Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogenic PTKs (original) (raw)
Abstract
Abnormal protein tyrosine kinases (PTKs) cause many human leukemias. For example, BCR/ABL causes chronic myelogenous leukemia (CML), whereas FLT3 mutations contribute to the pathogenesis of acute myelogenous leukemia. The ABL inhibitor Imatinib (Gleevec, STI571) has remarkable efficacy for treating chronic phase CML, and FLT3 inhibitors (e.g., PKC412) show similar promise in preclinical studies. However, resistance to PTK inhibitors is a major emerging problem that may limit long-term therapeutic efficacy. Development of rational combination therapies will probably be required to effect cures of these and other neoplastic disorders. Here, we report that the mTOR inhibitor rapamycin synergizes with Imatinib against BCR/ABL-transformed myeloid and lymphoid cells and increases survival in a murine CML model. Rapamycin/Imatinib combinations also inhibit Imatinib-resistant mutants of BCR/ABL, and rapamycin plus PKC412 synergistically inhibits cells expressing PKC412-sensitive or -resistant leukemogenic FLT3 mutants. Biochemical analyses raise the possibility that inhibition of 4E-BP1 phosphorylation may be particularly important for the synergistic effects of PTK inhibitor/rapamycin combinations. Addition of a mitogen-activated protein kinase kinase inhibitor to rapamycin or rapamycin plus PTK inhibitor further increases efficacy. Our results suggest that simultaneous targeting of more than one signaling pathway required by leukemogenic PTKs may improve the treatment of primary and relapsed CML and/or acute myelogenous leukemia caused by FLT3 mutations. Similar strategies may be useful for treating solid tumors associated with mutant and/or overexpressed PTKs.
Many leukemias are caused by oncogenic versions of protein tyrosine kinases (PTKs). BCR/ABL, encoded by the Philadelphia chromosome, in which a portion of BCR is fused to the gene for the tyrosine kinase ABL, typically causes chronic myelogenous leukemia (CML), but also causes acute lymphoblastic leukemia (ALL), and rarely, chronic neutrophilic leukemia (1). The Ets protein TEL can be fused to various PTKs; e.g., TEL-platelet-derived growth factor receptor β (PDGFRβ) is associated with chronic myelomonocytic leukemia (2), whereas TEL-JAK2 is found in childhood ALL and atypical CML (3). Internal tandem duplications (ITD) of the juxtamembrane domain and/or point mutations in the kinase domain of FLT3 occur in >30% of acute myelogenous leukemia (AML) (4). Many of these oncogenic PTKs cause myeloid leukemias in rodent models, providing firm evidence of their causal role (5).
Leukemogenic PTKs have deregulated kinase activity, and subvert normal growth factor, cytokine, and/or integrin signaling (4). Because PTK activity is required for pathogenesis, leukemogenic PTKs are attractive drug targets. Imatinib (IM, Gleevec/STI571), a relatively selective ABL inhibitor, inhibits BCR/ABL in hematopoietic cell lines and blocks its effects on cell signaling, proliferation, and survival (6). IM monotherapy produces a complete hematologic response in nearly all (≈95%) chronic-phase CML patients (7, 8). IM also inhibits kit and PDGFR, and is effective in treating gastrointestinal stromal tumor associated with kit mutations (9, 10), chronic myelomonocytic leukemia associated with TEL-PDGFRβ fusions (11), and hypereosinophilic syndrome associated with the FIP1L1-PDGFRα fusion (12). Small molecule inhibitors of FLT3 also have been developed (13-16), including PKC412, a staurosporine derivative that selectively induces G1 arrest and apoptosis in cells expressing FLT3-ITD (13). FLT3 inhibitors also can treat FLT3-ITD-evoked disease in mice.
Nevertheless, PTK inhibitors (PTK-Is) alone are unlikely to be curative. Only 52% of CML blast crisis patients respond to IM. Few such patients exhibit complete hematologic or cytogenetic responses, and most (≈60%) responders relapse within 6 months of therapy (8, 17). Even in chronic-phase patients with major cytogenetic response rates of 41-74% (7, 8, 18), almost all retain the BCR/ABL translocation (assessed by PCR) after 12 months of IM treatment (19).
Several mechanisms of IM resistance have been defined, including BCR/ABL amplification and, in ≈50% of cases, point mutations in the ABL kinase domain that interfere with drug-protein interaction. Conceivably, mutations in other domains of BCR/ABL will account for most, if not all, of the remaining cases of IM resistance (20). Resistance probably will occur in other settings as well. FLT3-ITD-expressing cells exposed to PKC412 for several months exhibited PKC412 resistance, accompanied by FLT3-ITD overexpression (13). Moreover, IM resistance caused by mutations in the FIP1L1-PDGFRα fusion protein has developed in some hypereosinophilic syndrome patients (12).
These findings suggest that additional agents, used in combination with PTK-Is, will be required for curative therapy. A rational approach to devising such combinations might capitalize on emerging information on how oncogenic PTKs perturb cell signaling. We showed earlier that the scaffolding adapter Gab2 is required for BCR/ABL transformation of myeloid and lymphoid cells, by virtue of its ability to mediate activation of the Erk and phosphatidylinositol 3-kinase (PI3K)/Akt pathways (21). Other studies also indicate that these pathways are vital for survival/proliferation of BCR/ABL-transformed cells (21-23), and they also are activated in FLT3 mutant-expressing cells (14). Although PI3K inhibitors exist, their efficacy and safety have not been tested. Neither Akt nor Erk inhibitors are available, although several inhibitors of the upstream mitogen-activated protein kinase kinase (MEK) exist, and at least one is in clinical trials (24).
The serine/threonine kinase mTOR (mammalian target of rapamycin) is downstream of PI3K/Akt (25, 26) and regulates cell growth and proliferation (27). mTOR phosphorylates p70 S6 kinase (p70 S6K) and eukaryotic initiation factor 4E-binding protein-1 (4E-BP1), both of which regulate mRNA translation (28). The macrolide rapamycin (Rap) binds to the immunophilin FKBP12, and this complex inhibits mTOR. Rap is approved by the Food and Drug Administration for the prevention of allograft rejection (27), but it and its ester analog CCI-779 also show antitumor activity (29). We tested Rap alone or in combination with PTK and/or MEK inhibitors in ex vivo and in vivo leukemia models. Our results suggest that such combinations may represent an improved therapeutic approach for diseases caused by oncogenic PTKs.
Materials and Methods
Cell Culture. K562 cells and Ba/F3 cell lines expressing WT p210 BCR/ABL (Ba/F-BCR/ABL WT), p210 BCR/ABL T315I (Ba/F-BCR/ABL T315I), FLT3-ITD (Ba/F-FLT3-ITD), or FLT3-ITD F691I (Ba/F-FLT3-ITD F691I) were grown in RPMI medium 1640 with 10% (vol/vol) FBS plus antibiotics. BCR/ABL-B-lymphoblasts were maintained as described (21). Retroviral transduction of bone marrow (BM) cells and colony assays were performed as described (21).
Reagents. IM (Novartis), Rap (Sigma), PKC412 (Novartis), and UO126 (Calbiochem) solutions were prepared in DMSO and stored at -20°C. Rapamune 1 mg tablets (Wyeth) were used for animal studies.
Proliferation/Apoptosis Assays. BCR/ABL-transformed primary B-lymphoblasts (1 × 104 cells per well) were cultured in 96-well plates in RPMI medium 1640/20% FBS for 24 h. Ba/F3 derivatives and K562 cells (3.5 × 103 cells per well) were cultured in 96-well plates in growth medium for 48 or 60 h. [3H]thymidine (1 μCi per well) was added 4-6 h before harvesting, and [3H]thymidine incorporation was determined by using a Cell Harvester (Skatron). Viable cell number was assessed by trypan blue exclusion after 48-h drug exposure. The combination index (30) was determined by using calcusyn software (Biosoft). Cell cycle and apoptosis assays were as described (21).
Immunoblotting. Immunoblotting was performed as described (31), using phospho-specific antibodies reactive with Thr-389 of p70 S6K, Thr-37, Ser-65, or Thr-70 of 4E-BP1 or Thr-202/Tyr-204 of p44/42 Erk (Cell Signaling Technology). For loading controls, blots were reprobed with anti-total Erk2 antibodies (Santa Cruz Biotechnology).
Animal Studies. CML-like disease was generated in Balb/C mice by retroviral gene transduction of 5′-fluorouracil-primed BM with MSCV p210 (BCR/ABL)-IRES-GFP, followed by BM transplantation (BMT) (13). Drug trials contained four treatment arms (nine mice each): IM (70 mg/kg per day), Rap (7 mg/kg per day), IM (70 mg/kg per day) plus Rap (7 mg/kg per day), and double placebo. Treatment began when animals had established disease (day 10 after transplant) and continued until all animals were dead. IM, resuspended in 0.5% methylcellulose (MC) solution, was administered by gavage every 12 h. Rapamune tablets were crushed, suspended in 0.2% MC, and 400 μl were administered i.p. every 24 h. Mice were assessed twice daily, and survival was measured from the day of BMT in half-day units. The Kaplan and Meier method was used to estimate survival curves, and the log rank test was used to assign a significance level to the difference between any two survival curves. Nominal P values are provided with no adjustments for multiple comparisons.
Results
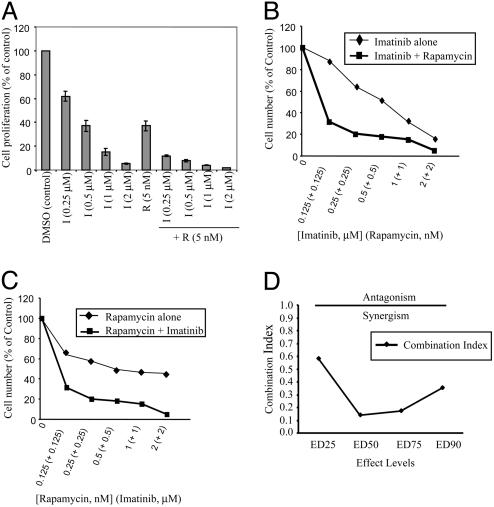
Rap and IM Synergistically Inhibit BCR/ABL-Transformed Ba/F3 Cells. Ba/F3 cells expressing p210 BCR/ABL WT (Ba/F-BCR/ABL WT) were treated with various doses of IM in the presence or absence of 5 nM Rap, and proliferation was assessed by thymidine incorporation. As expected, IM inhibited Ba/F-BCR/ABL WT proliferation in a dose-dependent manner, but combining the drugs markedly decreased proliferation (Fig. 1_A_). Similar results were obtained when cell number was used to monitor cell proliferation (Fig. 1 B and C). Rap alone also inhibited Ba/F-BCR/ABL WT cells, although combining Rap with IM showed markedly increased inhibition over a wide dose range (Fig. 1 B and C). The doses of each agent that, when used in combination, caused profound inhibition were below typical therapeutic serum levels of each drug (8, 32). Drug interaction analyses indicated that inhibition was synergistic (Fig. 1_D_).
Fig. 1.
Rap synergizes with IM to inhibit BCR/ABL-transformed Ba/F3 cells. (A) Ba/F-BCR/ABL WT cells were treated with the indicated doses of IM or Rap (5 nM) alone or in combination for 48 h, and [3H]thymidine incorporation was determined. Control cells were treated with DMSO vehicle. (_B_-D) Ba/F-BCR/ABL WT cells were exposed to varying concentrations of Rap and IM at a constant ratio of 1:1,000 for 48 h. Viable cells were counted by trypan blue exclusion, and the combination index was determined. Values <1.0 correspond to synergy, with values of 0.1-0.3 corresponding to strong synergy. Points are the means of triplicate determinations; bars ± SD.
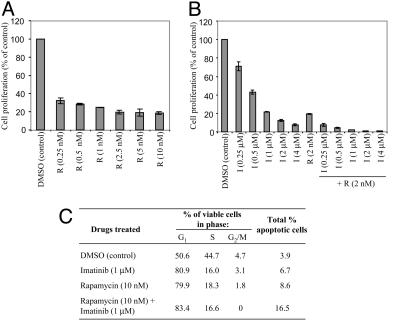
Inhibition of BCR/ABL-B Lymphoblasts by IM/Rap Combination. Rap also inhibited the proliferation of BCR/ABL-transformed B lymphoblasts (Fig. 2_A_), as did IM (Fig. 2_B_). Again, combining Rap with IM resulted in markedly enhanced inhibition. Decreased proliferation could reflect a lower rate of cell cycle progression and/or increased cell death. Low-dose IM (1 μM) or Rap (10 nM) alone caused G1 arrest with little apoptosis. However, combining these drugs increased apoptosis, as indicated by sub-G1 DNA content (Fig. 2_C_), providing further evidence of their synergistic action.
Fig. 2.
Effects of Rap and/or IM on BCR-ABL-transformed primary B lymphoblasts. (A) BCR/ABL-B lymphoblasts were treated with the indicated concentrations of Rap or DMSO vehicle for 24 h, and proliferation was assessed by [3H]thymidine incorporation. Lower drug doses and shorter exposure times were used here compared with Fig. 1 because these primary cells are more sensitive than Ba/F3 cells. (B) BCR/ABL-B lymphoblasts were exposed to IM (0.25-4 μM) alone or in combination with Rap (2 nM) for 24 h followed by measurement of [3H]thymidine incorporation. The results shown are representative of three independent experiments. (C) Cell cycle distribution of treated cells after 24-h exposure to the indicated agents. One of two experiments with similar results is shown.
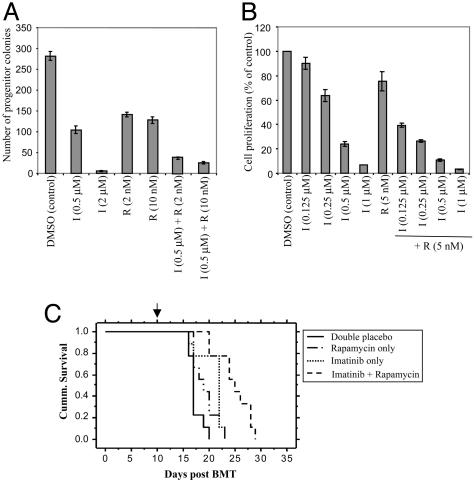
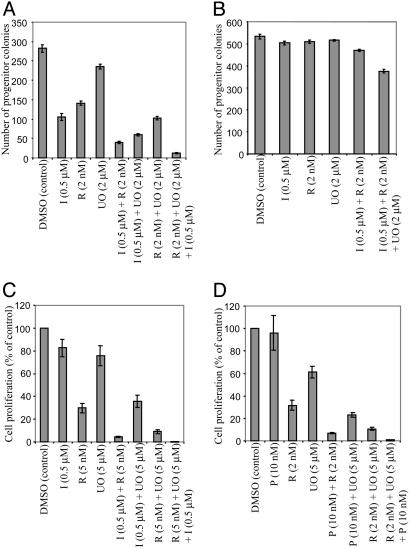
Rap Potentiates Effects of IM on BCR/ABL-Evoked Myeloid Transformation. Because BCR/ABL typically causes myeloid leukemia, we tested whether Rap alone or in combination with IM inhibits BCR/ABL-evoked myeloid colony formation. As expected, BM transduced with BCR/ABL retrovirus exhibited cytokine-independent myeloid colony outgrowth (33). Rap (2-10 nM) or IM (0.5 μM) inhibited myeloid colony formation by 50-60%, but combining the agents resulted in >90% fewer myeloid colonies (Fig. 3_A_).
Fig. 3.
Rap/IM inhibits myeloid transformation by BCR/ABL. (A) Effects of Rap/IM on BCR/ABL-evoked myeloid colony outgrowth. Similar results were obtained in two independent experiments. (B) Proliferation of K562 cells exposed to IM alone or in combination with Rap for 60 h. (C) Kaplan-Meier plot showing survival of mice transplanted with BM transduced with BCR/ABL retrovirus and subsequently treated with double placebo (solid line), Rap 7 mg/kg per day plus placebo (dots and dashes), IM 70 mg/kg per day plus placebo (dotted line), or IM 70 mg/kg per day plus Rap 7 mg/kg per day (dashed line); n = 9 mice per group. Initiation of drug therapy is indicated (arrow). The log rank test was used to determine the difference between each single drug curve and the double drug curve: IM vs. double placebo (P = 0.002), Rap vs. double placebo (P = 0.04), double drug vs. IM alone (P = 0.003), double drug vs. Rap alone (P = 0.0003).
To ensure that the effects of IM/Rap were not restricted to murine cells, we assayed K562 cells, which are derived from a blast crisis CML patient (34). In this highly transformed line, Rap alone had some (but minimal) antiproliferative effects, but again, coadministration of Rap and IM resulted in increased inhibition (Fig. 3_B_). Thus, combination therapy with these two approved drugs has broad efficacy against BCR/ABL-transformed cells, and may have activity in blast crisis.
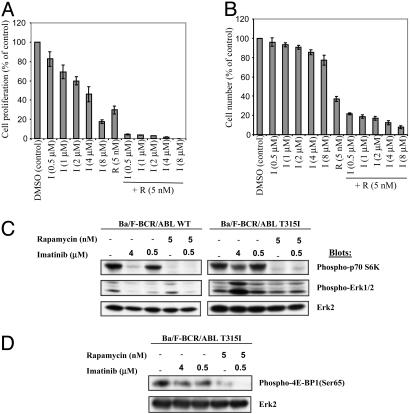
Rap Enhances the Effect of IM on Cells Expressing IM-Resistant Mutants. Mutations in the BCR/ABL kinase domain are a frequent cause of IM-resistance (35). We tested the effects of Rap on Ba/F3 cells expressing the markedly IM-resistant mutant BCR/ABL T315I. Rap alone showed comparable inhibition of proliferation of Ba/F3 cells expressing BCR/ABL WT and BCR/ABL T315I (compare Figs. 1 A and 4_A_), as predicted if mTOR were a critical downstream effector of BCR/ABL. Consistent with the IM resistance of the T315I mutant, IM doses (0.5-1 μM) that inhibited Ba/F-BCR/ABL WT cells by >50% caused little or no inhibition of Ba/F-BCR/ABL T315I (compare Figs. 1 A and 4_A_). Remarkably, combining low-dose Rap with IM markedly enhanced inhibition of Ba/F-BCR/ABL T315I cells, when measured by thymidine uptake (Fig. 4_A_) or cell number (Fig. 4_B_). The effective IC50 was lower in the latter assay, consistent with the ability of Rap to inhibit S phase entry (Fig. 2_C_). Analogous results were obtained with two other IM-resistant BCR/ABL mutants, G250E and M351T (data not shown), suggesting that Rap/IM combinations may be useful in IM-resistant CML.
Fig. 4.
Rap increases IM efficacy against IM-resistant cells. (A) Ba/F-BCR/ABL T315I cells were exposed to the indicated concentrations of IM and/or Rap and [3H]thymidine incorporation was assessed after 48 h. Values are the means of triplicate determinations; bars ± SD. (B) Viable cell number determined by trypan blue exclusion after treatment of Ba/F-BCR/ABL T315I cells with the indicated agents for 48 h. Values are expressed as percentages of controls (DMSO-treated). (C and D) The effects of IM and/or Rap on phosphorylation of p70 S6K, Erk 1/2 (C) and 4E-BP1 (D) in Ba/F-BCR/ABL WT and T315I cells were determined after 18 h of drug treatment by SDS/PAGE followed by immunoblotting with phospho-specific antibodies. Total Erk2 served as a loading control.
Rap Inhibits mTOR Targets in Ba/F-BCR/ABL WT and Ba/F-BCR/ABL T315I Cells. To begin to assess the mechanism of IM/Rap synergy, we performed immunoblotting experiments. As expected, IM markedly inhibited overall tyrosyl phosphorylation and tyrosyl phosphorylation of the BCR/ABL substrate Crk-L (36, 37) in Ba/F-BCR/ABL WT cells. In contrast, IM alone or in combination with Rap had virtually no effect on total or Crk-L tyrosyl phosphorylation in Ba/F-BCR/ABL T315I cells (Fig. 7_A_, which is published as supporting information on the PNAS web site). There was no effect of either drug alone or in combination on BCR/ABL protein levels (data not shown). Thus, Rap does not enhance inhibition of WT or IM-resistant BCR/ABL by increasing IM-mediated inhibition of BCR/ABL itself.
Treatment of Ba/F-BCR/ABL WT cells with an IM dose (0.5 μM) that causes ≈70% inhibition of proliferation (Fig. 1 A) partially inhibited p70 S6K activation (Fig. 4_C_). Not surprisingly, this dose failed to inhibit p70 S6K in Ba/F-BCR/ABL T315I cells. At higher doses of IM (4 μM), p70 S6K activation was inhibited nearly completely in Ba/F-BCR/ABL WT cells, whereas in Ba/F-BCR/ABL T315I cells, p70 S6K was inhibited only partially. Thus, the partial inhibition of proliferation in Ba/F-BCR/ABL T315I cells treated with 4 μM IM was paralleled by partial inhibition of p70 S6K. However, Rap at doses between 0.5-5 nM almost completely inhibited activation of p70 S6K in both Ba/F-BCR/ABL WT and Ba/F-BCR/ABL T315I cells (Fig. 4_C_). Interestingly, 4E-BP1 phosphorylation was more resistant than p70 S6K to treatment with either drug. Treatment of Ba/F-BCR/ABL T315I cells with IM (0.5-4 μM) or Rap (5 nM) alone partially inhibited 4E-BP1phosphorylation, as measured by multiple phospho-specific antibodies (Figs. 4_D_ and 7_B_); at the same dose, Rap totally inhibited p70 S6K phosphorylation. Combining IM with Rap led to complete inhibition of 4E-BP1 phosphorylation in Ba/F-BCR/ABL T315I cells (Figs. 4_D_ and 7_B_). Thus, suboptimal doses of IM (e.g., low-dose treatment of Ba/F-BCR/ABL WT cells or high-dose treatment of IM-resistant cells) leave the p70 S6K arm of the mTOR pathway partially active and susceptible to further inhibition by Rap. Even at significant doses of IM and Rap, however, detectable 4E-BP1 phosphorylation remains.
As expected, Rap did not inhibit Erk activation in either cell line. Although IM potently inhibited Erk activation in Ba/F-BCR/ABL WT cells, it failed to inhibit in IM-resistant cells (Fig. 4_C_). Instead, Erk activation actually increased in Ba/F-BCR/ABL T315I cells at higher IM doses. The reason for this paradoxical effect of high dose IM is unclear, although similar results were obtained in studies of IM-resistant K562 cells (38).
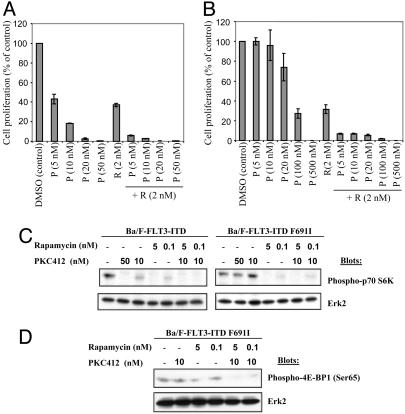
Rap in Combination with PKC412 Markedly Reduces Ba/F-FLT3-ITD Cell Proliferation. We also tested Rap alone or in combination with the FLT3 inhibitor PKC412 (13) on Ba/F3 cell lines expressing FLT3-ITD. As expected, full-dose PKC412 inhibited Ba/F-FLT3-ITD proliferation. But whereas PKC412 (5 nM) or Rap (2 nM) alone caused ≈55-60% inhibition, the drug combination inhibited proliferation by >90% (Fig. 5_A_). A PKC412-resistant cell line was derived by transduction of the PKC412-resistant FLT3-ITD mutant, F691I (D.G.G., unpublished observations), into Ba/F3 cells; these cells also express FLT3-ITD F691I at higher levels. Ba/F-FLT3-ITD F691I cells remained sensitive to the inhibitory effects of Rap alone, but were resistant to doses of PKC412 as high as 20 nM (Fig. 5_B_). Nevertheless, Rap (2 nM) plus PKC412 (5 nM) dramatically inhibited Ba/F-FLT3-ITD F691I proliferation (>90%, Fig. 5_B_). PKC412 plus Rap enhanced the inhibition of p70 S6K phosphorylation in Ba/F-FLT3-ITD F691I cells (Fig. 5_C_). Likewise, 4E-BP1 phosphorylation was >10-fold more resistant to Rap than p70 S6K in these cells (Fig. 5_D_ and Fig. 8, which is published as supporting information on the PNAS web site), and could only be substantially inhibited by the drug combination.
Fig. 5.
Effects of PKC412 and Rap on Ba/F-FLT3-ITD cell proliferation. PKC412-sensitive (Ba/F-FLT3-ITD) (A) and -resistant (Ba/F-FLT3-ITD F691I) (B) cells were exposed to the indicated concentrations of PKC412 for 48 h with or without 2 nM Rap, and proliferation was assessed. Values are the means of triplicate determinations; bars ± SD. (C and D) PKC412-sensitive or -resistant cells (as indicated) were treated with Rap, PKC412, or both, and cell lysates were subjected to immunoblotting with phospho-specific p70 S6 K (C) and 4E-BP1 (D) antibodies, followed by reprobes for Erk2 to monitor loading.
MEK Inhibition Enhances Inhibition by Rap Alone or with PTK-Is. MEK inhibitors (e.g., PD184352 or UO126) synergize with IM to induce K562 cell apoptosis (38). Erk activation paradoxically increases upon IM treatment of IM-resistant cells (Fig. 4_D_ and ref. 38), raising the possibility that residual Erk activation promotes survival of cells treated with IM/Rap. Low-dose Rap (2 nM) or IM (0.5 μM) inhibited BCR/ABL-evoked myeloid colony formation by ≈50-60%, whereas low-dose UO126 (2 μM) was only slightly (16%) inhibitory. UO126 plus Rap was additive, whereas UO126 plus IM or Rap plus IM synergistically inhibited myeloid colony outgrowth. Combining low doses of all three agents caused profound inhibition (96%) in this assay (Fig. 6_A_). These combinations had either no or minimal inhibitory effects on cytokine-evoked colony formation (Fig. 6_B_).
Fig. 6.
MEK inhibition increases effects of Rap/PTK-I combinations. (A) Murine BM cells, transduced with BCR/ABL-expressing retroviruses, were treated with IM, Rap, and/or UO126, and myeloid colonies were quantified at day 10. (B) Murine BM cells transduced with control GFP-expressing retroviruses were plated in methylcellulose media in the presence of a cytokine mixture [IL-3 (0.5 ng/ml), granulocyte/macrophage colony-stimulating factor (1 ng/ml), stem cell factor (1 ng/ml), and IL-6 (1 ng/ml)]. The indicated drugs were added, and myeloid colonies were counted at day 10. Ba/F-BCR/ABL T315I (C) or Ba/F-FLT3-ITD F691I (D) cells were exposed to the indicated drugs alone or in various combinations for 48 h, followed by measurement of cell proliferation.
In Ba/F-BCR/ABL T315I cells, UO126 (at 5 μM) or IM (at 0.5 μM) were only modestly inhibitory, although Rap (5 nM) inhibited proliferation by ≈70% (Fig. 6_C_). Inhibition by UO126 plus IM was additive, whereas UO126 plus Rap or IM plus Rap synergistically inhibited Ba/F-BCR/ABL T315I proliferation (Fig. 6_C_); inhibition by Rap plus UO126 plus IM was even more robust.
MEK inhibition also enhanced the potency of Rap and/or PKC412 in FLT3-ITD-transformed cells. Whereas PKC412 at 10 nM had almost no effect, Rap (2 nM) or UO126 (5 μM) inhibited PKC412-resistant Ba/F-FLT3-ITD F691I proliferation by 68% or 40%, respectively. Proliferation of these cells was profoundly inhibited when UO126 was added to Rap (Fig. 6_D_). Inhibition by Rap plus PKC412 also was synergistic, whereas UO126 plus PKC412 exhibited additive effects. The three-drug combination almost completely (>99%) inhibited proliferation and survival. Thus, Rap together with a FLT3 inhibitor and/or a MEK inhibitor may be useful for the treatment of disease caused by FLT3 mutants.
Rap Increases Efficacy of IM in Mice. BCR/ABL causes a CML-like myeloproliferative disease (MPD) in mice (5). Unlike human CML, murine BCR/ABL-evoked MPD is aggressive, with death typically ensuing within 3-4 weeks. In this model, mice treated with therapeutic doses of IM (150 mg/kg per day) survive indefinitely if therapy is continued (39).
Therefore, we tested whether Rap enhances the efficacy of a subtherapeutic dose of IM on this disease. Mice subjected to BCR/ABL retroviral gene transduction/BMT, followed by placebo treatment, died by day 20 after transplantation, with a median survival of 17 days (Fig. 3_C_). Suboptimal IM (70 mg/kg per day) extends survival (median 21.5 days) significantly compared to placebo-treated controls (P = 0.002). Mice treated with Rap alone (Rap plus placebo) also survived longer (median 19 days) than those treated with placebo (P = 0.04). However, animals treated with IM plus Rap showed improved survival (median 25.5 days), compared to those treated with either IM alone (P = 0.003) or Rap alone, (P = 0.0003), indicating that the IM/Rap combination improves efficacy in vivo.
Discussion
The success of IM shows that targeted therapies can have dramatic efficacy with low toxicity. However, drug resistance probably will impede curative monotherapy with signal transduction inhibitors. Combining inhibitors of different signaling pathways may circumvent this resistance. Our data show that Rap synergizes with IM to inhibit a wide variety of BCR/ABL-transformed cells and with PKC412 against FLT3-ITD-transformed cells. Even more potent inhibition is achieved by adding a MEK inhibitor to this regimen. Such combinations may improve the therapy of primary and relapsed CML and/or acute myelogenous leukemia (AML) caused by FLT3 mutations. Indeed, Rap improves the survival of mice with BCR/ABL-evoked myeloproliferative disease treated with suboptimal doses of IM.
We tested IM/Rap for several reasons. The drugs have known mechanisms of action and are already used clinically. Both are well tolerated at serum levels above those in our studies. The developmental path for two approved drugs, and thus the potential for benefiting patients rapidly, is likely to be shorter than for two novel agents. The PI3K/Akt pathway is critical for transformation (21, 22, 40), and mTOR is a component of this pathway (25, 26, 41). But mTOR also receives inputs from other pathways (i.e., mTOR is downstream of and parallel to BCR/ABL), so one might imagine improved efficacy of combined inhibition of leukemogenic PTKs and mTOR.
Rap alone significantly inhibits BCR/ABL-transformed primary lymphoid and myeloid cells (Figs. 2 A and 3_A_), demonstrating a requirement for mTOR in BCR/ABL transformation. More importantly, Rap plus IM is synergistic (Fig. 1 _B_-D). Also, whereas low doses of either agent alone cause G1 arrest, cells exposed to IM/Rap exhibit substantial apoptosis (Fig. 2_C_). Any attempt to improve on IM monotherapy must circumvent IM-resistant mutations. Rap not only remains active as a single agent against the most IM-resistant mutant reported (T315I) and several other BCR/ABL mutants, but also enhances the effect of IM on resistant mutant-expressing cells. After our work was completed, Ly et al. (42) reported increased inhibition of Ba/F-BCR/ABL cells by IM plus Rap. Our work substantially extends these findings by showing that the combination also has efficacy against primary BCR/ABL-transformed myeloid and lymphoid cells and a murine model of CML, and extends to FLT3-ITD transformed cells. Ly et al. did not observe any effect of the drug combination on Ba/F-BCR/ABL T315I cells. We cannot account for this discrepancy, as we have observed enhanced inhibition by Rap/IM on several IM-resistant mutants, and Rap also increases inhibition of PKC412-resistant FLT3-ITD cells (Fig. 5_B_).
Combining IM with chemotherapy also results in increased inhibition of BCR/ABL-transformed cell lines (43-46). The efficacy of these drug combinations for treating IM-resistant mutants remains to be assessed. Other signaling inhibitors also have been combined with IM. The farnesyl transferase inhibitor SCH66336 plus IM increased apoptosis in Ba/F3 cells resistant to IM because of BCR/ABL amplification, but not BCR/ABL T315I mutation (47). Hsp90 inhibitors inhibit Ba/F3 cells expressing WT and IM-resistant BCR/ABL, and induce the degradation of WT and IM-resistant mutant BCR/ABL (48). Their efficacy in combination with IM remains to be evaluated. Also, MEK inhibitors synergize with IM to induce K562 cell apoptosis (38).
We tested IM/UO126, Rap/UO126, and the three drugs together in several systems. Like IM/UO126 (or PD184352), Rap/IM potently inhibited K562 cells, but Rap (2 nM) is a better inhibitor of BCR/ABL-evoked myeloid colony formation than UO126 (2 μM) (Fig. 6_A_). Whereas both combinations are synergistic, IM/Rap is superior to IM/UO126. Most impressive was the three-drug combination: submaximal doses of each essentially eliminated BCR/ABL-evoked myeloid colonies. This is not nonspecific toxicity, given the minimal effect on cytokine-evoked colonies (Fig. 6_B_). Analogous results were obtained with FLT3-ITD cells.
Biochemical analyses provide some clues to the enhanced efficacy of these combinations and reveal previously undescribed features of Rap action. Rap (at 0.5-5 nM) inhibited p70 S6K in Ba/F-BCR/ABL WT and T315I cells. IM also inhibits p70 S6K in Ba/F-BCR/ABL WT cells, but consistent with their resistant phenotype, there is far less (but detectable) inhibition of p70 S6K by IM in T315I cells. Thus, addition of Rap to resistant cells enhances inhibition of p70 S6K. However, 4E-BP1 phosphorylation may be a better correlate for the inhibitory effects of Rap alone or with PTK-Is. Whereas p70 S6K is inhibited almost completely by doses of Rap as low as 0.05 nM (data not shown), 4E-BP1 phosphorylation was inhibited only partially at 5 nM Rap (Figs. 4_D_ and 7_B_). Thus, low doses of IM and Rap alone, even in BCR/ABL WT cells, but certainly in IM-resistant cells, are insufficient to eliminate 4E-BP1 phosphorylation, yet the drug combination completely blocks this phosphorylation. Whether the effects of IM (in combination with Rap) on IM-resistant cells reflects some low level of residual BCR/ABL inhibition, or rather, that Rap “exposes” some cryptic dependence on other IM targets (e.g., Kit, c-Abl) in these cells remains unknown.
Why Rap's potency against mTOR-dependent pathways is different also is unclear. Consistent with in vitro studies (49), FKBP12-Rap may more potently inhibit mTOR phosphorylation of p70 S6K. This may reflect distinct mTOR complexes for phosphorylation of p70 S6K and 4E-BP1 with differential sensitivity to FKBP12-Rap (50, 51). Alternatively, 4E-BP1 may be a better mTOR substrate than p70 S6K, so that low levels of residual mTOR activity phosphorylates the former, but not the latter. mTOR reportedly regulates protein phosphatase 2A (PP2A) (52); PP2A regulation by mTOR could differentially affect p70 S6K and 4E-BP1.
The ultimate test of any therapy is efficacy and toxicity in vivo. In a murine model of CML, addition of Rap to a subtherapeutic dose of IM extended survival by almost 2-fold (Fig. 3_C_). Although treated mice still die, the outcome may be far more favorable when both agents are used at therapeutic doses. The strong synergy between Rap (and MEK inhibitors) and IM suggests that clinical trials of these combinations be considered for treatment of CML and gastrointestinal stromal tumor. Likewise, tests of Rap (and possibly MEK inhibitors) plus PKC412 in acute myelogenous leukemia (AML) seem warranted. Although we only studied two leukemogenic PTKs in detail, our data suggest that combined mTOR/PTK inhibition also may be useful for other neoplasms. Indeed, Rap potentiates Herceptin inhibition of ErbB2+ breast cancer cells (M.G.M., L. Harris, and B.G.N., unpublished data) and the effect of IM or the epidermal growth factor receptor inhibitor PD 168393 on prostate cancer cells (M.G.M., D. Masiello, S. Balk, B.G.N., and G. Bubley, unpublished data).
Supplementary Material
Supporting Figures
Acknowledgments
We thank Novartis for providing PKC412, Doriano Fabbro (Novartis) for providing BCR/ABL T315I, and John Blenis, Diane Fingar (Harvard Medical School), and Ellen Weisberg (Dana-Farber Cancer Institute) for helpful comments. This work was supported by National Institutes of Health Grants R01 DK50654 and PO1 DK50693 (to B.G.N.) and PO1 CA66996 (to J.D.G. and D.G.G.). D.G.G. is an Associate Investigator of the Howard Hughes Medical Institute. M.G.M. is supported by a fellowship from the Leukemia and Lymphoma Society.
Abbreviations: PTK, protein tyrosine kinase; CML, chronic myelogenous leukemia; PDGFR, platelet-derived growth factor receptor; ITD, internal tandem duplication; IM, Imatinib; PTK-I, PTK inhibitor; PI3K, phosphatidylinositol 3-kinase; MEK, mitogen-activated protein kinase kinase; Rap, rapamycin, p70 S6K, p70 S6 kinase; BM, bone marrow; BMT, BM transplantation.
References
- 1.Chopra, R., Pu, Q. Q. & Elefanty, A. G. (1999) Blood Rev. 13**,** 211-229. [DOI] [PubMed] [Google Scholar]
- 2.Golub, T. R., Barker, G. F., Lovett, M. & Gilliland, D. G. (1994) Cell 77**,** 307-316. [DOI] [PubMed] [Google Scholar]
- 3.Gilliland, D. G. (2001) Curr. Opin. Hematol. 8**,** 189-191. [DOI] [PubMed] [Google Scholar]
- 4.Gilliland, D. G. & Griffin, J. D. (2002) Curr. Opin. Hematol. 9**,** 274-281. [DOI] [PubMed] [Google Scholar]
- 5.Van Etten, R. A. (2002) Oncogene 21**,** 8643-8651. [DOI] [PubMed] [Google Scholar]
- 6.Druker, B. J. & Lydon, N. B. (2000) J. Clin. Invest. 105**,** 3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantarjian, H., Sawyers, C., Hochhaus, A., Guilhot, F., Schiffer, C., Gambaccorti-Passerini, C., Niederwieser, D., Resta, D., Capdeville, R. & Zoellner, U. E. (2002) N. Engl. J. Med. 346**,** 645-652. [DOI] [PubMed] [Google Scholar]
- 8.Druker, B. J. (2002) Oncogene 21**,** 8541-8546. [DOI] [PubMed] [Google Scholar]
- 9.Joensuu, H., Roberts, P. J., Sarlomo-Rikala, M., Anderson, L. C., Tervahartiala, P., Tuveson, D., Silberman, S., Capdeville, R., Dimitrijevic, S., Druker, B. & Demetri, G. D. (2001) N. Engl. J. Med. 344**,** 1052-1056. [DOI] [PubMed] [Google Scholar]
- 10.Demetri, G. D., von Mehren, M., Blanke, C. D., Van den Abbeele, A. D., Eisenberg, B., Roberts, P. J., Heinrich, M. C., Tuveson, D. A., Singer, S., Janicek, M., et al. (2002) N. Engl. J. Med. 347**,** 462-463. [DOI] [PubMed] [Google Scholar]
- 11.Apperley, J. F., Gardembas, M., Melo, J. V., Russell-Jones, R., Bain, B. J., Baxter, E. J., Chase, A., Chessells, J. M., Colombat, M., Dearden, C. E., et al. (2002) N. Engl. J. Med. 347**,** 481-487. [DOI] [PubMed] [Google Scholar]
- 12.Cools, J., DeAngelo, D. J., Gotlib, J., Stover, E. H., Legare, R. D., Cortes, J., Kutok, J., Clark, J., Galinsky, I., Griffin, J. D., et al. (2003) N. Engl. J. Med. 348**,** 1199-1200. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg, E., Boulton, C., Kelly, L. M., Manley, P., Fabbro, D., Meyer, T., Gilliland, D. G. & Griffin, J. D. (2002) Cancer Cell 1**,** 433-443. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, L. M., Yu, J. C., Boulton, C., Apatira, M., Li, J., Sullivan, C. M., Williums, I., Amaral, S. M., Curley, D. P., Duclos, N., et al. (2002) Cancer Cell 1**,** 421-432. [DOI] [PubMed] [Google Scholar]
- 15.Levis, M., Allebach, J., Tse, K. F., Zheng, R., Baldwin, B. R., Smith, B. D., Jones-Bolin, S., Ruggeri, B., Dionne, C. & Small, D. (2002) Blood 99**,** 3885-3891. [DOI] [PubMed] [Google Scholar]
- 16.O'Farrell, A. M., Abrams, T. J., Yuen, H. A., Ngai, T. J., Louie, S. G., Yee, K. W., Wong, L. M., Hong, W., Lee, L. B., Town, A., et al. (2003) Blood 101**,** 3597-3605. [DOI] [PubMed] [Google Scholar]
- 17.Druker, B. J., Sawyers, C. L., Kantarjian, H., Resta, D. J., Reese, S. F., Ford, J. M., Capdeville, R. & Talpaz, M. (2001) N. Engl. J. Med. 344**,** 1038-1042. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien, S. G., Guilhot, F., Larson, R. A., Gathmann, I., Baccarani, M., Cervantes, F., Cornelissen, J. J., Fischer, T., Hochhaus, A., Hughes, T., et al. (2003) N. Engl. J. Med. 348**,** 994-1004. [DOI] [PubMed] [Google Scholar]
- 19.Wu, C. J., Neuberg, D., Chillemi, A., McLaughlin, S., Hochberg, E. P., Galinsky, I., DeAngelo, D., Soiffer, R. J., Alyea, E. P., Capdeville, R., et al. (2002) Leuk. Lymphoma 43**,** 2281-2289. [DOI] [PubMed] [Google Scholar]
- 20.Azam, M., Latek, R. R. & Daley, G. Q. (2003) Cell 112**,** 831-843. [DOI] [PubMed] [Google Scholar]
- 21.Sattler, M., Mohi, M. G., Pride, Y. B., Quinnan, L. R., Malouf, N. A., Podar, K., Gesbert, F., Iwaski, H., Li, S., Van Etten, R. A., et al. (2002) Cancer Cell 1**,** 479-492. [DOI] [PubMed] [Google Scholar]
- 22.Skorski, T., Kanakaraj, P., Nieborowskaskorska, M., Ratajczak, M. Z., Wen, S. C., Zon, G., Gewirtz, A. M., Perussia, B. & Calabretta, B. (1995) Blood 86**,** 726-736. [PubMed] [Google Scholar]
- 23.Cortez, D., Reuther, G. & Pendergast, A. M. (1997) Oncogene 15**,** 2333-2342. [DOI] [PubMed] [Google Scholar]
- 24.Sebolt-Leopold, J. (2000) Oncogene 19**,** 6594-6599. [DOI] [PubMed] [Google Scholar]
- 25.Sekulic, A., Hudson, C. C., Homme, J. L., Yin, P., Otterness, D. M., Karnitz, L. M. & Abraham, R. T. (2000) Cancer Res. 60**,** 3504-3513. [PubMed] [Google Scholar]
- 26.Manning, B. D., Tee, A. R., Logsdon, M. N., Blenis, J. & Cantley, L. C. (2002) Mol. Cell 10**,** 151-162. [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo, M. & Rowinsky, E. K. (2000) Oncogene 19**,** 6680-6686. [DOI] [PubMed] [Google Scholar]
- 28.Gingras, A.-C., Raught, B. & Sonenberg, N. (2001) Genes Dev. 15**,** 807-826. [DOI] [PubMed] [Google Scholar]
- 29.Sawyers, C. L. (2003) Cancer Cell 4**,** 343-348. [DOI] [PubMed] [Google Scholar]
- 30.Chou, T.-C. & Talalay, P. (1983) Trends Pharmacol. Sci. 4**,** 450-454. [Google Scholar]
- 31.Gu, H., Maeda, H., Moon, J. J., Lord, J. D., Yoakim, M., Nelson, B. H. & Neel, B. G. (2000) Mol. Cell. Biol. 20**,** 7109-7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald, A., Scarola, J., Burke, J. T. & Zimmerman, J. J. (2000) Clin. Ther. 22**,** Suppl. B**,** B101-B121. [DOI] [PubMed] [Google Scholar]
- 33.Gishizky, M. L. & Witte, O. N. (1992) Science 256**,** 836-839. [DOI] [PubMed] [Google Scholar]
- 34.Lozzio, C. B. & Lozzio, B. B. (1975) Blood 45**,** 321-334. [PubMed] [Google Scholar]
- 35.Shah, N. P., Nicoll, J. M., Nagar, B., Gorre, M. E., Paquette, R. L., Kuriyan, J. & Sawyers, C. L. (2002) Cancer Cell 2**,** 117-125. [DOI] [PubMed] [Google Scholar]
- 36.Nichols, G. L., Raines, M. A., Vera, J. C., Lacomis, L., Tempst, P. & Golde, D. W. (1994) Blood 84**,** 2912-2918. [PubMed] [Google Scholar]
- 37.Oda, T., Heaney, C., Hagopian, J. R., Okuda, K., Griffin, J. D. & Druker, B. J. (1994) J. Biol. Chem. 269**,** 22925-22928. [PubMed] [Google Scholar]
- 38.Yu, C., Krystal, G., Varticovksi, L., McKinstry, R., Rahmani, M., Dent, P. & Grant, S. (2002) Cancer Res. 62**,** 188-199. [PubMed] [Google Scholar]
- 39.Wolff, N. C. & Ilaria, R. L., Jr. (2001) Blood 98**,** 2808-2816. [DOI] [PubMed] [Google Scholar]
- 40.Skorski, T., Bellacosa, A., Nieborowska-Skorska, M., Majewski, M., Martinez, R., Choi, J. K., Trotta, R., Wlodarski, P., Perrotti, D., Chan, T. O., et al. (1997) EMBO J. 16**,** 6151-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tee, A. R., Fingar, D. C., Manning, B. D., Kwiatkowski, D. J., Cantley, L. C. & Blenis, J. (2002) Proc. Natl. Acad. Sci. USA 99**,** 13571-13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ly, C., Arechiga, A. F., Melo, J. V., Walsh, C. M. & Ong, S. T. (2003) Cancer Res. 63**,** 5716-5722. [PubMed] [Google Scholar]
- 43.Thiesing, J. T., Ohno-Jones, S., Kolibaba, K. S. & Druker, B. J. (2000) Blood 96**,** 3195-3199. [PubMed] [Google Scholar]
- 44.Topaly, J., Zeller, W. J. & Fruehauf, S. (2001) Leukemia 15**,** 342-347. [DOI] [PubMed] [Google Scholar]
- 45.Marley, S. B., Davidson, R. J., Goldman, J. M. & Gordon, M. Y. (2002) Br. J. Haematol. 116**,** 162-165. [DOI] [PubMed] [Google Scholar]
- 46.Porosnicu, M., Nimmanapalli, R., Nguyen, D., Worthington, E., Perkins, C. & Bhalla, K. N. (2001) Leukemia 15**,** 772-778. [DOI] [PubMed] [Google Scholar]
- 47.Hoover, R. R., Mahon, F.-X., Melo, J. V. & Daley, G. Q. (2002) Blood 100**,** 1068-1071. [DOI] [PubMed] [Google Scholar]
- 48.Gorre, M. E., Ellwood-Yen, K., Chiosis, G., Rosen, N. & Sawyers, C. L. (2002) Blood 100**,** 3041-3044. [DOI] [PubMed] [Google Scholar]
- 49.McMahon, L. P., Choi, K. M., Lin, T.-A., Abraham, R. T. & Lawrence, J. C., Jr. (2002) Mol. Cell. Biol. 22**,** 7428-7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loewith, R., Jacinto, E., Wullschleger, S., Lorberg, A., Crespo, J. L., Bonenfant, D., Oppliger, W., Jenoe, P. & Hall, M. N. (2002) Mol. Cell 10**,** 457-468. [DOI] [PubMed] [Google Scholar]
- 51.Hara, K., Maruki, Y., Long, X., Yoshino, K., Oshiro, N., Hidayat, S., Tokunaga, C., Avruch, J. & Yonezawa, K. (2002) Cell 110**,** 177-189. [DOI] [PubMed] [Google Scholar]
- 52.Peterson, R. T., Desai, B. N., Hardwick, J. S. & Schreiber, S. L. (1999) Proc. Natl. Acad. Sci. USA 96**,** 4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figures