Phase I Trial of Bevacizumab Plus Escalated Doses of Sunitinib in Patients With Metastatic Renal Cell Carcinoma (original) (raw)
Abstract
Purpose
Both bevacizumab and sunitinib target the vascular endothelial growth factor pathway and demonstrate activity against advanced renal cell carcinoma (RCC). In this phase I study, the maximum-tolerated dose (MTD) and safety of sunitinib in combination with bevacizumab were examined in patients with advanced RCC.
Patients and Methods
Three cohorts of three to six patients were treated with escalated doses of daily oral sunitinib (ie, 25 mg, 37.5 mg, 50 mg) for 4 weeks followed by a 2-week break and with fixed doses of bevacizumab (10 mg/kg) intravenously once every 2 weeks. Dose-limiting toxicities (DLTs) were assessed during the first cycle to determine the MTD, and an expanded cohort was treated to obtain additional safety information.
Results
Of 26 study participants, 25 received treatment at one of three dose levels. Grade 4 hemorrhage, identified as a DLT, occurred in one patient in each of cohorts 2 and 3. The MTD was determined to be sunitinib 50 mg/bevacizumab 10 mg/kg, but chronic therapy at this dose level frequently resulted in grades 3 to 4 hypertension and hematologic and vascular toxicities. Overall, 48% of patients discontinued treatment because of adverse events. One complete and 12 partial responses were observed, which provided an objective response rate of 52%.
Conclusion
In this phase I trial of patients with metastatic RCC, the combination of sunitinib and bevacizumab caused a high degree of hypertension and vascular and hematologic toxicities at the highest dose level. We do not plan to pursue additional study of this regimen at these doses in patients with RCC.
INTRODUCTION
Until recently, treatment options for metastatic renal cell carcinoma (RCC) were limited to cytokines with only modest clinical benefit. Insight into the role of angiogenesis prompted the study of several new therapies in this cancer. Both sunitinib, which targets the vascular endothelial growth factor (VEGF) receptor and other tyrosine kinases, and bevacizumab, which is a monoclonal antibody to VEGF, have produced prolonged progression-free survival (PFS) in patients with treatment-naïve or cytokine-pretreated RCC.1–5
Combination programs are being actively studied in RCC with the hope of additionally increasing the efficacy of targeted therapies.6 Because sunitinib and bevacizumab each inhibit a different target of the VEGF pathway, we hypothesized that their combination might provide more effective blockade and might enhance antitumor activity. In addition, studies have shown that patients who progress after bevacizumab may respond to sunitinib, which suggests a lack of cross resistance.7,8 This study was designed to evaluate the safety and to identify the maximum-tolerated dose (MTD) of sunitinib when administered in combination with fixed-dose bevacizumab.
PATIENTS AND METHODS
Patients
Eligible patients had progressive metastatic RCC of any histology and had received no more than two prior systemic therapy regimens. Prior sunitinib or bevacizumab was not allowed. Other eligibility criteria included measurable disease per Response Evaluation Criteria in Solid Tumors and adequate hepatic (AST/ALT ≤ 2× upper limit of normal [ULN]), renal (serum creatinine ≤ 2× ULN), coagulation (PT ≤ 1.5× ULN), and bone marrow (leukocyte count ≥ 3,000 cells/μL, absolute neutrophil count ≥ 1,500 cells/μL, hemoglobin ≥ 9.0 g/dL, and platelet count ≥ 100,000 cells/μL) function, and a serum calcium level ≤ 12.0 mg/dL. Patients were excluded for inadequately controlled blood pressure, significant proteinuria (urine protein:creatinine > 1.0), or any history of brain metastases. Patients with a history of an acute cardiac event or those who underwent intervention for coronary disease or stroke in the prior 6 months were not enrolled. Concurrent therapeutic doses of warfarin, ongoing atrial fibrillation, other arrhythmias of grade ≥ 2, and prolongation of the corrected QT (QTc) interval (> 450 milliseconds for men; > 470 milliseconds for women) were additional exclusion criteria.
Study Design
This was a single-center, investigator-initiated, phase I trial that used a standard 3 + 3 design. Cohorts of three to six patients were sequentially enrolled to receive one of three escalated doses of sunitinib in combination with fixed-dose bevacizumab to establish the MTD (ie, highest dose level at which zero or one of six experienced a dose-limiting toxicity [DLT]). Patients who experienced progressive disease (PD) before completion of cycle 1 without a DLT were replaced. Six additional patients were planned for treatment at the MTD for additional safety and efficacy information. Patients were allowed to remain on therapy if treatment was tolerated and if there was no evidence of disease progression for a maximum of 2 years.
Treatment and Dose Escalation Plan
Treatment was administered in 42-day cycles, during which patients received oral sunitinib once daily from days 1 to 28 and bevacizumab intravenously every 2 weeks (on days 0, 14, 28). Bevacizumab was administered at 10 mg/kg in all three cohorts. Sunitinib doses varied by cohort (ie, 25, 37.5, and 50 mg).
Patients were evaluated for adverse events on the basis of National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Escalation to a new dose cohort was based on safety evaluation of the previous cohort after one cycle of treatment. Hematologic DLTs included grade 4 neutropenia that lasted 7 days or longer, any febrile neutropenia, and grade ≥ 3 thrombocytopenia that lasted 7 days or longer or that was associated with bleeding. Specific nonhematologic DLTs included grade 4 hypertension or grade 3 hypertension uncontrolled with medications, clinical evidence of congestive heart failure, grade 4 hemorrhage, grade 4 proteinuria, grade 4 venous thrombosis, and any arterial thromboembolic event. Any other grade ≥ 3 toxicity that lasted 7 days or longer was also considered a DLT, with the following exceptions: asymptomatic hyperamylasemia, hyperlipasemia, hyperuricemia, and fatigue.
Patients who experienced grades 3 to 4 adverse events had dose adjustments to one or both drugs, as specified in the protocol. Dose reductions to sunitinib were made in 12.5-mg decrements. Bevacizumab could be reduced from 10 mg/kg to 5 mg/kg. Patients with toxicities that warranted reductions at either sunitinib 25 mg or bevacizumab 5 mg/kg were withdrawn from the study. Intrapatient dose escalation was not allowed.
Antitumor Activity Assessment
Response was assessed by Response Evaluation Criteria in Solid Tumors9 during the last 2 weeks of each of the first four cycles and on even cycles thereafter. PFS was measured from the first bevacizumab dose until the date of documented disease progression, death, or study termination because of toxicity, whichever occurred first. Patients who did not meet one of these end points had PFS censored at their last tumor assessment. Overall survival (OS) was measured from the first bevacizumab dose until death or until the last date the patient was known to be alive. For these time-to-event end points, medians and 95% CIs were estimated by using Kaplan-Meier methods.
Pharmacodynamics
Serum levels of VEGF were measured by the clinical chemistry laboratory at our institution on days 0 and 28 of cycles 1 and 2 and at the time of disease progression if possible. The assay used was specific for the VEGF-A 165 and 121 amino acid isoforms and had no cross-interference with VEGF-B VEGF-C, VEGF-D, and VEGF-206 during control testing. Initial serum VEGF values, changes with treatment, and values at the time of disease progression were compared with clinical outcomes (response, PFS).
Statistics
The primary objectives were to evaluate the safety and to establish the MTD of sunitinib in combination with bevacizumab; as such, patients with all RCC histologies were included. Toxicities were described by frequency and grade. Secondary end points included the overall response rate (ORR), PFS, OS, and correlations between serum VEGF levels and outcomes (response). Changes in VEGF levels were described by using graphical methods, and differences in VEGF levels between response groups were examined by using the Wilcoxon rank sum test.
RESULTS
The study enrolled 26 patients between April 2006 and June 2007; most (88%) had undergone prior nephrectomy. Only three (12%) had received prior systemic therapy with interferon-alfa or interleukin-2, and none had previously received inhibitors of receptor tyrosine kinases or angiogenesis (Table 1).
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 56 | |
| Range | 32-76 | |
| Sex | ||
| Male | 16 | 62 |
| Female | 10 | 38 |
| Histology | ||
| Clear cell | 18 | 69 |
| Unclassified | 4 | 15 |
| Chromophobe | 3 | 12 |
| Papillary | 1 | 4 |
| Prior therapy | ||
| Nephrectomy | 23 | 88 |
| Radiation | 3 | 12 |
| Cytokine treatment | 3 | 12 |
| No. of metastatic sites | ||
| 1 | 8 | 31 |
| 2 | 8 | 31 |
| ≥ 3 | 10 | 38 |
| Select sites of metastatic disease | ||
| Lymph node | 16 | 62 |
| Lung | 12 | 46 |
| Liver | 7 | 27 |
| Adrenal gland | 3 | 12 |
| Bone | 2 | 8 |
| Pancreas | 2 | 8 |
| MSKCC risk group* | ||
| Good | 13 | 50 |
| Intermediate | 11 | 42 |
| Poor | 2 | 8 |
Treatment
Of 26 patients enrolled, one (in cohort 1) never received study therapy because of acute renal failure. A total of 127 cycles (median, five cycles per patient) were administered to the remaining 25 patients who were assessable for toxicity and response. Seven patients were treated at the initial dose level of sunitinib 25 mg; one of seven was replaced as a result of progression of disease (PD) before completion of cycle 1, which precluded full DLT evaluation. No DLTs occurred in the remaining six patients in cohort 1.
One patient each in cohorts 2 and 3 developed a DLT that consisted of grade 4 hemorrhage. The patient in cohort 2 was taking therapeutic doses of low molecular weight heparin for a pre-existing lower-extremity deep venous thrombosis and developed a subcutaneous hematoma at sites of heparin injection (ie, right arm and abdomen). The patient in cohort 3 developed epistaxis in the setting of grade 3 thrombocytopenia.
According to protocol design, the MTD was sunitinib 50 mg and bevacizumab 10 mg/kg. Six additional patients were enrolled at this dose level for additional safety and efficacy information. None of these six developed a DLT during cycle 1.
Adverse Events
The most frequently reported adverse events (any grade) for all patients included fatigue (92%), hypertension (92%), proteinuria (88%), diarrhea (76%), hand-foot-skin reaction (72%), and bleeding (72%; Table 2). The most frequently reported grades 3 to 4 adverse events included hypertension (60%), proteinuria (36%), and thrombocytopenia (24%) (Table 3). The majority of grades 3 to 4 events occurred at the highest dose level.
Table 2.
Adverse Events and Laboratory Toxicities of All Grades
| Event or Toxicity | Grade | |||||
|---|---|---|---|---|---|---|
| 1(No.) | 2(No.) | 3(No.) | 4(No.) | All | ||
| No. | % | |||||
| Fatigue | 14 | 6 | 2 | 1 | 23 | 92 |
| Hypertension | 3 | 5 | 12 | 3 | 23 | 92 |
| Proteinuria | 10 | 3 | 6 | 3 | 22 | 88 |
| Diarrhea | 9 | 10 | 0 | 0 | 19 | 76 |
| Decreased taste | 15 | 4 | 0 | 0 | 19 | 76 |
| Hand-foot-skin reaction | 8 | 6 | 4 | 0 | 18 | 72 |
| Hemorrhage* | 13 | 2 | 1 | 2 | 18 | 72 |
| Mucositis† | 12 | 5 | 0 | 0 | 17 | 68 |
| Nausea | 11 | 4 | 0 | 0 | 15 | 60 |
| Constipation | 14 | 0 | 0 | 0 | 14 | 56 |
| Rash | 8 | 3 | 2 | 0 | 13 | 52 |
| Dyspnea | 9 | 1 | 1 | 0 | 11 | 44 |
| Vomiting | 6 | 4 | 0 | 0 | 10 | 40 |
| Heartburn/dyspepsia | 4 | 4 | 1 | 0 | 9 | 36 |
| Headaches | 5 | 2 | 1 | 0 | 8 | 32 |
| Pain, other | 8 | 0 | 0 | 0 | 8 | 32 |
| Cough | 5 | 1 | 1 | 0 | 7 | 28 |
| Abdominal pain, NOS | 1 | 4 | 2 | 0 | 7 | 28 |
| Sensory neuropathy | 5 | 1 | 0 | 0 | 6 | 24 |
| Back pain | 6 | 0 | 0 | 0 | 6 | 24 |
| Hemorrhoids | 4 | 2 | 0 | 0 | 6 | 24 |
| Colitis | 5 | 1 | 0 | 0 | 6 | 24 |
| Peripheral edema | 2 | 3 | 1 | 0 | 6 | 24 |
| Anorexia | 4 | 1 | 0 | 0 | 5 | 20 |
| Dry skin | 5 | 0 | 0 | 0 | 5 | 20 |
| Non-neutropenic fever | 4 | 1 | 0 | 0 | 5 | 20 |
| MAHA (schistocytes)‡ | 3 | 0 | 2 | 0 | 5 | 20 |
| Oral cavity pain | 3 | 1 | 0 | 0 | 4 | 16 |
| Dry mouth | 2 | 1 | 0 | 0 | 3 | 12 |
| MAHA-like features§ | 3 | 0 | 0 | 0 | 3 | 12 |
| Weight loss | 2 | 1 | 0 | 0 | 3 | 12 |
| Hyperglycemia | 13 | 10 | 1 | 0 | 24 | 96 |
| Elevated AST | 18 | 3 | 1 | 0 | 22 | 88 |
| Thrombocytopenia | 10 | 5 | 6 | 0 | 21 | 84 |
| Anemia | 10 | 9 | 1 | 0 | 20 | 80 |
| Leukopenia | 11 | 9 | 0 | 0 | 20 | 80 |
| Hypoalbuminemia | 16 | 1 | 1 | 0 | 18 | 72 |
| Elevated alkaline phosphatase | 16 | 1 | 0 | 0 | 17 | 68 |
| Creatinine elevation | 10 | 6 | 0 | 1 | 17 | 68 |
| Elevated ALT | 11 | 5 | 0 | 0 | 16 | 64 |
| Elevated amylase | 11 | 3 | 2 | 0 | 16 | 64 |
| Elevated lipase | 4 | 1 | 6 | 1 | 12 | 48 |
| Hyperkalemia | 6 | 5 | 0 | 1 | 12 | 48 |
| Hyponatremia | 9 | 0 | 3 | 0 | 12 | 48 |
| Hypothyroidism | 1 | 10 | 0 | 0 | 11 | 44 |
| Neutropenia | 0 | 10 | 1 | 0 | 11 | 44 |
| Hypothyroidism | 1 | 10 | 0 | 0 | 11 | 44 |
| Prolonged PTT | 4 | 1 | 1 | 0 | 6 | 24 |
| Hypernatremia | 6 | 0 | 0 | 0 | 6 | 24 |
| Hypophosphatemia | 0 | 5 | 0 | 0 | 5 | 20 |
| Hypercholesterolemia | 2 | 2 | 0 | 0 | 4 | 16 |
| Hypertriglyceridemia | 2 | 2 | 0 | 0 | 4 | 16 |
| Hypoglycemia | 3 | 0 | 0 | 0 | 3 | 12 |
| Hyperthyroidism | 2 | 1 | 0 | 0 | 3 | 12 |
Table 3.
Grade 3 and Higher Adverse Events and Laboratory Toxicities
| Event or Toxicity | Incidence per Adverse Event Grade by Cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 7) | 2 (n = 6) | 3 (n = 12) | All (N = 25) | |||||||||
| 3 (No.) | 4 (No.) | 3-4 (%) | 3 (No.) | 4 (No.) | 3-4 (%) | 3 (No.) | 4 (No.) | 3-4 (%) | 3 (No.) | 4 (No.) | 3-4 (%) | |
| Hypertension | 2 | 0 | 29 | 3 | 0 | 50 | 7 | 3 | 83 | 12 | 3 | 60 |
| Proteinuria | 3 | 0 | 43 | 0 | 0 | 0 | 3 | 3 | 50 | 6 | 3 | 36 |
| Elevated lipase | 2 | 1 | 43 | 3 | 0 | 50 | 1 | 0 | 8 | 6 | 1 | 28 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 50 | 6 | 0 | 24 |
| Hand-foot-skin reaction | 2 | 0 | 29 | 2 | 0 | 33 | 0 | 0 | 0 | 4 | 0 | 16 |
| Fatigue | 1 | 0 | 14 | 1 | 0 | 17 | 0 | 1 | 8 | 2 | 1 | 12 |
| Hyponatremia | 1 | 0 | 14 | 0 | 0 | 0 | 2 | 0 | 17 | 3 | 0 | 12 |
| Hemorrhage | 0 | 0 | 0 | 0 | 1 | 17 | 1 | 1 | 17 | 1 | 2 | 12 |
| RPLS | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 17 | 2 | 0 | 8 |
| Elevated amylase | 1 | 0 | 14 | 1 | 0 | 17 | 0 | 0 | 0 | 2 | 0 | 8 |
| Rash | 1 | 0 | 14 | 0 | 0 | 0 | 1 | 0 | 8 | 2 | 0 | 8 |
| MAHA* | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 17 | 2 | 0 | 8 |
| Hyperuricemia | 0 | 0 | 0 | 1 | 0 | 17 | 0 | 1 | 8 | 1 | 1 | 8 |
| Abdominal pain | 2 | 0 | 29 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 8 |
| Lymphopenia | 1 | 0 | 14 | 0 | 0 | 0 | 1 | 0 | 8 | 2 | 0 | 8 |
| MI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1† | 8 | 0 | 1† | 4 |
One death occurred during treatment in a 51-year-old man with widely metastatic RCC who suffered a cardiac arrest during cycle 2 that was related to a large anterior wall MI. Autopsy demonstrated multifocal tumor involvement of the epicardium and myocardium and 70% to 100% necrosis, which is consistent with treatment effect. No significant coronary artery obstruction was seen.
Two patients (described in Case Reports of Severe MAHA) in cohort 3 developed neurologic symptoms associated with hypertension, thrombocytopenia, renal insufficiency, and proteinuria. Investigation revealed laboratory features of hemolysis (ie, elevated LDH, low haptoglobin, elevated reticulocyte count, decreased hemoglobin) and schistocytes on peripheral smear consistent with microangiopathic hemolytic anemia (MAHA). Both patients also developed reversible posterior leukoencephalopathy syndrome (RPLS). Based on these two index patient cases, we investigated prospectively patients who were still on study and retrospectively those who were treated previously for evidence of MAHA.
Smears were available for review in 13 of 25 patients, including 10 of 11 with grade 2 or greater thrombocytopenia and 9 of 12 in cohort 3. Schistocytes were graded from 0 to 4+ by a board-certified hematologist (H.H.). Three additional patients (all cohort 3) were found to have schistocytes and some laboratory features that resembled the two index patient cases, which conferred a diagnosis of MAHA. Table 4 lists the most extreme laboratory results obtained on study for each of these five patients who developed MAHA. In several patients, the most abnormal value for one test was obtained at a different time point from the most abnormal value of another test. Therefore, although all patients who developed MAHA were in cohort 3, several were receiving dose-reduced sunitinib (ie, 37.5 or 25 mg) at the time MAHA was detected because of an isolated toxicity, such as thrombocytopenia, that preceded development of associated features. Three other patients (also all cohort 3) had thrombocytopenia and features of hemolysis but without schistocytes; two of these had grade 3 proteinuria; one of these patients also developed reversible mild renal insufficiency. None of the patients exhibited fever associated with these findings. Coagulation profiles were evaluated in seven of these eight patients without any prolongation; five of eight also had fibrinogen level tests, all of which were normal or elevated, making disseminated intravascular coagulation unlikely. Study therapy was discontinued in all patients treated in this dose cohort. Clinical symptoms and laboratory features reversed in all patients on treatment discontinuation, and no patients required plasmapheresis.
Table 4.
Characteristics of Patients Who Developed MAHA
| Clinical Characteristic | Reference Range | Patient | ||||
|---|---|---|---|---|---|---|
| 1* | 2† | 3 | 4 | 5 | ||
| Age, years | 57 | 76 | 56 | 41 | 51 | |
| Sex | F | F | F | M | F | |
| RCC histology | Unclassified | Clear cell | Clear cell | Chromophobe | Clear cell | |
| PLT nadir, K/μL | 160-400 | 43 | 39 | 26 | 52 | 37 |
| Hgb, g/dL‡ | ||||||
| Nadir | 8.4 | 7.8 | 8.7 | 10.9 | 11.2 | |
| Baseline | 12.3 | 12.7 | 14.4 | 15.2 | 12.5 | |
| LDH, units/L | 60-200 | |||||
| Peak | 398 | 547 | 531 | 387 | 466 | |
| Baseline | 198 | 181 | 152 | 150 | 207 | |
| Peak reticulocyte count, % | 0.5-2.5 | 4.4 | 4.4 | 2.8 | 1.6 | 1.9 |
| Haptoglobin nadir, mg/dL | 30-200 | < 6 | < 6 | < 6 | < 6 | < 6 |
| Schistocytes | 0+ | 2+ | 4+ | 2+ | 1+ | 1+ |
| Creatinine, mg/dL | 0.6-1.3 | |||||
| Peak | 2.6 | 2.8 | 1.5 | 1.8 | 1.6 | |
| Baseline | 1.4 | 1.0 | 1.3 | 1.7 | 1.3 | |
| Worst proteinuria grade | 4 | 4 | 2 | 3 | 3 | |
| Worst hypertension grade | 4 | 4 | 3 | 3 | 3 | |
| Neurologic signs or symptoms | Seizure (RPLS) | Seizure, confusion (RPLS) | None | None | None | |
| MAHA grade‖ | 3 | 3 | 1 | 1 | 1 | |
| Best response | PR | SD | PR | PR | PR |
Nineteen of 25 patients underwent thyroid function testing; 14 had one or more abnormalities detected, but none were greater than grade 2. Both hyperthyroidism and hypothyroidism were observed (Table 2).
Response and Survival
Of 25 treated patients, one (4%) achieved a CR, and 12 (48%) achieved a PR, which provided a cumulative ORR of 52% (95% CI, 31% to 72%). Nine additional patients (36%) had stable disease (SD), and three (12%) had PD as the best response. Responses occurred at all dose levels and in a variety of histologies, including clear cell, unclassified, and chromophobe tumors.
The most common reason for discontinuation of treatment was toxicity (48%); only seven patients (28%; Table 5) developed PD while on study (one of 13 responders). Another reason for study discontinuation was patient choice (24%), primarily because of concerns regarding toxicities in other patients disclosed during an update of the informed consent. The median PFS was 11 months (95% CI, 6 months to not reached).
Table 5.
Dose Reductions and Discontinuations Because of Toxicity
| Cohort | No. of Assessable Patients | Discontinuation for Toxicity | Dose Reduction | ||||
|---|---|---|---|---|---|---|---|
| Sunitinib | Bevacizumab | ||||||
| No. | % | No. | % | No. | % | ||
| 1 | 7 | 3 | 43 | NA | NA | 2 | 29 |
| 2 | 6 | 2 | 33 | 2 | 33 | 0 | 0 |
| 3 | 12 | 7 | 58 | 8 | 67 | 2 | 17 |
| Total | 25 | 12 | 48 | 10 | 40 | 4 | 16 |
With eight deaths among the 25 treated patients (32%), median OS has not yet been reached. Death occurred in all seven patients who progressed on study and in the patient who suffered cardiac arrest while responding to treatment. Of the remaining 17 patients, two (one each in cohorts 1 and 2) are alive with no evidence of disease, which includes the patient with a CR (who continues on sunitinib prophylactically and has had no evidence of disease for longer than 9 months) and another who underwent surgical resection for residual disease after achieving a prolonged PR with a plateau in response after 1 year of treatment.
Serum VEGF Levels
Pretreatment serum VEGF levels were obtained at baseline in 23 of 25 patients. Levels decreased during the first 4 weeks (during exposure to both sunitinib and bevacizumab) and additionally decreased during the 2-week sunitinib break. VEGF levels increased slightly during the first 4 weeks of cycle 2, which reflected the return of sunitinib to the regimen (Appendix Fig A1A, online only). Baseline VEGF levels inversely correlated with response (P = .02; Appendix Fig A1B). No correlations were observed between degree of change and outcome, and there were no differences in degree of VEGF change between the three cohorts.
Case Reports of Severe MAHA
Patient 1.
A 57-year-old woman with metastatic clear cell RCC and a history of well-controlled hypertension began treatment in cohort 3 after recovery from cytoreductive nephrectomy. She developed grade 3 hypertension during cycle 1 and grade 3 thrombocytopenia and grade 4 hyperuricemia at the end of cycle 3. Allopurinol was started, and her sunitinib dose was lowered to 37.5 mg for cycle 4. One week later, she developed a diffuse rash. Both allopurinol and study therapy were discontinued, and steroids were initiated. However, her rash worsened with mucosal involvement; she was diagnosed with Stevens-Johnson syndrome (SJS) and required hospitalization. Her rash persisted for longer than 1 week after allopurinol was stopped; concern that some of her antihypertensive medications could be contributing to SJS led to change or discontinuation of most of these. She was discharged, but she represented 1 week later with severe hypertension uncontrolled with medications, and she suffered a seizure in the urgent care center. Laboratory studies and peripheral smear were consistent with MAHA (Fig 1A), and brain magnetic resonance imagine (MRI; Fig 1B) confirmed the diagnosis of RPLS. She improved rapidly with diuresis and hypertensive control. Repeat MRI 6 weeks later demonstrated complete resolution of RPLS (Fig 1C). She restarted sunitinib monotherapy at 25 mg and has remained on this dose with SD for 8 months.
Fig 1.
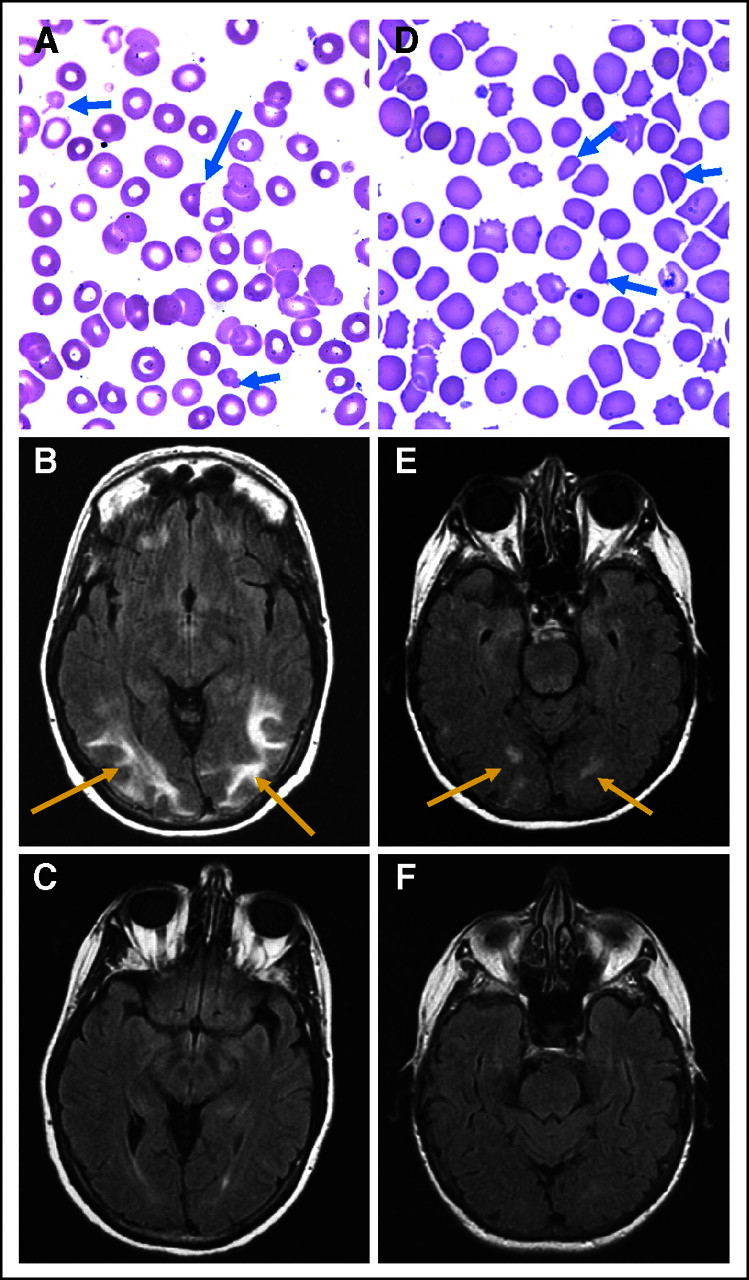
Two patients on study developed severe microangiopathic hemolytic anemia and reversible posterior leukoencephalopathy syndrome (RPLS). (A) The peripheral blood smear for patient 1 showed schistocytes (blue arrows) consistent with microangiopathy. (B) Brain magnetic resonance imagine (MRI; flair images) obtained within a few days of the peripheral smear was consistent with RPLS (red arrows denote flair intensity). (C) Repeat MRI taken 6 weeks later after a break from therapy and with hypertension controlled showed complete resolution of RPLS. (D) The peripheral smear of patient 2 again showed schistocytes (blue arrows), and (E) brain MRI (flair images) showed RPLS (red arrows denote flair intensity). (F) Eight weeks later, a repeat brain MRI confirmed resolution of RPLS concurrent with the patient's clinical recovery.
Patient 2.
A 76-year-old woman with a history of well-controlled hypertension and a nephrectomy for unclassified RCC developed recurrent metastatic disease and was enrolled into cohort 3. During cycle 1, she developed grade 4 fatigue, grade 3 thrombocytopenia, and grade 2 tremors. Brain MRI at that time was normal, and she recovered completely with a 1-week delay in the initiation of cycle 2, at which time both sunitinib and bevacizumab doses were reduced. Nevertheless, she developed grade 3 hypertension and recurrent fatigue, which required further sunitinib reduction to 25 mg. The patient then self-discontinued her antihypertensive medications and sunitinib without consulting her physician. A few days later, she developed confusion and was admitted for severe hypertension, nephrotic syndrome, and MAHA (Fig 1D). She suffered a syncopal episode, and brain MRI documented RPLS (Fig 1E). She also developed multiple deep vein thromboses that were followed by bleeding with anticoagulation. Fibrinogen was normal. She recovered within 3 weeks, and brain MRI 8 weeks later demonstrated resolution of RPLS (Fig 1F). She has remained off systemic therapy with SD since that time.
DISCUSSION
To improve on the low cure rates with single agents in metastatic RCC, sunitinib and bevacizumab were combined in this trial, which demonstrated activity at the cost of significant toxicity, particularly at the highest dose level. At the MTD, hypertension occurred almost universally along with a high proportion of other significant hematologic and vascular toxicities. Grades 3 to 4 hypertension, proteinuria, and thrombocytopenia occurred in 50%, 36%, and 24% of all patients, respectively, and in 83%, 50%, and 50%, respectively, of patients in cohort 3. In addition, 11 of 12 patients in cohort 3 required at least one dose reduction, and nearly half of all patients were withdrawn because of serious toxicities. Another one fourth of the patients withdrew because of difficulty tolerating treatment or concerns about toxicities. A recent report highlighted similar difficulties in tolerability with the combination of sorafenib and bevacizumab despite administration of both agents at less than full doses.10
We observed adverse events thought to occur rarely with either agent alone. The constellation of thrombocytopenia, hemolytic anemia, hypertension, proteinuria, and renal insufficiency with microangiopathy (schistocytes) was observed in five patients, and an additional three patients had some of these features without schistocytes. Recent reports highlighted a similar syndrome with bevacizumab (alone or in combination with traditional chemotherapeutic agents) in a variety of malignancies11,12 and, less commonly, with sunitinib.13,14 Renal biopsies performed in some instances helped establish the diagnosis of thrombotic microangiopathy. However, the microangiopathy that occurred with bevacizumab in these other malignancies was primarily isolated to the kidney, whereas our patients had systemic findings similar to classic MAHA. Thus, in our experience, this appears to be a frequent and severe toxicity when these two agents are combined in RCC. These clinical findings are similar to the hemolytic anemia, elevated liver enzymes, and low platelets (HELLP) syndrome and pre-eclamptic states observed during pregnancy that recently were demonstrated to result from systemic VEGF depletion from excess placental secretion of soluble VEGF receptor (fms-like tyrosine kinase-1).15–18
Two patients with MAHA developed RPLS with hypertension and neurologic manifestations, including seizures. This toxicity is believed to occur at a rate of less than 0.1% with bevacizumab19,20 and occurs even more rarely with sunitinib.13 Our finding of RPLS in two of 12 patients in cohort 3 (two of 25 overall), therefore, suggests a high incidence. Of note, both patients had preexisting hypertension and had discontinued some of their antihypertensive medications before RPLS developed.
Investigators conducting a separate trial21 to evaluate this combination did not report the same degree of hypertension (and its consequences), MAHA, or other toxicities. Both studies included modest numbers of patients and had different eligibility criteria and design. One important distinction is that our trial was specific to RCC, and nearly all patients had undergone nephrectomy. The other trial included patients with multiple tumor types (including RCC), many of whom had not undergone nephrectomy. It is possible that preexisting hypertension, the mononephric state, or RCC itself might enhance susceptibility to treatment-related hypertension, proteinuria, renal impairment, and—perhaps—vascular and hematologic toxicities. This hypothesis is supported by a recent publication that described a mathematical model of bevacizumab-induced renal thrombotic microangiopathy, in which glomerular hyperperfusion and hyperfiltration from chronic renal insufficiency were identified as predisposing risk factors.22
Pharmacodynamic studies demonstrated a predictable change in serum VEGF levels during therapy with a pattern that differs from that reported with sunitinib alone.23 In addition, pretreatment serum VEGF levels were significantly lower among patients with RCC who responded (CR or PR) to study therapy compared with those who did not (SD or PD). Higher baseline serum VEGF levels have been demonstrated previously to portend a worse prognosis in some,24–27 but not all,28,29 studies of RCC.
In conclusion, in this phase I trial of patients with metastatic RCC, the combination of sunitinib and bevacizumab caused a high degree of hypertension and vascular and hematologic toxicities at the highest dose level. We do not plan to pursue additional study of this regimen at these doses in patients with RCC.
Appendix
Fig A1.
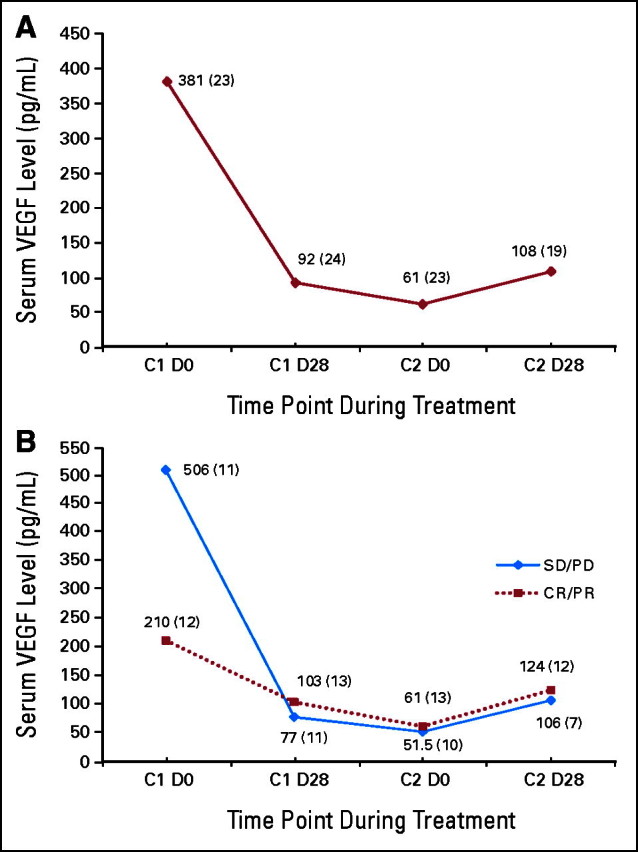
Changes in the (A) median serum vascular endothelial growth factor (VEGF) level of the entire study population during treatment with the combination of sunitinib and bevacizumab; and (B) median serum VEGF level for patients separated by response type. The blue line corresponds to patients whose best responses were either stable disease (SD) or progressive disease (PD), whereas the red line corresponds to patients who achieved a partial (PR) or complete response (CR). The baseline median VEGF level was significantly lower for patients who achieved a PR or CR compared with those who experienced SD or PD. The median value for each time point is provided along the data line; the number of patients who had VEGF levels drawn at each time point is in parentheses. C, cycle; D, day.
Footnotes
Supported by Genentech Inc, South San Francisco, CA.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL; and at the 44th Annual Meeting of the American Society of Clinical Oncology Meeting, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Darren R. Feldman, Genentech; Michelle S. Ginsberg, Genentech; Hani Hassoun, Genentech; Susanne Velasco, Genentech; Patricia Fischer, Genentech; Robert J. Motzer, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Darren R. Feldman, Michael S. Baum, Patricia Fischer, Ellen Ronnen, Nicole Ishill, Sujata Patil, Robert J. Motzer
Administrative support: Susanne Velasco, Patricia Fischer, Robert J. Motzer
Provision of study materials or patients: Darren R. Feldman, Susanne Velasco, Patricia Fischer, Robert J. Motzer
Collection and assembly of data: Darren R. Feldman, Michael S. Baum, Michelle S. Ginsberg, Hani Hassoun, Carlos D. Flombaum, Susanne Velasco, Patricia Fischer, Ellen Ronnen, Nicole Ishill, Sujata Patil, Robert J. Motzer
Data analysis and interpretation: Darren R. Feldman, Michael S. Baum, Michelle S. Ginsberg, Hani Hassoun, Carlos D. Flombaum, Susanne Velasco, Patricia Fischer, Ellen Ronnen, Nicole Ishill, Sujata Patil, Robert J. Motzer
Manuscript writing: Darren R. Feldman, Nicole Ishill, Sujata Patil, Robert J. Motzer
Final approval of manuscript: Darren R. Feldman, Michael S. Baum, Michelle S. Ginsberg, Hani Hassoun, Carlos D. Flombaum, Susanne Velasco, Patricia Fischer, Ellen Ronnen, Nicole Ishill, Sujata Patil, Robert J. Motzer
REFERENCES
- 1.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Rini B, Halabi S, Rosenberg JE, et al. CALGB 90206: A phase III trial of bevacizumab plus interferon-alfa versus interferon-alfa monotherapy in metastatic renal cell carcinoma. the Genitourinary Cancers Symposium; February 14-16, 2008; San Francisco, CA. Presented at. abstr 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaelin WG., Jr The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res. 2004;10:6290S–6295S. doi: 10.1158/1078-0432.CCR-sup-040025. [DOI] [PubMed] [Google Scholar]
- 7.Tamaskar I, Garcia JA, Elson P, et al. Antitumor effects of sunitinib or sorafenib in patients with metastatic renal cell carcinoma who received prior antiangiogenic therapy. J Urol. 2008;179:81–86. doi: 10.1016/j.juro.2007.08.127. discussion 86. [DOI] [PubMed] [Google Scholar]
- 8.George DJ, Michaelson MD, Rosenberg JE, et al. Phase II trial of sunitinib in bevacizumab-refractory metastatic renal cell carcinoma (mRCC): Updated results and analysis of circulating biomarkers. J Clin Oncol. 2007;25:5035–5306. [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangie C, Lefaucheur C, Medioni J, et al. Renal thrombotic microangiopathy caused by anti-VEGF-antibody treatment for metastatic renal-cell carcinoma. Lancet Oncol. 2007;8:177–178. doi: 10.1016/S1470-2045(07)70037-2. [DOI] [PubMed] [Google Scholar]
- 12.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapiteijn E, Brand A, Kroep J, et al. Sunitinib induced hypertension, thrombotic microangiopathy and reversible posterior leukencephalopathy syndrome. Ann Oncol. 2007;18:1745–1747. doi: 10.1093/annonc/mdm454. [DOI] [PubMed] [Google Scholar]
- 14.Levey SA, Bajwa RS, Picken MM, et al. Thrombotic microangiopathy associated with sunitinib, a VEGF inhibitor, in a patient with factor V Leiden mutation. NDT Plus. 2008;1:154–156. doi: 10.1093/ndtplus/sfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumwell S, Karumanchi SA. Pre-eclampsia: Clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106:c72–81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 17.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 18.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 19.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. doi: 10.1056/NEJMc052954. discussion 980-982. [DOI] [PubMed] [Google Scholar]
- 20.Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. discussion 980-982. [PubMed] [Google Scholar]
- 21.Cooney MM, Garcia J, Brell J, et al. A phase I study of bevacizumab in combination with sunitinib in advanced solid tumors. J Clin Oncol. 2007;25(suppl):652s. abstr 15532. [Google Scholar]
- 22.Katavetin P, Katavetin P, Nochy D, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;359:205–206. doi: 10.1056/NEJMc080770. author reply 206-207. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 24.O'Byrne KJ, Dobbs N, Propper D, et al. Vascular endothelial growth factor platelet counts, and prognosis in renal cancer. Lancet. 1999;353:1494–1495. doi: 10.1016/S0140-6736(99)00471-7. [DOI] [PubMed] [Google Scholar]
- 25.Ljungberg B, Jacobsen J, Haggstrom-Rudolfssson S, et al. Tumour vascular endothelial growth factor (VEGF) mRNA in relation to serum VEGF protein levels and tumour progression in human renal cell carcinoma. Urol Res. 2003;31:335–340. doi: 10.1007/s00240-003-0346-x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsen J, Grankvist K, Rasmuson T, et al. Prognostic importance of serum vascular endothelial growth factor in relation to platelet and leukocyte counts in human renal cell carcinoma. Eur J Cancer Prev. 2002;11:245–252. doi: 10.1097/00008469-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen J, Rasmuson T, Grankvist K, et al. Vascular endothelial growth factor as prognostic factor in renal cell carcinoma. J Urol. 2000;163:343–347. [PubMed] [Google Scholar]
- 28.Dosquet C, Coudert MC, Lepage E, et al. Are angiogenic factors, cytokines, and soluble adhesion molecules prognostic factors in patients with renal cell carcinoma? Clinical Cancer Res. 1997;3:2451–2458. [PubMed] [Google Scholar]
- 29.Schips L, Dalpiaz O, Lipsky K, et al. Serum levels of vascular endothelial growth factor (VEGF) and endostatin in renal cell carcinoma patients compared to a control group. Eur Urol. 2007;51:168–173. doi: 10.1016/j.eururo.2006.06.026. discussion 174. [DOI] [PubMed] [Google Scholar]