Neurod1 regulates survival and formation of connections in mouse ear and brain (original) (raw)
. Author manuscript; available in PMC: 2013 May 20.
Published in final edited form as: Cell Tissue Res. 2010 May 30;341(1):95–110. doi: 10.1007/s00441-010-0984-6
Abstract
The developing sensory neurons of the mammalian ear require two sequentially activated bHLH genes, Neurog1 and Neurod1, for their development. Neurons never develop in Neurog1 null mice, and most neurons die in Neurod1 null mutants, a gene upregulated by Neurog1. The surviving neurons of Neurod1 null mice are incompletely characterized in postnatal mice because of the early lethality of mutants and the possible compromising effect of the absence of insulin on peripheral neuropathies. Using Tg (Pax2-cre), we have generated a conditional deletion of floxed Neurod1 for the ear; this mouse is viable and allows us to investigate ear innervation defects of Neurod1 absence only in the ear. We have compared the defects in embryos and show an ear phenotype in conditional Neurod1 null mice comparable with the systemic Neurod1 null mouse. By studying postnatal animals, we show that Neurod1 not only is necessary for the survival of most spiral and many vestibular neurons, but is also essential for a segregated central projection of vestibular and cochlear afferents. In the absence of Neurod1 in the ear, vestibular and cochlear afferents enter the cochlear nucleus as a single mixed nerve. Neurites coming from vestibular and cochlear sensory epithelia project centrally to both cochlear and vestibular nuclei, in addition to their designated target projections. The peripheral innervation of the remaining sensory neurons is disorganized and shows collaterals of single neurons projecting to multiple endorgans, displaying no tonotopic organization of the organ of Corti or the nucleus. Pending elucidation of the molecular details these Neurod1 functions, these data demonstrate Neurod1 is not only a major factor for the survival neurons but is crucial for the development of normal connections, both in the ear and in the central system.
Keywords: Ear development, Neuronal development, Afferent connections, Cochlea, Vestibular ganglion, Organ of Corti, Mouse (Neurod1 conditional knockout)
Introduction
Basic helix-loop-helix (bHLH) genes contribute to proliferation, cell cycle exit, cell fate acquisition, and differentiation of neuron and glia cell types (Guillemot 2007; Ohsawa and Kageyama 2008) by interacting with various suppressors (Hes, Id), by combining in various ways (Neurog2/Neurod1), and by cooperating to regulate downstream genes (Kageyama et al. 2007). The absence of bHLH genes can result in phenotype switch (Ma et al. 1999; Shroyer et al. 2007), lack of differentiation (Bermingham et al. 1999), or cell death (Chen et al. 2002). Acquisition of cellular identity depends on proneural bHLH genes, whereas inhibitors of their function (Hes, Id) retain cells in an unspecified proliferative stage or promote the differentiation of glia/supporting cells.
Three bHLH genes are important for the ear: Neurog1 (Ma et al. 1998), Atoh1 (Bermingham et al. 1999), and Neurod1 (Kim et al. 2001). Neurog1 null mice lack inner ear neurons, Atoh1 null mice lack differentiated hair cells, and Neurod1 null mice lack most spiral and many vestibular neurons. In addition, lack of Neurog1 results in massive hair cell loss in the saccule and cochlea (Ma et al. 2000; Matei et al. 2005), and the absence of Atoh1 results not only in hair cell death (Chen et al. 2002), but also the remaining _Atoh1_-lacZ-positive cells remain epithelial (Dabdoub et al. 2008; Fritzsch et al. 2005a). Other than the initial description of neuronal loss (Kim et al. 2001; Liu et al. 2000) and the more recent verification that the deafness in these mice is related to the disorganization of the cochlea (Xia et al. 2007), no detailed analysis has been performed on the remaining neurons and their peripheral and central innervations in Neurod1 null mice. The lack of further studies is in large measure related to the limited viability of these mice, as they also lack pancreatic beta cells and thus are insulin-deficient.
We have generated a conditional Neurod1 null mouse by crossing a floxed Neurod1 line (Goebbels et al. 2005; Pan et al. 2009) with a Tg(Pax2-cre) line (Ohyama and Groves 2004) thus avoiding insulin deficiency. We show that this conditional deletion of Neurod1 has embryonic ear defects comparable with the simple Neurod1 null mouse, but that these mice can be used to investigate postnatal effects in an otherwise uncompromised animal. Our data suggest that Neurod1 is essential for the proper migration and segregation of spiral and vestibular neurons and for the differential peripheral and central projections of vestibular and cochlear afferents.
Materials and methods
Mice and genotyping for generation of conditional Neurod1 knockout mice
Neurod1 systemic null (KO) mice die within a few days after birth because of severe hyperglycemia. To overcome this problem, we extended our analysis in the inner ear by using Neurod1 conditional knockout (CKO) mice [Neurod1f/f,Tg(Pax2-cre)].
To generate the Neurod1 CKO mice, we crossed a _Pax2_-cre line (Ohyama and Groves 2004) with the floxed Neurod1 line (Goebbels et al. 2005). For this study, we crossed homozygotic floxed Neurod1 mice (Neurod1f/f) with heterozygous Neurod1f/+,Tg(Pax2-cre) mice. The resulting Neurod1f/f,Tg(Pax2-cre) mice were CKO mutants, and the Neurod1f/+,Tg(Pax2-cre) heterozygous siblings served as controls, here referred to as wildtypes. To monitor endogenous Neurod1 expression, we used Neurod1f/z,Tg(Pax2-cre) mice as mutants and Neurod1+/z mice as controls, in which a LacZ reporter replaced the Neurod1 coding region (Kim et al. 2001). Expression of the lacZ reporter was monitored by β-galactosidase staining. To compare the Neurod1 CKO effect with the systemic Neurod1 null mice (Neurod1 KO mice), we examined the Neurod1 z/z mice. Offspring was genotyped by polymerase chain reaction analysis of tail DNA by using _cre_-specific primers, which produce a 280-bp product, and _Neurod1_-specific primers, which produce a 400-bp product from the _Neurod1_-coding region and a 600-bp product from the floxed allele. Embryos were collected from timed pregnant females at embryonic day 9.5 ( E9.5), E11.5, E12.5, E13.5, and E14.5, counting noon of the day the vaginal plug was found as E0.5. We also analyzed mice at postnatal day 0 (P0), P7, P14, and P30. Pregnant mothers or juvenile mice were anesthetized with a lethal dose of Avertin (1.25% 2.2.2-tribromoethanol at a dose of 0.025 ml/g body weight). Embryos were dissected from the uterus and perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4) by using a peristaltic pump. Heads were isolated and fixed in 4% PFA for further analysis. All animal procedures were approved by the University of Iowa Animal Care and Use Committee (IACUC) (ACURF #0804066).
X-gal staining
Heads of mice perfused with 4% PFA were hemisected. Ears were dissected and then briefly washed with 0.1 M phosphate buffer. The samples were stained in a solution containing 0.1 M phosphate buffer, 0.01% deoxycholic acid, 0.02% NP40, 2 mM magnesium chloride, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 0.1 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactoside) for about 24 h at room temperature (Matei et al. 2006).
In situ hybridization
In situ hybridization was performed by using an RNA probe labeled with digoxigenin. The plasmids containing the cDNAs were used to generate the RNA probe by in vitro transcription. The dissected ears were dehydrated in 100% methanol and rehydrated in graded methanol and digested briefly with 20 μg/ml proteinase K (Ambion, Austin, Tex., USA) for 15–20 min. The samples were then hybridized overnight at 60°C to the riboprobe in hybridization solution containing 50% (v/v) formamide, 50% (v/v) 2× saline sodium citrate (Roche), and 6% (w/v) dextran sulfate. After the unbound probe had been washed off, the samples were incubated overnight with an anti-digoxigenin antibody (Roche Diagnostics, Mannheim, Germany) conjugated with alkaline phosphatase. Following a series of washes, the samples were reacted with nitroblue phosphate/5-bromo, 4-chloro, 3-indolyl phosphate (BM purple substrate; Roche Diagnostics, Germany) which is enzymatically converted to a purple-colored product. The ears were mounted flat in glycerol and viewed with a Nikon Eclipse 800 microscope by differential interference contrast microscopy, and images were captured with MetaMorph software. All probes used in this study are listed in Table 1.
Table 1.
List of probes
| Probe | Source |
|---|---|
| Neurod1 | Lee et al. 1995 |
| Neurog1 | Ma et al. 1998 |
| Nhlh1 and Nhlh2 | Gift from Dr. Thomas Braun, Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany |
| Prox1 | Oliver et al. 1993 |
Immunofluorescence
For immunofluorescence staining, the ears were dehydrated in 100% ethanol overnight and rehydrated and blocked with 0.25% normal goat serum in phosphate-buffered saline (PBS) containing 0.01% Triton X-100 for 1 h. The primary antibodies for tubulin (Sigma), Myo VII (Myosin VIIa, Proteus Biosciences), and caspase 3 (Cell Signaling Technology) were used at dilutions of 1:800, 1:200, and 1:100, respectively. Ears were incubated with antibodies for 48 h at 4°C. After several washes with PBS, corresponding secondary antibodies (Alexa fluor molecular probe 647 or 532 or 488; Invitrogen) were added at a dilution of 1:500 and incubated overnight at 4°C. The ears were washed with PBS and mounted in glycerol. Images were taken with a Leica TCS SP5 confocal microscope.
Lipophilic dye tracing
The heads of the mice were cut sagittally along the midline, and three different colored dyes were inserted to label the afferent and efferent fibers from and to the inner ear. Lipophilic-dye-soaked filter strips (Fritzsch et al. 2005b) were inserted into the alar plate of the brainstem to label the afferent fibers of the eighth cranial nerve. Efferent fibers were labeled by applying dye into the olivo-cochlear efferent bundle as it crosses the floor plate in rhombomere 4. Disregarding the type of dye inserted into the brainstem, the afferents were shown with a green color and efferents with a red color. Half heads were kept in an oven at 60°C for about 3–7 days depending on the age of the mice to allow proper diffusion. In the E14.5 mice the dyes were injected into rhombomeres 2, 5, and 7 to label the afferent and efferent fibers. The ears, vestibular ganglia, and brains were subsequently dissected out for analysis, and images were taken via the Leica TCS SP5 confocal microscope. Afferent tracing from the ear was performed by selectively placing the dye-soaked filter strips into the vestibular and cochlear sensory epithelia. For double- and triple-color tracing from the ear, NeuroVue red dye inserted into the cochlear apex was coded as red and NeuroVue maroon, a blue color dye inserted into the base was coded as blue. NeuroVue Jade dye, which gives a yellow color with white light, was applied to the anterior crista/horizontal crista or utricle and was shown with a false color code as green in epifluorescent light. Brains were viewed as whole-mounts or as coronal sections.
Results
KO and CKO Neurod1 null mice have a similar ear phenotype
The near complete early fatality of the Neurod1 KO mice (Kim et al. 2001) combined with the possibility of additional ear-specific phenotypes attributable to insulin deficiency (Sanchez-Calderon et al. 2007) has arrested analysis in postnatal mice as proinsulin plays a neuro-protective role (Sanchez-Calderon et al. 2007). Moreover, one characteristic of the lack of insulin is peripheral neuropathy, a problem that could compromise later stage analysis of innervation in Neurod1 null mice. We have sidestepped this problem by crossing a Tg(Pax2-cre) line (Ohyama and Groves 2004) with a conditional deletion of Neurod1 (Goebbels et al. 2005) to generate a new line that is viable and fertile. _Pax2_-cre is expressed in the early somite stage in the ear before and during Neurod1 expression (Kim et al. 2001; Ohyama and Groves 2004). In contrast to late-expressing Tg(Atoh1-cre) that had no effect on inner ear neurons (Pan et al. 2009), Tg(Pax2-cre) has been used to effectively recombine floxed genes such as Dicer (Soukup et al. 2009) in the ear.
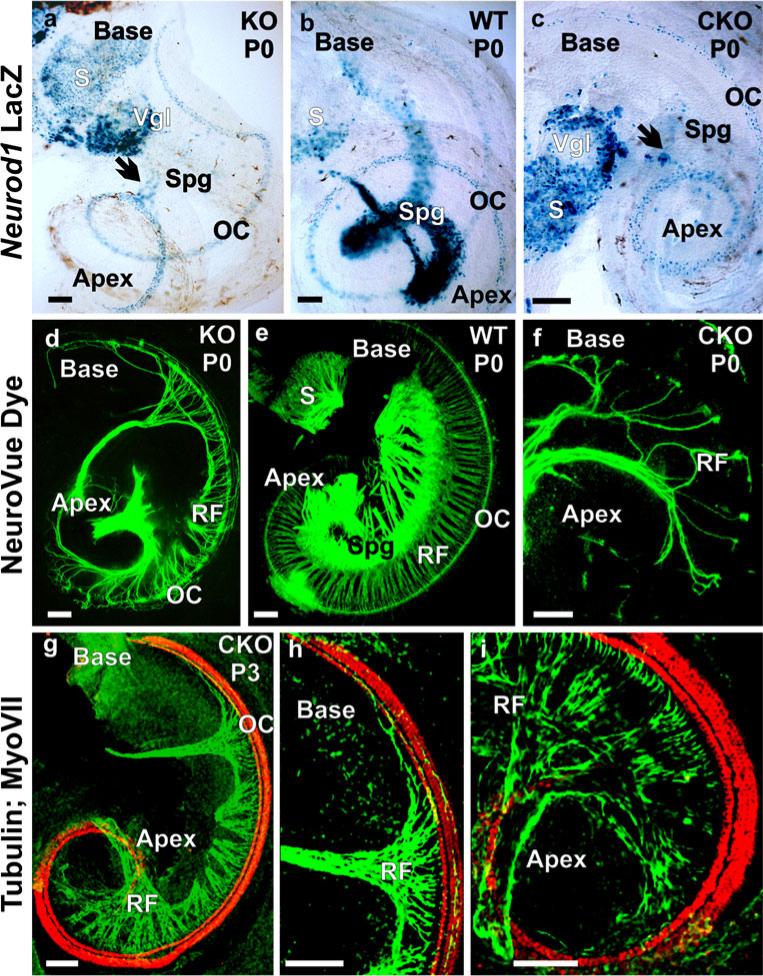
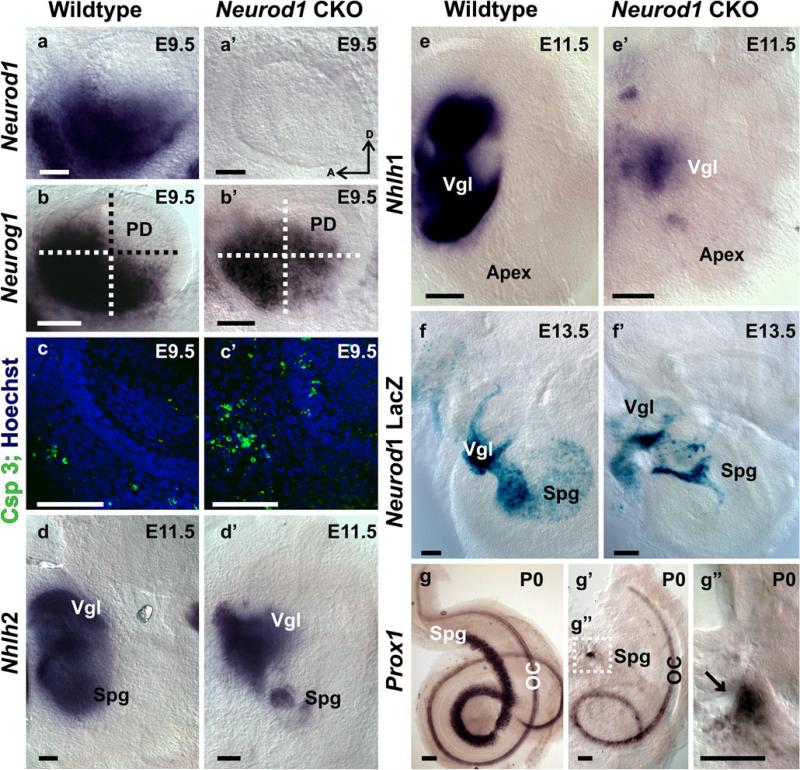
To ascertain the effectiveness of conditionally deleted Neurod1 with Tg(Pax2-cre), we compared the phenotypes of Neurod1 CKO mice with those of Neurod1 KO mice. The loss of spiral ganglia was remarkable and indistinguishable for Neurod1 CKO and KO mice (Fig. 1) with only a few scattered surviving ganglion neurons near the middle turn of the cochlea (Fig. 1a–c). _Neurod1_-lacZ showed a massive loss of spiral ganglia with some vestibular ganglia in both types of mutants, compared with heterozygotic control mice (Fig. 1a–c). In addition to sensory neurons, _Neurod1_-lacZ was expressed in many hair cells of all sensory epithelia of both CKO and KO mice (Fig. 1a, c). In control mice, _Neurod1_-lacZ expression was also detected in hair cells but with a more prominent expression in the spiral and vestibular ganglia (Fig. 1b).
Fig. 1.
Neurod1 conditional knockout (CKO) and knockout (KO) mice have a similar phenotype. In newborn mice, _Neurod1_-LacZ reaction shows expression in spiral ganglion (Spg) in wildtype (WT) mice (b), whereas only a few scattered spiral ganglion neurons are positive for _Neurod1_-LacZ in the Neurod1 KO and CKO (arrows in a, c). Additional expression of _Neurod1_-LacZ is seen in the hair cells of the organ of Corti (OC). The near complete loss of spiral ganglion neurons results in alterations of the projections of afferents in both mutants (d, f). The wildtype (e) has a well-developed spiral ganglion, and lipophilic dyes show regularly spaced radial fibers that carry both afferent and efferent fibers to the OC. In contrast, fewer and disorganized afferents reach the OC in both Neurod1 KO and CKO mice. The fiber reduction in the CKO mice is more severe in the apex and base, with some retained innervation in the middle turn, consistent with the region of remaining spiral ganglion neurons shown with tubulin (green) and Myo VII (red) immunofluorescence staining (g–i). (RF radial fibers, S saccule, Vgl vestibular ganglion). Bars 100 μm
Absence of Neurod1 causes massive reduction and disorganization of innervations
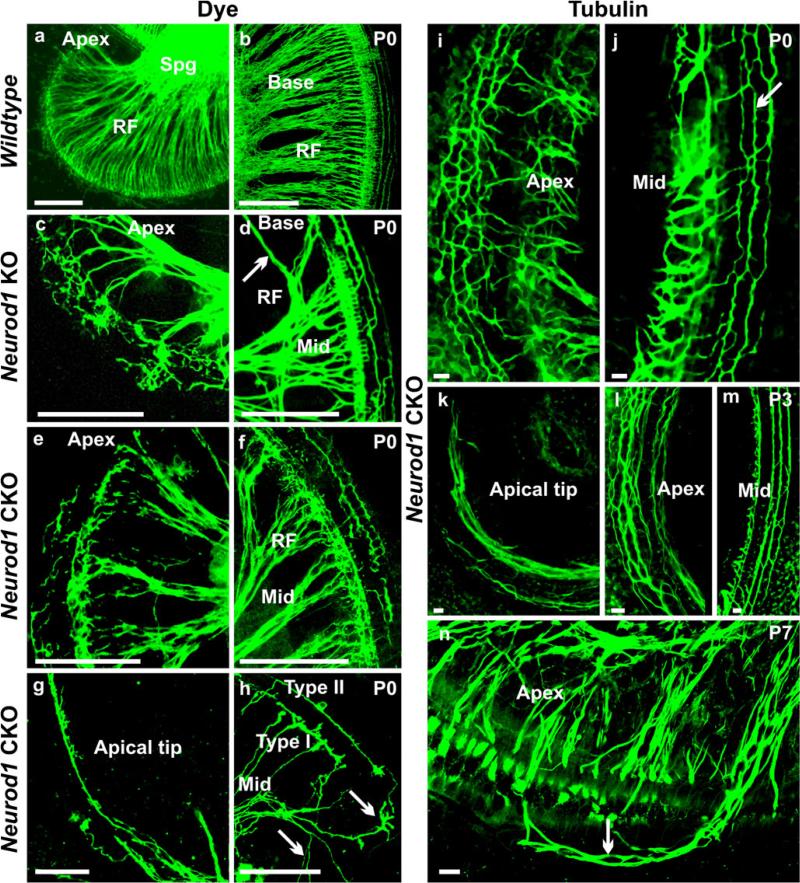
Consistent with the previous study of Neurod1 KO mice (Kim et al. 2001), we found distorted and diminished innervation of the inner ear in Neurod1 CKO mice by using lipophilic dye tracing (Figs. 1d, f, 2c–h) and nerve fiber labeling with anti-tubulin (Figs. 1g–i, 2i–n). Only a few afferent fibers extended to the cochlea of Neurod1 CKO mice (Figs. 1f–i, 2e–j) compared with the dense innervation in control littermates (Figs. 1e, 2a, b). Whereas radial fibers were tightly spaced in control mice, both KO and CKO Neurod1 mice had severely reduced radial fibers (Figs. 1d, f–i, 2c–n). Cochlear innervation was restricted to type I radial fibers to inner hair cells in the apex and base of the Neurod1 KO mice without any recognizable type II fiber projections to outer hair cells. Type II fibers preserved some normal orientation, including the innervation to three rows of outer hair cells, only in the middle turn of this mutant cochlea (Fig. 2d). In newborn Neurod1 CKO mice, we observed a similar reduction of afferent fibers and incomplete formation of radial fibers (Figs. 1f–i, 2e–n). In both mutants, afferent fibers were comparatively more in the middle turn of the cochlea consistent with the topology of remaining spiral ganglion neurons. The basal part of the middle turn showed some type II fibers, which extended randomly toward the base and apex of the cochlea (Fig. 2f, j, m). The fibers near the basal and apical tip were extremely reduced with only a few collaterals from the middle turn extending along inner hair cells (Figs. 1g–i, 2d, g, h, k). In contrast, the apical part of the middle turn (Fig. 2c, e, i, l, n) showed numerous disorganized projections to inner and outer hair cells and a disorganization of type II fibers, as recently described in various mutants affecting supporting cell development (Fritzsch et al. 2010; Puligilla et al. 2007).
Fig. 2.
Afferents are disorganized in Neurod1 mutant mice. Afferents form tightly spaced radial fiber bundles to the organ of Corti (a, b) in wildtype animals (RF radial fibers, Spg spiral ganglion). In Neurod1 KO and CKO mice, radial fibers are severely reduced and more widely spaced with profuse collateral branching (c–n). Both mutants have few recognizable type II fibers in the middle turn of the cochlea (d, m), but most of these fibers near the apical region are reduced or disorganized with random extension toward the base (arrow in d) or apex (f, i, arrow in j, l). The type I fibers have extensive branching to the inner hair cells near the lower middle turn (c, e, h, i, n). The basal and apical tips are innervated by a single fiber projecting from the inner spiral bundles with progressive abolition of type II fibers (g, k). Lipophilic dye applications to the apex of the Neurod1 CKO mice shows fibers in the middle turn bearing characteristics of type II fibers extending along the rows of outer hair cells (h). In addition, some fibers to the inner hair cells are labeled and resemble normal type I afferents, but many other fibers branch profusely (arrows in h) as they reach inner hair cells near the apical middle turn (h). This abnormal branching and overshooting of type I fibers in the apical region is observed more clearly in later stages in which they appear to innervate hair cells in the rows of outer hair cells (arrow in n). Bars 100 μm (a–h), 10 μm (i–n)
To further assess the correlation between the defects in innervations to the reduced number of spiral neurons, we injected dyes into the apex of the cochlea (Fig. 2h). In wildtype control mice, such injections led at best to efferent fibers going to different parts of the same epithelium and, only in the vestibular system efferent fibers reached nearby sensory epithelia (data not shown). Our injections into the apex labeled unusually projecting type I and type II afferent fibers in the middle turn (Fig. 2h). Although type II fibers showed some extent of normal trajectory in the upper middle turn, type I fibers had multiple terminal branches in the lower middle turn and apex of the cochlea (Fig. 2h), rather having single type I fibers innervating single inner hair cells, as in the wildtype. Moreover, in the lower middle turn, type II fibers seemed to be branches of type I fibers, which finally ran as a single fiber to the apical tip (Fig. 2g, k). Combined, these data suggest that the remaining innervation of the ear in Neurod1 CKO mice comes about through extensive branching of individual neurons so that type II neurons branch to both the apex and the base, whereas type I neurons reach multiple inner hair cells within the same area and between different parts of the cochlea.
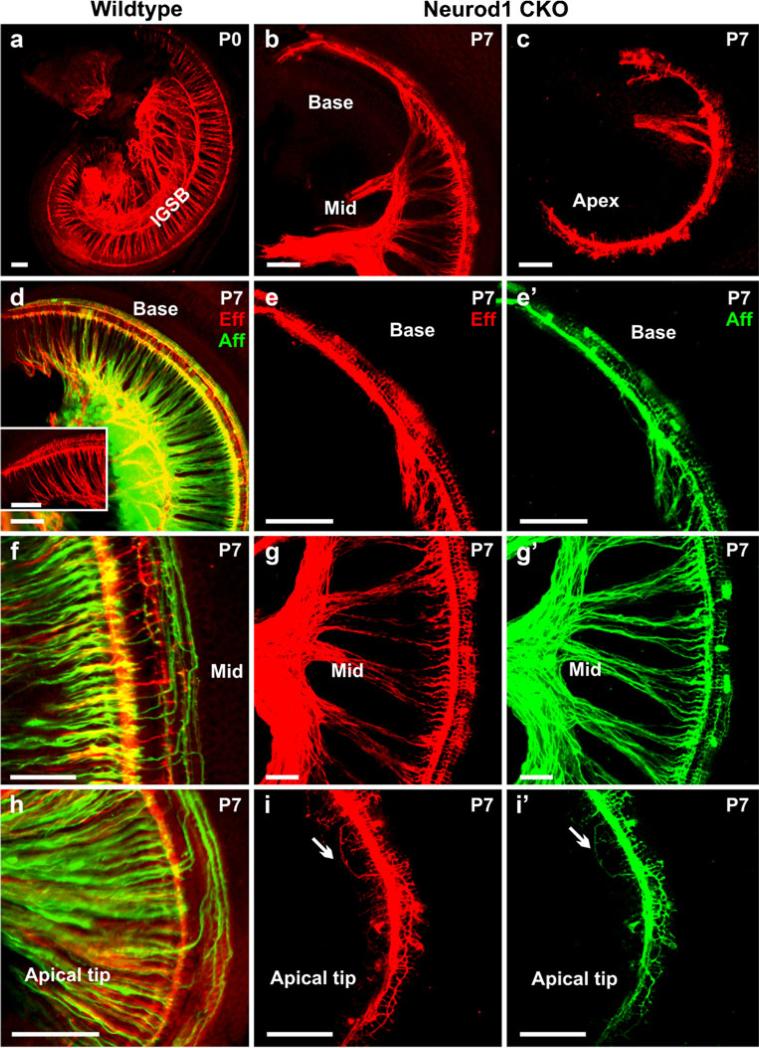
We have previously shown that the efferent system is severely reduced in the Neurod1 KO mouse (Kim et al. 2001); we obtained similar information in Neurod1 CKO mice (Fig. 3). The reduction and disorganization of efferent fibers were closely matching that of afferent fibers consistent with previous data suggesting that the efferent fibers navigate along afferents (Fritzsch et al. 1999). We showed that in control mice, the efferent fibers from the lateral superior olivocochlear nucleus projected to the inner hair cells, which exclusively make contacts with the afferent fibers in the inner hair cells, and that the fibers from the medial olivocochlear nucleus innervate the outer hair cells after crossing the tunnel of Corti (Fig. 3a, d, insert in d, f, h). Only wildtype efferent fibers exhibited the formation of the intraganglionic spiral bundle (IGSB in Fig. 3a). The efferent fibers in the middle turn of Neurod1 CKO mice projected directly to the inner and outer hair cells following the afferent fibers (Fig. 3g, g’). However, the base and the apex were innervated by fibers emanating from inner spiral bundles that extended along the inner hair cells or toward the outer hair cells (Fig. 3b, c, e, i).
Fig. 3.
Defects in efferent innervation are comparable with those in afferents in Neurod1 CKO mice. Efferent fibers (red, Eff) in the Neurod1 CKO mice are massively reduced and disorganized, comparable with the afferent fibers (green, Aff) shown with dye application in the olivo-cochlear efferent bundles and cochlear nucleus. Only the wildtype mice show the formation of the intraganglionic spiral bundle (IGSB) together with the efferents to inner and outer hair cells (a, d, inset in d, f, h). The efferent organization is disrupted in the absence of Neurod1. Near the middle turn of Neurod1 CKO mice, some efferent fibers innervate the inner hair cells along with the afferent fibers (g, g’). A collateral branch from this efferent extends as a single fiber along type I afferent fiber to the inner hair cells without any formation of radial fibers (b, c, e, e’). In the apical tip, both efferents and afferents are reduced, with random overshooting of type II fibers, which extend toward the apex (arrows in i, i’). Some efferent fibers cross the tunnel of Corti innervating only the first row of outer hair cells rather than forming three parallel bundles along three rows of outer hair cells (b, c, e, g, i). The disorganization of afferents in the cochlea is even more severe in the later stage, with progressive reduction of type II neurons (e’, g’, i’). Bars 100 μm (a–e’, h–i’), 50 μm (f–g’)
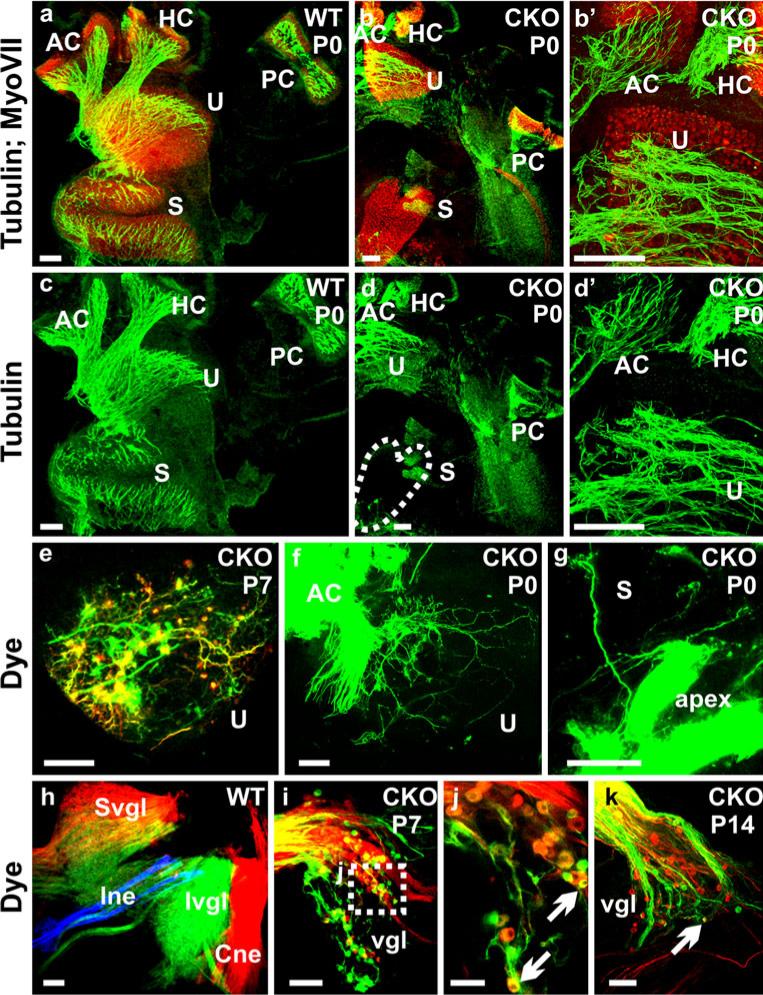
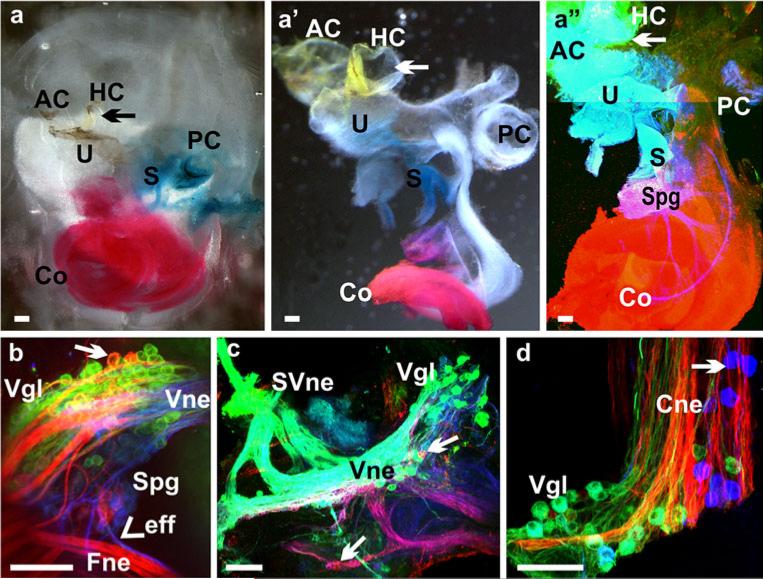
Consistent with the retention of the vestibular ganglia, we found a persistence of more profound projections to the vestibular sensory epithelia, which we analyzed by both tubulin immunofluorescence labeling and dye tracing (Fig. 4). We found some variability among those remaining fibers. Among the five vestibular sensory epithelia, the saccule was the most affected in terms of fiber reduction (Fig. 4b, d). We demonstrated the size reduction of vestibular ganglia and of their survival near adulthood in Neurod1 CKO mice (Fig. 4i–k). Canal crista and utricular fibers were also severely disorganized in contrast to those of control littermates (Fig. 4a–d). Dye injections in the anterior canal labeled many fibers to the utricle displaying an unusual trajectory parallel to the striola region with both bouton and calyceal endings (Fig. 4f). Few fibers in the saccule were labeled from the injection in the apex of the cochlea (Fig. 4g). This mixed peripheral innervation was consistent with the mixed neuron labeling from both vestibular and cochlear nucleus injections (Fig. 4i–k). As previously shown in Neurod1 KO mice (Kim et al. 2001), the surviving vestibular and spiral ganglia were displaced from their original position. Our observation in CKO mice revealed that these ganglia were not only displaced, but also remained as mixed ganglia of both vestibular and spiral neurons (Fig. 4i–k); this correlates with the lost specificity of topological fiber projection.
Fig. 4.
Absence of Neurod1 causes reduction of innervation to vestibular end organs with more profound loss in the saccule (S). The innervation of vestibular end organs in Neurod1 CKO mice are reduced and disorganized (b, b’, d, d’, e) in comparison with the control littermate (a, c) shown with the merged tubulin (green) and Myo VII (red) immunofluorescence staining (HC horizontal crista, PC posterior crista). In addition, the abnormal branching of fibers to reach other sensory epithelia is observed by dye application to the anterior crista (AC); this results in fibers reaching the utricle (f, U). These fibers take unusual trajectories and form both calyceal and bouton endings on hair cells (f). Such branching is also obvious in dye applications in the apex of the cochlea which labels few fibers to the saccule (g). The vestibular ganglia (vgl) in Neurod1 CKO mice (i–k) are reduced in comparison with the control ganglia (h), which are labeled from the brainstem dye injection (Cne cochlear nerve, Ine intermediate nerve, Ivgl inferior vestibular ganglion, Svgl superior vestibular ganglion). Moreover, these ganglia represent mixed types of neurons, which label both the cochlear and vestibular nuclei (arrows in j, k). These mixed ganglia may therefore be responsible for fiber projection in between the different sensory epithelia (f, g). Bars 100 μm (a–i, k), 50 μm (j)
In summary, our data suggest that Tg(Pax2-cre) can effectively recombine floxed Neurod1 early enough in ear development to generate a viable Neurod1 null mouse with an ear phenotype that is nearly identical in terms of loss of spiral afferents presumably through neuronal apoptosis. The Neurod1 CKO mouse can thus be used to assess in depth the phenotype of ear-specific Neurod1 loss after birth without any additional systemic problems related to the absence of insulin. In both KO and CKO of Neurod1, we cannot rule out that additional effects through the local production of Neurod1 expression-mediated proinsulin (Sanchez-Calderon et al. 2007) adds to the Neurod1 phenotype.
Loss of inner ear sensory neurons correlates with expression changes in other bHLH genes
To assess further the timeline of neuronal loss, we studied the onset of apoptosis in delaminating sensory neurons of E9.5 otocysts with a conditional deletion of Neurod1 (Fig. 5). We showed a rapid and complete elimination of the floxed Neurod1 via Cre-mediated recombination by demonstrating absence of any in situ signal for Neurod1 in the Neurod1f/f,Tg(Pax2-cre) mice (Fig. 5a, a’). Consistent with this early and complete loss of Neurod1, we found an enhanced upregulation of Neurog1 nearly throughout the otocyst (Fig. 5b’). Neurog1 is expressed in the proliferating precursor neuronal cells and is responsible for the formation and delamination of inner ear sensory neurons (Ma et al. 1998) but is rapidly downregulated in differentiating neurons. We observed prominent Neurog1 gene expression in the ventral region of the otic vesicle in E9.5 control mice (Fig. 5b). We found less intense Neurog1 expression in the ventral region of the Neurod1 CKO otocyst, whereas in the postero-dorsal region, there was an aberrant upregulation of Neurog1 expression, suggesting a negative feedback loop of Neurod1 on Neurog1 (compare Fig. 5b, b’). The reduction in the ventral part of the otocyst could indicate a loss of neuronal precursors attributable to apoptosis in Neurod1 CKO mice. We tested the latter assumption by using an antibody against activated Caspase 3 and found additional labeled cells inside and outside the otocyst in the Neurod1 CKO mice (Fig. 5c, c’), suggesting an increased cell death of delaminating and intra-otocyst cells in the absence of Neurod1. These data were consistent with a progressive loss of sensory neurons in Neurod1 CKO mice, resulting in a much reduced number of _Neurod1_-positive neurons (Fig. 5).
Fig. 5.
Conditional deletion of Neurod1 is early and complete and induces gene expression changes. In situ hybridization with a Neurod1 probe results in massive labeling in the otocyst as early as E9.5 in wildtype mice (a), whereas no Neurod1 in situ hybridization signal can be seen in Neurod1 CKO mice (a’). The bHLH gene Neurog1 shows changes in expression with upregulation in the postero-dorsal quadrant and a reduction in the ventral half (b, b’) in the absence of Neurod1 (PD postero-dorsal). These changes imply that Neurod1 has been effectively recombined at least a few hours before our investigation of these expression changes. The distribution of activated Caspase 3, a marker for apoptotic cell death, shows greater apoptotic cells both inside and even more profoundly outside the otocyst in the forming vestibular ganglion (c, c’). These data suggest that Neurod1 absence leads to a rapid loss of neuronal precursors through apoptosis and could be related to the signal reduction of Neurog1 in the ventral half (b’). Nhlh2 is another early marker of developing sensory neurons. This gene shows expression changes with a reduction in spiral ganglion (Spg) neurons as early as E11.5 (d, d’; Vgl vestibular ganglion). Similar changes are also seen in another early neuronal marker, Nhlh1, which makes the loss of sensory neurons expression more obvious (e, e’). A direct comparison of wildtype and Neurod1 CKO or KO (shown here) mice shows that, by E13.5, the loss of spiral ganglion neurons is completed (f, f’) validating the observations of an early onset of apoptosis. A specific marker for spiral ganglion neurons, Prox1, confirms the loss of spiral ganglia in a later stage (g’, g”; OC organ of Corti). Bars 100 μm
The bHLH genes Nhlh1 and Nhlh2 are expressed in all cranial ganglia including the vestibulo-cochlear ganglia and also in cochlear and vestibular hair cells (Kruger et al. 2006). Previous work has demonstrated that Neurog1, Neurod1, and Nhlh1 are involved as a cascade in sensory neurogenesis (Ma et al. 1997). Moreover, Neurod1 and Nhlh1 have been suggested to play redundant roles in the development of vestibular and spiral ganglion neurons (Kruger et al. 2006). To improve our understanding of this aspect of possibly multiple interacting bHLH genes being involved in the differentiation of inner ear sensory neurons, we investigated Nhlh1 and Nhlh2 gene expression in Neurod1 CKO mice by in situ hybridization. In control mice, both Nhlh1 and Nhlh2 were massively expressed in the vestibular and spiral ganglia in the early embryonic stage (Fig. 5d, e). Nhlh2 expression remained exclusively in the sensory neurons and was greatly reduced in the mutant spiral ganglion and, to a lesser extent, in the vestibular ganglion (Fig. 5d, d’). At later stages, Nhlh2 was almost absent in the region of the spiral ganglion but continued to be expressed in a reduced set of vestibular ganglion neurons in Neurod1 CKO mice (data not shown). Likewise, Nhlh1 expression was progressively reduced in these ganglia in the absence of Neurod1 as early as E11.5 (Fig. 5e’). The reduction in Nhlh1 and Nhlh2 expression in sensory neurons of the ear closely followed the progressive reduction of sensory neurons as demonstrated by Neurod1 expression with the β-galactosidase reporter system (Fig. 5f’).
In combination, these data indicate that a rapid loss of sensory neurons occurs in Neurod1 CKO mice with minor expression changes in Neurog1, indicating a possible feedback loop of Neurod1 on Neurog1, as suggested in the development of the olfactory system (Kawauchi et al. 2004). Both Nhlh1 and Nhlh2 genes might be able to rescue the remaining sensory neurons as previously proposed (Kruger et al. 2006), but additional double-null mutants are required to determine which of the two Nhlh genes is more important for this possible rescue of inner ear sensory neurons in the absence of Neurod1.
In addition to Nhlh genes, we also observed the expression of Prox1 in Neurod1 CKO mice. Prox1 is expressed in inner ear supporting cells and spiral neurons (Bermingham-McDonogh et al. 2006) and is required for the differentiation of type II spiral neurons, hair cells, and supporting cells (Fritzsch et al. 2010; Kirjavainen et al. 2008). With in situ hybridization, we showed dominant Prox1 expression in the spiral ganglia in wildtype newborn mice (Fig. 5g), whereas in the mutant, only a few _Prox1_-positive neurons remained near the modiolus (Fig. 5g’, g”). The downregulation of Prox1 may be involved in the disorganization of cochlear afferents in Neurod1 CKO mice, as recent evidence supports the role of Prox1 in guiding at least the extension of type II spiral fibers (Fritzsch et al. 2010).
Absence of Neurod1 results in overlapping projections of vestibular and cochlear afferents
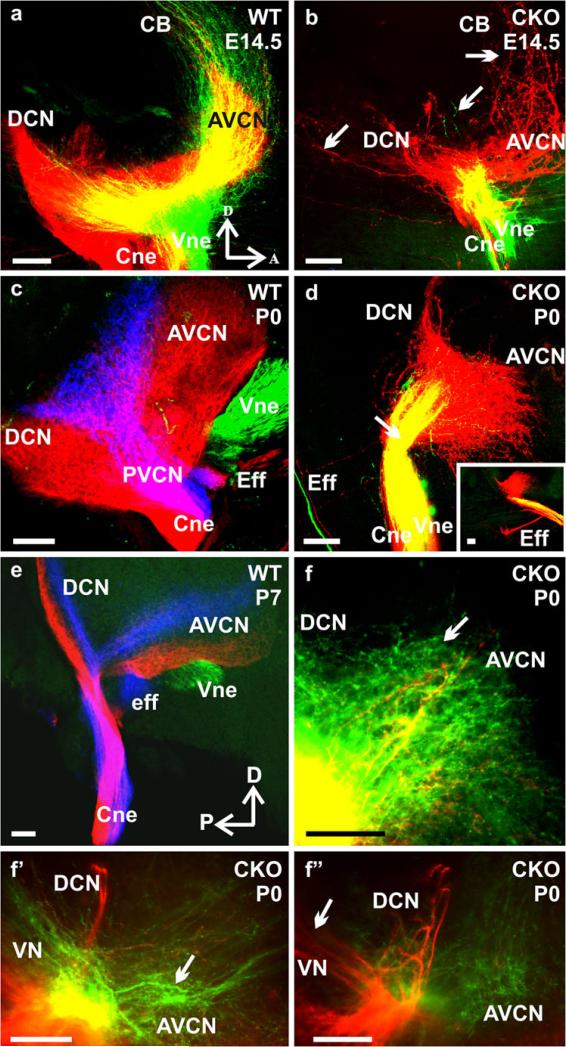
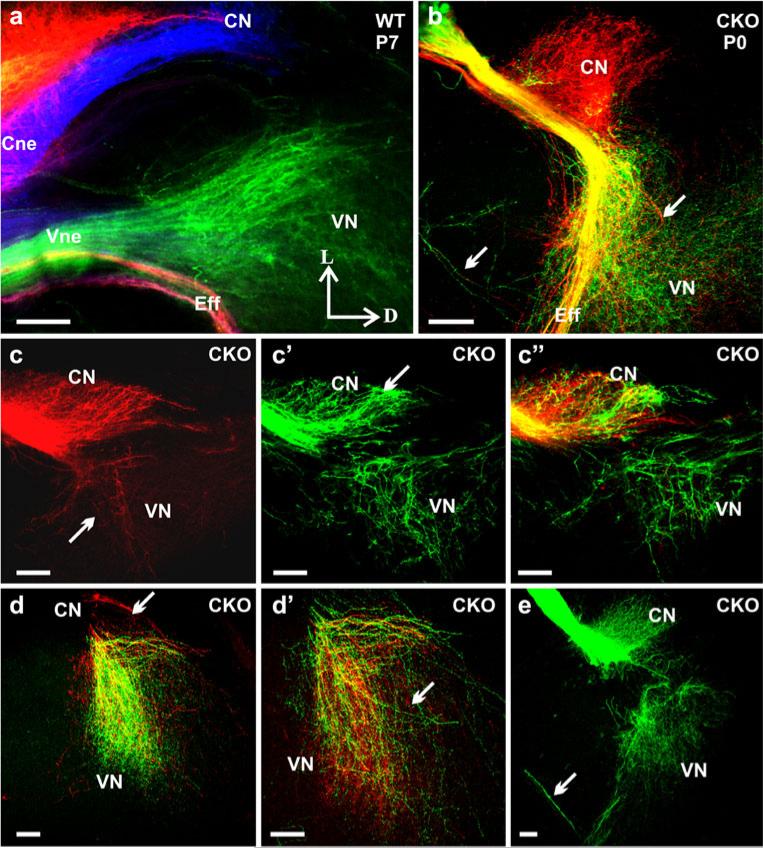
Using lipophilic dye tracing, we have previously demonstrated that vestibular and cochlear afferents develop segregated central projections into the vestibular and cochlear nuclei, respectively, as early as E12.5 (Maklad and Fritzsch 2003). We injected lipophilic dyes in the cochlea and vestibular organs (Fig. 6a–a”) of Neurod1 CKO mutant mice and showed here, for the first time, that the absence of Neurod1 resulted in overlapping vestibular and cochlear afferent projections to the vestibular and cochlear nuclei (Fig. 7). This overlapping central and peripheral projection was consistent with the finding of mixed vestibular and cochlear neurons in the surviving ganglia of Neurod1 CKO mice (Figs. 4j, 6c). Lipophilic dyes with different chromatic properties were applied to the cochlea and utricle and showed that, as early as E14.5, the vestibular and cochlear fibers were segregated and reached their target nuclei in the brain in control littermates (Fig. 7a). In contrast, in Neurod1 CKO mice, fibers from both end organs reached the brain as a single nerve that entered through the cochlear nucleus instead of having two divisions (Figs. 7b, d, f, 9b). In E16.5 (data not shown), newborn (P0), and P7 control mice, we demonstrated not only the complete segregation between vestibular and cochlear fibers, but also fibers from the basal and apical region of the cochlea reaching distinct aspects of the dorsal and ventral cochlear nuclei (Fig. 7c, e). In the absence of Neurod1, these fibers displayed no tonotopic organization in the target nuclei, and fibers labeled from vestibular injections overlapped with those from cochlear injections (Fig. 7b, d, f). Moreover, the efferent bundles were also misguided in Neurod1 CKO mice in which some efferent fibers were rerouted and exited with the facial nerve or cochlear nerve, instead of exiting only with the vestibular division (insert in Fig. 7d). This suggests that the absence of a vestibular division results in a rerouting of efferent fibers, which sometimes reach the ear after having exited through the facial nerve (Fig. 6b)
Fig. 6.
Afferent disorganization may correlate with the mixed ganglia in Neurod1 CKO mice. Afferents of the inner ear were labeled by using filter strips that were soaked with chromatically different lipophilic dye tracers and that were inserted into the various sensory epithelia of inner ear (AC anterior crista, HC horizontal crista, PC posterior crista, U utricle, S saccule, Co cochlea, Spg spiral ganglion, Vgl vestibular ganglion, Cne cochlear nerve, Vne vestibular nerve, Fne facial nerve, Eff efferent fibers, SVne superior vestibular nerve). NeuroVue red (direct and false-colored red) dye was applied into the apex of cochlea, with NeuroVue jade (direct color yellow, false-colored green; arrows in a–a”) dye into the anterior canal crista/horizontal canal crista/utricle and NeuroVue maroon (direct and false-colored blue) into the base of cochlea or into the saccule (a-a”). Vestibular ganglion neurons with vestibular nerve fibers were labeled by the dye inserted into the canal crista and were shown to branch unusually from the vestibular nerve to innervate the canal crista and utricle (c). In addition, these ganglion neurons were labeled not only from the vestibular end organs, but also from the dye injected into the cochlea (red neurons, arrow in b, c). These mixed ganglion neurons migrated unusually near the nerve entry site to the brainstem (arrow in d). Moreover, efferents entered into the ear together with the facial nerve (arrowhead in b). Bars 100 μm
Fig. 7.
Loss of Neurod1 results in overlapping central projections. Various dyes were applied into the different sensory epithelia of the inner ear to label their central projections. This approach showed segregated central projections of vestibular and cochlear afferents to the vestibular and cochlear nuclei and other targets such as the cerebellum as early as E14.5 (a). In contrast, afferent fibers entered into the brain as a single root in Neurod1 CKO mice (b, d, f-f”), and afferents labeled from the cochlea projected to the cerebellum instead of stopping at the antero-ventral cochlear nucleus (b). In newborn and P7 wildtype mice, central projections from the base (blue), apex (red), and vestibular endorgans (green) and all afferents entered the hindbrain as discrete fascicles, terminating exclusively in cochlear and vestibular nuclei (c, e). In Neurod1 CKO mice, afferents to the cochlear nuclei were greatly reduced, showing overlapping projections from both the apex and base, with little extension toward the dorsal cochlear nucleus, and both vestibular and cochlear afferents entered through the same root (arrow in d). Vestibular afferents not only entered through the cochlear nucleus, but also projected variably to the antero-ventral cochlear nucleus (arrow in f) in addition to the vestibular nucleus (f’). Similarly cochlear afferents projected to vestibular nucleus (arrow in f”) in addition to cochlear nucleus.. Efferent fibers to the ear normally exited with the vestibular nerve root (c) but instead exited through the facial nerve in the mutant (d, insert in d) and were rerouted outside the brain to the vestibulo-cochlear nerve or the “cochlear nerve root” (AVCN antero-ventral cochlear nucleus, PVCN postero-ventral cochlear nucleus, DCN dorsal cochlear nucleus, CB cerebellum, Cne cochlear nerve, Vne vestibular nerve, Eff efferent nerve fibers, VN vestibular nucleus, A anterior, D dorsal, P posterior). Bars 100 μm
Fig. 9.
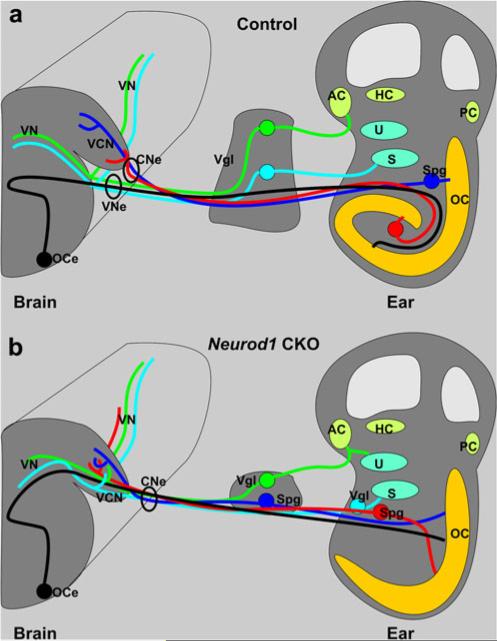
Illustration comparing the neuronal connection of the wildtype ear with the brain (a) with that of the Neurod1 conditional null (CKO) mouse (b). All major features can be distinguished, but the cochlea is shortened and misshapen in Neurod1 CKO mice. Whereas the normal organ of Corti (OC) has multiple turns and an accompanying spiral ganglion (Spg), there is typically no spiral ganglion found near the shortened organ of Corti in Neurod1 CKO mice. However, spiral ganglion neurons as verified by their backfilling from the organ of Corti can be found near the saccule (S) or in the much reduced vestibular ganglion. A comparable but less severe disorganization is found in the vestibular ganglia (Vgl), which normally lie outside the ear but are scattered both inside and outside the ear in Neurod1 CKO mice. As a consequence, nearby neurons may project to both vestibular and cochlear sensory epithelia. As fibers approach the brain, spiral afferents normally segregate from vestibular afferents, so that each fascicle enters as distinct vestibular and cochlear nerves into the vestibular and cochlear nuclei (a). In contrast, in Neurod1 CKO mice, all afferents enter the brain together as a single root into the ventral cochlear nucleus (VCN). Despite this common entry point, fibers nevertheless project to the vestibular nuclei (VN) but also end up without any apparent topology in the cochlear nuclei. These data show that Neurod1 expression is needed for proper migration of peripheral and central projection of all spiral neurons and to a lesser extent of vestibular neurons. Rerouting to the periphery is also observed in olivocochlear and vestibular efferents, which normally exit the brain via the vestibular nerve to reroute to the cochlea but instead exit the brain via the cochlear nerve or, less often, via the facial nerve to enter into the ear (AC anterior crista, HC horizontal crista, PC posterior crista, U utricle, CNe cochlear nerve, OCe olivocochlear efferent, VNe vestibular nerve)
In Neurod1 CKO mutant mice, vestibular and cochlear afferents not only entered as a single nerve root, but their fibers also projected to both cochlear and vestibular nuclei, respectively, in addition to their original target nuclei projections (Figs. 7, 8). For example, at E14.5, some cochlear fibers were found projecting not only to the DCN and AVCN, but also caudally to the vestibular nuclei and rostrally to the cerebellum (Fig. 7b). Some vestibular fibers reached the cochlear nuclei, and some projected through the cochlear nuclei to reach vestibular nuclei (Figs. 7d, f–f’, 8b, c’). These differential projections of cochlear and vestibular afferents were most apparent in later stages and in coronal sections (Fig. 8). Vestibular fibers projected to both vestibular and cochlear nuclei in Neurod1 CKO mice (Figs. 7f, f’, 8b, c’, c”, e), whereas these were limited entirely to the vestibular nuclei in control animals (Fig. 8a). Likewise, cochlear afferents projected exclusively to the cochlear nuclei in wildtype mice (Fig. 8a), whereas many cochlear afferents projected to vestibular nuclei in Neurod1 CKO mice (Figs. 7f”, 8b, c, c”, d, d’). This altered central projection of the vestibulo-cochlear fibers was confirmed by applying dye only to the anterior crista of the inner ear. This labeling showed that the vestibular afferent fibers projected to both the vestibular and cochlear nucleus, rather than being restricted to the vestibular nucleus (Fig. 8e).
Fig. 8.
Vestibular and cochlear fibers project to both nuclei in Neurod1 CKO mice. Coronal sections from the cochlear and vestibular nuclei with same dye injections as shown in Fig. 7 reveal that afferents labeled from the cochlea (red) target mostly the cochlear nucleus but extend also to the vestibular nuclei (right arrow in b, arrows in c, d, d’) in the absence of Neurod1. Likewise, afferents labeled from vestibular endorgans (green) project into the cochlear nucleus in addition to their original vestibular nuclei projections (b, c’, c”, d, d’, e). This altered and overlapping projection is confirmed by specific dye application only in vestibular or cochlear epithelia showing that the fibers from vestibular end organs reach to the cochlear nucleus and vice versa (vestibular afferents labeling shown in e). The sections from wildtypes demonstrate the distinct topology of afferents from vestibular epithelia (green), cochlear apex (red), and cochlear base (blue) and the efferents that exit with the vestibular nerve root (a), whereas they exit through the cochlear nerve root (b) or through the facial nerve (left arrows in b, arrow in e) in Neurod1 CKO mice (CN cochlear nucleus, Cne cochlear nerve, Eff efferent nerve fibers, VN vestibular nucleus, Vne vestibular nerve, L lateral, D dorsal). Bars 100 μm
In summary, these data demonstrate that, in the absence of Neurod1, the cochlear and vestibular fibers fail to segregate and reach distinct parts of their target nuclei (Fig. 9b). Neurod1 is therefore not only necessary for the survival of most cochlear and many vestibular neurons, but is also required to regulate the expression of genes that allow organ-specific fiber projections such as Brn3a (Huang et al. 2001). Further work with restricted injections to all six sensory organs is in progress to show the lack of segregation among vestibular fibers in more detail.
Discussion
Neurod1 is essential for neuronal differentiation (Pan et al. 2009) and can convert non-neuronal cells into neurons (Lee et al. 1995) through the regulation of over 500 downstream genes (Seo et al. 2007). We have analyzed the role of Neurod1 in inner ear neurosensory cell development by using a newly generated Neurod1 CKO mouse. Consistent with our previous report (Kim et al. 2001), we have found substantial loss of inner ear sensory neurons and the remaining afferents and efferents are disorganized in Neurod1 CKO mice but not completely absent as previously suggested (Liu et al. 2000). In addition, we have identified a novel role: Neurod1 is required for segregating and organizing central and peripheral projections in the surviving sensory neurons.
Neuron formation of the inner ear depends on a number of bHLH genes (Neurog1, Neurod1, Nhlh1, and Nhlh2). Neurog1 null mice never form neurons and show a reduction of hair cells, suggesting a lineage and some clonal neurosensory relationship (Satoh and Fekete 2005). Neurod1 CKO mutants show loss of spiral and vestibular neurons and truncation of sensory epithelia. Nhlh1 and Neurod1 play a redundant role in the development of sensory neurons in Nhlh1-Neurod1 compound null mice (Kruger et al. 2006). We suggest that residual neurons might survive by compensatory gene activation of Nhlh1 and/or Nhlh2. Crossing our viable Neurod1 CKO mice with existing mutants of both Nhlh1 and Nhlh2 might show that these three genes are necessary for the survival of all inner ear sensory neurons. Clearly, neither Nhlh1 nor Nhlh2 can substitute for Neurod1 to upregulate the program needed for nerve guidance. Neurog1 null mice show no neuronal migration and can be compared with Neurod1 null mice to establish the expression profiles of genes necessary for migration.
Neurod1 is required for segregated projections in the peripheral ear and for proper afferent and efferent organization within sensory epithelia
Previous work has identified several genes relevant for some aspects of pathfinding of nerve fibers, such as neurotrophins (Tessarollo et al. 2004), POU domain factors (Huang et al. 2001), semaphorins (Gu et al. 2003), Erbb2 (Morris et al. 2006), Slitrk6 (Katayama et al. 2009), Foxg1 (Pauley et al. 2006), and Prox1 (Fritzsch et al. 2010). In addition, defects in supporting cell development affect sensory neuron projections (Puligilla et al. 2007; Shim et al. 2005). Neurod1 is known to lie upstream to Prox1 in the developing hippocampus and is involved in directed growth of neuronal processes (Roybon et al. 2009). Consistent with a possible regulation of Prox1 by Neurod1 in the ear, we have found little Prox1 expression left in only a few sensory neurons in the mutant mouse (Fig. 5g’). The unusual and random fiber growth to the area of outer hair cells (Fig. 2i, l, n) is therefore possibly in part mediated by the downregulation of Prox1, since the Prox1 null phenotype has derailed innervation of outer hair cells (Fritzsch et al. 2010). We have previously shown that Neurod1 regulates the neurotrophin receptor tyrosine kinase Ntrk3, and that the reduction in innervation closely mimics the loss of spiral neurons in Ntf3 and Ntrk3 null mutant mice (Fritzsch et al. 2004). The derailment of peripheral innervation including the unusual branching of what appears to be single fibers will allow us to correlate the Neurod1 phenotype with specific genes with similar defects and ultimately to establish causality comparable with that of Ntrk3 and Prox1.
Neurod1 promoter analysis has revealed many downstream target genes including Prox1, Ntrk3, semaphorin 3a, neuropilin2, plexin A2, Ephb2, Robo1, and Slit2/3, which are directly involved in fiber guidance (Seo et al. 2007). In addition to the role of chemoattractants for fiber growth, the repellents play an equally important role in generating the correct innervation pattern. A class of axon repellents, the semaphorins, prevent the growth of neurons in the dorsal part of otocyst, if the neuropilin receptor is present (Gu et al. 2003). Semaphorin signaling of axon repulsion requires the plexin A2 co-receptor which is expressed in the vestibular and spiral ganglia of developing inner ear (Murakami et al. 2001). Moreover, Slit/Robo signaling can affect axon guidance, axon branching, and neuronal migration (Wong et al. 2002). Ephb2 guides the contralateral efferent growth cones, which show inappropriate pathway selection in the midline with a defect in the vestibular efferent in the absence of Ephb2 (Cowan et al. 2000). The way in which these known and suspected (Fekete and Campero 2007) guidance molecules in the ear depend on Neurod1 requires further investigation. A comparison of Neurod1 with wildtype ears by using deep sequencing might allow to elucidate, in more detail, the expression differences related to the aberrant projections reported here for Neurod1 null mice.
Neurod1 is required for segregated central projection of cochlear and vestibular neurons
We describe here, for the first time, the disorganization of centrally and peripherally projecting afferents in Neurod1 null mice. In the ear of Neurod1 null mice, individual afferents branch profusely to reach most hair cells despite their highly reduced numbers. This expansion is comparable to the only other case of severe reduction of afferents, the Ntf3 null mouse (Farinas et al. 2001). The exuberant branching of remaining afferents implies that competition with other afferents may normally block such expansion, but a genuine direct effect of Neurod1 is also possible. The efferents are also reduced in Neurod1 CKO mice; the remaining efferents never form the normal intra-ganglionic spiral bundle, and the inner spiral bundle provides branches that run along the inner hair cells.
In Neurod1 CKO mice, cochlear and vestibular fibers enter the cochlear nucleus as a single bundle, and both cochlear and vestibular fibers project to both cochlear and vestibular nuclei. Neurod1 therefore regulates aspects of central and peripheral branching and pathfinding in sensory neurons. This effect is related to the nerve fibers, not to the Neurod1 expression in the dorsal cochlear nucleus (DCN) (Fritzsch et al. 2006) as Tg (Pax2-cre) do not express in the DCN, thereby cannot eliminate Neurod1 in the DCN. However, even a severe reduction of cochlear nuclei in mutants with conditional deletion of Atoh1 has only minor effects on afferent projections (Maricich et al. 2009). Conditional deletions of Neurod1 at a later stage are now needed to uncouple neuronal survival in the ear from the proposed pathfinding regulation. We demonstrate here that Neurod1 is responsible not only for survival, but also for neuronal differentiation such as the segregation of cochlear and vestibular afferent projections. Indeed, Neurod1 expression in cochlear spiral neurons is, in addition to Gata3 (Karis et al. 2001) and Prox1 (Fritzsch et al. 2010), the only approximately specific marker for spiral neurons.
In summary, our findings show a more sophisticated action of Neurod1 in the developing ear than the previously suggested simple role in neuronal survival. These data are in line with the 500 genes directly regulated by Neurod1 (Seo et al. 2007), most of which have a known or suspected function in neuronal guidance. More work on putative neuronal guidance genes is now needed in these viable mutants in order to understand the precise way in which Neurod1 can regulate features of inner ear neuronal development including targeted peripheral and central projections that are so disorganized in Neurod1 null mice.
Acknowledgements
We express our thanks to Drs. K.-A. Nave and S. Goebbels, Max Planck Institute of Experimental Medicine, for providing the floxed Neurod1 mice used for this study and to Drs. T. Ohyama and A. Groves for providing the Pax2-Cre line. We thank K. Elliot for carefully checking the English and the Office of the Vice President for Research (OVPR) for support.
This work was supported by an NIH grant (R01 DC 005590 to B.F.). The Leica TCS SP5 confocal microscope used in this study was purchased in part with a grant from the Roy J. Carver foundation.
References
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, Caprona DC de, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Campero AM. Axon guidance in the inner ear. Int J Dev Biol. 2007;51:549–556. doi: 10.1387/ijdb.072341df. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295:369–382. doi: 10.1007/s004410051244. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005a;233:570–583. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue Maroon, NeuroVue Red and NeuroVue Green and their use for double and triple labeling of neuronal profile. Brain Res Bull. 2005b;66:249–258. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Feng F, Matei V, Nichols DH. The evolution of the vertebrate auditory system: transformations of vestibular mechanosensory cells for sound processing is combined with newly generated central processing neurons. Int J Comp Psychol. 2006;19:1–24. [Google Scholar]
- Fritzsch B, Dillard M, Lavado A, Harvey NL, Jahan I. Canal cristae growth and fiber extension to the outer hair cells of the mouse ear require Prox1 activity. PLoS ONE. 2010;5:e9377. doi: 10.1371/journal.pone.0009377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Bode U, Pieper A, Funfschilling U, Schwab MH, Nave KA. Cre/loxP-mediated inactivation of the bHLH transcription factor gene NeuroD/BETA2. Genesis. 2005;42:247–252. doi: 10.1002/gene.20138. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys Semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F. Spatial and temporal specification of neural fates by transcription factor codes. Development. 2007;134:3771–3780. doi: 10.1242/dev.006379. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- Karis A, Pata I, Doorninck JH van, Grosveld F, Zeeuw CI de, Caprona D de, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morpho-genesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Katayama K, Zine A, Ota M, Matsumoto Y, Inoue T, Fritzsch B, Aruga J. Disorganized innervation and neuronal loss in the inner ear of Slitrk6-deficient mice. PLoS ONE. 2009;4:e7786. doi: 10.1371/journal.pone.0007786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, Calof AL. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26:166–180. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen A, Sulg M, Heyd F, Alitalo K, Yla-Herttuala S, Moroy T, Petrova TV, Pirvola U. Prox1 interacts with Atoh1 and Gfi1, and regulates cellular differentiation in the inner ear sensory epithelia. Dev Biol. 2008;322:33–45. doi: 10.1016/j.ydbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Kruger M, Schmid T, Kruger S, Bober E, Braun T. Functional redundancy of NSCL-1 and NeuroD during development of the petrosal and vestibulocochlear ganglia. Eur J Neurosci. 2006;24:1581–1590. doi: 10.1111/j.1460-9568.2006.05051.x. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Sommer L, Cserjesi P, Anderson DJ. Mash1 and neurogenin1 expression patterns define complementary domains of neuroepithelium in the developing CNS and are correlated with regions expressing notch ligands. J Neurosci. 1997;17:3644–3652. doi: 10.1523/JNEUROSCI.17-10-03644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, Barco BI del, Pompa JL de la, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull. 2003;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, Xia A, Mathes EL, Wang VY, Oghalai JS, Fritzsch B, Zoghbi HY. Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. J Neurosci. 2009;29:11123–11133. doi: 10.1523/JNEUROSCI.2232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei VA, Feng F, Pauley S, Beisel KW, Nichols MG, Fritzsch B. Near-infrared laser illumination transforms the fluorescence absorbing X-Gal reaction product BCI into a transparent, yet brightly fluorescent substance. Brain Res Bull. 2006;70:33–43. doi: 10.1016/j.brainresbull.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Maklad A, Hansen LA, Feng F, Sorensen C, Lee KF, Macklin WB, Fritzsch B. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–199. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Suto F, Shimizu M, Shinoda T, Kameyama T, Fujisawa H. Differential expression of plexin-A subfamily members in the mouse nervous system. Dev Dyn. 2001;220:246–258. doi: 10.1002/1097-0177(20010301)220:3<246::AID-DVDY1112>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;1192:90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- Pan N, Jahan I, Lee JE, Fritzsch B. Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg(Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res. 2009;337:407–428. doi: 10.1007/s00441-009-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, Brundin P. Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE. 2009;4:e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Milo M, Leon Y, Varela-Nieto I. A network of growth and transcription factors controls neuronal differentiation and survival in the developing ear. Int J Dev Biol. 2007;51:557–570. doi: 10.1387/ijdb.072373hs. [DOI] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Soukup GA, Fritzsch B, Pierce ML, Weston MD, Jahan I, McManus MT, Harfe BD. Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Dev Biol. 2009;328:328–341. doi: 10.1016/j.ydbio.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Park HT, Wu JY, Rao Y. Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev. 2002;12:583–591. doi: 10.1016/s0959-437x(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Xia A, Visosky AM, Cho JH, Tsai MJ, Pereira FA, Oghalai JS. Altered traveling wave propagation and reduced endocochlear potential associated with cochlear dysplasia in the BETA2/NeuroD1 null mouse. J Assoc Res Otolaryngol. 2007;8:447–463. doi: 10.1007/s10162-007-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]