Constitutively-active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin (original) (raw)
. Author manuscript; available in PMC: 2013 Mar 22.
Abstract
Rationale
Calcium entry is pivotal in the heart and blood vessels but its significance and mechanisms in adipose tissue are largely unknown. An important factor produced by adipocytes is adiponectin, which confers myocardial protection, insulin-sensitisation, and anti-atherosclerotic effects.
Objective
To investigate the relevance of calcium channels to adipocytes and the production of adiponectin.
Methods and Results
Micro-array analysis led to identification of TRPC1 and TRPC5 as channel subunits that are induced when adipocytes mature. Both subunits were found in perivascular fat of patients with atherosclerosis. Intracellular calcium and patch-clamp measurements showed that adipocytes exhibit constitutively-active calcium-permeable non-selective cationic channels that depend on TRPC1 and TRPC5. The activity could be enhanced by lanthanum or rosiglitazone, known stimulators of TRPC5 and TRPC5-containing channels. Screening identified lipid modulators of the channels that are relevant to adipose biology. Dietary ω-3 fatty acids (e.g. α-linolenic acid) were inhibitory at concentrations that are achieved by ingestion. The adipocyte TRPC1/TRPC5-containing channel was functionally negative for the generation of adiponectin because channel blockade by antibodies, knock-down of TRPC1-TRPC5 in vitro, or conditional disruption of calcium permeability in TRPC5-incorporating channels in vivo increased the generation of adiponectin. The previously recognised capability of α-linolenic acid to stimulate the generation of adiponectin was lost when calcium permeability in the channels was disrupted.
Conclusions
The data suggest that TRPC1 and TRPC5 contribute a constitutively-active heteromultimeric channel of adipocytes that negatively regulates adiponectin and through which ω-3 fatty acids enhance the anti-inflammatory adipokine, adiponectin.
Keywords: calcium channel, transient receptor potential, α-linolenic acid, adipocyte, adiponectin
Introduction
Adipocytes are sites for metabolism, storage, and effects of fatty acids. The cells are also pivotal in generating the endocrine organ of adipose tissue, which impacts on whole body metabolism and inflammation through secretion of adipokines1. A key adipokine is adiponectin, which is anti-inflammatory, insulin-sensitising, and protective against atherosclerosis and myocardial decline2. Decreased concentrations of adiponectin occur in obesity-induced insulin resistance and are associated with endothelial dysfunction, diabetes, and hypertension. Diminished adiponectin secretion from adipose tissue of human coronary arteries has been suggested to be an initiator of atherosclerosis3, 4.
The concentration of free cytoplasmic calcium (Ca2+) and the amplitude and rhythmicity of its fluctuations have primary importance in a plethora of cell types5. For many cells there has been extensive study of intracellular Ca2+ signals, including investigation of the plasma membrane ion channels that directly permit Ca2+ influx or control Ca2+ influx indirectly. There is, by contrast, relatively little known about Ca2+-signalling in adipocytes, despite its suggested importance6, 7.
A major class of Ca2+-permeable channels is formed by Transient Receptor Potential (TRP) proteins, which are encoded by twenty eight genes in mammals8, 9. The proteins span the plasma or intracellular membranes, assembling around central ion pores as mono- or hetero-multimers to allow influx of cations such as Ca2+ and Na+. The proteins are classified into subfamilies based on amino acid sequence; one of these is the canonical (C) subfamily, which contains six members in humans (TRPC1, 3-7). Unlike many other ion channels, they are not voltage- or neurotransmitter- gated. Instead, they couple relatively slow chemical and physical activators to intracellular Ca2+-signalling. Activator chemicals include dietary factors such as capsaicin which activates TRPV1, and menthol which activates TRPM810. Several TRP channels are expressed, albeit not exclusively, in sensory neurones, supporting the concept of TRP channels as mechanisms by which animals detect external chemical signals9. Although there is potential for importance of chemical-sensing ion channels in adipocyte biology, there are only two reports on TRP channel function in this context, both addressing TRPV1: One of the reports suggested function of TRPV1 in pre-adipocytes, while the other suggested no function in pre-adipocytes or adipocytes but a role in sensory nerves of adipose tissue11, 12. Here we sought Ca2+ channels that are important in adipocyte function and have potential relevance to cardiovascular health and disease. The investigation highlights TRPs from the C subfamily.
Methods
Human and mouse tissues
Transgenic mice
DNT5 cDNA was cloned into the pTRE vector from Clontech (Online Figure I). After AseI restriction digestion transgene was purified and microinjected into the pronucleus of C57BL/6 mouse embryos (MRC Harwell). Double transgenics were generated by breeding with mice carrying transgene encoding reverse tetracycline transactivator (rtTA) at the ROSA26 locus13. ROSA 26 mice were provided by G Belteki, J Haigh and A Nagy. Male animals were weaned onto high fat diet (lard, fat calories 60 %; BioServ) at 3 weeks of age; 5 weeks later, animals were supplied with doxycycline (1mg/ml and 2% sucrose in the drinking water); 1 week later, animals were culled and blood/tissue samples removed for analysis. All procedures were carried out with ethical approval under UK Home Office licence.
Cell culture and transfection
HEK 293 cells stably expressing human TRPC5 under a tetracycline inducible promoter and expression of TRPC1 using FuGene HD (Roche, UK) have been described13. The 3T3-L1 cell line was obtained from the American Type Culture Collection (ATCC) and cultured in DMEM-F12 containing 10 % fetal calf serum (FCS), 100 U/ml penicillin and 100 μg/ml streptomycin. To induce differentiation, cells were grown to confluence and 2 days post confluence, the medium was changed to medium containing 5 μg/ml insulin, 0.25 μmole/L dexamethasone and 0.5 mmole/L IBMX with 10 % FCS and antibiotics. After 48 hr, medium was changed to medium containing 5 μg/ml insulin, 10 % FCS, and antibiotics. Cells were fed with fresh maintenance medium every 2 days until the day of experiments. Cells were differentiated for 12-16 days. Accell siRNA delivery was according to the manufacturer’s protocol (Dharmacon, UK). siRNA sequences are in Online Table I. For investigation of adipocytes from mice, preadipocytes were isolated using methods adapted from previous studies14. Epididymal fat pad was dissected and digested in collagenase II (500μg/50mg tissue) for 1 hr at 4 °C and 2 hr at 37 °C and then centrifuged at 200g for 10 min. The pellet was dissolved in erythrocyte lysis buffer14, filtered and centrifuged again. Preadipocytes were cultured and differentiated as described for 3T3-L1 cells but, in addition, all media contained 5 μg/mL doxycycline. Cells were differentiated for 9 days. For Ca2+ measurement, cells were plated on fibronectin-coated glass bottom dishes (Fluorodish, WPI, USA).
Intracellular Ca2+ measurement and electrophysiology
3T3-L1 cells were plated in 96-well biocoat plates (Corning) to 80-90 % confluence for 24 hr. Prior to recordings, cells were incubated for 1 hr at 37 °C in 4 μmole/L fluo-4AM in standard bath solution (SBS) containing (mmole/L): 140 NaCl, 5 KCl, 1.2 MgCl2, 1.5 CaCl2, 8 glucose and 10 HEPES titrated to pH 7.4 using NaOH. Cells were washed for 0.5 hr in SBS at 37 °C. Except for measurements from mouse adipocytes, recordings used the FlexStation II in 96-well mode (Molecular Devices, USA). Mouse adipocytes were studied using a Nikon Eclipse TE2000 microscope equipped with a 40× objective and confocal fluorescence system (Thorlabs, Sterling, VA). Images from approximately 20 cells per dish were collected using ThorImageLS (Thorlabs) and analysed using ImageJ software. Consistent with a previous report15, a fluorescence artefact between fura-2 and the lipid droplets of mature adipocytes prevented ratiometric Ca2+ measurements. Therefore, the non-ratiometric fluo-4 Ca2+ indicator was used with 3T3-L1 cells or mouse adipocytes. Fluo-4 was excited at 485 nm (FlexStation) or by a 488 nm laser (microscope) and emission was collected at 525 nm. Experiments were at room temperature (21±2 °C). For HEK 293 cells the protocol was similar except fluo-4AM was used with 0.01 % pluronic acid and 2.5 mmole/L probenecid, or 2 μmole/L fura-2AM was used. Fura-2 was excited at 340 and 380 nm and emitted light was collected at 510 nm; intracellular Ca2+ was indicated by the ratio of emission intensities for the excitation wavelengths. For electrophysiology methods see Supplemental Material.
Adipokine measurement
3T3-L1 cells were differentiated in 6-well plates. On day 12, cells were serum-starved for 24 hr and then treated with dialysed anti-TRPC1 (T1E3) and/or anti-TRPC5 (T5E3) antisera for 24 hr. For α-linolenic acid (lino.) treatment, cells were incubated with 50 μmole/L lino. or its vehicle (0.5 % DMSO). After 24 hr the supernatant was collected and centrifuged at 1000 rpm for 10 min. Full length adiponectin and soluble leptin were measured using ELISA kits (R&D Systems, UK). For organ cultures, epididymal fat tissue was harvested from 8-12 week old male C57BL/6 mice and about 0.5 cm3 pieces were kept in DMEM-F12 containing 10 % fetal calf serum (FCS), 100 U/ml penicillin and 100 μg/ml streptomycin for 24 hr. The tissues were treated with agents (as in 3T3-L1 experiments) and the supernatant collected. For tissue from transgenic mice the medium was supplemented with 5 μg/mL doxycycline. For mouse plasma adiponectin or leptin levels, the mice were terminally bled and anti-coagulant (EDTA) containing blood was centrifuged at 7000 rpm for 7 min and the supernatant plasma was used.
Immunostaining, western blotting, RNA isolation, RT-PCR and microfluidic cards
See Supplemental Material and Online Table II for PCR primer sequences.
Chemicals and antibody reagents
All chemicals were from Sigma (UK) except for fura-2AM and fluo-4AM (Invitrogen) and the fatty acid library (Biomol, Enzo Life Sciences, UK). For functional antibody experiments cells were pre-treated with anti-TRPC1 T1E3 (1:500) or anti-TRPC5 T5E3 (1:100) antisera with or without preadsorption to the relevant antigenic peptide (10 μmole/L)16. T1N1 was custom-made rabbit anti-TRPC1 antibody targeted to intracellular N-terminal sequence (EVMALKDVREVKEENTC) of TRPC1. Dialysed antisera were diluted in DMEM medium and incubated with cells for 2-3.5 hr at 37 °C prior to recordings. Chemical identity and purity of α-linolenic acid was confirmed by liquid chromatography-mass spectrometry.
Data analysis
Data were collected in control and test pairs, expressed as mean ± s.e.mean and compared statistically using Student’s _t_-tests; n is the number of independent experiments and N is the number of wells in multi-well assays (when only N is stated, the data are from one 96-well plate). Probability (P) <0.05 (*) indicates statistically significant difference; n.s. indicates no significant difference. All results were from at least 3 independent experiments. Origin software was used for data analysis and presentation.
Results
TRPC1 and TRPC5 are expressed when adipocytes mature
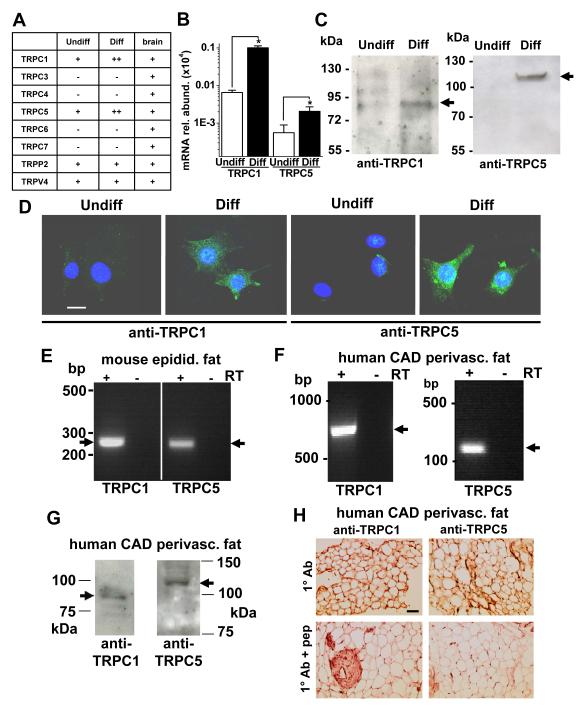
As a first step towards elucidating ion channel types that are important in adipocytes we performed an unbiased screen to identify ion channel transcript expression that up-regulates on maturation of pre-adipocytes to adipocytes. As a basis for the screen we chose mouse 3T3-L1 cells which have been extensively characterised as a model of in vivo adipocytes and can be compared in two groups: pre-adipocytes and differentiated mature adipocytes. Appropriate differentiation of the cells was validated by Oil-red O staining and expression of the adipocyte markers PPARγ, aP2, adiponectin and leptin (Online Figure II). Total RNA was isolated from each group of cells and ion channel expression was investigated in micro-fluidic PCR array cards representing 185 ion channel genes. Expression of 51 ion channel genes was indicated. Of these, 18 are known to confer Ca2+-permeability and 6 are TRPs; the most highly up-regulated in adipocyte maturation was TRPC1. TRPC mRNAs were therefore investigated in independent quantitative RT-PCR reactions. Expression of TRPC1 mRNA was confirmed and TRPC5 mRNA was also detected, whereas mRNAs encoding TRPC3-4/6-7 were not detected (Figure 1A; Online Figure III). Notable was the marked up-regulation of TRPC1 (15.5 times) and TRPC5 (36.9 times) mRNAs as the cells differentiated (Figure 1A, B). TRPV4 and TRPP2 mRNAs were also detected on the array card and are potentially relevant, but neither was up-regulated on differentiation (Online Figure III).
Figure 1. TRPC1 and TRPC5 in mature adipocytes.
A, Summary of mRNA detection in undifferentiated (Undiff) and differentiated (Diff) 3T3-L1 cells and mouse brain (+, expression; ++, higher expression; −, undetected). B, Abundances of TRPC1 and TRPC5 mRNAs in 3T3-L1 cells relative to 18S. C, 3T3-L1 proteins labelled with T1E3 anti-TRPC1 and T5E3 anti-TRPC5 antibodies. Expected masses are indicated by arrows. Equal total protein was loaded in each lane. A β-actin blot is shown in Online Figure II. D, Labelling of 3T3-L1 cells with T1E3 or T5E3 (green). Cell nuclei were stained with DAPI (blue). Representative of 3 experiments. The scale bar is 20 μm and for all images. Controls are shown in Online Figure IV. E, Products from RT-PCR on RNA from mouse epididymal fat tissue. F, Analysis of mRNA from human coronary artery disease (CAD) perivascular fat by RT-PCR. G, Lysates showing single protein bands of the expected sizes for TRPC1 and TRPC5. H, As for (G) but protein analysis by immunostaining (brown colour in the upper panels indicates channel detection). The control was the antibody (Ab) preadsorbed to its antigenic peptide (+pep). Scale bar, 100 μm.
Western blotting and immunostaining were used to investigate TRPC1 and TRPC5 proteins. Neither protein was detectable in undifferentiated 3T3-L1 cells but both were expressed after differentiation (Figure 1C). Similarly, immunofluorescence experiments showed that TRPC1 and TRPC5 were expressed on differentiation (Figure 1D; Online Figure IV). These TRP proteins were not only expressed in 3T3-L1 cells but also in native mature adipocytes of mice and humans. In mice, TRPC1 and TRPC5 mRNAs were detected in native epididymal fat (Figure 1E). We also investigated perivascular fat because it is considered to be crucial in atherosclerosis3. TRPC1 and TRPC5 were detected in perivascular fat of the mouse aorta (Online Figure V). To investigate perivascular fat in humans we obtained internal mammary artery during coronary artery bypass surgery. TRPC1 and TRPC5 mRNAs (Figure 1F) and proteins (Figure 1G) were detected and localised to adipocytes (Figure 1H). The data suggest that expression of TRPC1 and TRPC5 is induced in mature adipocytes and relevant to endogenous fat of mice and humans, including perivascular fat.
TRPC1 and TRPC5 confer constitutive calcium entry in adipocytes
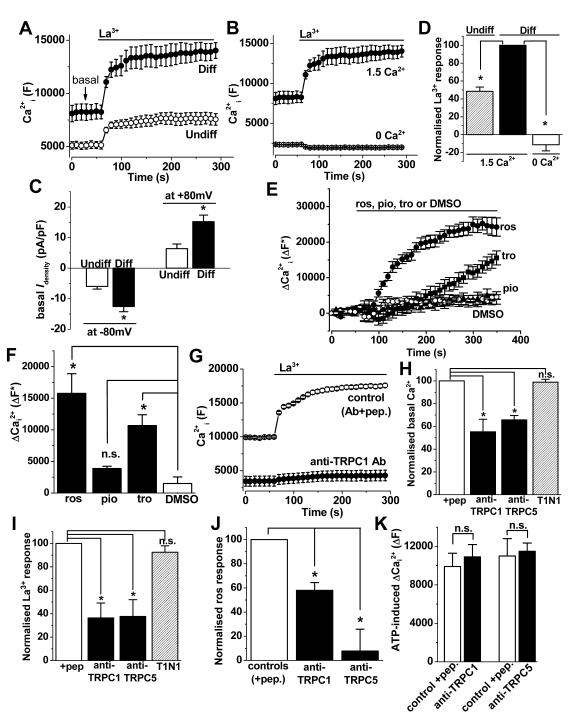
To investigate if TRPC1 and TRPC5 are functionally relevant we performed intracellular Ca2+ measurements. Differentiated 3T3-L1 cells showed higher basal fluo-4 signal (Figure 2A) which depended on extracellular Ca2+ (Figure 2B), suggesting the presence of constitutively-active Ca2+ entry channels. Moreover, whole-cell patch-clamp recordings revealed larger basal currents in differentiated 3T3-L1 cells (Figure 2C). We tested the effect of extracellular lanthanum ions (La3+) because a distinguishing feature of TRPC5-containing channels is that they may be stimulated by lanthanides such as La3+ or gadolinium (Gd3+)16. Consistent with the presence of functional TRPC5-containing channels, La3+ stimulated Ca2+-entry in differentiated 3T3-L1 cells (Figure 2A, B, D). Another unusual property of TRPC5 is that it is stimulated by the PPARγ agonist rosiglitazone but not by a related thiazolidinedione pioglitazone and only slightly but not significantly by troglitazone17. In differentiated 3T3-L1 cells, rosiglitazone stimulated Ca2+ entry whereas pioglitazone had no effect, and troglitazone caused a delayed increase in Ca2+ (Figure 2E, F).
Figure 2. Ca2+ entry mediated by endogenous channels of differentiated 3T3-L1 cells.
(A, B, D-K) 96-well FlexStation fluo-4 intracellular Ca2+ data from pre-adipocytes (Undiff) and mature adipocytes (differentiated 3T3-L1 cells, Diff). A, From a single 96-well plate, example basal Ca2+ and La3+ (20 μmole/L)-evoked Ca2+ elevation in Undiff and Diff 3T3-L1 cells in the presence of 1.5 mmole/L extracellular Ca2+. B, for Diff 3T3-L1 cells, 20 μmole/L La3+ responses in the presence of 0 or 1.5 mmole/L extracellular Ca2+ (N=4 each; from the same experiment as A). C, Mean constitutive basal whole-cell current (I) densities for Undiff and Diff 3T3-L1 cells (n=5 for each). Further data from these cells are in Figure 5C. D, Mean normalised data across different 96-well plate experiments for La3+ responses (n=3), as shown in A, B. E, for Diff 3T3-L1 cells, changes (Δ) in Ca2+i in response to 100 μmole/L rosiglitazone (ros), pioglitazone (pio) and troglitazone (tro) in the presence of 1.5 mmole/L Ca2+ (N=3 each). F, Mean data from across 3 plates for experiments of the type shown in (E). G, Typical La3+ responses of Diff 3T3-L1 cells pre-treated with anti-TRPC1 (T1E3) antibody or control T1E3 preadsorbed to its antigenic peptide (+pep). H&I, For the type of experiment illustrated in (G), mean data normalised to controls showing suppression of basal (H) and La3+-evoked (I) intracellular Ca2+ responses by T1E3 and T5E3 compared with controls of antibodies preadsorbed to antigenic peptide (+pep) (n/N=3/24, n/N=3/20). T1N1 was a different antibody targeted to TRPC1 N-terminus that did not block the channel (n/N=3/12). J, Mean data normalised to controls showing suppression of rosiglitazone-evoked Ca2+ responses by T1E3 and T5E3 compared with the controls of antibodies preadsorbed to antigenic peptides (+pep) (n/N=3/9, n/N=3/9). K, Mean data for responses to extracellular 100 μmole/L ATP, showing no effects of T1E3 and T5E3 (n=3 for each).
To investigate more directly if Ca2+ signals related to TRPC1 and TRPC5 we used antibodies that target extracellular peptides in TRPC1 or TRPC5 and acutely inhibit channel function16, 18. Antibody to either TRPC1 or TRPC5 suppressed constitutive and La3+- or rosiglitazone-evoked Ca2+ signals in differentiated 3T3-L1 cells (Figure 2G-J). There was a trend towards anti-TRPC5 antibody having a greater effect, compared with anti-TRPC1 antibody, on the rosiglitazone response (Figure 2J). Control antibody targeted to the N-terminus of TRPC1 (which is intracellular and therefore not accessible to extracellular agents) had no effect (Figure 2H, I). The anti-TRPC blocking antibodies had no effects on ATP-evoked Ca2+-release, consistent with them being specific (Figure 2K).
The data suggest that ion channels containing both TRPC1 and TRPC5 generate constitutive Ca2+ entry that is up-regulated in differentiated 3T3-L1 cells. The channel activity may be further enhanced by La3+ or rosiglitazone.
Identification of negative impact on adiponectin
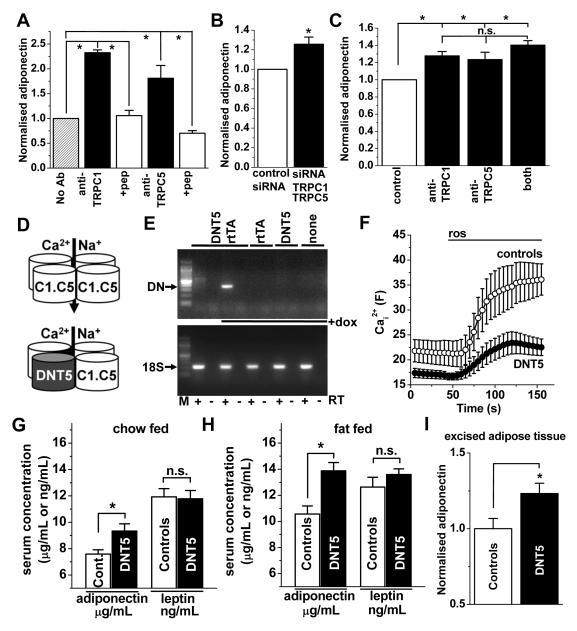
To investigate whether there is a relationship of TRPC1 and TRPC5 channels to adiponectin we first incubated differentiated 3T3-L1 cells with blocking antibodies targeted to TRPC1 or TRPC5. Anti-TRPC1 or anti-TRPC5 antibody enhanced the generation of adiponectin (Figure 3A). As an independent test, differentiated 3T3-L1 cells were transfected with siRNAs to knock-down TRPC1 and TRPC5 expression. Cellular delivery of siRNAs by standard transfection methods was inefficient but cell-permeable Accell siRNA achieved 70-90 % knock-down (Online Figure VI). Combined knock-down of TRPC1 and TRPC5 increased adiponectin generation (Figure 3B). There was less effect compared with the blocking antibodies (Figure 3B cf 3A), possibly because the antibodies inhibited the channels more effectively than the siRNA. To investigate the relevance of the channels to native adipocytes, organ-cultured mouse fat tissue was incubated with anti-TRPC blocking antibodies, and again there was increased adiponectin (Figure 3C). Addition of both antibodies together did not generate a significantly greater effect than either antibody alone (Figure 3C). The antibodies had less effect than in 3T3-L1 cells (Figure 3C cf 3A), which may reflect inadequate penetration of the tissue by antibodies. Collectively the data suggest that channels comprising TRPC1 and TRPC5 impact negatively on the generation of adiponectin.
Figure 3. Negative coupling to adiponectin.
(A-B) Adiponectin secreted from differentiated 3T3-L1 cells. (C) Adiponectin secreted from organ-cultured mouse fat tissue. A, Effects of T1E3 anti-TRPC1 and T5E3 anti-TRPC5 antibodies on adiponectin (n=3 for each). B, As for (A) but the effect of TRPC1 and TRPC5 siRNAs compared with control siRNA (n=3). C, As for (A) but intact excised fat and including data for both antibodies combined (n=4 for each). D, Diagram of the wild-type TRPC1-TRPC5 channel with Ca2+ and Na+ permeability (top) and the channel after incorporation of DNTRPC5 (DNT5) , which inhibits ion permeability (bottom). E, RT-PCR analysis for mRNA from adipose tissue of mice containing the DNT5 and rtTA transgenes, DNT5 transgene only, rtTA transgene only, or neither transgene (none). The PCR primers were for DNT5 or 18S. +dox: doxycycline. Expected product sizes are indicated by arrows. M: DNA marker ladder. F, Confocal fluo-4 Ca2+ measurements for adipocytes from transgenic mice expressing DNT5 (n=4) or controls (n=5). G&H, Serum adiponectin and leptin concentrations in mice expressing the DNT5 transgene (n=6 and 6 chow-fed, n=13 and 9 fat-fed) compared with matched control mice (n=9 and 6 chow-fed, n=11 and 11 fat-fed). I, Adiponectin secreted from fat excised from chow-fed mice, shown for mice expressing DNT5 normalised to controls (n=5 each). Controls were litter mates expressing neither transgene, rtTA only, or DNT5 only. See Supplemental Material for more details.
Regulation of adiponectin in vivo
To determine the relevance of the above findings to endogenous channels in vivo we used a dominant negative (DN) ion pore mutant of TRPC5 (DNT5) to engage with and disrupt channel complexes that can accept TRPC5 (Figure 3D; Online Figure I)18, 19. The specificity of DNT5 was validated by showing its lack of effect on Ca2+ entry through TRPM2 or TRPM3 channels or K+ efflux through endogenous K+ channels (Online Figure I). DNT5 was therefore generated as an in vivo transgene for global inducible expression in the adult mouse (Online Figure I). Expression depended on doxycycline-regulation of an additional co-expressed transgene encoding reverse tetracycline transactivator (rtTA) from the ROSA26 locus, which confers broad expression across multiple cell types13. As predicted, DNT5 expression occurred in adipose tissue of doxycycline-treated double transgenic mice but not doxycycline-treated single transgenics or mice carrying neither transgene (controls) or non-induced double transgenics (Figure 3E). Expression of DNT5 suppressed rosiglitazone-evoked Ca2+ entry by 62 % in adipocytes from the mice (Figure 3F), and so DNT5 acted as we expected.
Because of the association of TRPC5-containing channels with adversity8 we studied mice that were either fed chow diet or high-fat diet for 6 weeks, the latter inducing expression of inflammatory indicators (Online Figure VII) but not obesity. In each litter there was a mixture of genotypes: double transgenics (DNT5+rtTA), single transgenics (DNT5 only or rtTA only), and mice carrying neither transgene. At 8 weeks of age, doxycycline was administered to all of the mice for 1 week. Double transgenic (DNT5, test) and single transgenic and no transgene (controls) mice were compared. No differences in weight or well-being of the mice in each group were observed. However, in chow-fed and fat-fed mice, DNT5 significantly increased the circulating adiponectin concentration without affecting leptin (Figure 3G, H). In the fat-fed mice, insulin was measured and found to be unchanged by DNT5 (_P_>0.05, data not shown). Further details are provided in the Supplemental Material. To test if the effect on adiponectin arose because of an effect of DNT5 on adipose tissue, we excised the tissue from mice expressing double (DNT5) or single (controls) transgenes and analysed the supernatant after organ culture. The adiponectin was significantly higher in the DNT5 group (Figure 3I).
The data suggest that constitutive Ca2+ entry through TRPC1/TRPC5-containing channels suppresses the generation of adiponectin by adipose tissue in vivo.
TRPC inhibition by dietary fatty acids
We hypothesised that TRPC1/TRPC5-containing channels might act as sensors of chemical factors that are important in adipocyte biology and coronary artery disease. We therefore screened for novel activators or inhibitors of the channels, first testing chemicals against signals arising from TRPC5 expressed alone in HEK 293 cells.
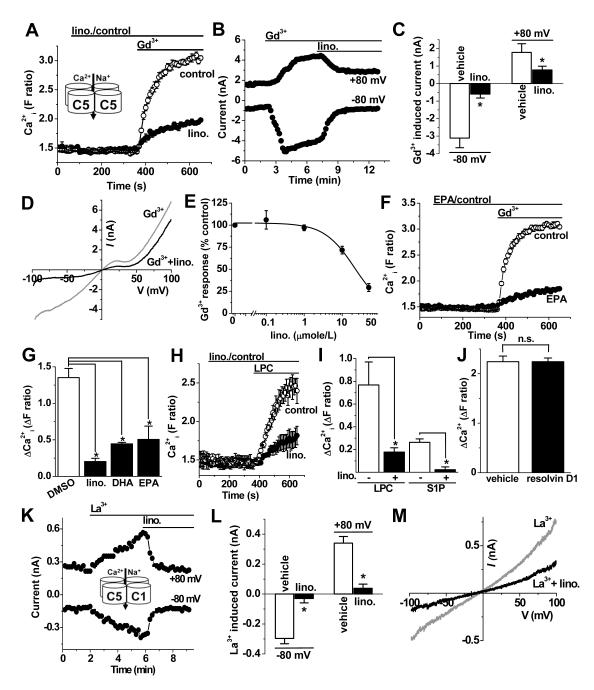
Using an intracellular Ca2+ indicator as the read-out of channel function, 66 fatty acids (Online Tables III, IV) were screened against TRPC5. A two-step addition protocol first delivered the fatty acid and then the TRPC5 stimulator, Gd3+ (Figure 4A). None of the fatty acids stimulated TRPC5 but 19 had inhibitory effects (Figure 4A, Online Table III). A relationship of TRPC5 to anti-inflammatory fatty acids was indicated. Included were dietary ω-3 fatty acids, lino. and DHA, which are present in oily plants and fish20, 21. Inhibitory action of these fatty acids was confirmed in voltage-clamp recordings of membrane current where TRPC5 activity was evoked by Gd3+ (Figure 4B, C) and the defining TRPC5 current-voltage relationship (I-V) was observed (Figure 4D). Lino. inhibited TRPC5 with a threshold at 1 μmole/L and IC50 of 21.5 μmole/L (Figure 4E), which is in the concentration range achieved after ingestion20, 21. Another dietary ω-3 fatty acid, EPA, was also an inhibitor of TRPC5 (Figures 4F, G). Inhibition occurred independently of the type of TRPC5 activator because TRPC5 activity evoked by other, non-lanthanide, agonists was also inhibited (Figure 4H, I). Resolvin D1, an endogenous substance that is related to the dietary ω-3 fatty acids, had no effect when applied at the putative physiological concentration of 50 nmole/L (Fig 4J). TRPC1 and TRPC5 mix together to form a heteromultimeric channel that has different electrophysiological characteristics compared with TRPC5 alone, showing an almost linear I-V16. We therefore investigated if lino. inhibited the heteromultimeric channel. Figure 4K-M show that there was strong inhibition of co-expressed TRPC1-TRPC5.
Figure 4. Inhibition by dietary fatty acids.
Fura-2 intracellular Ca2+ (A, F-J) and whole-cell voltage-clamp (B-E) data from HEK 293 cells expressing TRPC5. A, Effect of 50 μmole/L α-linolenic acid (lino.) compared with vehicle control (Gd3+, 100 μmole/L) (N=4 each). B, Typical effect of 50 μmole/L lino. on ionic current evoked by 100 μmole/L Gd3+ at +80 and −80 mV. C, Mean data for the type of experiment shown in (B) (n=5). D, I-V for the effect of lino. shown in (B). E, Concentration-response data for lino. fitted with a Hill equation. Current was measured at −80 mV (n≥3 for each point). F, As for (A) but using EPA (N=4 each). G, As for (A) except mean data for inhibition of Gd3+ responses by 50 μmole/L lino., DHA and EPA (n=3 each). H, As for (A) except TRPC5 activity was evoked by 5 μmole/L LPC (N=4 each). I, Mean data for the type of experiment shown in (H) with LPC or 10 μmole/L S1P as the TRPC5 stimulator (n=3 each). J, As for (A) except mean data for the effect of 50 nmole/L resolvin-D1 (n/N=4/16 each). (K-M) Data from HEK 293 cells over-expressing TRPC5 plus TRPC1. K, Effect of 50 μmole/L lino. on ionic current evoked by 20 μmole/L La3+. L, Mean data for the type of experiment shown in (K) (n=5). M, I-V for the effect of lino. shown in (K).
The data suggest that the dietary ω-3 fatty acids lino., DHA and EPA inhibit the TRPC5 homomeric and TRPC1-TRPC5 heteromeric channels.
Inhibition of endogenous adipocyte channels by fatty acids
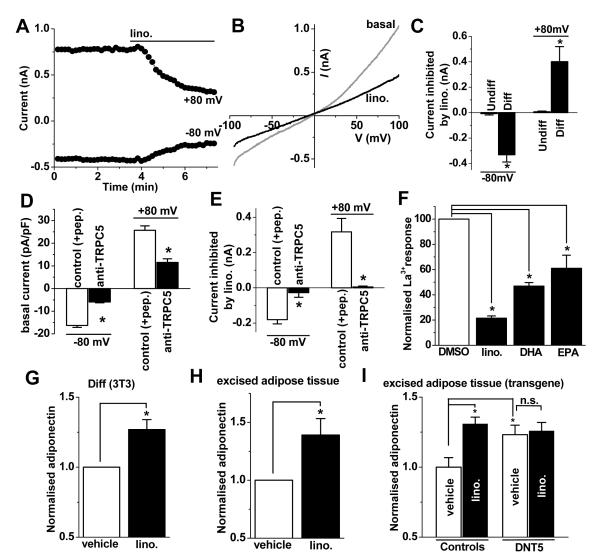
Whole-cell patch-clamp recording from differentiated 3T3-L1 cells revealed a constitutively-active ionic current that averaged about −300 pA at −80 mV (Figure 5A). The I-V of the inhibited current was similar to that of the TRPC1-TRPC5 heteromultimeric channels in HEK 293 cells (Figure 5B cf 4M). The current was inhibited by lino. in differentiated but not in undifferentiated 3T3-L1 cells (Figure 5C). Anti-TRPC5 antibody suppressed the constitutive ionic current and no effect of lino. was seen (Figure 5D, E), showing that the effect of lino. depended on the presence of functional TRPC5-containing channels. The dietary ω-3 fatty acids also inhibited La3+-evoked Ca2+-entry in differentiated 3T3-L1 cells (Figure 5F). The fatty acid profile of the Ca2+ signal was similar to that of over-expressed TRPC5 channels (Figure 5F cf 4G). Rosiglitazone-evoked Ca2+ entry in mouse adipocytes was also suppressed by lino. (Online Figure VIII). The data suggest that ω-3 fatty acids are inhibitors of endogenous TRPC1/TRPC5-containing channels of differentiated 3T3-L1 cells.
Figure 5. Inhibition of endogenous adipocyte channels by dietary factors.
Data arose from differentiated (A-G) or undifferentiated (C, Undiff) 3T3-L1 cells. A&B, Typical time-series graph (A) and I-Vs (B) showing 50 μmole/L lino. effects on constitutive ionic current. C-E, Mean constitutive (basal) current density (D) and inhibition of current by lino (C, E). The data of (C) were from the same experiments as those of Figure 2C. C, n=5 for each. D&E, Cells were pre-treated with T5E3 anti-TRPC5 antibody or the control of T5E3 preadsorbed to antigenic peptide (+pep) (n=4 for each group). F, Mean data for inhibition of La3+-evoked Ca2+ responses by 50 μmole/L lino., DHA or EPA (n=3 each). (G-I) Adiponectin secreted from 3T3-L1 cells (G) or adipose tissue excised from chow-fed wild-type (H) or transgenic (I) mice, showing effects of 50 μmole/L lino. compared with vehicle (DMSO). Transgenic mice expressed DNT5 or were single transgenic controls. All data groups are n=5.
Because lino. inhibited the TRPC channels we hypothesised that it should stimulate the production of adiponectin, consistent with prior reports22, 23. In support of this, lino. enhanced the generation of adiponectin by differentiated 3T3-L1 cells (Figure 5G) and adipose tissue excised from wild-type mice (Figure 5H). Strikingly, in excised adipose tissue from transgenic mice, lino. failed to enhance the generation of adiponectin if it had already been enhanced by DNT5 (Figure 5I). The data suggest that the capability of lino. to stimulate adiponectin production depended on its ability to suppress Ca2+ entry through TRPC5-incorporating channels.
Discussion
This study gives insight into a Ca2+ entry mechanism of adipocytes. Molecular components, TRPC1 and TRPC5, were up-regulated as mature adipocytes formed, leading to constitutively-active heteromeric Ca2+-permeable channels. The arising Ca2+ influx inhibited the generation of adiponectin, without effect on leptin. Most assays showed about 25 % increase in the generation of adiponectin when the TRPC channels were inhibited. While TRP channels in general have been found to be chemically-activated, the constitutive nature of the adipocyte channels conferred significance to chemical inhibition. Dietary ω-3 fatty acids were identified as inhibitors with strong relevance to adipocyte biology, metabolic syndrome, and cardiovascular disease. The findings of the study are summarised schematically in Online Figure IX.
TRPC1 and TRPC5 have multiple functions in addition to those in adipocytes, including roles in vascular and cardiac remodelling24, 25. Striking vascular up-regulation has been observed in metabolic syndrome, with protection conferred by exercise26. Channel activity has been shown to be stimulated acutely by factors associated with cardiovascular disease, such as oxidised phospholipids18. Therefore, suppression of adiponectin by TRPC channels may be part of a general effect of the channels as drivers or facilitators of inflammatory responses such as those occurring in the metabolic syndrome.
The fatty acids identified as TRPC inhibitors included the ω-3 polyunsaturated fatty acids that derive primarily from the diet. α-Linolenic acid is found mostly in vegetable oils, including those from rapeseed and soybean. DHA and EPA are in oily fishes that consume marine microorganisms. Depending on the diet, ω-3 fatty acids occur at plasma concentrations of 1-100 μmole/L20, 21, which would be sufficient to affect TRPC1/TRPC5-containing channels. Large-scale trials suggest that ω-3 fatty acids decrease the risk of major diseases or disease-related events, including coronary heart disease, insulin resistance, myocardial infarction, atrial fibrillation, and heart failure22, 27. ω-3 fatty acid therapy shows promise for disease prevention22, 28.
Our data suggest that ω-3 fatty acids elevate adiponectin substantially by acting through a mechanism that depends on TRPC1/TRPC5-containing channels. Molecular targets of ω-3 fatty acids are not, however, restricted to TRPC channels. They bind or indirectly affect PPAR-γ, the GPR120 receptor, voltage-dependent Na+ and Ca2+ channels, and TRPV1 channels29-31. The mechanism by which ω-3 fatty acids suppress TRPC channels has not been elucidated but it was not a transcriptional effect (because the effect occurred within a few minutes) and is unlikely to have occurred through GPR120 because this receptor couples via Gq/11, which stimulates TRPC channel activity31, 32. TRPV1 modulation by ω-3 fatty acids was suggested to occur via protein kinase C33, which inhibits TRPC534. Therefore, protein kinase C is a putative transduction mechanism. More direct effects are possible, although lipid effects on TRPC5 have previously been found to be stimulatory35. Intriguingly, the Drosophila TRP channel is activated directly by polyunsaturated fatty acids36; our data indicate that mammalian orthologues (i.e. TRPC1/TRPC5) are also sensitive to such fatty acids but that the functional consequence is the opposite (i.e. inhibition). Substantial sequence differences between the mammalian and Drosophila channels make it difficult to predict which residues are responsible for the reversal of polarity.
Fatty acid inhibitors of TRPC1-TRPC5 channels are predicted to oppose the adverse effects of TRPC channel activation in inflammation and cardiovascular disease. There may be additional inhibitory factors acting similarly on TRPCs, such as resveratrol, vitamin C, and gallic acid37 (Online Figure IX). These factors are exogenous to the body, suggesting that a general function of TRPC channels may be to enable coupling between external chemicals and the internal biology of the body. Previously studies have focused on TRP channels other than TRPCs as integrators of cells with external signals10.
The study used 3T3-L1 cells as a foundation, but data obtained using human tissue and mouse samples and through genetic manipulation in vivo supported the 3T3-L1 findings, and studies of over-expressed TRPCs supported the conclusion that the specified channel is a target of ω-3 fatty acids. There was technical difficulty in measuring intracellular Ca2+ in the mature adipocytes, but independent electrophysiological studies supported the data obtained with the fluo-4 Ca2+ indicator.
This study identified a Ca2+-permeable cationic channel (TRPC1/5) mechanism of adipocytes. Inhibition of the mechanism raised circulating adiponectin levels and would thus be expected to confer cardiovascular protection. Constitutive activity of the channels was significant, suggesting that inhibitors are likely to be important even in the absence of an activator. Novel inhibitors of the channels were identified (i.e. ω-3 fatty acids), adding to previously identified TRPC inhibitors which are associated with protection against major cardiovascular diseases.
Supplementary Material
Supplemental material
Novelty and Significance.
What Is Known?
- Adiponectin secreted by adipocytes confers protection against cardiovascular disease.
- Secretion from adipocytes is calcium regulated.
- TRPC proteins form calcium-permeable channels that promote cardiac and vascular smooth muscle cell hypertrophy, migration, and proliferation.
What New Information Does This Article Contribute?
- TRPC1 and TRPC5 form calcium-permeable channels in adipocytes.
- Inhibition of adipocyte TRPC channels increases the circulating concentration of adiponectin.
- ω-3 fatty acids inhibit adipocyte TRPC channels.
The calcium ion is arguably the most important intracellular messenger in mammalian cells. Therefore, many different types of calcium channel have evolved to control calcium entry into cells, depending on the context. A cell type in which we know almost nothing of these calcium systems is the adipocyte, which is important for storing fats and regulating the health of the cardiovascular system. Our study reveals a calcium channel in adipocytes that controls the generation of the cardiovascular protector, adiponectin. The study also reveals that dietary ω-3 fatty acids are inhibitors of the adipocyte TRPC channels, suggesting a mechanism by which they confer cardiovascular benefit. We initially identified the TRPC system through molecular and cell biology experiments in vitro but to explore the relevance in the living animal we generated a transgenic mouse for conditional inhibition of calcium permeation through the channels in vivo. Inhibition led to an increased concentration of adiponectin arising from adipose tissue and in the plasma. Up-regulation of adiponectin by ω-3 fatty acids was prevented by inhibition of the channels. The study reveals a previously unrecognised mechanism in adipocytes that is relevant to cardiovascular health. The results are consistent with the idea that inhibition of the channels confers protection against some of the major cardiovascular disease problems.
Acknowledgments
Sources of Funding The work was supported by the Wellcome Trust. PS was an Overseas and University of Leeds Research Scholar. LAW was supported by a BBSRC-AstraZeneca PhD Studentship.
Non-standard abbreviations and acronyms
TRPC
(Transient Receptor Potential Canonical)
HEK
(human embryonic kidney)
siRNA
(short-interfering RNA)
DNT5
(dominant negative TRPC5 mutant)
lino
(α-linolenic acid)
DHA
(docosahexaenoic acid)
EPA
(eicosapentaenoic acid)
S1P
(sphingosine-1-phosphate)
LPC
(lysophosphatidylcholine)
T1E3
(anti-TRPC1 blocking antibody)
T5E3
(anti-TRPC5 blocking antibody)
Footnotes
References
- 1.Trayhurn P, Wood IS. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 2.Smith CC, Yellon DM. Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharmacol Ther. 2011;129:206–219. doi: 10.1016/j.pharmthera.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 5.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Cammisotto PG, Bukowiecki LJ. Role of calcium in the secretion of leptin from white adipocytes. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1380–1386. doi: 10.1152/ajpregu.00368.2004. [DOI] [PubMed] [Google Scholar]
- 7.Worrall DS, Olefsky JM. The effects of intracellular calcium depletion on insulin signaling in 3T3-L1 adipocytes. Mol Endocrinol. 2002;16:378–389. doi: 10.1210/mend.16.2.0776. [DOI] [PubMed] [Google Scholar]
- 8.Jiang LH, Gamper N, Beech DJ. Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: Focus on TRPC5, TRPM2 and TRPA1. Curr Drug Targets. 2011;12:724–736. doi: 10.2174/138945011795378568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol. 2008;18:R880–889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 10.Vriens J, Nilius B, Vennekens R. Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol. 2008;6:79–96. doi: 10.2174/157015908783769644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- 13.Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Harmelen V, Rohrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism: clinical and experimental. 2004;53:632–637. doi: 10.1016/j.metabol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein RL, Hyun WC, Davis JH, Fulwyler MJ, Pershadsingh HA. Flow cytometric analysis of mature adipocytes. Cytometry. 1989;10:469–474. doi: 10.1002/cyto.990100416. [DOI] [PubMed] [Google Scholar]
- 16.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majeed Y, Bahnasi Y, Seymour VA, Wilson LA, Milligan CJ, Agarwal AK, Sukumar P, Naylor J, Beech DJ. Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels. Mol Pharmacol. 2011;79:1023–1030. doi: 10.1124/mol.110.069922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O’Regan D, Porter KE, Li J, Beech DJ. Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2010;30:1453–1459. doi: 10.1161/ATVBAHA.110.205666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell KJ, Fielding BA, Samra JS, Humphreys SM, Frayn KN. Release of individual fatty acids from human adipose tissue in vivo after an overnight fast. J Lipid Res. 1996;37:1842–1848. [PubMed] [Google Scholar]
- 21.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–1265. [PubMed] [Google Scholar]
- 22.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 23.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, Shimatsu A, Kuzuya H, Kamei Y, Ogawa Y. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27:1918–1925. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 24.Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards JM, Neeb ZP, Alloosh MA, Long X, Bratz IN, Peller CR, Byrd JP, Kumar S, Obukhov AG, Sturek M. Exercise training decreases store-operated Ca2+ entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc Res. 2010;85:631–640. doi: 10.1093/cvr/cvp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–146. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- 28.Belalcazar LM, Reboussin DM, Haffner SM, Reeves RS, Schwenke DC, Hoogeveen RC, Pi-Sunyer FX, Ballantyne CM. Marine omega-3 fatty acid intake: Associations with cardiometabolic risk and response to weight loss intervention in the look ahead (action for health in diabetes) study. Diabetes Care. 2010;33:197–199. doi: 10.2337/dc09-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng DD, Zhao YF, Luo ZQ, Keating DJ, Chen C. Linoleic acid induces Ca2+-induced inactivation of voltage-dependent Ca2+ currents in rat pancreatic beta-cells. J Endocrinol. 2008;196:377–384. doi: 10.1677/JOE-07-0426. [DOI] [PubMed] [Google Scholar]
- 30.Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, Gillum M, Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 31.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanki H, Kinoshita M, Akaike A, Satoh M, Mori Y, Kaneko S. Activation of inositol 1,4,5-trisphosphate receptor is essential for the opening of mouse TRP5 channels. Mol Pharmacol. 2001;60:989–998. doi: 10.1124/mol.60.5.989. [DOI] [PubMed] [Google Scholar]
- 33.Matta JA, Miyares RL, Ahern GP. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J Physiol. 2007;578:397–411. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkatachalam K, Zheng F, Gill DL. Regulation of Canonical Transient Receptor Potential (TRPC) channel function by diacylglycerol and protein kinase c. J Biol Chem. 2003;278:29031–29040. doi: 10.1074/jbc.M302751200. [DOI] [PubMed] [Google Scholar]
- 35.Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, Benham CD, Bateson AN, Muraki K, Beech DJ. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- 36.Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the drosophila light-sensitive channels TRP and TRPl. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 37.Naylor J, Al-Shawaf E, McKeown L, Manna PT, Porter KE, O’Regan D, Muraki K, Beech DJ. TRPC5 channel sensitivities to antioxidants and hydroxylated stilbenes. J Biol Chem. 2011;286:5078–5086. doi: 10.1074/jbc.M110.196956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material