Higher Levels of Neanderthal Ancestry in East Asians than in Europeans (original) (raw)
Abstract
Neanderthals were a group of archaic hominins that occupied most of Europe and parts of Western Asia from ∼30,000 to 300,000 years ago (KYA). They coexisted with modern humans during part of this time. Previous genetic analyses that compared a draft sequence of the Neanderthal genome with genomes of several modern humans concluded that Neanderthals made a small (1–4%) contribution to the gene pools of all non-African populations. This observation was consistent with a single episode of admixture from Neanderthals into the ancestors of all non-Africans when the two groups coexisted in the Middle East 50–80 KYA. We examined the relationship between Neanderthals and modern humans in greater detail by applying two complementary methods to the published draft Neanderthal genome and an expanded set of high-coverage modern human genome sequences. We find that, consistent with the recent finding of Meyer et al. (2012), Neanderthals contributed more DNA to modern East Asians than to modern Europeans. Furthermore we find that the Maasai of East Africa have a small but significant fraction of Neanderthal DNA. Because our analysis is of several genomic samples from each modern human population considered, we are able to document the extent of variation in Neanderthal ancestry within and among populations. Our results combined with those previously published show that a more complex model of admixture between Neanderthals and modern humans is necessary to account for the different levels of Neanderthal ancestry among human populations. In particular, at least some Neanderthal–modern human admixture must postdate the separation of the ancestors of modern European and modern East Asian populations.
Keywords: human evolution, Neanderthals, ancient admixture
NEANDERTHALS were a group of archaic hominins that occupied large parts of Europe and West Asia from ∼30,000 to 300,000 years ago (KYA) (Stringer and Hublin 1999; Hublin 2009). Their disappearance in the fossil record often coincides with the first appearance of anatomically modern humans (AMH) in that region (Finlayson 2004). Where, when, and how often Neanderthals interbred with expanding AMH populations is still an open question. Morphological studies have generally concluded that Neanderthals made little or no contribution to present-day human populations (Stringer and Andrews 1988; Lahr 1994), but others have suggested there was some admixture (Duarte et al. 1999; Trinkaus 2007). Initial comparisons of Neanderthal and modern human DNA found no evidence for a Neanderthal contribution to the modern human gene pool (Krings et al. 1997; Serre et al. 2004; Noonan et al. 2006). However, indirect studies of patterns of linkage disequilibrium (LD) in contemporary human populations have consistently found support for admixture between “archaic” human groups (such as Neanderthals) and modern humans (Garrigan et al. 2005a,b; Plagnol and Wall 2006; Wall et al. 2009; Hammer et al. 2011; Lachance et al. 2012).
A detailed analysis of a draft Neanderthal genome and five low-coverage (4×) human sequences estimated that Neanderthals made a 1–4% contribution to the gene pool of modern non-African populations (Green et al. 2010). The presence of “Neanderthal DNA” in East Asians and Melanesians was initially surprising because the archaeological record shows that Neanderthals and early modern humans coexisted only in Europe and western Asia. Green and colleagues hypothesized that Neanderthals and modern humans came into contact and interbred in the Middle East ∼50–80 KYA, prior to the divergence of modern-day European and Asian populations.
Green et al. (2010) presented three kinds of evidence in favor of interbreeding. First, they found (using _D_-statistics, a new measure of genetic similarity introduced in that article) that the three sampled non-African genome sequences (from a French, a Han Chinese, and a Papua New Guinean) are more similar to the Neanderthal sequence than is either of the two sampled African sequences (from a San and a Yoruban). Second, they identified several haplotypes that are in low frequency in Europeans, absent from Africans, and present in the Neanderthal sequence, which suggests those haplotypes were derived from Neanderthals. Third, they found many more genomic fragments in a European genome than in an African genome that have low divergence to the Neanderthal genome.
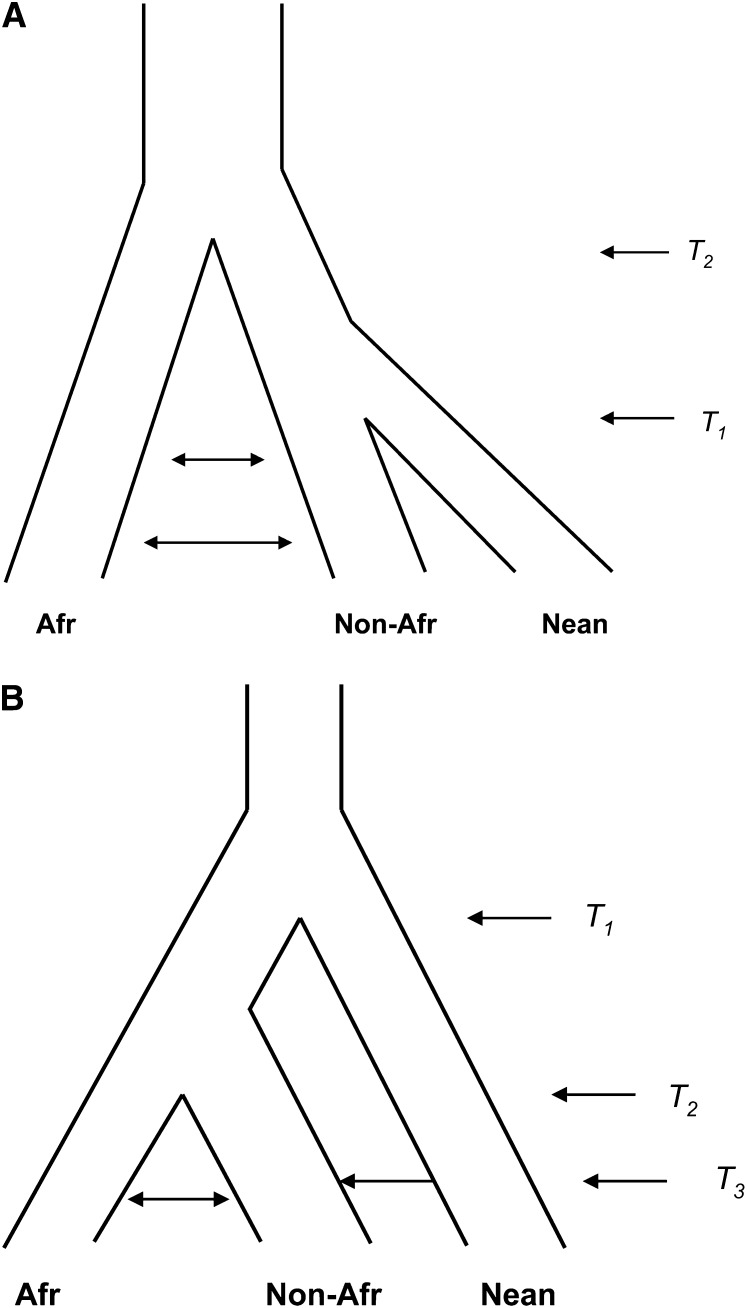
Admixture between modern humans and Neanderthals within the past 100,000 years (Kyr) is only one possible explanation for these _D_-statistic patterns. Green et al. noted that another potential explanation is ancient population subdivision within Africa before both Neanderthals and modern humans left Africa (cf. Green et al. 2010, figure 6). If there had been long-lived (e.g., >500 Kyr) population structure within Africa, and both Neanderthals and non-African AMH came from the same “source” subpopulation, then Neanderthals would be more similar to non-Africans in the absence of any recent admixture between AMH and Neanderthals (see Figure 1A). This intuitive argument was confirmed by the simulation studies of Durand et al. (2011) and Eriksson and Manica (2012), but these studies did not account for the other two lines of evidence summarized above. Two other studies have shown that the ancient-subdivision model is incompatible with other aspects of the data. Yang et al. (2012) demonstrated that recent admixture (Figure 1B) could be distinguished from ancient subdivision (Figure 1A) by computing the frequency spectrum of modern humans, conditioned on the Neanderthal sequence having the derived allele and an African sequence having the ancestral allele. This double conditioning enriches for alleles introduced by recent admixture if it occurred. Yang and colleagues found that the doubly conditioned frequency spectrum in Europeans and in East Asians is consistent with recent admixture, not with ancient subdivision. Separately, an analysis of the extent of LD at closely linked sites also concluded that the data were consistent with recent admixture and not with ancient subdivision (Sankararaman et al. 2012).
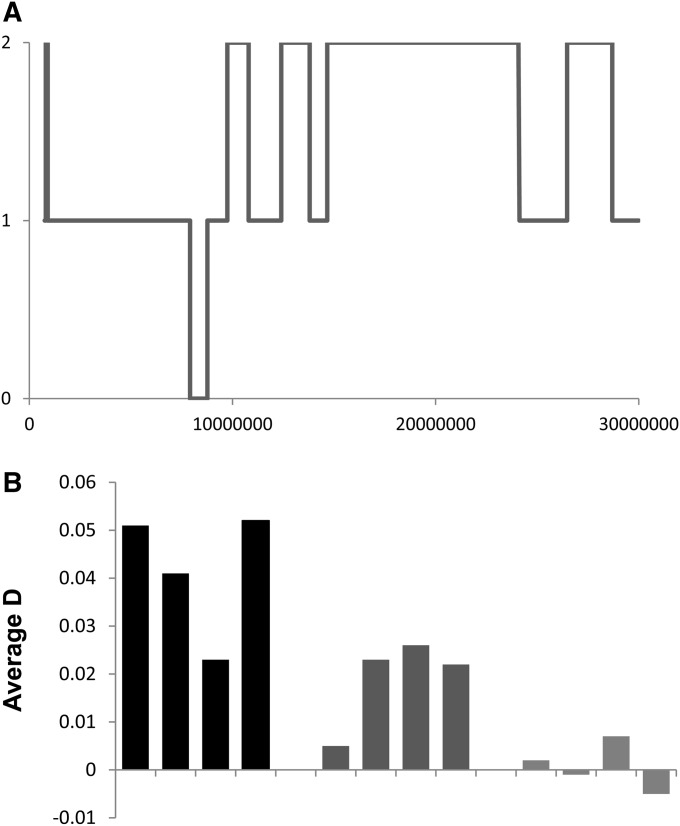
Figure 6.
Recent and ancient admixture in the Maasai. (A) Representative plot of the number of estimated “African” alleles across the first 30 Mb of chromosome 1 in one of the Maasai genomes. (B) Estimated values of D for portions of the genome estimated to contain zero, one, or two “non-African” alleles.
Figure 1.
Simplified versions of models of ancient population structure (A) or recent admixture (B) that can explain the observed levels of divergence between modern human genomes and the draft Neanderthal genome. Here T1 is the time when Neanderthals and modern humans first split, T2 is the time when African and non-African modern human populations split, and T3 is the time when Neanderthals mixed with modern humans.
In this study, we revisit the question of Neanderthal admixture, using an expanded data set of 42 high-coverage (>45×) modern human genomic sequences, and we take advantage of the recent high-coverage Denisova genome (Meyer et al. 2012) to obtain more refined estimates of admixture proportions. We use two complementary methods of analysis. One is the _D_-statistic method introduced by Green et al. (2010). _D_-statistics reflect site-by-site differences. Because we have multiple individuals from each of several populations, we can quantify the extent of variation in _D_-statistics among pairs of individuals from the same two populations and obtain greater statistical power by combining estimates among all pairs. The second method is an LD-based method similar to one introduced by Wall (2000) and Plagnol and Wall (2006) for identifying putatively introgressed regions in modern human genomes. We use the draft Neanderthal genome to identify segments in the modern human genome that were derived from admixture with Neanderthals. This method is similar to the one used by Green et al. (2010) but is less restrictive and allows quantification of the differences in the number of admixed segments in different populations.
Using both of these methods, we show there was more Neanderthal admixture into East Asian populations than into European populations. This conclusion is consistent with that of Meyer et al. (2012), which was based on the analysis of a smaller number of modern human sequences. By using the high-coverage Denisova genome, we are able to show that the admixture rate into East Asians is 40% higher than into Europeans. We conclude that admixture between Neanderthals and modern humans did not occur at a single time and place, as suggested by Green et al. (2010). Some of it had to have occurred after the separation of East Asians and Europeans. Further, we show that there was significant Neanderthal admixture into the Maasai population of East Africa, probably because of secondary contact with a non-African population rather than admixture directly from Neanderthals.
Materials and Methods
Complete genomics data
We downloaded data from 69 publicly available genome sequences from the Complete Genomics (CGI) website (http://www.completegenomics.com/public-data/). Complete Genomics sequenced a Yoruba (YRI) trio, a Centre d'Etude du Polymorphisme Humain (CEPH)/Utah (CEU) pedigree family of 17 family members, a Puerto Rican (PUR) trio, and a diversity panel from 10 different populations. Combining these data sets and using only nonrelated, nonadmixed individuals, we have a sample size of 42 individuals representing nine different populations (Table 1). In addition to 36 members of the diversity panel, we also used the parents from the YRI trio and the maternal and paternal grandparents in the CEU pedigree. The individual genomes were sequenced to a minimum 45-fold coverage (Drmanac et al. 2010). The eight populations are Utah residents with Northern and Western European ancestry from the CEPH collection (CEU); Han Chinese from Beijing, China (CHB); Gujarati Indians from Houston (GIH); Japanese from Tokyo (JPT); Luhya from Webuye, Kenya (LWK); Maasai from Kinyawa, Kenya (MKK); Toscani from Italy (TSI); and Yoruba from Ibadan, Nigeria (YRI). Samples from three other populations were also available from Complete Genomics, those of Mexican ancestry in Los Angeles (MXL), African-Americans from southwest Arizona (ASW), and Puerto Ricans from Puerto Rico (PUR), but these were excluded from our analysis because of recent intercontinental admixture. All genomic data were downloaded from Complete Genomics’ ftp site (ftp://ftp2.completegenomics.com/). We used two separate pipelines for filtering and processing the data, optimized for the different analyses performed (see below).
Table 1. Forty-two individual genome sequences from Complete Genomics included in our study.
| ID | Population | ID | Population |
|---|---|---|---|
| NA06985 | CEU | NA21732 | MKK |
| NA06994 | CEU | NA21733 | MKK |
| NA07357 | CEU | NA21737 | MKK |
| NA10851 | CEU | NA21767 | MKK |
| NA12004 | CEU | NA18940 | JPT |
| NA12889 | CEU | NA18942 | JPT |
| NA12890 | CEU | NA18947 | JPT |
| NA12891 | CEU | NA18956 | JPT |
| NA12892 | CEU | NA20502 | TSI |
| NA18526 | CHB | NA20509 | TSI |
| NA18537 | CHB | NA20510 | TSI |
| NA18555 | CHB | NA20511 | TSI |
| NA18558 | CHB | NA18501 | YRI |
| NA20845 | GIH | NA18502 | YRI |
| NA20846 | GIH | NA18504 | YRI |
| NA20847 | GIH | NA18505 | YRI |
| NA20850 | GIH | NA18508 | YRI |
| NA19017 | LWK | NA18517 | YRI |
| NA19020 | LWK | NA19129 | YRI |
| NA19025 | LWK | NA19238 | YRI |
| NA19026 | LWK | NA19239 | YRI |
_D_-statistic filtering
For the _D_-statistic analyses, each individual genome was aligned with the human genome assembly hg19 for consistency with the available assembly of the Neanderthal genome. Since our results were somewhat unexpected, we prepared the data for analysis in two different ways to check for consistency. We denote these analysis A and analysis B.
For analysis A, we used the release of the file format version 2.0 (software version 2.0.0.26) that was generated in September 2011. This version was mapped to the human reference genome hg19. We also downloaded the chimpanzee genome pantro2 aligned to hg19 from the University of California, Santa Cruz (UCSC) Genome Browser (http://hgdownload.cse.ucsc.edu/goldenPath/hg18/vsPanTro2/). The Neanderthal sequence was obtained by pooling reads from the three Vindija bones (SL Vi33.16, SL Vi33.25, and SL Vi33.26) that were aligned to the reference human genome (Green et al. 2010). The Neanderthal data were downloaded from the UCSC genome browser (http://genome.ucsc.edu/Neandertal/). To match the filtering used in the original Green et al. (2010) study, we used only sites with a mapping quality score (MAPQ) of at least 90 and a sequence quality >40. On average, the coverage of the Neanderthal genome was ∼1.3-fold. We kept only sites that had one, two, or three reads.
After filtering out any insertions, deletions, or ambiguously called sites in the Complete Genomics data, we merged them with the chimpanzee and Neanderthal genomes. We kept only sites that had no more than two alleles in any of the human genomes and at which alleles were called for each human, the chimp, and the Neanderthal. Furthermore, we considered only transversion differences.
We also obtained the high-coverage Denisova genome from Meyer et al. (2012). The genome was aligned to the human reference genome (hg19) and the average coverage was ∼30x. We filtered out all sites that had <16 reads or >46 reads. We merged these data with the data from analysis A to compute the _D_-statistic and _f_-statistic.
For analysis B, we redownloaded the genomic data from the Complete Genomics website (ftp://ftp2.completegenomics.com/, software version 2.0.2.15, file format version 2.0, February 2012). These sequences were aligned to hg18. We applied a less stringent filter of the Neanderthal data: the filtering for mapping quality and sequence quality remained the same as in analysis A, but there were no restrictions on the number of reads per site. Finally, instead of considering the chimp genome as the outgroup, we used the ancestral alleles defined by the 1000 Genomes Project from the Enredo-Pecan-Ortheus (EPO) pipeline (Paten et al. 2008a,b) (data downloaded from ftp://ftp.1000genomes.ebi.ac.uk/). We refer to this outgroup as the reconstructed common ancestor (RCA).
For samples from any two populations compared, we filtered out any insertions, deletions, or ambiguously called sites. These genomic samples were then merged with the Neanderthal genome and the RCA outgroup. This differs from analysis A, where all populations were merged with the Neanderthal, Denisova, and chimp genome prior to any comparisons between populations. We considered only sites where the difference between the ancestral allele from the RCA and the alternate allele is a transversion, as we did in analysis A.
LD-based analysis filters
Since the LD-based analyses primarily utilize patterns of extant genetic variation (and only secondarily use the draft Neanderthal genome), we aligned variant calls to the updated human genome assembly (hg19), included both transitions and transversions, and imposed more stringent filters to throw out repetitive regions. Specifically, a custom series of Perl/C scripts and cgatools v1.3.0.9 were used to get a common set of variants from each individual. Using the CGI’s variant file, all polymorphic regions containing SNPs were identified and reconstructed according to CGI’s descriptions. These regions were then filtered for SNPs in such a way that both alleles were known for a given individual and were not part of a complex variant (for example, a SNP on one haploid phase and a deletion on the other phase). We then pooled all unique SNP positions from the full panel of samples and removed all SNPs located within repeats and segmental duplications with a minimum size of 50 bp. Structural variants (dgv track on UCSC), self chain (identity <90%, UCSC self-chain track), segmental duplications (UCSC), microsatellites (UCSC), simple tandem repeats (UCSC), and repeat masked sequence (UCSC) were also excluded. The final list of SNPs was then used by CGI’s “snpdiff” tool to extract each sample’s base calls relative to the human reference genome (hg19, Build 37). The snpdiff output was then reformatted to ms, PLINK, and other text-based formats for further analyses.
Subsequently, we identified numerous regions where all/most individuals had heterozygous SNP calls but only one homozygous genotype was present. These regions likely reflect either alignment errors due to the Complete Genomics short-read sequencing technology or errors in the human reference genome sequence. We excluded all regions that included sites where over half of the individuals are heterozygous and only one homozygous genotype is present. The coordinates for these regions are available from the authors upon request.
Denisova sequence reads (Reich et al. 2010), mapped to the human reference genome hg18, were downloaded from the UCSC genome browser (http://genome.ucsc.edu/cgi-bin/hgTrackUi?db=hg18&c=chrX&g=bamSLDenisova). Consensus Neanderthal sequence generated from three bones and aligned to the human reference genome hg18 was downloaded from the Ensembl genome browser (http://neandertal.ensemblgenomes.org/data_info.html). Samtools 0.1.18 (Li et al. 2009) was used to convert the BAM files into a pileup alignment (mpileup arguments: -B -q5 -Q30) of each ancient hominin genome and hg18 for the region of interest. To compare modern human sequence tracks to ancient hominin sequences, hg19 coordinates of interest were converted to hg18 coordinates using the UCSC genome browser tool liftOver and extracted from the pileup alignments via custom perl scripts. To further compare the human sequences to sequences of other primate genomes, another custom perl script was used to extract the same hg19 coordinates of interest from a subset of the genomes in the UCSC MultiZ alignments found at http://hgdownload.cse.ucsc.edu/goldenPath/hg19/multiz46way/. Computations were performed using the University of California, San Francisco, Biostatistics High-Performance Computing System.
_D_-statistics and estimates of admixture rates
_D_-statistics, introduced by Green et al. (2010), are summary statistics for genome sequences from four populations. Two populations, _P_1 and _P_2, are compared to a test population, _P_3. The fourth population _P_4 is used as an outgroup to determine which allele is ancestral at each site. In our case, _P_4 is the chimpanzee reference sequence (pantro2) denoted by C, and _P_3 is the Neanderthal sequence, denoted by N. _P_1 and _P_2 are two human sequences. The chimp reference sequence is assumed to have the ancestral allele, denoted by A. D is computed only for sites at which both of the Neanderthal and one but not both of the human sequences have a different allele, assumed to be derived and denoted by B. That is, only those sites with configurations ABBA and BABA are used, where the order is _P_1, _P_2, _P_3, _P_4. The requirement that two copies of both the derived and the ancestral alleles be present greatly reduces the effect of sequencing error (Durand et al. 2011).
When only a single sequence from each population is available,
| D(P1,P2,P3,P4)=nABBA−nBABAnABBA+nBABA, | (1) |
|---|
where nABBA and nBABA are the numbers of sites with each of the two configurations. When diploid sequences from each individual from _P_1 and _P_2 are available, then
| D(P1,P2,P3,P4)=∑i(1−pi(1))pi(2)−∑pi(1)(1−pi(2))∑i(1−pi(1))pi(2)+∑pi(1)(1−pi(2)), | (2) |
|---|
where pi(1) and pi(2) are the frequencies of the derived allele (0, 0.5, 1) in the individual in _P_1 and _P_2, respectively at site i. Equation 2 is equivalent to sampling one of the chromosomes at random from _P_1 and _P_2 and then using Equation 1.
Green et al. (2010) and Durand et al. (2011) showed that the expected value of D is 0 if _P_1 and _P_2 form a clade and _P_3 is the outgroup. These articles also showed that if there was admixture from _P_3 into _P_2, then E(D) > 0. The magnitude of D depends on the admixture proportion f and on the population divergence times and various effective population sizes.
Reich et al. (2010) showed that if there is a sister group of _P_3, which we call _P_5, that has not admixed with _P_1, _P_2, or _P_3, then it is possible to estimate f directly. In our case, _P_5 is the Denisovan genome. To estimate f, we define S(P1,P2,P3,P4) to be the numerator of either Equation 1 or Equation 2. Then
| f^=S(P1,P2,P5,P4)S(P1,P3,P5,P4). | (3) |
|---|
The intuition behind this estimator is that the denominator quantifies the excess coalescent events that occur between lineages in _P_3 and _P_5 because they are sister groups. Lineages in _P_2 that are introduced by admixture have the same coalescent history as all lineages from _P_3. Hence, the ratio is the fraction of lineages in _P_2 that trace their ancestry to _P_3 because of admixture (Reich et al. 2010). In our application of this method, we are assuming that there is no admixture from Denisovans (_P_5) into the other populations (_P_1, … , _P_4). Although Skoglund and Jacobsson (2011) have argued that there was admixture from Denisovans into East Asians, our results described below did not find evidence of this admixture for the Han Chinese and Japanese samples we analyzed. For analysis A, we explored the variation in estimated _D_-statistics and admixture rates (f) for all pairs of individuals of different human populations. For analysis B, since we did not include the Denisova genome, we estimated only _D_-statistics.
Randomization tests
We computed D for each pair of individuals, both within populations and between populations. We developed two randomization tests of statistical significance. Both are similar to the Mantel test. Test 1 tests whether the average D computed for one pair of populations is significantly larger than for another pair, and test 2 tests whether the average D for a pair of populations differs significantly from 0.
For test 1, we start with sequences from three human populations, _G_1, _G_2, and _G_3, each containing _k_1, _k_2, and _k_3 diploid sequences. We compute two matrices of D values. The elements of _M_1 are D(_G_1,i, _G_3,j, N, C), where _G_1,i and _G_3,j are the _i_th and _j_th individuals in _G_1 and G3 (i = 1, … , _k_1; j = 1, … , _k_3). The elements of _M_2 are D(_G_2,i, _G_3,j, N, C). _M_1 has _k_3 rows and _k_1 columns, and _M_2 has _k_3 rows and _k_2 columns. From _M_1 and _M_2 the average _D_’s are computed, _D_1 and _D_2. The problem is to test whether _D_1 = _D_2. A _t_-test cannot be used because the elements within each matrix are not independent of each other and because the same reference population (_G_3) is used to compute both matrices. Instead, we combine _M_1 and _M_2 into a single matrix with _k_3 rows and _k_1 + _k_2 columns. Then we randomize the columns and compute _D_1 for the matrix containing the first _k_1 columns and _D_2 for the matrix containing the last _k_2 columns. Then we compare the observed _D_1 – _D_2 with the distribution of differences from the randomized matrices. We used a two-tailed test and 1 million replicates for each test.
Test 2 is similar to test 1, but because we compare only _G_1 and _G_2, a subset of one population is used in place of the reference population, _G_3. For the population with the larger sample size (say _G_1), we create a random partition (G1a,G1b) subject to the constraint that they differ in number by no more than one. For _M_1, we compute D for all pairs of individuals in G1a and _G_2. The elements of _M_2 are D(G1,ia,G1,jb,N,C), where G1,ia and G1,jb are the _i_th and _j_th individuals in the two subpopulations created by the partition. Test 1 is then applied to _M_1 and _M_2.
We also calculated the _f_-statistics for each pair of individuals. Using the same randomization tests as described above, we determined whether there were significant differences between populations in estimates of the admixture rate. Significant differences observed using the admixture rate suggest that the effect is truly due to the Neanderthal and not admixture with Denisovans.
Identifying putative archaic human regions
Previous work has shown that archaic admixture often leads to long, divergent haplotypes at low frequency (Wall 2000; Plagnol and Wall 2006). We define two SNPs to be “congruent” if their diploid allele counts (i.e., zero, one, or two counts of a particular allele) across individuals are completely correlated (i.e., _r_2 = 1). We define the maximum number of pairwise congruent SNPs to be _l_d and denote the collection of rarer (minor allele frequency ≤ 0.5) alleles at each of these pairwise congruent sites to be the putative archaic haplotype. From the filtered Complete Genomics data, we then identified all regions from 8 to 100 kb in length where _l_d ≥ 30 and _l_d/S ≥ 0.1, where S is the total number of polymorphic sites in the region. When identified regions overlapped, we took the region with the largest value of _l_d/S. We also required that neighboring regions with putative archaic haplotypes congruent with each other be separated by at least 200 kb, to avoid double counting long archaic haplotypes. A total of 2254 regions were identified. Of these, 411 were private to the non-African samples.
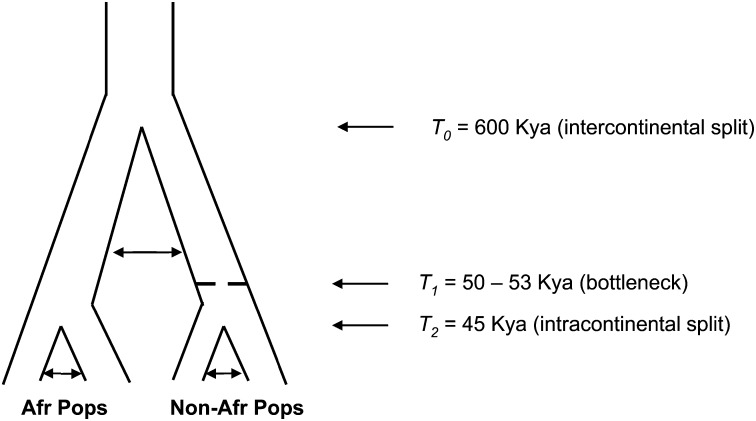
To estimate what proportion of these regions might be false positives, we simulated whole-chromosome sequence data (Chen et al. 2009) under a model that incorporated both recent (intracontinental) and ancient (intercontinental) population structure (Figure 2). Specifically, we assume a panmictic ancestral population split into two daughter populations at time T_0 = 0.6 (using the standard coalescent scaling of 4_N generations), with (symmetric) scaled migration rate of _M_0 = 5. At time _T_1 = 0.05 – 0.053, one of the ancestral populations (i.e., the “non-African” one) experiences a population bottleneck resulting in a 100-fold reduction in population size. Then, at time _T_2 = 0.045, each population splits into two descendant populations, connected by migration rate _M_1 = 8. While arbitrary, this model attempts to incorporate the major features of human demographic history, including intra- and intercontinental population structure and a bottleneck in the history of non-African populations, and is similar to the model used by Yang et al. (2012). The results described below are qualitatively similar if other plausible values for the times and migration rates are used (results not shown). Using N = 10,000 and an average generation time of 25 years, each unit of scaled time corresponds to 1 million years.
Figure 2.
Schematic of a model of recent and ancient population structure without admixture used in simulations. See text for details.
We simulated 30 different 100-Mb chromosomes, using the model described above with mutation parameter θ = 3.5 × 10−4/bp, recombination parameter ρ = 4 × 10−4/bp, and 10 individuals sampled from each of the four extant populations. The simulated number of segregating sites was substantially higher than the actual number in our filtered data. Since average _l_d values are positively correlated with levels of diversity, the simulated _l_d values are higher on average than expected in real data, and our choice of θ is conservative. Also, standard estimates of ρ are generally higher than the value we took (Myers et al. 2005), which is also conservative for our purposes. We then tabulated the total number of regions with _l_d ≥ 30, _l_d/S ≥ 0.1, and with divergent haplotype SNPs private to the simulated non-African samples. We identified a total of 3 regions that satisfied these criteria, compared with 411 regions that were identified from the actual data. This leads to an estimate of a false discovery rate of q < 0.01.
Identifying putative Neanderthal regions
To identify which of the 2254 regions described above were likely to reflect recent Neanderthal admixture, we imposed the following additional criteria on the putative archaic human haplotypes:
- The Neanderthal allele must be called at ≥12 SNPs and match the putative archaic haplotype at ≥70% of these SNPs.
- The Neanderthal allele and the chimp allele must be called at ≥8 SNPs and the Neanderthal allele must be derived (relative to chimp) at ≥60% of these sites.
- The putative archaic haplotype must be at low frequency (<5%) in the sub-Saharan African samples.
The motivation for criterion 1 is obvious, and we note that a more stringent cutoff was not used due to the poor quality of the Neanderthal genome sequence. Criterion 2 was implemented to cut down on regions that reflect shared ancestral polymorphism between modern humans and Neanderthals; it is based on an observation of Noonan et al. (2006) that recent Neanderthal admixture will lead to an increase in SNPs where Neanderthals have the derived allele. Finally, criterion 3 reflects our prior belief that admixture with Neanderthals did not occur in Africa and that the presence of Neanderthal alleles in Africa could reflect only more recent migration patterns. A total of 226 regions were identified that meet these additional criteria. We note in passing that the specific cutoffs used in criteria 1–3 are somewhat arbitrary, but our qualitative conclusions are unchanged under a range of similar criteria (results not shown).
We implemented a simple permutation test to assess the statistical significance of the observed difference in frequencies of Neanderthal regions in East and South Asians and Europeans. Specifically, we kept the presence/absence of Neanderthal regions for each individual constant and randomly permuted the geographic label (i.e., “European” vs. “East Asian”) of the sample 100,000 times. Similar analyses were used to compare the frequency of Neanderthal regions in Maasai vs. other sub-Saharan African samples.
Identifying putative Denisovan regions
Excluding the 226 Neanderthal regions identified above, we screened the remaining 2028 putative archaic regions for Denisovan admixture, using the same criteria as for Neanderthals. Thirty total regions fit these criteria.
Estimating local ancestry in the Maasai
We took the filtered Complete Genomics data described at the start of this section and estimated SNP allele frequencies separately in the 13 European samples and the 13 non-Maasai African samples. These were used as proxies for the (unknown) non-African and African ancestral populations. We then included only those SNPs with allele frequencies that differ by at least 0.3 in our analyses. We calculated the likelihood of each ancestral configuration (i.e., zero, one, or two alleles inherited from the non-African population) separately for each SNP. Then, over sliding windows of 1 Mb, we formed a composite likelihood by multiplying together all of the single-SNP likelihoods contained in the window and tabulated which ancestral configuration had the highest (composite) likelihood. For each SNP, we then used majority rule to make ancestry calls, using all windows containing the SNP in question. See Wall et al. (2011) for further details.
Results
_D_-statistics and estimates of f
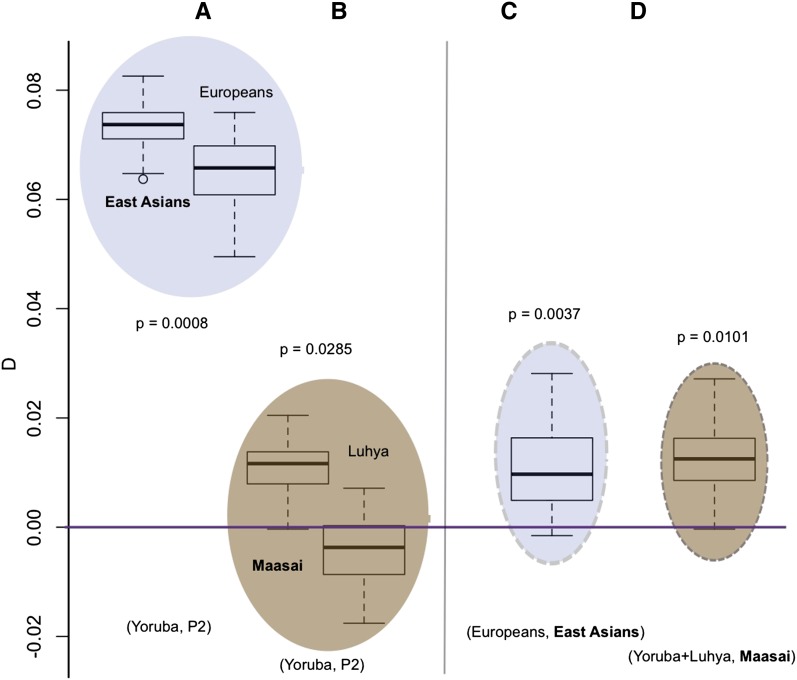
The _D_-statistics and estimates of f we computed are summarized in Figure 3 and Supporting Information, File S1, Table S1, Table S2, Table S3, Table S4, Table S5, Table S6, Table S7, Table S8, Table S9, Figure S1, Figure S2, Figure S3, Figure S4, Figure S5, Figure S6, Figure S7, and Figure S8. Several features of the results are notable. First, we find evidence for more Neanderthal admixture into the East Asian samples than into the European samples (P = 0.001)—consistently higher D values result when East Asians are compared to one of the African populations than when Europeans are compared (Figure 3A, Table S4), and the average D is positive when East Asians are compared to Europeans (Figure 3C, Table S5). In analysis B, comparisons with the South Asian samples are intermediate with respect to the European and East Asian samples but not in analysis A, indicating that the South Asian sample differs from the East Asian ones but the degree of similarity to Europeans remains to be established. Also, we find evidence for a small but significant amount of Neanderthal admixture into the Maasai genomes (P ∼ 0.03, Table S4). When compared to the Yoruba, the Maasai have a higher average D than the Luhya (Figure 3B, Table S4). When the Maasai are compared to all other African samples, the average D is positive (Figure 3D). In addition, when East Asians and Europeans are compared to the Maasai, the average _D_’s are somewhat lower than when they are compared to either the Yoruba or the Luhya. The _P_-values shown in Figure 3, A and B are from test 1 and those in Figure 3, C and D are from test 2.
Figure 3.
Summary of significance tests for average values of D. Positive values indicate that the second sequence is more similar to the Neanderthal genome than the first sequence. In all parts, the box plots indicate the range of D values obtained for pairs of individuals from the populations indicated. A and B are box plots of individual _D_-statistics computed for each individual from the specified population compared with each Yoruban. _P_-values are from the randomization test, test 1, of significant differences in the average D values for different pairs of populations. C and D show box plots of individual _D_-statistics computed for every pair of individuals in the specified populations. _P_-values are from the randomization test, test 2, of significant differences of the average D from 0. See also Table S2.
Table S1, Table S2, and Table S3 show estimated values of f. The estimates of the admixture rate show that when we incorporate the Denisovan genome into our analysis, the admixture rate between East Asians and Neanderthals remains significantly higher than the admixture rate between Europeans and Neanderthals (P ∼ 0.001, Table S7). The Maasai remain significantly more genetically similar to the Neanderthals when compared to the Luhya (P ∼ 0.03, Table S7), but the observed significant difference for the _D_-statistic when comparing the Maasai and the Yoruba is not observed for the _f_-statistic (P ∼ 0.34, Table S7), which probably reflects the lower power of using f as a test statistic. The admixture rates for the South Asians give the same results as those for the _D_-statistic (Table S9).
Identifying “Neanderthal haplotypes”
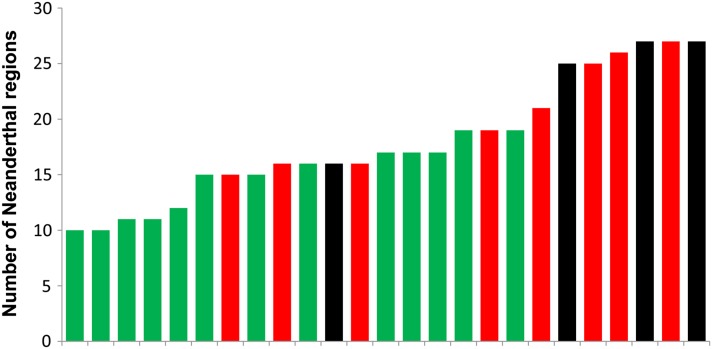
Our new method for identifying introgressed Neanderthal fragments in human populations detected 226 different putative Neanderthal regions. The relative frequencies of these putative Neanderthal haplotypes in the 42 sampled modern human individuals then provide estimates of the relative contributions of Neanderthal DNA to the gene pools of contemporary human populations. We found that on average the “Neanderthal haplotypes” were at higher frequency in the East Asians than in the Europeans (9.6% vs. 6.4%; P = 3.0 × 10−4, permutation test), consistent with the _D_-statistic results presented in Figure 3 (Figure 4). We also found evidence for a small, but statistically significant, Neanderthal contribution to the genomes of the Maasai (P = 4.9 × 10−4), but did not find a significant difference in Neanderthal haplotype frequency between the East Asian and South Asian samples (P > 0.05).
Figure 4.
Distribution of the number of putative Neanderthal regions for each Eurasian individual. European genomes are colored in green, East Asian genomes are colored in red, and South Asian genomes are colored in black.
Additional test of ancient population structure
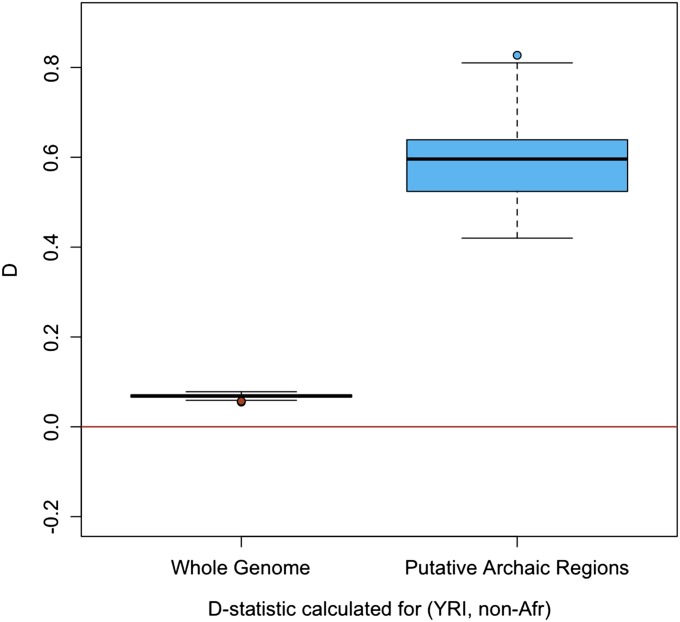
As reviewed in the Introduction, there is already evidence against the hypothesis that the extra similarity of non-African populations to Neanderthals is accounted for by ancient population subdivision. To explore this point further, we took the 411 regions from our whole-genome analyses that were identified purely on the basis of their LD patterns (i.e., without using any information from the Neanderthal genome sequence). Then, for each non-African individual, we calculated the _D_-statistic for those regions where the individual contained a rare, diverged haplotype. If this haplotype were recently inherited from Neanderthals, we would expect the D values to be strongly positive. If instead there were no recent admixture between modern humans and Neanderthals, then there is no a priori reason why these regions would show D values significantly different from 0. Recombination acting over the past 300 Kyr would break up local patterns due to shared ancestral polymorphisms to scales <0.01 cM (i.e., <10 kb on average). The D values that we observe are strongly positive (average D = 0.594, compared with an average D = 0.068 for the whole genome), providing additional evidence that most of the unusual haplotypes from these 411 regions are indeed the result of recent introgression from the Neanderthal gene pool (P << 10−8, Figure 5).
Figure 5.
Box plot showing the average D across the whole genomes of the non-African individuals compared with the average D (for the same individuals) across regions identified as having unusual patterns of LD (i.e., putative archaic regions).
Identifying “Denisovan haplotypes”
Excluding the 226 Neanderthal regions described above, we used the same criteria to identify regions likely inherited from Denisovans. We identified a total of 30 regions, all at low frequency, with no significant difference in frequency between populations.
Maasai admixture
Previous genetic studies have suggested that the Maasai may be an admixed population with a substantial proportion of non-African ancestry (Henn et al. 2011). If the non-African ancestry were due to recent (i.e., post-Neanderthal) admixture, then the observation of Neanderthal ancestry in the Maasai would not be unexpected. Alternatively, spatially explicit models of ancient population structure might explain the greater similarity between Maasai and Neanderthals relative to other sub-Saharan African groups (A. Manica, personal communication). One difference between these alternative explanations is what they predict about the patterns of similarity across the genomes of Maasai individuals. Under a model of recent admixture, we expect Maasai genomes to show large, distinct blocks of sequence with different genetic patterns, corresponding to blocks with non-African vs. African ancestry. The average size of the non-African blocks (in morgans) is roughly the inverse of the time (in generations) since admixture. In contrast, under a model of ancient admixture the similarity of Maasai genomes to the Neanderthal genome will be spread throughout the genome because the admixture happened much longer ago.
To distinguish between these two possibilities, we employed a composite-likelihood–based approach to identifying African and non-African regions of ancestry across the genomes of the four Maasai samples (Wall et al. 2011). Briefly, we used the European (CEU and TSI) and other African (YRI and LWK) samples (Table 1) to estimate allele frequencies in non-African and African ancestral populations and then estimated the number of alleles inherited from each ancestral population at each SNP in the genome. These extant samples may not be perfect proxies for the true ancestral populations, but the qualitative results presented below are likely to be valid.
In summary, we estimate an average of ∼30% non-African ancestry in each Maasai genome, and the sizes of the ancestral blocks are consistent with admixture that happened ∼100 generations ago (Figure 6A). We then partitioned each Maasai genome into regions with zero, one, or two inferred African alleles and calculated D separately for each partition. We found that the D values are significantly more negative with increasing numbers of inferred non-African alleles (P = 2.0 × 10−4; Figure 6B). This observation provides strong support for recent non-African gene flow into the Maasai, with the non-African alleles bringing with them low levels of Neanderthal ancestry.
Discussion
Our results confirm and reinforce several conclusions about admixture between Neanderthals and the ancestors of modern humans. Using a much larger number of high-coverage genome sequences than were previously analyzed for this purpose and using two complementary methods of analysis (_D_-statistics and detection of introgressed Neanderthal segments), we confirm the conclusion of Meyer et al. (2012) that East Asians (Han Chinese and Japanese) are more similar to the published Neanderthal sequence than are Europeans. Because we have analyzed more modern human sequences than Meyer et al. (2012) did, we are able to show the extent of variation within both Asian and African populations. We also confirm the conclusions of Yang et al. (2012) and Sankararaman et al. (2012) that the similarity of both Europeans and East Asians to Neanderthals is the result of recent admixture and not ancient population subdivision. Finally, we used the high-coverage Denisova sequence of Meyer et al. (2012) to determine that the admixture rate (f) into East Asians is ∼40% higher than into Europeans.
We were not able to confirm the conclusion of Skoglund and Jakobsson (2011) that there was Denisovan admixture into East Asians. We did not detect any difference in the number of apparent Denisovan segments in Europeans and East Asians. The East Asian genomes analyzed, however, were from northern East Asia (Beijing and Tokyo), not from southern East Asia where Skoglund and Jakobsson found the strongest signal of admixture with Denisovans.
Our results and those of Meyer et al. (2012) imply that the relatively simple admixture scenario proposed by Green et al. (2010) needs to be altered. At least two separate episodes of admixture between Neanderthals and modern humans must have occurred, and at least one of those episodes must have occurred after the separation of the ancestors of modern Europeans and East Asians. Rather than have two distinct episodes of admixture, it seems more plausible that admixture took place over a protracted period 50–80 KYA. During that period the ancestors of Europeans diverged and subsequently experienced less admixture than the ancestors of East Asians. This scenario is consistent with the simulation models of Currat and Excoffier (2011) and Skoglund and Jakobsson (2011).
If this scenario is correct, the time of separation of the ancestors of modern European and East Asian populations is constrained. Since there is no archaeological record of Neanderthals in the past ∼30 Kyr, it follows that the separation of Europeans from East Asians had to have occurred before Neanderthals went extinct. Consequently, estimates of East Asian–European population divergence of <30 KYA (Gutenkunst et al. 2009; Gravel et al. 2011) are unlikely to be correct. This timeframe is also supported by a 40- to 50-KYA modern human fossil recently found in China (Fu et al. 2013).
Our two analyses yielded slightly different results for the Gujarati (South Asian) samples. However, it would not be surprising if the true level of Neanderthal ancestry in South Asians was intermediate between Europeans and East Asians because previous studies have shown gradients in genetic ancestry across Eurasia (Rosenberg et al. 2002).
Our finding of Neanderthal admixture into the Maasai was initially surprising, given the lack of evidence that Neanderthals ever crossed into Africa or that the ancestors of the Maasai were ever in the Middle East. Although direct contact between the two groups in the past is theoretically possible, our results are more consistent with a scenario involving recent admixture between the ancestors of the Maasai and one or more (historically) non-African groups with Neanderthal ancestry several thousand years ago. This interpretation is broadly consistent with recent findings of African admixture into Middle Eastern and Southern European populations during the same timescale (Moorjani et al. 2011) and a greater genetic similarity between East African and non-African samples than between West African and non-African samples (Tishkoff et al. 2009). Together these studies provide additional support for the hypothesis that admixture between genetically diverged groups is a common feature of human demographic history.
The new picture of human and Neanderthal ancestry that emerges from our results is almost certainly not complete, and our results suggest that intracontinental variation in levels of Neanderthal ancestry may be common. With the current rate of progress in whole-genome sequencing and the possibility of additional draft genomes from specimens of archaic individuals, we will soon learn more about the admixture process. In particular, the construction of “archaic admixture maps” detailing the distribution of archaic DNA segments in different modern human populations will help us to infer the timing, locations, and exact numbers of introgression events and the role that archaic admixture may have played in the evolution of the AMH genome.
Supplementary Material
Supporting Information
Acknowledgments
This work was supported in part by National Institutes of Health grants R01-GM40282 (to M.S.), R01-HG005226 (to J.D.W. and M.F.H.), and T32 HG 00047 (training grant to M.A.Y.), as well as by National Science Foundation Graduate Research Fellowship Program Division of Graduate Education grant 1106400 (to M.A.Y.).
Footnotes
Communicating editor: A. Di Rienzo
Literature Cited
- Chen G. K., Marjoram P., Wall J. D., 2009. Fast and flexible simulation of DNA sequence data. Genome Res. 19: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currat M., Excoffier L., 2011. Strong reproductive isolation between humans and Neanderthals inferred from observed patterns of introgression. Proc. Natl. Acad. Sci. USA 108: 15129–15134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drmanac R., Sparks A. B., Callow M. J., Halpern A. L., Burns N. L., et al. , 2010. Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science 327: 78–81. [DOI] [PubMed] [Google Scholar]
- Duarte C., Mauricio J., Pettitt P. B., Souto P., Trinkaus E., et al. , 1999. The early Upper Paleolithic human skeleton from the Abrigo do Lagar Velho (Portugal) and modern human emergence in Iberia. Proc. Natl. Acad. Sci. USA 96: 7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E. Y., Patterson N., Reich D., Slatkin M., 2011. Testing for ancient admixture between closely related populations. Mol. Biol. Evol. 28: 2239–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson A., Manica A., 2012. Effect of ancient population structure on the degree of polymorphism shared between modern human populations and ancient hominins. Proc. Natl. Acad. Sci. USA 109: 13956–13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson C., 2004. Neanderthals and Modern Humans: An Ecological and Evolutionary Perspective. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Fu Q., Meyer M., Gao X., Stenzel U., Burbano H. A., et al. , 2013. DNA analysis of an early modern human from Tianyuan Cave, China. Proc. Natl. Acad. Sci. USA 110: 2223–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigan D., Mobasher Z., Kingan S. B., Wilder J. A., Hammer M. F., 2005a Deep haplotype divergence and long-range linkage disequilibrium at xp21.1 provide evidence that humans descend from a structured ancestral population. Genetics 170: 1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigan D., Mobasher Z., Severson T., Wilder J. A., Hammer M. F., 2005b Evidence for archaic Asian ancestry on the human X chromosome. Mol. Biol. Evol. 22: 189–192. [DOI] [PubMed] [Google Scholar]
- Gravel S., Henn B. M., Gutenkunst R. N., Indap A. R., Marth G. T., et al. , 2011. Demographic history and rare allele sharing among human populations. Proc. Natl. Acad. Sci. USA 108: 11983–11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. E., Krause J., Briggs A. W., Maricic T., Stenzel U., et al. , 2010. A draft sequence of the Neandertal genome. Science 328: 710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutenkunst R. N., Hernandez R. D., Williamson S. H., Bustamante C. D., 2009. Inferring the joint demographic history of multiple populations from multidimensional SNP frequency data. PLoS Genet. 5: e1000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M. F., Woerner A. E., Mendez F. L., Watkins J. C., Wall J. D., 2011. Genetic evidence for archaic admixture in Africa. Proc. Natl. Acad. Sci. USA 108: 15123–15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn B. M., Gignoux C. R., Jobin M., Granka J. M., Macpherson J. M., et al. , 2011. Hunter-gatherer genomic diversity suggests a southern African origin for modern humans. Proc. Natl. Acad. Sci. USA 108: 5154–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hublin J. J., 2009. Out of Africa: modern human origins special feature: the origin of Neandertals. Proc. Natl. Acad. Sci. USA 106: 16022–16027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings M., Stone A., Schmitz R. W., Krainitzki H., Stoneking M., et al. , 1997. Neandertal DNA sequences and the origin of modern humans. Cell 90: 19–30. [DOI] [PubMed] [Google Scholar]
- Lachance J., Vernot B., Elbers C. C., Ferwerda B., Froment A., et al. , 2012. Evolutionary history and adaptation from high-coverage whole-genome sequences of diverse African hunter-gatherers. Cell 150: 457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahr M. M., 1994. The multiregional model of modern human origins - a reassessment of its morphological basis. J. Hum. Evol. 26: 23–56. [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Kircher M., Gansauge M. T., Li H., Racimo F., et al. , 2012. A high-coverage genome sequence from an archaic Denisovan individual. Science 338: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorjani P., Patterson N., Hirschhorn J. N., Keinan A., Hao L., et al. , 2011. The history of African gene flow into Southern Europeans, Levantines, and Jews. PLoS Genet. 7: e1001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S., Bottolo L., Freeman C., McVean G., Donnelly P., 2005. A fine-scale map of recombination rates and hotspots across the human genome. Science 310: 321–324. [DOI] [PubMed] [Google Scholar]
- Noonan J. P., Coop G., Kudaravalli S., Smith D., Krause J., et al. , 2006. Sequencing and analysis of Neanderthal genomic DNA. Science 314: 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paten B., Herrero J., Beal K., Fitzgerald S., Birney E., 2008a Enredo and Pecan: genome-wide mammalian consistency-based multiple alignment with paralogs. Genome Res. 18: 1814–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paten B., Herrero J., Fitzgerald S., Beal K., Flicek P., et al. , 2008b Genome-wide nucleotide-level mammalian ancestor reconstruction. Genome Res. 18: 1829–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagnol V., Wall J. D., 2006. Possible ancestral structure in human populations. PLoS Genet. 2: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich D., Green R. E., Kircher M., Krause J., Patterson N., et al. , 2010. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 468: 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. A., Pritchard J. K., Weber J. L., Cann H. M., Kidd K. K., et al. , 2002. Genetic structure of human populations. Science 298: 2381–2385. [DOI] [PubMed] [Google Scholar]
- Sankararaman S., Patterson N., Li H., Paabo S., Reich D., 2012. The date of interbreeding between Neandertals and modern humans. PLoS Genet. 8: e1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre D., Langaney A., Chech M., Teschler-Nicola M., Paunovic M., et al. , 2004. No evidence of Neandertal mtDNA contribution to early modern humans. PLoS Biol. 2: E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P., Jakobsson M., 2011. Archaic human ancestry in East Asia. Proc. Natl. Acad. Sci. USA 108: 18301–18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C. B., Andrews P., 1988. Genetic and fossil evidence for the origin of modern humans. Science 239: 1263–1268. [DOI] [PubMed] [Google Scholar]
- Stringer C. B., Hublin J., 1999. New age estimates for the Swanscombe hominid, and their significance for human evolution. J. Hum. Evol. 37: 873–877. [DOI] [PubMed] [Google Scholar]
- Tishkoff S. A., Reed F. A., Friedlaender F. R., Ehret C., Ranciaro A., et al. , 2009. The genetic structure and history of Africans and African Americans. Science 324: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkaus E., 2007. European early modern humans and the fate of the Neandertals. Proc. Natl. Acad. Sci. USA 104: 7367–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., 2000. Detecting ancient admixture in humans using sequence polymorphism data. Genetics 154: 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Lohmueller K. E., Plagnol V., 2009. Detecting ancient admixture and estimating demographic parameters in multiple human populations. Mol. Biol. Evol. 26: 1823–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Jiang R., Gignoux C., Chen G. K., Eng C., et al. , 2011. Genetic variation in Native Americans, inferred from Latino SNP and resequencing data. Mol. Biol. Evol. 28: 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. A., Malaspinas A. S., Durand E. Y., Slatkin M., 2012. Ancient structure in Africa unlikely to explain Neanderthal and non-African genetic similarity. Mol. Biol. Evol. 29: 2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information