Greater Cortical Gray Matter Density in Lithium-Treated Patients with Bipolar Disorder (original) (raw)
. Author manuscript; available in PMC: 2013 Mar 4.
Abstract
Background
The neurobiological underpinnings of bipolar disorder are not well understood. Previous neuroimaging findings have been inconsistent; however, new methods for three-dimensional (3-D) computational image analysis may better characterize neuroanatomic changes than standard volumetric measures.
Methods
We used high-resolution magnetic resonance imaging and cortical pattern matching methods to map gray matter differences in 28 adults with bipolar disorder, 70% of whom were lithium-treated (mean age = 36.1 ± 10.5; 13 female subject), and 28 healthy control subjects (mean age = 35.9 ± 8.5; 11 female subjects). Detailed spatial analyses of gray matter density (GMD) were conducted by measuring local proportions of gray matter at thousands of homologous cortical locations.
Results
Gray matter density was significantly greater in bipolar patients relative to control subjects in diffuse cortical regions. Greatest differences were found in bilateral cingulate and paralimbic cortices, brain regions critical for attentional, motivational, and emotional modulation. Secondary region of interest (ROI) analyses indicated significantly greater GMD in the right anterior cingulate among lithium-treated bipolar patients (n = 20) relative to those not taking lithium (n = 8).
Conclusions
These brain maps are consistent with previous voxel-based morphometry reports of greater GMD in portions of the anterior limbic network in bipolar patients and suggest neurotrophic effects of lithium as a possible etiology of these neuroanatomic differences.
Keywords: Bipolar disorder, cortical pattern matching, lithium, magnetic resonance imaging, mood disorders, neuroprotection
Conventional volumetric magnetic resonance imaging (MRI) studies and postmortem brain studies suggest that at least a proportion of patients with bipolar disorder have reductions in regional brain volumes, accompanied by reductions in neuronal and glial density, particularly in the ventral frontal cortex (Drevets 2000; Rajkowska 2000) and anterior cingulate (Lochhead et al. 2004; Lyoo et al. 2004). However, previous findings have been inconsistent, with both volumetric increases and decreases reported (see Brambilla et al. 2005 for a review). As such, it is not yet known whether these changes reflect neurodevelopmental anomalies, part of the disease progression, or functional sequelae of biochemical changes that accompany repeated illness episodes.
A recent meta-analysis of regional morphometry in bipolar disorder found no significant differences in total brain volume or whole brain gray or white matter between bipolar and comparison subjects or in the volume of individual cortical, subcortical, or limbic structures (McDonald et al. 2004). However, the authors noted significant heterogeneity across studies for several brain structures, including the amygdala, left subgenual prefrontal cortex, and thalamus. Psychotropic medication usage may be a potentially important contributor to the observed heterogeneity.
In particular, lithium has been the reference standard medication treatment for bipolar disorder for over 50 years (Brambilla et al. 2001). While lithium’s mechanism of therapeutic action is currently unknown, recent human studies offer evidence that pharmacologically induced increases in cortical gray matter may occur with chronic lithium use in patients with bipolar disorder. Notably, Moore et al. (2000b) observed that lithium significantly increased total gray matter volume in bipolar patients by 3%, on average, after 4 weeks. More recently, Sassi et al. (2002) found larger total gray matter volume in lithium-treated bipolar patients, as compared with both untreated patients and control subjects. In a partially overlapping sample, decreased left anterior cingulate volumes were observed in untreated bipolar patients, whereas lithium-treated patients were not significantly different from healthy control subjects (Sassi et al. 2004). In addition, magnetic resonance spectroscopy (MRS) has revealed increases in cortical _N_-acetyl-aspartate (NAA), a putative marker of neuronal integrity, in both bipolar patients and normal control subjects following lithium administration (Moore et al. 2000a; Silverstone et al. 2003).
While these studies suggest potential neuroprotective effects of this agent, the few in vivo neuroimaging studies published to date have used relatively crude or region-specific volumetric measures. With advances in computational image analysis, differences in cortical anatomy can be examined at high spatial resolution. Here, we apply these algorithms to map gray matter abnormalities over the entire cortical surface in bipolar patients and to determine whether, if present, these changes are influenced by lithium treatment.
Methods and Materials
Participants
This study was approved by the University of Pittsburgh Biomedical Institutional Review Board, and written informed consent was obtained from all subjects prior to participation. The sample included 28 patients with bipolar disorder and 28 healthy comparison subjects matched for age, sex, handedness, and level of education; a subset of this sample was previously reported on in Sassi et al. (2004). Patients were recruited through the outpatient facilities of the University of Pittsburgh Medical Center or through advertisements in the local media. The inclusion criteria were a DSM-IV diagnosis of bipolar disorder, subtype I or II, as determined by the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al. 1998), in any mood state, age between 18 and 65 years, and no lithium treatment for at least 1 month at the time of the MRI scan (for the untreated group) or receiving lithium for at least 2 weeks for the lithium-treated group. The Bech-Rafaelsen Mania Scale (Bech et al. 1979) and the Hamilton Depression Rating Scale (25-item version) (HDRS) (Hamilton 1960) were used to rate clinical symptoms and were administered within 1 week of the scan. All subjects had normal physical examination results and no history of neurologic problems. Exclusion criteria were any comorbid psychiatric disorder, current medical problems, and/or alcohol or substance abuse or dependence within 6 months preceding the study (see Sassi et al. 2004 for further details).
Healthy comparison subjects were recruited through local advertisements, according to the same exclusion criteria used for patients. Healthy control subjects had no DSM-IV Axis I disorders, as determined by the SCID-IV (First et al. 1998); no current medical problems; and no history of psychiatric disorders among first-degree relatives. The ethnic and racial makeup of the groups did not differ from each other, and there were no differences in level of education (Table 1).
Table 1.
Sample Demographics
| Lithium-Positive Patients withBipolar Disorder (n = 20) | Lithium-Negative Patients withBipolar Disorder (n = 8) | Healthy ComparisonSubjects (n = 28) | Between-GroupDifferences | |
|---|---|---|---|---|
| Age (Mean ± SD) | 35.1 ± 10.8 | 38.6 ± 10.0 | 35.9 ± 8.5 | F = .40, p = .67 |
| % Female (n) | 45% (9) | 4 (50%) | 39% (11) | χ2 = .35, p = .84 |
| % Right Handed (n) | 100% (20) | 100% (8) | 100% (28) | χ2 = .00, p = 1.0 |
| Education Level | 15.0 ± 2.7 | 15.5 ± 3.3 | 15.4 ± 2.7 | F = .20, p = .82 |
| Race | ||||
| % Caucasian (n) | 100% (20) | 88% (7) | 89% (25) | χ2 = 4.1, p = .25 |
| % Asian (n) | 0 | 0 | 4% (1) | |
| % African American (n) | 0 | 0 | 7% (2) | |
| % Biracial (n) | 0 | 4% (1) | 0 | |
| % Bipolar I Disorder | 90% (18) | 50% (4) | χ2 = 5.43, p = .04 | |
| % Bipolar II Disorder | 10% (2) | 50% (4) | NA | |
| HDRS | 6.9 ± 8.6 | 15.3 ± 11.8 | NA | F = 3.76, p = .07 |
| Bech-Rafaelsen Mania Scale | .67 ± 1.7 | 1.63 ± 2.0 | NA | F = 1.31, p = .27 |
| Duration of Illness (Years) | 15.1 ± 8.2 | 15.1 ± 8.3 | NA | F = .00, p = .99 |
| Age at Onset | 18.6 ± 6.1 | 23.5 ± 7.8 | NA | F = 3.03, p = .094 |
| Number of Episodes | 20.5 ± 25.9 | 16.3 ± 17.5 | NA | F = .16, p = .70 |
| Current Mood State | χ2 = 4.13, p = .13 | |||
| Depressed | 30% (6) | 50% (4) | NA | |
| Hypomanic | 0% (0) | 12% (1) | ||
| Euthymic | 70% (14) | 38% (3) | ||
| % with Previous (Lifetime) | ||||
| Antipsychotic Use (n) | 25% (5) | 12% (1) | NA | χ2 = .89, p = .64 |
Bipolar patients were outpatients at the time of assessment, with treatment histories of varying lengths (mean age of onset of 20 ± 6.9 years). At the time of MRI scan, 36% of patients (n = 10) were in a depressed state, 61% (n = 17) were euthymic, and 3% (n = 1) was hypomanic. Six patients (21%) had bipolar II disorder, whereas 22 (79%) had bipolar I disorder. Bipolar I disorder patients did not differ from bipolar II disorder patients in terms of length of illness [F(1,26) = 2.84, p = .11], age at onset [F(1,26) = .47, p = .50], depression or mania severity scores [HDRS: F(1,26) = 2.56, p = .12; Bech-Rafaelsen Mania Scale: F(1,26) = .43, p = .52], or mood state at scan time [χ2(2) = .019, p = .89]. All analyses were run with and without the six bipolar II disorder subjects. Although p values changed slightly, this did not affect whether each result was statistically significant, so we present results for the full sample.
The majority (n = 20, 71%) were taking lithium at the time of evaluation (Table 1) for a mean duration of 128 weeks (±230 weeks; range 3–1000 weeks), at a mean dosage of 1158.8 mg/day (±362.1; range 675–2100 mg/day). In addition to lithium, two patients were also taking tranylcypromine (10 to 20 mg), one was taking levothyroxine, and one was taking citalopram (60 mg) plus levothyroxine. The other patients in the lithium-treated group were all on lithium monotherapy. Of the eight patients that were not taking lithium, one was taking citalopram (20 mg), and the others were on no medication. Further information regarding clinical and medication history for each patient is provided in Supplement 1.
MRI Scanning
Magnetic resonance imaging scans were acquired with a 1.5T GE Signa Imaging System (GE Medical Systems, Waukesha, Wisconsin) running Signa version 5.4.3 software. The scanning protocol was identical to that used in Sassi et al. (2004). All magnetic resonance (MR) images were processed with a series of manual and automated procedures developed at the UCLA Laboratory of NeuroImaging that are described in detail in other reports (Thompson et al. 2004) and summarized below.
Image Processing
First, each image volume was resliced into a standard orientation by trained image analysts (M.D., M.N., M.T.) who “tagged” 20 standardized anatomical landmarks in each subject’s image dataset corresponding to the same 20 anatomical landmarks defined on the ICBM-53 average brain (Thompson et al. 2003). Next, brain image volumes were more carefully spatially registered to each other by defining 80 standardized, manually defined anatomical landmarks on each brain (Sowell et al. 2001). A least-squares, rigid-body transformation spatially matched each individual to the average of all the control subjects. In this manner, all individual brains were matched in space, but global differences in brain size and shape remained intact. Automated tissue classification used a partial volume correction method (Shattuck et al. 2001) to classify voxels as most representative of gray matter, white matter, or cerebrospinal fluid (CSF). Nonbrain tissue (i.e., scalp, orbits) was removed from the spatially transformed, tissue-segmented images. Then, each individual cortical surface was extracted and three-dimensionally rendered using automated software (MacDonald et al. 2000). Each resulting cortical surface was represented as a high-resolution mesh of 131,072 surface triangles spanning 65,536 surface points.
Anatomical Analysis
Cortical pattern matching methods (Thompson et al. 2003, 2004) were used to localize disease effects on cortical anatomy. Image analysts (M.D., M.N., M.T.), blind to all subject information, traced each of 30 sulci in each hemisphere on the surface rendering of each subject’s brain (13 on the medial surface, 17 on the lateral surface), employing previously validated anatomic delineation protocols (Sowell et al. 2003; Thompson et al. 2003). In addition to contouring the major sulci, six midline landmark curves bordering the longitudinal fissure were outlined in each hemisphere to establish hemispheric gyral limits. Spatially registered image volumes in coronal, axial, and sagittal planes were available simultaneously to help disambiguate brain anatomy. Landmarks were defined according to a detailed anatomical protocol (Sowell et al. 2001) based on the Ono et al. (1990) sulcal atlas. These criteria, along with methods for assessing interrater reliability, have been described previously (Sowell et al. 2001) and the written anatomical protocols are available via the Internet (http://www.loni.ucla.edu/~khayashi/Public/medial_surface/ and http://www.loni.ucla.edu/~esowell/new_sulcvar.html). Briefly, interrater variability of manual outlining was measured as the three-dimensional (3-D) root mean square difference in millimeters between 100 equidistant points from each sulcal landmark traced in six test brains by each rater, relative to a gold standard arrived at by a consensus of raters, as previously reported (Sowell et al. 2003). Interrater reliability was determined by comparing the three-dimensional root mean square distance between equidistant surface points from sulcal landmarks from one test brain traced six times by each rater (M.D., M.N., M.T.). Three-dimensional root mean square disparities were < 2 mm, and on average < 1 mm, between points for all landmarks within and between raters. This equates to interrater reliability coefficient (intraclass correlation coefficient [ICC]) values ranging from .95 to .975.
Cortical Gray Matter Maps
Points on the cortical surfaces were calculated using the averaged sulcal contours as anchors to drive into correspondence the 3-D cortical surface mesh models from each subject (Thompson et al. 2003). Because deformation maps (acquired during matching of the cortical surfaces) associate corresponding anatomical features of the cortex across patients and control subjects based on sulcal contours drawn in every individual, a local measurement termed gray matter density (GMD) can be calculated at every point over the surface of the brain for every patient and control subject and averaged across corresponding regions of cortex (Ashburner and Friston 2000; Thompson et al. 2003). Briefly, a sphere with a radius of 15 mm centered at every cortical surface point was made and referenced to the same spatial location in the gray matter maps for every participant derived earlier in the tissue classification. The proportion of segmented grey matter voxels relative to the total number of voxels in this sphere is calculated at every point and stored as a map of proportional gray matter—with possible values ranging from .0 to 1.0—for every patient and control subject. The proportion of gray matter in each sphere at every point in every participant indicates, in part, local cortical thickness that varies over different regions of the brain. Given the large anatomic variability in some cortical regions, high-dimensional elastic matching of cortical patterns (Thompson et al. 2004) was used to associate GMD measures from homologous cortical regions across subjects. One advantage of cortical matching is that it localizes deficits relative to gyral landmarks; it also averages data from corresponding gyri, which would be impossible if data were only linearly mapped into stereotaxic space.
Mapping Gray Matter Differences
Statistical maps were generated indicating group differences in local GMD. To do this, at each cortical point, a multiple regression was run to assess whether the quantity of gray matter at that point depended on group membership. The _p_-value describing the significance of this linkage was plotted at each cortical point using a color code to produce a statistical map (e.g., Figure 2). Permutation methods (Bullmore et al. 1999; Thompson et al. 2003) assessed the significance of the statistical maps and corrected for multiple comparisons. In each case, the covariate (group membership) was permuted 1,000,000 times on a Reality Monster supercomputer (Silicon Graphics, Inc, Mountain View, California) with 32 internal R10000 processors, and a null distribution was developed for the area of the average cortex with group difference statistics above a fixed threshold in the significance maps. An algorithm was then used to determine the significance probability for the overall difference patterns in each map (Thompson et al. 2003). In addition, based on our a priori hypotheses regarding specific brain regions that might be affected in bipolar disorder patients, we conducted permutation tests in two specific regions of interest (ROIs), the anterior cingulate gyrus and frontal lobe, considering only effects within that ROI when compiling a reference distribution from the randomized data. The anterior cingulate ROI consisted of the entire anterior cingulate gyrus and was defined with boundaries encompassing the cingulate sulcus (anterior, superior, and inferior boundaries); the paracentral sulcus (posterior boundary); and the pericallosal sulcus (inferior and posterior boundaries), while the frontal lobe ROI consisted of all portions of the frontal cortex anterior to the genu of the corpus callosum. These ROIs were traced by a single rater blind to diagnosis (K.M.H.) who had established excellent reliability (≥.98) relative to a gold standard arrived at by a consensus of raters. Anatomic criteria for these ROIs are defined in detail in previous publications (Ballmaier et al. 2004). The total supra-threshold volume, within the ROI, is assessed in the permutation test that corrects for multiple comparisons at all voxels within the ROI. This procedure is similar to the small-volume correction conducted in statistical parametric mapping (Ashburner and Friston 2000).
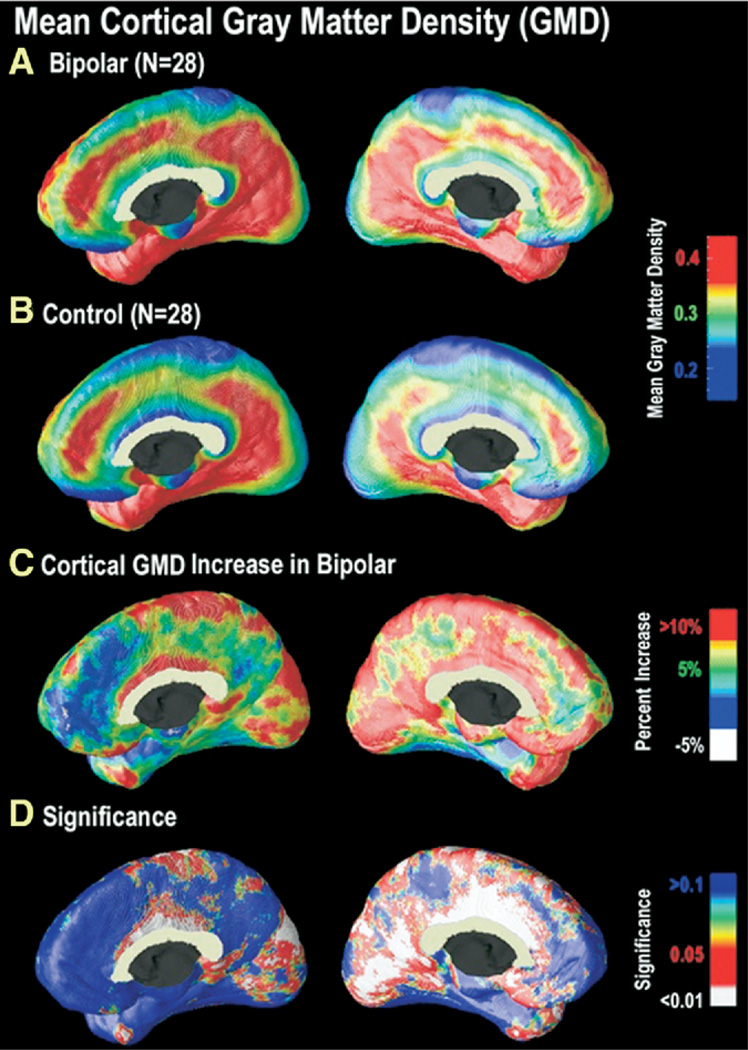
Figure 2.
Cortical GMD maps: gray matter differences on the medial brain surfaces. Group difference maps (C) show mean percent differences in gray matter density between the bipolar group average (A) and the control group average (B), according to the color bar. The significance of these differences is plotted in (D) as a map of _p_-values. As can be seen in (A) and (B), areas of relatively high cortical GMD are more widespread in the cingulate gyrus in bipolar patients as compared with control subjects. Group differences were maximal in the cingulate gyrus (bilaterally, but left more extensive than right), extending along the dorsomedial extent of the interhemispheric fissure. In these regions, GMD was 10% to 15% above the control average (p < .05). In the left hemisphere, the GM difference is highly significant in a broad area encompassing posterior and anterior cingulate cortex, extending posteriorly to extrastriate regions and anteriorly to ventromedial PFC (C) (right panel). In the right medial wall (C) (left panel), differences are more circumscribed to cingulate and occipital cortex. GMD, gray matter density; GM, gray matter; PFC, prefrontal cortex.
Results
Overall Volumetric Differences
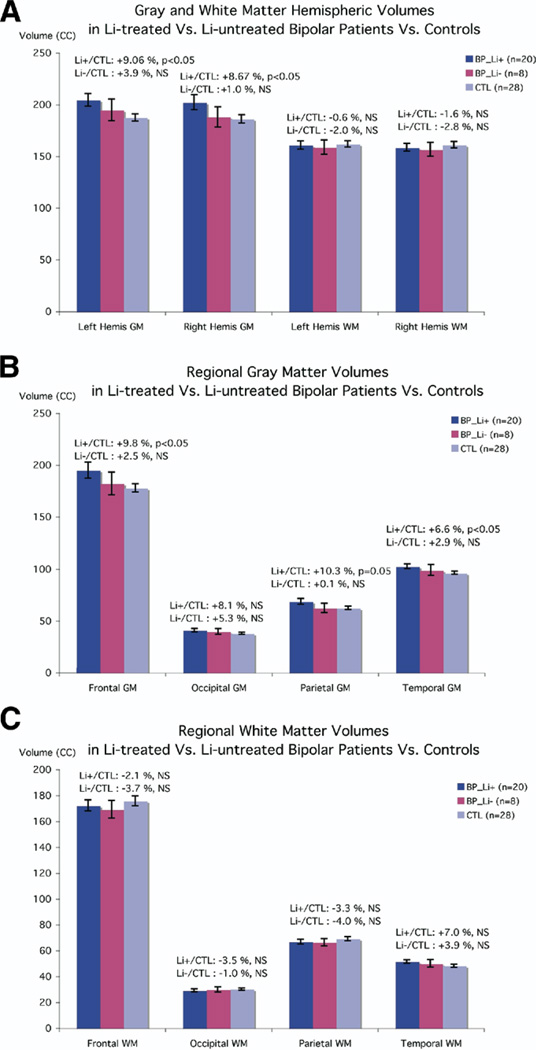
To provide context for the cortical gray matter maps, we first analyzed overall differences in total gray and white matter volume in patients with bipolar disorder, as compared with control subjects. The bipolar group overall did not differ from comparison subjects in total brain volume [F(1,54) = .069, p = .79] or total white matter volume [F(1,54) = .28, p = .60] but had significantly larger overall gray matter volume by 6.6% [F(1,54) = 4.01, p ≤ .05]. Left and right hemisphere gray matter volumes were significantly greater by 7.6% (p < .05) and 6.5% (p < .01), respectively, but there were no differences in hemispheric white matter volumes (left: 1% smaller, p = .72, ns; right: 1.9% smaller, p = .60, ns).
Mapping Cortical Gray Matter Differences
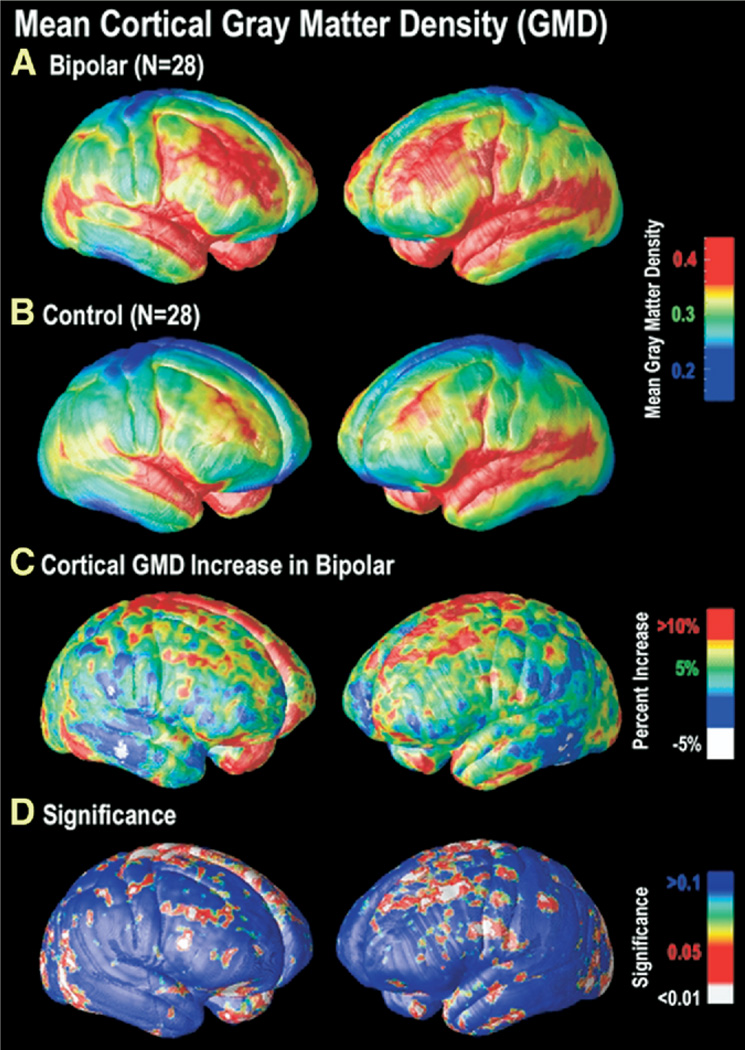
In bipolar patients, highly significant gray matter enlargement was observed in a broad anatomical region encompassing the medial frontal and parietal cortices and portions of the temporal and occipital cortex (left hemisphere: p = .025, right hemisphere: p = .0075, corrected). On the lateral brain surface, GMD was greater by 10% or more in bilateral superior and middle frontal gyri, somatosensory cortex, and left ventrolateral prefrontal and superior temporal cortices (Figure 1). These brain regions comprise heteromodal association cortices, in which information from lower-level sensory systems is integrated into higher-order percepts and functions (Sowell et al. 2003). Together, these regions are thought to form a distributed system responsible for attentional, motivational, and emotional modulation (Mesulam 1998).
Figure 1.
Cortical GMD maps: gray matter differences on the lateral brain surfaces. Maps of the mean GMD for bipolar subjects (n = 28) (A) and control subjects (n = 28) (B) are shown on a color coded scale where red colors denote areas of greater GMD at the cortex and blue colors relatively lower GMD. While both groups show relatively higher GMD in temporal regions, particularly perisylvian cortex, greater GMD in widespread areas of frontal cortex appears specific to the bipolar group (A, B). In (C), the mean difference in gray matter in the bipolar group, relative to healthy control subjects, is expressed as a percentage and shown color coded (blue colors: no difference; red colors: greater increase). On the right lateral (left panel) and left lateral (right panel) cortical surfaces, gray matter differences are seen primarily in superior and middle frontal gyri (BA 4, 6, 8, and 9), the inferior temporal gyrus, and superior parietal cortex surrounding the central sulcus. The significance of these differences is plotted in (D) as a map of _p_-values. GMD, gray matter density; BA, Brodmann area.
As evident in Figure 2, differences were most striking on the medial brain surface, with substantial gray matter enlargement in bipolar patients in the retrosplenial and paracingulate cortices, the subgenual cortex, and paralimbic belts, which form a ring of cortex that encircles the corpus callosum (red colors, Figure 2D). In the cingulate gyrus (bilaterally but left more extensive than right), GMD was 10% to 15% above the control average (p < .05). No significant reductions in GMD in bipolar patients compared with control subjects were observed in any cortical location.
Effects of Lithium Treatment
Given that most patients in this study were lithium treated (n = 20; 71%), we hypothesized that these gray matter differences may reflect medication effects. To investigate whether the findings in the overall sample were primarily accounted for by patients currently treated with lithium, we subdivided the sample into bipolar patients who were being treated with lithium (Li+) and those who were not being treated with lithium (Li−). The resulting subject groups (healthy control subjects, Li+, and Li−) did not differ with regard to age, gender, race, or educational attainment (Table 1). Lithium-treated bipolar patients did not differ from nonlithium-treated patients in terms of length of illness, age at onset, number of previous episodes, mania severity scores, or mood state at scan time. However, depression severity scores were nonsignificantly higher in the untreated group (p = .07), and bipolar II disorder patients were overrepresented in the Li− group (p = .04; Table 1).
Figure 3 depicts volumetric differences for the three groups (Li+, Li−, and control subjects). While there were no differences in white matter volume between the groups [left hemisphere: F(2,53) = .107, p = .90; right hemisphere: F(2,53) = .266, p = .77), the gray matter differences observed in the bipolar group overall were entirely attributable to patients treated with lithium. Lithium-treated bipolar patients had significantly greater overall gray matter volumes compared with normal control subjects [left: 9.06%, t(46) = −2.6; p = .017; right: 8.68%, t(46) = −2.09; p = .042], although patients not on lithium did not differ from normal control subjects [_t_(34) = .873, _p_ = .44; _t_(34) = .21, _p_ = .86, for left and right hemisphere, respectively; Figure 3A]. Lobar gray matter volumes were significantly greater in lithium-treated bipolar patients versus control subjects in frontal (by 9.8%, p = .04), temporal (by 6.6%, p = .04), and parietal lobes (by 10.3%, p = .05), but this difference was not significant in the occipital lobe (8.1% larger, p = .14, ns; Figure 3B). Lobar white matter volumes did not significantly differ between Li+, Li−, and control subjects (Figure 3C). Concomitant with larger gray matter volumes, lithium-treated bipolar patients had significantly smaller cerebrospinal fluid volumes than both control subjects [t = 2.40(46), p = .02] and patients not on lithium [t = 1.82(26), p = .04], resulting in similar total cerebral volumes across groups. In contrast, CSF values in bipolar patients not on lithium did not differ from normal control subjects (t = −.61(34), p = .55, ns).
Figure 3.
Comparison of brain volumes in lithium-treated versus untreated patients with bipolar disorder and healthy control subjects. Means and standard error measures (error bars) are shown for the three groups (Li+, Li−, and control subjects), for gray and white matter volumes of the left and right cerebral hemispheres (A), lobar gray matter (B), and lobar white matter (C). There were no differences in white matter volume between the groups, but lithium-treated bipolar patients had significantly greater gray matter volumes (9.1%, left; 8.7%, right), although patients not on lithium did not differ from normal control subjects (A). In addition, lobar gray matter volumes were significantly increased in lithium-treated bipolar patients versus control subjects (B), whereas lobar white matter volumes did not significantly differ between Li+, Li−, and control subjects (C). Li+, treated with lithium; Li−, not treated with lithium.
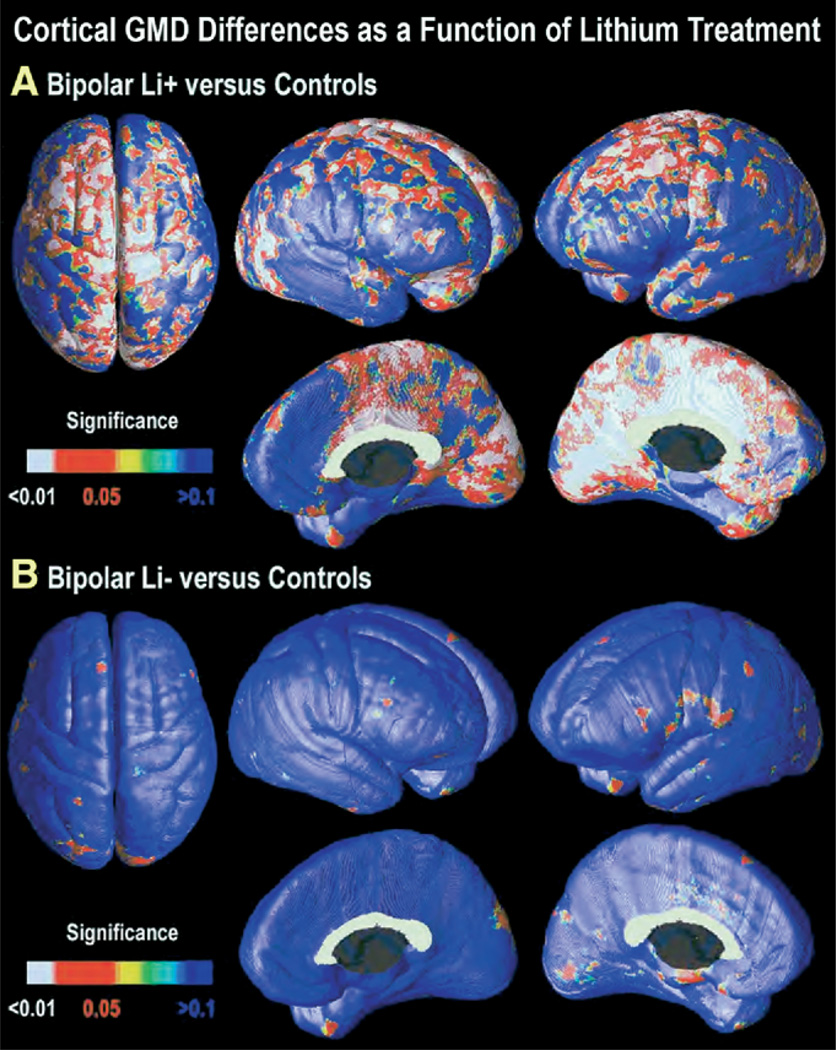
Cortical surface maps were generated to characterize regionally specific differences in gray matter across groups. Maps of GMD for Li+ versus control subjects indicated a more robust effect than that seen in the group of bipolar patients as a whole (Figure 4A). After multiple-comparison correction, GMD was significantly greater in Li+ bipolar patients for both whole brain (left: p = .0082, right: p = .0015) and in frontal and anterior cingulate ROIs (Table 2). Gray matter differences were most marked in the superior aspects of primary motor cortex bilaterally, with greater extent into dorsolateral prefrontal cortex in the left hemisphere. Areas of significantly greater gray matter concentration extended onto the medial surface of almost the entire left hemisphere, while in the right hemisphere, the effects were more circumscribed to the cingulate, sensorimotor, and occipitoparietal cortices. However, in nonlithium-treated patients, GMD did not differ from normal control subjects for both the whole brain (left: p = .87, right: p = .45, ns; Figure 4B) and in frontal and anterior cingulate ROIs (Table 2). These maps are consistent with the volumetric data, indicating the observed gray matter differences in the bipolar group overall were accounted for by higher GMD in patients treated with lithium.
Figure 4.
Cortical GMD increase as a function of lithium usage. Statistical significance maps of GMD for bipolar patients treated with lithium versus control subjects (A). The previously observed differences in GMD become even more robust when the analysis is restricted to patients treated with lithium only (n = 20) versus control subjects (n = 28). Widespread areas of greater gray matter concentration are observed in diffuse cortical areas, particularly in left cingulate and paralimbic association cortices, and bilaterally in visual association cortex (BA 18 and 19). However, GMD in bipolar patients who were not taking lithium (Li−; n = 8) did not differ significantly from control subjects in any cortical region (B). GMD, gray matter density; BA, Brodmann area; Li−, not treated with lithium.
Table 2.
Summary of Results of Permutation Tests: Significance Levels for Group Comparisons of Patients with Bipolar Disorder and Control Subjects
| Permutation Results Summary | ||||||
|---|---|---|---|---|---|---|
| Measure | Hemisphere | Significance Levels | Anterior Cingulate ROI | Frontal ROI | ||
| GMD CTL vs. BIP Li+ | Left | p = .0082 | Left | p = .01 | Left | p = .01 |
| Right | p = .0015 | Right | p = .004 | Right | p = .003 | |
| GMD BIP Li+ vs. BIP Li− | Left | p = .06 | Left | p = .06 | Left | p = .06 |
| Right | p = .06 | Right | p = .02 | Right | p = .06 | |
| GMD CTL vs. BIP Li− | Left | p = .87, ns | Left | p = .35, ns | Left | p = .70, ns |
| Right | p = .45, ns | Right | p = .65, ns | Right | p = .42, ns |
Direct comparison between the lithium-treated versus untreated groups revealed nonsignificantly greater GMD in the Li+ versus Li− group overall (p = .06, for both right and left hemispheres; Supplement 2). After permutation analysis to correct for multiple comparisons, this GMD difference in the Li+ versus Li− group was significant in the right hemisphere ROI in the anterior cingulate gyrus (p = .02) but not in the left anterior cingulate (p = .06). Permutation analyses for the frontal lobe ROI also indicated nonsignificantly greater GMD in the Li+ versus Li− group (p = .06, for both right and left hemispheres).
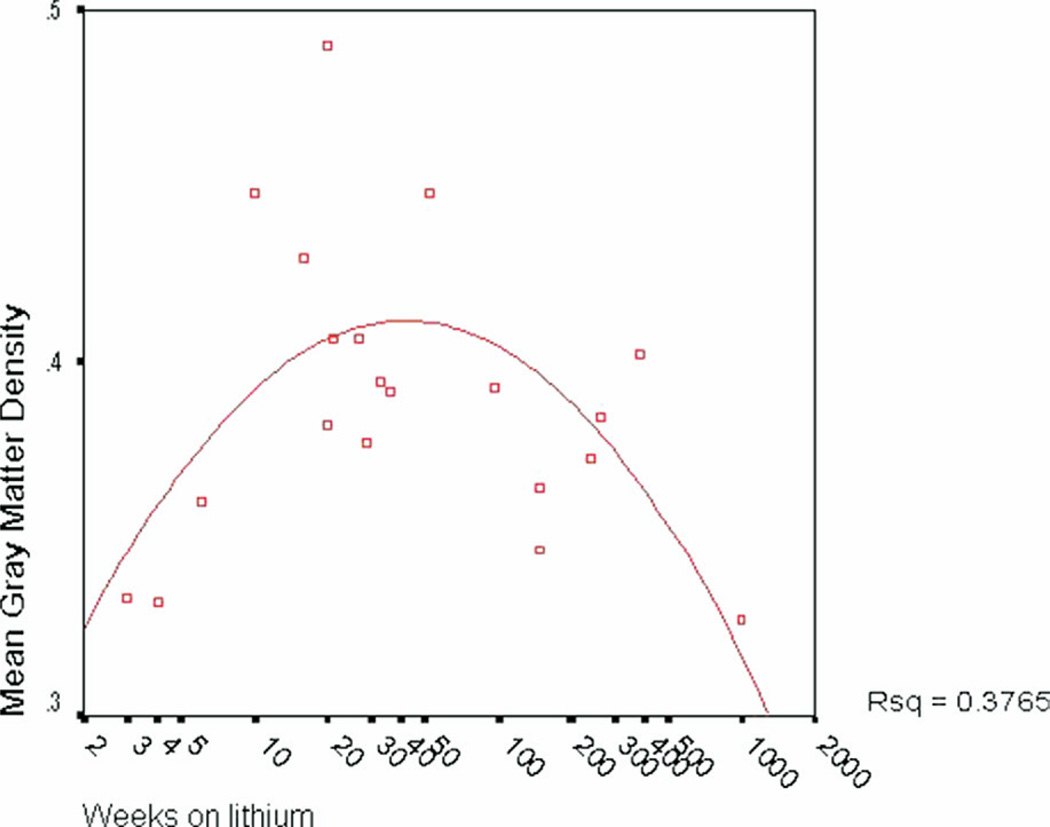
After normalizing the duration of lithium treatment variable using a log transformation, there was not a significant linear correlation between duration of lithium use and GMD (left hemisphere: p = .51, ns; right hemisphere: p = .78, ns, corrected). However, given that a linear relationship would not be anticipated, we further examined the relationship between duration of lithium treatment and gray matter using curve estimation to determine the model best fitting the relationship. A significant quadratic relationship between weeks on lithium and overall gray matter density was observed [_R_2 = .38, _F_(17) = 5.13; _p_ = .018; Figure 5], indicating that duration of lithium treatment accounts for 38% percent of the variance in gray matter volume in lithium-treated bipolar patients. No significant relationships were observed between GMD and lithium dosage or lithium blood level within the lithium-treated group (all p > .25).
Figure 5.
Scatterplot depicting quadratic relationship between duration of lithium usage (in weeks) and mean cortical GMD. A significant quadratic relationship was observed between weeks on lithium and overall gray matter density [_R_2 = .38, F = 5.13(17), p = .018], indicating that duration of lithium treatment accounts for 38% percent of the variance in gray matter volume in lithium-treated bipolar patients. GMD, gray matter density.
To determine whether the correlation between weeks on lithium and overall GMD was better accounted for by other disorder-related variables, we also examined the relationship between gray matter density and other illness variables simultaneously in a multiple regression analysis (HDRS and Bech-Rafaelsen Mania Scale scores, duration of illness, age at onset of illness, number of previous mood episodes). Results indicated that when considered together in the regression model, none of the individual predictors bore a significant relationship to overall GMD [_R_2 = .39, F(5,8) = 1.02, p = .47]. However, age was significantly inversely correlated with GMD in both bipolar patients (for Li+ bipolar patients: r = −.49; p = .03; for Li− bipolar patients: r = −.73; p = .04) and control subjects (r = −.54; p = .003). Thus, to examine whether there was an interactive effect of age with lithium treatment that might be accounting for the observed relationship, we added age to the model. This analysis indicated that after accounting for the effects of age, weeks on lithium remained a significant predictor of GMD [_R_2 = .34; F(17) = 4.39, p = .03].
Effects of Other Medications
Because a small number of bipolar patients in this study were taking other medications at the time of the scan (n = 5; see Supplement 1), we reanalyzed group differences in total GMD after excluding these patients from the analysis and group differences remained significant. One-way analysis of variance (ANOVA) analyses comparing the three groups (using the least significant difference correction for multiple comparisons) indicated gray matter density was significantly greater in lithium-treated bipolar patients (n = 16), as compared with both control subjects [n = 28; t(42) = −.036; p = .001] and unmedicated patients [n = 7; t(21) = −.27; p = .03]. However, unmedicated patients and control subjects did not differ from each other [t(33) = −.003; p = .82, ns).
Effects of Current Mood State
Overall GMD values did not differ between depressed (n = 10) and euthymic (n = 17) bipolar patients, whether controlling for weeks on lithium [F(1,24) = 1.37, p = .25, ns] or not [F(1,25) = 1.87, p = .18, ns]. Similarly, GMD values did not differ between depressed and euthymic bipolar patients for either the left [F(1,25) = .38, p = .54, ns] or right anterior cingulate ROI [F(1,25) = 1.6, p = .21, ns].
Discussion
These maps provide novel information regarding regionally specific gray matter enlargement associated with lithium treatment in bipolar patients. Cortical pattern matching methods revealed significantly greater gray matter concentration, particularly within the medial walls of the cerebral hemispheres, in the anterior cingulate, ventral prefrontal cortex, and paralimbic association cortex in lithium-treated patients with bipolar disorder, as compared with healthy control subjects. These brain regions are part of a complex interconnected neural circuitry involved in mood and cognitive regulation, memory, and the pathophysiology of both unipolar and bipolar mood disorders (Brambilla et al. 2005; Phan et al. 2002). Given that this effect was not found in unmedicated bipolar patients, these results suggest changes in regional gray matter brain content related to lithium treatment and may reflect its postulated effect of neuropil increase manifested as increases in gray matter volume (Moore et al. 2000b). Further corroborating these data in a prospective MRI study with healthy volunteers, we found significant increases in gray matter concentration in the left cingulate, left precuneus, and right superior frontal gyrus after 4 weeks of lithium treatment (Monkul et al. 2004).
These findings are also consistent with a recent voxel-based morphometry investigation (Adler et al. 2005) that observed areas of significantly greater gray matter in several brain regions, including portions of the anterior cingulate, ventral prefrontal cortex, fusiform gyrus, and primary and supplementary motor cortex, in a sample of adult bipolar patients on a variety of medications. Although the authors interpret their findings as possibly reflective of preapoptotic osmotic changes or hypertrophy, they did not explicitly investigate differential medication effects, leaving open the question of the etiology of these neuroanatomic differences. In contrast, using similar methods, Lyoo et al. (2004) found reduced gray matter density in left anterior cingulate and right inferior frontal gyrus in bipolar patients relative to comparison subjects. In this study, only 25% of patients were currently taking lithium, while a slight majority (39%) were unmedicated.
While very little is currently known regarding the functional significance of lithium-associated brain changes, functional neuroimaging studies offer preliminary evidence that mood-stabilizing medications may normalize functional abnormalities within frontotemporal neural systems in bipolar illness (Blumberg et al. 2005). In addition, two recent functional neuroimaging studies have reported decreases in task-associated physiological activity following 2 weeks of lithium treatment in both euthymic bipolar patients (Silverstone et al. 2005) and healthy volunteers (Bell et al. 2005). The relationship of such physiological changes to structural neuroanatomic changes is unknown and clearly warrants further investigation.
We did not detect an effect of lithium dosage or a significant linear correlation between duration of lithium usage and GMD. However, in this naturalistic study, a quadratic (inverted U-shaped) relationship was observed between overall GMD and weeks of lithium treatment. The onset of lithium’s neuroprotective action appears to require about 7 days of treatment, at least in cultured cells (Manji and Lenox 2000). Moreover, the therapeutic effects of mood stabilizers are generally not immediately reversed on discontinuation, suggesting a progressive and complex biological response (Manji et al. 2000a). In rats treated by diet for 2 or 4 weeks, lithium at therapeutic doses has been shown to increase the activity of two prominent transcription factors, AP-1 and cyclic adenosine monophosphate (cAMP)-response element binding protein (CREB), in cultured cerebellar granule cells and in distinct brain regions, including the frontal cortex, amygdala, hippocampus, and cerebellum (Ozaki and Chuang 1997).
However, it is difficult to infer from these animal studies what might be predicted over a longer time course in humans. While prior studies in human subjects have detected effects of lithium treatment on gray matter volume within 4 weeks (Moore et al. 2000a, 2000b), it is unsurprising that the relationship between duration of treatment and brain changes is complex and nonlinear. Although gray matter increases attributable to lithium treatment would be expected to reach asymptote at some point, the inverted U-shaped function is somewhat unexpected and may possibly reflect a complex interaction between effects of lithium treatment and normal aging processes. Because these questions cannot be addressed in this naturalistic, cross-sectional study, we are actively investigating neuroanatomic, as well as neurocognitive, effects of duration and dosage of lithium treatment in a longitudinal follow-up study.
Our findings suggest that some of the inconsistencies between prior neuroanatomic studies of patients with bipolar disorder may be attributable to competing processes of disease-related atrophy and/or tissue reduction pitted against possible neurotrophic or neuroprotective effects of mood-stabilizing medication. Consistent with this possibility, Drevets et al. (1997) previously reported that a small subregion of the anterior cingulate, the subgenual prefrontal cortex (SGPFC), was about 40% smaller in patients with bipolar disorder than in matched control subjects; however, they subsequently found that the patients treated with lithium or valproate had significantly higher SGPFC volumes than nontreated patients and did not differ from control subjects.
Although the mechanism of action of lithium is still largely unknown, recent animal and human studies have provided converging evidence for its potential neuroprotective and neurotrophic effects (e.g., Manji et al. 2000a). At the molecular level, both lithium and valproate robustly increase levels of the neuroprotective protein B-cell lymphoma protein-2 (bcl-2) in the frontal cortex (Chen et al. 1999) and inhibit the proapoptotic protein glycogen synthase kinase-3β (GSK-3β) in the central nervous system (CNS) (Chen et al. 1999; Manji et al. 2000a). B-cell lymphoma protein-2 is part of a well-characterized protein family that regulates apoptotic cell death, acting on mitochondria to stabilize membrane integrity and prevent release of apoptogenic factors (Manji et al. 2000b). Glycogen synthase kinase-3β is implicated in regulation of various cytoskeletal processes and disease-related neuronal death (Chen et al. 1999; Jope 1999; Manji et al. 2000a). Its inhibition by lithium is thought to be protective against processes of programmed cell death (Hetman et al. 2000). It is unclear which of these cellular actions is related to lithium’s therapeutic effects; an increasing number of studies are now attempting to elucidate the processes that convert these second messenger-mediated events into long-term cellular phenotypic changes.
Notably, recent postmortem neuropathologic studies have documented reduced neuronal density in brain regions in which we found evidence for robustly increased gray matter density in lithium-treated patients, particularly the anterior cingulate (Benes et al. 2001; Bouras et al. 2001). Although effects of mood-stabilizing medications were not explicitly examined in these studies, the question of whether lithium treatment has long-term neuroprotective benefits that persist following termination of treatment is also of fundamental importance.
Certain limitations of the current study must be noted. Because we did not specifically design this study to examine the effects of lithium treatment, we were unable to match the patient groups on all clinical variables; specifically, there was a nonsignificant trend toward higher depression severity scores in the untreated group at the time of scanning. However, given that prior studies have not reported structural anatomic differences as a function of current mood state (Brambilla et al. 2005), we do not believe that this presents a significant confound. Further, while patients with bipolar II disorder were proportionally overrepresented in the untreated group, bipolar II disorder patients did not significantly differ from bipolar I disorder patients in terms of GMD [means: bipolar I disorder = .38 ± .03 versus .38 ± .06 for bipolar II disorder; F(1,26) = .12, p = .73]. Thus, the higher proportion of bipolar II disorder patients in the untreated group cannot account for this pattern of findings. With regard to medication treatment, our sample included patients who had been on lithium for varying time periods and dosages were not uniform across subjects. In the context of a clinical trial, such variables could be better controlled. In addition, while some subjects were taking more than one medication, results did not change when these subjects were excluded from analysis. These limitations notwithstanding, as this study is, to our knowledge, the first to characterize the pattern of gray matter differences across the cortical surface in lithium-treated bipolar patients, replication with a larger homogenous group of subjects may serve to confirm the results observed here.
Given the small sample size in the lithium-untreated group and the cross-sectional nature of this investigation, these findings do not conclusively demonstrate that greater gray matter concentration in the lithium-treated group results from medication effects alone. Although robust differences were detected between the lithium-treated group and normal control subjects, the group difference comparison for lithium-treated versus untreated bipolar patients reached statistical significance only for the right anterior cingulate after permutation analysis to correct for multiple comparisons. Thus, it cannot be ruled out that these regional increases were present prior to initiation of lithium and instead reflect neuronal pathology intrinsic to bipolar illness, perhaps resulting from a selective failure of these systems to myelinate, which could result in more tissue segmenting as gray matter in these brain regions. However, this explanation is unlikely, given the clear absence of differences between the untreated group and normal control subjects, as well as converging evidence demonstrating gray matter increases resulting from short-term lithium treatment in healthy volunteers (Monkul et al. 2004; Moore et al. 2000b). In addition, while we cannot exclude the possibility that the observed gray matter differences in the lithium-treated group are related to osmotic effects of lithium leading to changes in water content in the brain, a purely osmotic action would be unlikely to be restricted to gray matter alone (Sassi et al. 2002); the absence of any differences in white matter argues against this interpretation. Notably, other psychotropic agents, including valproate (Chen et al. 1999) and atypical antipsychotics (Braus et al. 2001), may also affect neuronal viability. These observations demonstrate the importance of taking medication effects into account when interpreting data from in vivo neuroimaging studies and postmortem reports. Furthermore, other factors that may contribute to GMD increases in bipolar patients should be systematically examined in future investigations.
In conclusion, the sensitive cortical pattern matching methods employed in this study were able to detect prominent gray matter enlargement, most pronounced in bilateral cingulate and paralimbic cortices, in lithium-treated patients with bipolar disorder relative to healthy control subjects. It is tempting to infer that the observed gray matter increases may suggest a mechanism of action for lithium’s therapeutic effects, but these findings clearly need to be replicated in other studies. The pattern of results observed here, combined with those of prior studies indicating structural and neurochemical differences as a function of lithium treatment, suggest that reanalysis of previously collected neuroimaging data may be an efficient way to substantially increase our current understanding of the magnitude and time course of lithium’s effects on the brain. Future studies should also assess whether valproate or other medications that effectively treat bipolar disorder lead to similar increases in brain gray matter in vivo and further investigate the clinical and functional relevance of these results.
Supplementary Material
Supplement Files
Acknowledgments
This work was partly supported by K23 MH074644-01 (CEB), MH 29618, MH 01736, MH 030915, MH 068662, MH 068766, RR020571, Krus Endowed Chair in Psychiatry (University of Texas Health Science Center San Antonio), Veterans Administration (VA Merit Review), National Alliance for Research on Schizophrenia and Depression (NARSAD), and CAPES Foundation (Brazil). Algorithm development was funded by the National Center for Research Resources, the National Institute for Biomedical Imaging and Bioengineering, and the National Institute on Aging (EB01651, RR019771, AG021431).
DJK has served on Advisory Boards of Eli Lilly & Company, Forest Pharmaceuticals, Inc., Pfizer, Inc., and Solvay/Wyeth Pharmaceuticals and also served as a Consultant for Servier Amerique.
EF serves on advisory boards for Pfizer, Inc., and Eli Lilly, as a consultant to Pfizer, Italia, and Novartis, USA, and is the recipient of an investigator-initiated grant from Forest Research Institute.
We thank Robert M. Bilder, Ph.D., ABPP, for helpful comments on the manuscript.
Footnotes
None of the other authors have financial disclosures pertinent to the contents of the manuscript.
Supplementary materials cited in this article are available online.
References
- Adler C, Levine A, DelBello M, Strakowski S. Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry. 2005;58:151–157. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Sowell ER, Thompson PM, Kumar A, Narr KL, Lavretsky H, et al. Mapping brain size and cortical gray matter changes in elderly depression. Biol Psychiatry. 2004;55(4):382–389. doi: 10.1016/j.biopsych.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bech P, Bolwig TG, Kramp P, Rafaelsen OJ. The Bech-Rafaelsen Mania Scale and the Hamilton Depression Scale. Acta Psychiatr Scand. 1979;59:420–430. doi: 10.1111/j.1600-0447.1979.tb04484.x. [DOI] [PubMed] [Google Scholar]
- Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Differential effects of chronic lithium and valproate on brain activation in healthy volunteers. Hum Psychopharmacol. 2005;20:415–424. doi: 10.1002/hup.710. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005;183(3):308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol (Berl) 2001;102:373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Barale F, Soares JC. Perspectives on the use of anticonvulsants in the treatment of bipolar disorder. Int J Neuropsychopharmacol. 2001;4:421–446. doi: 10.1017/S1461145701002668. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Glahn DC, Balestrieri M, Soares JC. Magnetic resonance findings in bipolar disorder. Psychiatr Clin North Am. 2005;28:443–467. doi: 10.1016/j.psc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Braus D, Ende G, Weber-Fahr W, Demirakca T, Henn F. Favorable effect on neuronal viability in the anterior cingulate gyrus due to long-term treatment with atypical antipsychotics: An MRSI study. Pharmacopsychiatry. 2001;34:251–253. doi: 10.1055/s-2001-18037. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structuralMRimages of the brain. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version. New York: New York State Psychiatric Institute; 1998. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Anti-bipolar therapy: Mechanism of action of lithium. Mol Psychiatry. 1999;4:117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Signaling: Cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry. 2000;48:518–530. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: Implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry. 2000a;48:740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Lithium up-regulates the cytoprotective protein Bcl-2 in the CNS in vivo: A role for neurotrophic and neuroprotective effects in manic depressive illness. J Clin Psychiatry. 2000b;61(suppl 9):82–96. [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Monkul E, Dalwani M, Nicoletti M, Soares J. Brain gray matter changes after lithium treatment: A voxel-based morphometry study in healthy individuals. Biol Psychiatry. 2004;55:202S. doi: 10.1016/j.neulet.2007.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB, et al. Lithium increases N-acetyl-aspartate in the human brain: In vivo evidence in support of bcl-2’s neurotrophic effects? Biol Psychiatry. 2000a;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000b;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey C. Atlas of the Cerebral Sulci. Stuttgart, Germany: Thieme; 1990. [Google Scholar]
- Ozaki N, Chuang DM. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J Neurochem. 1997;69(6):2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ, et al. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett. 2002;329:243–245. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Bell EC, Willson MC, Dave S, Wilman AH. Lithium alters brain activation in bipolar disorder in a task- and state-dependent manner: An fMRI study. Ann Gen Psychiatry. 2005;4:14. doi: 10.1186/1744-859X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone PH, Wu RH, O’Donnell T, Ulrich M, Asghar SJ, Hanstock CC. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol. 2003;18:73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, et al. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23(suppl 1):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Files