–7. doi: 10.1001/archsurg.1995.01430100040009. [DOI] [PubMed] [Google Scholar]
- 3.Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. 1997;40:15–24. doi: 10.1007/BF02055676. [DOI] [PubMed] [Google Scholar]
- 4.International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) Investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet. 1995;345:939–44. [PubMed] [Google Scholar]
- 5.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–8. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 6.Laurie JA, Moertel CG, Fleming TR, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. J Clin Oncol. 1989;7:1447–56. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- 7.Beart RW, Jr, Moertel CG, Wieand HS, et al. Adjuvant therapy for resectable colorectal carcinoma with fluorouracil administered by portal vein infusion: a study of the Mayo Clinic and the North Central Cancer Treatment Group. Arch Surg. 1990;125:897–901. doi: 10.1001/archsurg.1990.01410190095015. [DOI] [PubMed] [Google Scholar]
- 8.O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 9.Tempero M, Anderson J. Progress in colon cancer — do molecular markers matter? N Engl J Med. 1994;331:267–8. doi: 10.1056/NEJM199407283310410. [DOI] [PubMed] [Google Scholar]
- 10.Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes’ B2 colon cancer. J Clin Oncol. 1995;13:2936–43. doi: 10.1200/JCO.1995.13.12.2936. [DOI] [PubMed] [Google Scholar]
- 11.Nauta R, Stablein DM, Holyoke ED. Survival of patients with stage B2 colon carcinoma: the Gastrointestinal Tumor Study Group experience. Arch Surg. 1989;124:180–2. doi: 10.1001/archsurg.1989.01410020050008. [DOI] [PubMed] [Google Scholar]
- 12.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. J Clin Oncol. 1999;17:1356–63. [PubMed] [Google Scholar]
- 13.American Joint Committee on Cancer. Manual for staging of cancer. 4. Philadelphia: J.B. Lippincott; 1992. [Google Scholar]
- 14.Fielding LP, Pettigrew N. College of American Pathologists Conference XXVI on clinical relevance of prognostic markers in solid tumors: report of the Colorectal Cancer Working Group. Arch Pathol Lab Med. 1995;119:1115–21. [PubMed] [Google Scholar]
- 15.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 16.Lothe RA, Peltomaki P, Meling GI, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–52. [PubMed] [Google Scholar]
- 17.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 18.Vogelstein B, Fearon ER, Kern SE, et al. Allelotype of colorectal carcinomas. Science. 1989;244:207–11. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- 19.Lengauer C, Kinzler KW, Vogelstein B. DNA methylation and genetic instability in colorectal cancer cells. Proc Natl Acad Sci U S A. 1997;94:2545–50. doi: 10.1073/pnas.94.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 21.Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–7. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 22.Gryfe R, Kim H, Hsieh ETK, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 23.Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91:1295–303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- 24.Hemminki A, Mecklin JP, Jarvinen H, Aaltonen LA, Joensuu H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119:921–8. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 25.Aaltonen LA, Peltomaki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–6. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 26.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–9. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 27.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho MU. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–61. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 28.Strand M, Prolla TA, Liskay RM, Petes TD. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993;365:274–6. doi: 10.1038/365274a0. [Erratum, Nature 1994;368:569.] [DOI] [PubMed] [Google Scholar]
- 29.Leach FS, Nicolaides NC, Papadopoulos N, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–25. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 30.Fishel R, Lescoe MK, Rao MR, et al. The human mutator gene homologue MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–38. doi: 10.1016/0092-8674(93)90546-3. [Erratum, Cell 1994;77:167.] [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos N, Nicolaides NC, Wei YF, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263:1625–9. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 32.Bronner CE, Baker SM, Morrison PT, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994;368:258–61. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145:148–56. [PMC free article] [PubMed] [Google Scholar]
- 34.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 35.Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T-lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–13. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukish JR, Muro K, DeNobile J, et al. Prognostic significance of DNA replication errors in young patients with colorectal cancer. Ann Surg. 1998;227:51–6. doi: 10.1097/00000658-199801000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bubb VJ, Curtis LJ, Cunningham C, et al. Microsatellite instability and the role of hMSH2 in sporadic colorectal cancer. Oncogene. 1996;12:2641–9. [PubMed] [Google Scholar]
- 38.Wright CM, Dent OF, Barker M, et al. Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. Br J Surg. 2000;87:1197–202. doi: 10.1046/j.1365-2168.2000.01508.x. [DOI] [PubMed] [Google Scholar]
- 39.Elsaleh H, Powell B, Soontrapornchai P, et al. p53 Gene mutation, microsatellite instability and adjuvant chemotherapy: impact on survival of 388 patients with Duke’s C colon carcinoma. Oncology. 2000;58:52–9. doi: 10.1159/000012079. [DOI] [PubMed] [Google Scholar]
- 40.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355:1745–50. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe T, Wu T-T, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res. 1997;57:4749–56. [PubMed] [Google Scholar]
- 43.Mirabelli-Primdahl L, Gryfe R, Kim H, et al. Beta-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res. 1999;59:3346–51. [PubMed] [Google Scholar]
- 44.Rosty C, Chazal M, Etienne MC, et al. Determination of microsatellite instability, p53 and K-RAS mutations in hepatic metastases from patients with colorectal cancer: relationship with response to 5-fluorouracil and survival. Int J Cancer. 2001;95:162–7. doi: 10.1002/1097-0215(20010520)95:3<162::aid-ijc1028>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 45.Laiho P, Launonen V, Lahermo P, et al. Low-level microsatellite instability in most colorectal carcinomas. Cancer Res. 2002;62:1166–70. [PubMed] [Google Scholar]
- 46.Zhou XP, Hoang JM, Li YJ, et al. Determination of the replication error phenotype in human tumors without the requirement for matching normal DNA by analysis of mononucleotide repeat microsatellites. Genes Chromosomes Cancer. 1998;21:101–7. doi: 10.1002/(sici)1098-2264(199802)21:2<101::aid-gcc4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 48.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–202. [Google Scholar]
- 49.Claij N, te Riele H. Microsatellite instability in human cancer: a prognostic marker for chemotherapy? Exp Cell Res. 1999;246:1–10. doi: 10.1006/excr.1998.4299. [DOI] [PubMed] [Google Scholar]
- 50.Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6. [PubMed] [Google Scholar]
- 51.Anthoney DA, McIlwrath AJ, Gallagher WM, Edlin AR, Brown R. Microsatellite instability, apoptosis, and loss of p53 function in drug-resistant tumor cells. Cancer Res. 1996;56:1374–81. [PubMed] [Google Scholar]
- 52.Kat A, Thilly WG, Fang W-H, Longley MJ, Li GM, Modrich P. An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proc Natl Acad Sci U S A. 1993;90:6424–8. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Branch P, Aquilina G, Bignami M, Karran P. Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature. 1993;362:652–4. doi: 10.1038/362652a0. [DOI] [PubMed] [Google Scholar]
- 54.Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001;61:5193–201. [PubMed] [Google Scholar]
- 55.Bras-Goncalves RA, Rosty C, Laurent-Puig P, Soulie P, Dutrillaux B, Poupon M-F. Sensitivity to CPT-11 of xenografted human colorectal cancers as a function of microsatellite instability and p53 status. Br J Cancer. 2000;82:913–23. doi: 10.1054/bjoc.1999.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–14. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]