The epidemiology of clinical malaria among African children (original) (raw)
. Author manuscript; available in PMC: 2013 May 14.
Abstract
There is a resurgence of interest in the clinical epidemiology of malaria among African children. This renewed interest follows fifty years of failure to eradicate infection in Africa and redirected efforts toward disease control and prevention. We have a poor understanding of the mechanisms by which clinical immunity is acquired; however, several recent studies have provided new insights into how fast clinical protection is acquired under the varied transmission intensities common to Africa. What is clear is that the frequency with which individuals encounter infection from birth will determine the speed with which they become clinically immune and the patterns of severe pathology they are likely to experience. There remains doubt and concerns over the long-term consequences of reducing natural parasite exposure in several areas of Africa. New field studies are urgently required to tackle these issues so that control may be guided by an improved understanding of malaria as a disease that can lead to death.
Keywords: Malaria, Africa, Child, Immunity, Control, Review
Introduction
Recent debate over the long-term health consequences of interventions which aim to reduce Plasmodium falciparum exposure among endemic populations in Africa has received considerable attention in the scientific and the popular press. This reflects both the public-health significance of the proposed effects and the realization that the scientific community has yet to adequately define the relationship between parasite exposure and disease outcomes. Over the last ten years there has been a renaissance in the study of malaria as a disease that can lead to death. Previously our epidemiological understanding of malaria was dominated by studies of host and vector parasite infection. These studies continued despite the general acceptance that eradication had failed in Africa and that the new goals focused on “disease control”.
Evidence of changing risks of clinical malaria and malaria-specific mortality with increasing intensity of parasite exposure
Sub-Saharan Africa is able to support a wide range of malaria vector ecologies which result in a diverse pattern of basic reproduction rates of infection. Furthermore, it has been observed that parasite prevalence curves among populations exposed to different intensities of transmission will be similar in shape but shifted to the left or the right at higher or lower transmission pressures, respectively [1]. Under stable conditions of P. falciparum transmission, immunity to the fatal consequences of infection develops rapidly compared with an individual’s ability to control infection per se. Until recently there were few empirical observations of the patterns of clinical disease under different intensities of parasite exposure from birth. Defining the immunological components of clinical protection has proved difficult [2]. Most immunological markers examined under field conditions have shown no or weak associations with clinical protection [3]. An alternate, but non-specific, approach toward an epidemiological understanding of immunity is the direct observation of changing disease risk by age.
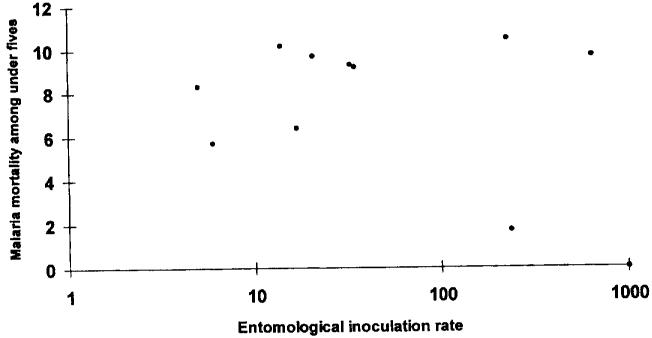
An attempt to examine the relationship between the risks of childhood malaria mortality and increasing transmission intensity was undertaken through the abstraction of all the available published and unpublished literature from community-based studies of disease-specific mortality conducted in Africa [4]. These studies were then related to estimates of annual sporozoite inoculation rates derived from entomological studies conducted within the same locality (fig. 1). Despite the intense controversy subsequent to the publication of these data [5-8] their principal purpose was to (1) highlight the paucity and inadequacy of existing data on such an important relation in malaria epidemiology; (2) indicate that mortality probably rises sharply under low rates of parasite transmission, after which it may form a plateau, rise or decline under increasing transmission intensity; and (3) emphasize the significance of this relationship to attempt to define appropriate control strategies for Africa.
Fig. 1.
Possible relationships between malaria-specific mortality among children under five years and the entomological inoculation rate (derived from studies in Africa) [4].
Several authors have chosen to examine this relationship using more sensitive markers of disease outcome. At one end of the disease continuum, a study of parasite densities among children living in a high-transmission area of Kenya showed that increased frequency of exposure to infected mosquitoes was related to an increasing risk of high parasite densities, although the authors were unable to distinguish whether this simply reflected increasing risks of superinfection [9]. Trape and Rogier [10] studied the lifetime frequency of mild malaria morbidity (fever associated with a qualifying parasite density) among two populations in Senegal exposed to markedly different annual sporozoite inoculation rates. Their study suggested that individuals living under different transmission settings experience similar rates of lifetime morbidity risk despite different patterns of age-specific risk. The protective mechanisms which operate to limit the risks of mild, transitory illness may be different from those which limit the risks of a fatal outcome given the differences in age patterns between severe and mild disease under similar transmission systems [11]. Two studies have assessed the entomological inoculation rate in relation to the incidence of severe disease within defined communities [12, 13]. Despite several limitations, neither of these studies demonstrated a linear rise in disease risk with increasing intensity of sporozoite inoculation.
In a similar approach to that taken in Senegal for mild disease, we selected two populations in East Africa to examine the risks of severe morbidity [14]. At Kilifi, on the Kenyan coast, the annual entomological inoculation rate is approximately 10 infectious bites per person, whereas at Ifakara, Tanzania entomological inoculation rates are 30 times higher. In this study the annual risk of admission to hospital with a primary diagnosis of malaria was similar between the two childhood communities (0-4 years) surrounding their respective district hospitals (46 per 1,000 children per annum (p.a.) in Kilifi compared with 51 per 1,000 children p.a. at Ifakara). Similarities persisted when the risks of admission among older children were included (26 per 1,000 children aged 0-9 years p.a. at Kilifi versus 27 per 1,000 children aged 0-9 years p.a. at Ifakara). However, the overall risks of malaria admission, particularly those due to severe anaemia, were considerably higher among infants living in the high-transmission setting compared with their counterparts living under low-to-moderate-transmission conditions. Conversely, cerebral malaria was much rarer under conditions of high transmission.
The studies described above suffer from a series of problems. First, studies of disease and infection risk within a single contiguous geographical area are unable to control for host mobility between exposure settings [9, 12, 13]. Furthermore, the wide areas selected for the passive case surveillance of severe disease at Ifakara [14] were not representative of estimates of infection risk derived from one area of the drainage population. Secondly, even within stable transmission settings in Africa there exists large, interannual variation in infection and disease risk, and studies conducted over single years are unlikely to capture these, often marked, year-to-year variations. And thirdly, defining parasite exposure through entomological studies requires exhaustive sampling over a wide geographic area for a minimum period of one year. Such studies are rare [4] and despite their rigor yield only an approximation of infection risk, given the inherent difficulties of effective sampling under low transmission [15]. There also remains the traditionally observed discrepancies between estimated sporozoite challenge and the emergence of blood-stage infection [16]. These limitations were recently addressed in a further series of ecological comparisons of severe malaria morbidity presenting to hospitals from a wide range of endemicities common to sub-Saharan Africa [17].
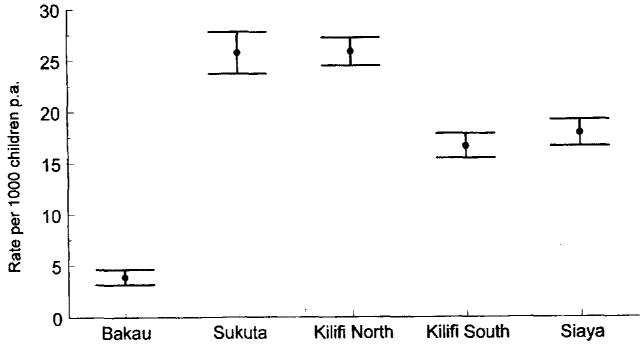
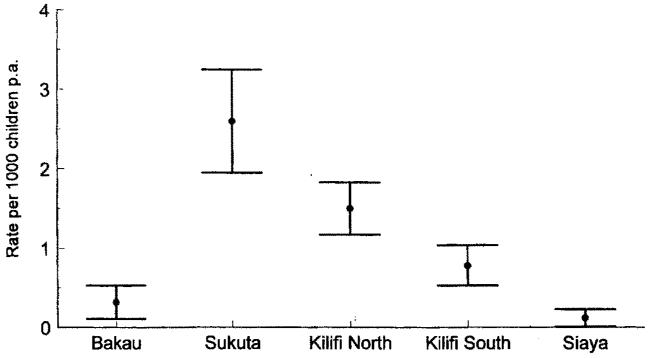
Prospective paediatric admission surveillance was maintained over 3-5 years at hospitals in The Gambia and Kenya situated next to five distinct populations with markedly different intensities of P. falciparum transmission [17]. The intensity of transmission was estimated using parasitological and serological markers of infection among infants and children aged 1-9 years sampled across the study communities. Infection risks ranged from 0.01 to 2.1 new infections p.a. Under the most intense levels of P. falciparum transmission (Kilifi south and Siaya, Kenya), the risks of developing severe disease were highest during the first two years of life, after which the risks declined rapidly. By contrast, communities which encountered low-to-moderate rates of P. falciparum challenge (Sukuta, The Gambia and Kilifi north, Kenya) experienced a much more extended period of risk in childhood, lasting from the first year of life through to the 5th birthday. At the lowest end of the transmission spectrum, disease risk appeared to be spread evenly across the entire childhood period (Bakau, The Gambia). The total disease risk for children below 10 years of age is shown in figure 2. These data do not support the notion that the higher the natural parasite exposure risk from birth, the higher the disease risk throughout childhood; on the contrary, they suggest a decline in overall malaria rates in childhood under the highest levels of P. falciparum challenge. Only at Bakau, where the highest mean and median ages of disease presentation and the lowest levels of P. falciparum transmission were recorded, did the risk of disease in childhood remain low. This paradoxical decline in disease risk under high transmission was much more pronounced for cerebral malaria, which was notable by its rarity under high transmission (fig. 3) — an observation made in other studies [14, 18].
Fig. 2.
Total rates of severe, clinical malaria among children aged 0-9 years at Bakau, The Gambia (low transmission), Sukuta, The Gambia (low-to-moderate transmission), Kilifi north, Kenya (low-to-moderate transmission), Kilifi south, Kenya (high transmission) and Siaya, Kenya (high transmission) [17].
Fig. 3.
Total rates of cerebral malaria among children aged 0-9 years at Bakau, The Gambia (low transmission), Sukuta, The Gambia (low-to-moderate transmission), Kilifi north, Kenya (low-to-moderate transmission), Kilifi south, Kenya (high transmission) and Siaya, Kenya (high transmission) [17].
Among the communities where haemoglobin levels were routinely recorded on all admissions (Kilifi north, Kilifi south and Siaya), there was little evidence that the absolute risks of severe malaria anaemia were higher the higher the intensity of transmission. This observation contrasts with those made between Kilifi north and Ifakara [14] and between two communities in Malawi [18]. Perhaps one explanation lies with the recent detailed quantification of the role of iron deficiency anaemia at Ifakara, which accounts for 28% of severe anaemia among infants [19], most of whom will be infected with P. falciparum because of the high prevalence of asymptomatic infection. The net result may be that severe “malaria” anaemia is overdiagnosed at the district hospital. Anaemia in malaria-endemic areas is a complex issue, and to interpret the true patterns of severe malaria anaemia probably requires a detailed understanding of the community-acquired competing risks for anaemia. Whilst it is convenient to consider severe malaria as being either cerebral malaria or severe malaria anaemia, it is important to recognize that there exists a myriad of potentially fatal pathologies in falciparum malaria whose end states may involve coma, anaemia or respiratory distress, but the pathways to these conditions are varied [20, 21].
Several authors have argued that the patterns of malaria morbidity shown in figures 2 and 3 could have arisen through differential use of the clinical services [22, 23]. This was examined through estimates of the risk of admission from other major causes of admission (acute respiratory tract infections, diarrhoeal-related admissions, meningitis and malnutrition), none of which showed any evidence of differences in cumulative risks by age between the sites. Furthermore, community survey data on the proportions of childhood deaths which occurred at home or in hospital were similar at each study site. Both observations are probably a direct result of the unique selection of each community in terms of its proximity to secondary-level clinical services: never further than 19 km away and served with regular local transport to and from the hospitals. These data add further support for the patterns of malaria admission risk being directly attributable to differences in transmission intensity. Nevertheless, these data were derived from facility-based passive surveillance and one cannot exclude the possibility that a very different pattern for mortality may be observed in the communities served by these hospitals.
Possible biological mechanisms of changing disease risks under different parasite exposure intensities
Epidemiological descriptions of disease risk provide an important framework for the understanding of mechanisms of protection. Similarly, without a biological basis for these observations the descriptions themselves lack plausibility. Host/parasite interactions are complex in semi-immune populations, involving genetic regulation of disease severity, parasite polymorphisms and nutritional and social susceptibility factors to disease progression. Against this background an individual’s ability to acquire mechanisms that render him or her clinically immune will depend upon their individual lifetime experiences with the parasite. The most plausible explanation for the patterns of clinical malaria described above is that a given amount of exposure is required to develop effective functional immunity. When infection rates are high, these experiences occur early in life, furnishing the child with an acquired resistance to the consequences of infection, A range of putative immunological and physical protective mechanisms conferring clinical protection during early infancy have been described, including maternally derived IgG [24], foetal haemoglobin [25] and micronutrient deficiency [26]; however, their relative contribution remains unclear. Nevertheless, it is striking how little epidemiological evidence there is on the degree and duration of any such protection in relation to clinical disease during this period of life. Further investigations on risks of clinical disease during infancy among the communities described above provide quantifiable evidence of clinical protection up to the third month of life [27]. Results from longitudinal morbidity studies in Ghana have demonstrated similar periods of clinical protection despite early parasitological exposure [28]. During this period of life active immunization occurs, reducing the size of the susceptible cohorts during the remaining period of infancy who continue to undergo active immunization. Under high rates of P. falciparum challenge there is also evidence that they already begin to demonstrate clinical immunity before the first birthday not apparent among infant populations exposed to low-to-moderate-intensity transmission [27].
Children exposed to very low risks of parasite exposure probably develop very little clinical immunity, but this is likely to be balanced by the inherent low risks of infection. Consequently disease risk is a direct function of parasite exposure, and this direct relation continues throughout life which, under stable conditions, would never achieve a cumulative risk seen among populations exposed to moderate or high transmission, because the host would not live long enough. However, under such conditions significant changes in the interannual parasite exposure could lead to apparent epidemics of clinical disease among all age-groups, as demonstrated in the “fringe” areas transmission in East Africa [29].
Consequences for malaria control
There appears to be a consensus view that the age patterns and clinical spectrum of disease are defined by the intensity of transmission within a given community [30]. Agreement on these points alone allows significant progress toward rational disease control. An understanding of the variations in endemicity between communities might influence the cost-effectiveness of a variety of interventions including targeted chemoprophylaxis or vaccine delivery, the provision of adequate peripheral services for the rapid treatment of early signs of cerebral malaria or specialist training in symptom-driven algorithms for diagnosis.
Perhaps the most significant implications from the observations presented above involve the potential effects of delaying the acquisition of clinical immunity among populations previously exposed to high transmission and the reequilibration of disease risk among older ages. The observed pattern of disease among high- versus low-to-moderate-transmission intensity communities suggests that there may be a rise in disease risk if repeated and early exposure to parasites is reduced. Insecticide-treated bed nets (ITBN) have been tested under a wide range of transmission settings [31-34], and the reductions in all-cause mortality, whilst less significant under the highest transmission settings [32, 34], are all consistent with a short-term gain under all transmission conditions. The recent epidemiological observations alone do not argue against implementing ITBN or chemoprophylaxis in these communities but argue strongly for intensive monitoring of the long-term consequences upon cumulative disease and risk following their introduction. Presently there is too little data to develop a rational strategy for targeted prevention of parasite exposure in Africa. This also applies to two hypothetical arguments proposed for the advantages associated with reducing parasite exposure and thus increasing the average age of first exposure: (1) reductions in “indirect” mortality and (2) age dependency.
Molineaux [34] argues that the reductions in malaria parasite exposure will significantly impact upon other competing causes of death in childhood to provide indirect benefits not solely attributed to malaria-specific mortality. This view derives from the observations that (1) under eradication conditions in Guyana and Sri Lanka during the first half of this century, all-cause mortality declined in parallel with markers of malaria morbidity; (2) studies of malaria-specific interventions have shown reductions in non-malaria mortality, as judged by verbal autopsy; and (3) there is an apparent disparity in mortality attributed to malaria through verbal autopsy findings, on the one hand, and demographic estimates of the global burden of malaria mortality recently calculated by Murray and Lopez [35], and the frequency of sickle cell trait among African populations, on the other hand.
It is to be expected that both long-term social and economic change will impact upon malaria and non-malaria mortality with increased knowledge, availability and access to effective treatment. It may be hard, therefore, to disassociate the malaria-specific effects of a large community intervention from the general effects. However, any argument based on verbal autopsy assessment of cause of death must be suspect because of the demonstrated inability to differentiate malaria from other conditions [36, 37]. Several pieces of evidence argue against the idea that specific antimalarial interventions lead to reductions in other causes of death. The studies cited by Molineaux include two studies in The Gambia which demonstrated a 24% [38] and 34 % [31] reduction in non-malaria mortality as judged by verbal autopsies during randomized controlled trials of chemoprohylaxis with pyrimethamine-dapsone (Maloprim). However, these same studies respectively demonstrated a rise of 70 and 14% in the incidence of fevers not associated with P. falciparum infection. Greenwood and colleagues [39] subsequently studied the impact of Maloprim chemoprophylaxis upon reported and observed symptoms of severe respiratory tract infections and acute gastroenteritis and concluded that there was no evidence that malaria plays a direct or indirect role in either cause of morbidity. Similarly, a community randomized controlled trial of insecticide-treated bed nets on the Kenyan coast was unable to show any significant impact upon the risks of hospitalization with respiratory tract, diarrhoeal or nutritional related conditions despite a 40 % reduction in the risks of malaria admission [33] following a 50% reduction in the risks of infection within the community [40]. The indirect consequences of reducing parasite exposure remain a plausible hypothesis which deserves further investigation; however, the present data do not support the notion that malaria infection plays a major role in determining the risks of non-malaria mortality, whilst the data perhaps are further confirmation of the inadequacy of verbal autopsy diagnosis [36, 37].
Several authors have argued that the risks of severe or fatal outcomes following first parasite exposures decrease with increasing age [7, 30]. Baird observed that non-immune adult migrants into a malaria-endemic area of Indonesia developed anti-parasitic immunity much quicker than their accompanying children [41]. A recent study in a high-transmission area of Tanzania [19] which provides conclusive evidence that chemoprophylaxis prevented over 60% of clinical episodes of malaria during the first year of life also showed a doubling of risk of clinical malaria during the second year of life when chemoprophylaxis was withheld. However, in support of the age-dependency claim, the authors suggest that the clinical events during the second year of life were less severe and involved lower geometric mean parasite densities. Unfortunately, the authors were unable to measure the risks of cause-specific admission or mortality given the size of the trial. Against the possibility that malaria severity lessens with age are recent reports of the severity of disease among non-immune migrants in Indonesia, where it appears that adults (aged > 15 years) have a 4.5 higher risk of cerebral malaria associated with first clinical attacks compared with children (< 10 years) (Baird, personal communication). These discrepancies in age-dependent risks of parasite density regulation versus severe pathology require further investigation through much larger and longer duration studies as described by Menendez [19] and among non-immune communities living under epidemic conditions in Africa.
Conclusions and recommendations
There is a general agreement that the intensity of parasite exposure will define the speed with which clinical immunity is acquired and the subsequent pathologies of severe, life-threatening disease a community is likely to experience. There is also a consensus view on the short-term health impact of insecticide-treated bed nets or chemoprophylaxis. However, disagreement prevails over the long-term consequences of reducing parasite exposure upon the development of acquired clinical immunity. Ironically fifty years ago the malaria research community was equally divided over the consequences of widespread use of DDT in hyperendemic areas of tropical Africa [42-44]. Today the debate is strengthened by empirical evidence from field-based studies of disease risk but appears to be no less controversial. Immunological mechanisms of disease protection, the significance of innate protection early in life, the relative contribution of P. falciparum infection as a risk for other causes of paediatric mortality and the risks of fatal outcomes on first encounters with infection later in life remain poorly defined. These features of the epidemiology of malaria’s health impact require further community-based investigation. It is widely accepted that health policy must be evidence-based, and to this end, the challenge is to examine carefully what limited evidence already exists and to identify ways in which unresolved questions may be tackled through appropriate field research.
Acknowledgements
The authors are grateful to the Wellcome Trust for their support as part of their senior fellowships programme (RWS: 033340 and KM: 631342).
References
- [1].Molineaux L, Gramiccia G. The Gharki project: research on the epidemiology and control of malaria in the Sudan savanna of West Africa. W. H. O.; Geneva: 1980. [Google Scholar]
- [2].Marsh K. Malaria — a neglected disease? Parasitology. 1992;104:S53–S69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- [3].Molineaux L. Plasmodium falciparum malaria: some epidemiological implications of parasite and host diversity. Ann. Trop. Med. Parasitol. 1997;90:379–393. doi: 10.1080/00034983.1996.11813067. [DOI] [PubMed] [Google Scholar]
- [4].Snow RW, Marsh K. Will reducing Plasmodium falciparum transmission alter malaria mortality among African children? Parasitol. Today. 1995;11:188–190. doi: 10.1016/0169-4758(95)80025-5. [DOI] [PubMed] [Google Scholar]
- [5].Lengeler C, Smith TA, Armstrong Schellenberg J. Focus on the effect of bednets on malaria morbidity and mortality. Parasitol. Today. 1997;13:123. doi: 10.1016/s0169-4758(97)84870-3. [DOI] [PubMed] [Google Scholar]
- [6].D’Alessandro U, Coosemans M. Concerns on long-term efficacy of an insecticide-treated bed net. Parasitol. Today. 1997;13:124. doi: 10.1016/s0169-4758(97)84871-5. [DOI] [PubMed] [Google Scholar]
- [7].Greenwood BM. Malaria transmission and vector control. Parasitol. Today. 1997;13:90–92. doi: 10.1016/s0169-4758(97)01002-8. [DOI] [PubMed] [Google Scholar]
- [8].Trape JF. Which strategy for malaria control in Africa? Parasitol. Today. 1997;13:125–126. [Google Scholar]
- [9].McElroy PD, Beier JC, Oster CN, et al. Predicting the outcome in malaria: correlation between rate of exposure to infected mosquitoes and level of Plasmodium falciparum parasitaemia. Am. J. Trop. Med. Hyg. 1994;51:523–532. [PubMed] [Google Scholar]
- [10].Trape JF, Rogier C. combating malaria morbidity and mortality by reducing transmission. Parasitol. Today. 1996;12:236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- [11].Snow RW, Marsh K. New insights into the epidemiology of malaria relevant to disease control. Brit. Med. Bull. 1998 doi: 10.1093/oxfordjournals.bmb.a011689. in press. [DOI] [PubMed] [Google Scholar]
- [12].Trape JF, Quinet MC, Nzingoula S, et al. Malaria and urbanization in Central Africa : the example of Brazzaville. Part V : Pernicious attacks and mortality, Trans. R. Soc. Trop. Med. Hyg. 1987;81(suppl 2):34–42. doi: 10.1016/0035-9203(87)90475-5. [DOI] [PubMed] [Google Scholar]
- [13].Mbogo CNM, Snow RW, Khamala CPM, et al. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at 9 sites on the Kenyan Coast. Am. J. Trop. Med. Hyg. 1995;52:201–206. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- [14].Snow RW, Bastos de Azevedo I, Lowe B, et al. Severe childhood malaria in two areas of markedly different falciparum malaria transmission in East Africa. Acta Trop. 1994;57:289–300. doi: 10.1016/0001-706x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- [15].Dye C, Hasibeder G. Mosquito-borne disease dynamics: control of flies that bite some people more than others. Trans. R. Soc. Trop. Med. Hyg. 1986;80:69–77. doi: 10.1016/0035-9203(86)90199-9. [DOI] [PubMed] [Google Scholar]
- [16].MacDonald G. The analysis of malaria parasite rates in infants. Trop. Dis. Bull. 1950;47:915–938. [PubMed] [Google Scholar]
- [17].Snow RW, Omumbo JA, Lowe B, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- [18].Slutsker L, Taylor TE, Wirima J, Steketee RW. In-hospital morbidity and mortality due to malaria-associated severe anaemia in two areas of Malawi with different patterns of malaria infection. Trans. R. Soc. Trop. Med. Hyg. 1994;88:548–551. doi: 10.1016/0035-9203(94)90157-0. [DOI] [PubMed] [Google Scholar]
- [19].Menendez C, Kahigwa E, Hirt R, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxsis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–850. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- [20].Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children: clinical spectrum and simplified prognostic criteria. N. Engl. J. Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- [21].English M, Wariuru C, Amukoye E, et al. Deep breathing reflects acidosis and is associated with poor prognosis in children with severe malaria and respiratory distress. Am. J. Trop. Med. Hyg. 1996;55:521–524. doi: 10.4269/ajtmh.1996.55.521. [DOI] [PubMed] [Google Scholar]
- [22].D’Alessandro U. Severity of malaria and level of Plasmodium falciparum transmission. Lancet. 1997;349:636–637. doi: 10.1016/S0140-6736(97)26031-9. [DOI] [PubMed] [Google Scholar]
- [23].Lines J. Severe malaria in children and transmission intensity. Lancet. 1997;350:813. doi: 10.1016/S0140-6736(05)62608-6. [DOI] [PubMed] [Google Scholar]
- [24].McGregor IA. The passive transfer of human immunity. Am. J. Trop. Med. Hyg. 1965;13:237–239. doi: 10.4269/ajtmh.1964.13.237. [DOI] [PubMed] [Google Scholar]
- [25].Gilles HM. The development of malarial infection in breast-fed Gambian infants. Ann. Trop. Med. Parasitol. 1957;51:58–62. doi: 10.1080/00034983.1957.11685795. [DOI] [PubMed] [Google Scholar]
- [26].Bates CJ, Prentice AM, Paul AA, Prentice A, Sutcliffe BA, Whitehead RG. Riboflavin status in infants born in rural Gambia and effect of weaning food supplement. Trans. R. Soc. Trop Med. Hyg. 1982;76:253–258. doi: 10.1016/0035-9203(82)90291-7. [DOI] [PubMed] [Google Scholar]
- [27].Snow RW, Nahlen B, Palmer A, Donnelly CA, Gupta S, Marsh K. Risks of severe malaria among African infants: direct evidence of clinical protection during early infancy. J. Infect. Dis. 1997 doi: 10.1086/517818. in press. [DOI] [PubMed] [Google Scholar]
- [28].Wagner G, Koram K, McGuinness D, Bennett S, Nkrumah F, Riley E. High incidence of asymptomatic malaria infections in children under 1 year of age in Ghana, detected by multicopy gene polymerase chain reaction. Am. J. Trop. Med., Hyg. 1998 doi: 10.4269/ajtmh.1998.59.115. in press. [DOI] [PubMed] [Google Scholar]
- [29].Garnham PCC. Malaria epidemics at exceptionally high altitudes in Kenya. Brit. Med. J. 1945;ii:45–47. [PubMed] [Google Scholar]
- [30].Molineaux L. Malaria and mortality: some epidemiological considerations. Ann. Trop. Med. Parasitol. 1997;91:811–825. doi: 10.1080/00034989760572. [DOI] [PubMed] [Google Scholar]
- [31].Alonso PL, Lindsay SW, Armstrong JRM, et al. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet. 1991;337:1499–1502. doi: 10.1016/0140-6736(91)93194-e. [DOI] [PubMed] [Google Scholar]
- [32].Binka FN, Kujabe A, Aduik M, et al. Impact of permethrin impregnated bednets on child mortality in Kassena-Nankana district, Ghana: a randomised controlled trial. Trop. Med. Int. Health. 1996;1:147–154. doi: 10.1111/j.1365-3156.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- [33].Nevill CG, Some ES, Mung’ala VO, et al. Insecticide-treated bed nets reduce mortality and severe morbidity from malaria among children on the Kenyan coast. Trop. Med. Int. Health. 1996;1:139–146. doi: 10.1111/j.1365-3156.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- [34].Habluetzel A, Diallo DA, Esposito F, et al. Do insecticide-treated curtains reduce all-cause child mortality in Burkina Faso? Trop. Med. Int. Health. 1997 doi: 10.1046/j.1365-3156.1997.d01-413.x. in press. [DOI] [PubMed] [Google Scholar]
- [35].Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- [36].Snow RW, Armstrong JRM, Forster D, et al. Childhood deaths in Africa: uses and limitations of verbal autopsies. Lancet. 1992;350:351–356. doi: 10.1016/0140-6736(92)91414-4. [DOI] [PubMed] [Google Scholar]
- [37].Todd JE, De Francisco A, O’Dempsey TJD, Greenwood BM. The limitations of verbal autopsy in a malaria endemic region. Ann. Trop. Paed. 1994;4:31–36. doi: 10.1080/02724936.1994.11747689. [DOI] [PubMed] [Google Scholar]
- [38].Greenwood BM, Greenwood AM, Bradley AK, Snow RW, Hayes RJ, N’jie ABH. Comparison of two strategies for control of malaria within a primary health care programme in The Gambia. Lancet. 1988;1:1121–l127. doi: 10.1016/s0140-6736(88)91949-6. [DOI] [PubMed] [Google Scholar]
- [39].Greenwood BM, Byass P, Greenwood AM, et al. Lack of association between acute gastroenteritis, acute respiratory infections and malaria in young Gambian children. Trans. R. Soc. Trop. Med. Hyg. 1989;83:595–598. doi: 10.1016/0035-9203(89)90364-7. [DOI] [PubMed] [Google Scholar]
- [40].Snow RW, Molyneux CS, Warn PA, et al. Infant parasite rates and IgM seroprevalence as a measure of exposure to Plasmodium falciparum during a randomised controlled trial of insecticide-treated bed nets on the Kenyan Coast. Am. J. Trop. Med. Hyg. 1996;55:144–149. [PubMed] [Google Scholar]
- [41].Baird JK. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol. Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- [42].World Health Organisation . Report of the malaria conference in Equatorial Africa. Vol. 38. W. H.O.; 1950. pp. l–72. Tech. Rep. Ser. [PubMed] [Google Scholar]
- [43].Wilson DB, Garnham PCC, Swellenberg NH. A review of hyperendemic malaria. Trop. Dis. Bull. 1950;47:677–698. [PubMed] [Google Scholar]
- [44].Garnham PCC. Malarial immunity in Africans: effects in infancy and early childhood. Ann. Trop. Med. Parasitol. 1949;43:47–61. doi: 10.1080/00034983.1949.11685394. [DOI] [PubMed] [Google Scholar]