Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche (original) (raw)
. Author manuscript; available in PMC: 2014 Aug 1.
Published in final edited form as: Curr Opin Neurobiol. 2013 Feb 4;23(4):500–506. doi: 10.1016/j.conb.2013.01.009
Abstract
Group I metabotropic glutamate receptors (mGluR) are important modulators of excitatory synaptic transmission and therefore potential targets for drug development. In several brain regions (ventral tegmental area, cerebellum, and amygdala), stimulation of mGluR1 selectively inhibits synaptic transmission mediated by calcium-permeable AMPA receptors (CP-AMPARs) and thus produces synaptic depression. The same relationship has now been demonstrated in the nucleus accumbens (NAc), a region that is critical for cocaine craving. CP-AMPAR levels in NAc synapses are normally low, but they increase after prolonged withdrawal from extended-access cocaine self-administration. These CP-AMPARs mediate the intensified (“incubated”) cue-induced cocaine craving observed under these conditions. Therefore, activation of mGluR1 with positive allosteric modulators may reduce cue-induced relapse in abstinent cocaine addicts.
Introduction
The group I metabotropic glutamate receptors (mGluR1 and mGluR5) are predominantly postsynaptic receptors that couple to the Gq-like class of G-proteins and are important in modulating neurotransmission and plasticity through their linkages with multiple signaling pathways as well as NMDA receptors [1]. Compounds that negatively or positively modulate group I mGluRs have been the focus of intense interest due to their potential to tune glutamate transmission up or down in disease states. Drug addiction has been recognized for many years as a disorder involving glutamate transmission and maladaptive plasticity [2,3], so it is not surprising that considerable effort has been directed at evaluating group I mGluR modulators in animal models of addiction [4,5].
This review will focus on group I mGluRs in the nucleus accumbens (NAc) and cocaine addiction. The NAc is a critical brain region for cocaine craving that expresses significant levels of both mGluR1 and mGluR5, mainly in extrasynaptic and perisynaptic regions [6,•7,•8,9]. While most studies on the role of group I mGluRs in addiction have focused on negative allosteric modulators (NAM) of mGluR5, we will argue that the optimal group I mGluR-based strategy for treating cocaine addiction depends on the nature of cocaine exposure which in turn defines the nature of adaptations in the NAc. In particular, emerging evidence reviewed herein suggests that positive allosteric modulators (PAM) of mGluR1 may prevent cue-induced relapse in abstinent cocaine addicts by removing Ca2+-permeable AMPA receptors (CP-AMPARs) from NAc synapses.
Negative allosteric modulators of group I mGluRs in animal models of cocaine addiction
The focus on negative modulation of mGluR5 dates from a report in 2001 that mGluR5 knockout mice do not exhibit increased locomotor activity after cocaine injection nor learn to self-administer cocaine [•10]. Subsequent studies extended these findings by showing that mGluR5 NAMs such as MPEP or MTEP prevented the development of cocaine conditioned place preference, reduced motivation to self-administer cocaine in progressive ratio experiments, and reduced reinstatement of cocaine seeking in models of relapse [4,5]. Much less attention has been paid to mGluR1, although a few studies have found that its negative modulation also opposes effects of cocaine exposure. Thus, mGluR1 NAMs reduced context-induced reinstatement of cocaine seeking when infused into the NAc core [11] or dorsal hippocampus [12], while systemic administration of an mGluR1 antagonist blocked the expression of locomotor sensitization to cocaine [13].
CP-AMPARs and mGluR1: A unique relationship
AMPARs are tetramers comprised of GluA1–4 subunits. In most brain regions of the adult drug-naïve rat, including the NAc [14,15,••16,17], the majority of AMPARs on principal neurons contain the GluA2 subunit. However, there is a minority population that lacks GluA2. Compared to GluA2-containing receptors, this population exhibits Ca2+-permeability, larger single channel conductance and faster kinetics, and voltage-dependent block by intracellular polyamines resulting in inward rectification. These CP-AMPARs have emerged as a highly regulated AMPAR subtype that mediates diverse types of neuronal plasticity [18,19,20,21].
There are many forms of group I mGluR-dependent long-term depression (mGluR-LTD), some of which are implicated in disease states [1,22,23]. As described below, when CP-AMPARs are present in synapses, stimulation of mGluR1 produces a form of mGluR-LTD that is mediated by CP-AMPAR removal. We will review evidence for this form of mGluR1-LTD in the ventral tegmental area (VTA), cerebellum and amygdala before considering mGluR1’s role in the NAc.
VTA dopamine neurons
Over 10 years ago it was shown that exposure to cocaine (even a single injection) rapidly increases the AMPA/NMDA ratio at excitatory synapses onto VTA dopamine neurons [24,25]. This occurs because high conductance CP-AMPARs are inserted into synapses and lower conductance GluA2-containing Ca2+-impermeable AMPARs (CI-AMPARs) are removed [26,••27,28,29]. The insertion of CP-AMPARs is accompanied by decreased NMDAR transmission, further contributing to elevation of the AMPA/NMDA ratio [30]. The functional significance of the increased AMPA/NMDA ratio may be related to the fact that CP-AMPAR incorporation alters the rules for subsequent induction of LTP [30], although the behavioral correlates of this alteration remain to be worked out [31]. Stimulation of mGluR1 leading to mGluR-LTD reverses this process --- CP-AMPARs are removed from synapses and replaced with CI-AMPARs through a mechanism that requires locally translated GluA2 [26,••27,28]. Thus, acute mGluR1 stimulation rapidly removes CP-AMPARs from VTA synapses. Subsequent in vivo studies showed that tonic mGluR1 activation in the VTA limits the duration of cocaine-induced CP-AMPAR synaptic incorporation, helping to restore these synapses to the precocaine state [•32]. A similar mechanism controls the maturation of VTA synapses during development [33].
Cerebellar stellate cells
CP-AMPARs are normally present in synapses on cerebellar stellate cells [34,35,••36]. A recent study showed that acute mGluR1 stimulation removes these CP-AMPARs and replaces them with GluA2-containing, CI-AMPARs. In addition, experiments with mGluR1 antagonists revealed that ongoing mGluR1 tone exerts a suppressive effect on CP-AMPAR levels in these synapses [••36]. While this is reminiscent of the VTA, there may be mechanistic differences. Thus, while protein synthesis is required to produce this form of mGluR1-LTD in both brain regions, it is not necessarily due to de novo GluA2 protein synthesis in stellate cells as it is in the VTA [28].
Instead, the increase in CI-AMPARs in stellate cells following mGluR1 stimulation may be attributable in part to their lateral movement from extrasynaptic sites [37]. Furthermore, CP-AMPARs may be mainly GluA3 homomers in stellate cells [35,38] but GluA1A3 receptors or GluA1 homomers in the VTA [29,39], which could alter the influence of some mechanisms that regulate AMPAR trafficking. Despite these differences, in both cell types mGluR1 activation produces a form of postsynaptically expressed LTD in which high conductance CP-AMPARs are removed and replaced by lower conductance CI-AMPARs.
Lateral amygdala
A related form of mGluR1-LTD has been implicated in extinction of fear memories [••40]. This study utilized a rodent model in which fear conditioning is produced by pairing an auditory tone (conditioned stimulus) with a footshock. Re-exposure to the conditioned stimulus (i.e., memory retrieval) during a critical interval after conditioning renders the memory labile and therefore vulnerable to erasure via extinction training. This process of memory erasure is termed reconsolidation update. The authors showed that fear conditioning leads to an increase in synaptic CP-AMPAR levels in the lateral amygdala that is detectable within hours of conditioning, peaks after 1 day and disappears by 7 days. They hypothesized that the presence of CP-AMPARs, by increasing the capacity for synaptic weakening, enables reconsolidation update. Confirming this, memory erasure via reconsolidation update could be elicited when CP-AMPARs were present, but not after they had dissipated. Furthermore, it depended on a form of mGluR1-LTD mediated by CP-AMPAR removal.
CP-AMPARs and mGluR1 in the NAc: regulators of cocaine craving
Our interest in the aforementioned effects of mGluR1 on CP-AMPAR transmission arose from our studies in a particular animal model of cocaine addiction called the incubation model. Incubation refers to the progressive intensification of cue-induced cocaine craving that occurs over the first two months of withdrawal from extended-access cocaine self-administration (SA); this reflects very persistent neuroadaptations, because craving remains elevated even 6 months after the last drug exposure [41,42].
In attempting to understand the mechanism of incubation, we evaluated AMPAR transmission in the NAc based on its demonstrated importance for cocaine seeking in other animal models [43,44]. Through a combination of biochemical and electrophysiological studies, we found that CP-AMPAR transmission onto NAc medium spiny neurons increases after ~4 weeks of withdrawal from a regimen leading to incubation (6 h/day of cocaine SA for 10 days) [••16,31]. Most importantly, intra-NAc administration of the selective CP-AMPAR antagonist naspm blocked the expression of incubated cue-induced cocaine craving on withdrawal day 45, indicating that expression of incubation after prolonged withdrawal is mediated by these CP-AMPARs [••16]. We interpret these results to indicate that the synaptic incorporation of high conductance CP-AMPARs increases the ability of medium spiny neurons to respond and engage the motor circuitry when presentation of a cocaine-related cue leads to glutamate release in the NAc. At early withdrawal times prior to CP-AMPAR accumulation, adaptations in other brain regions may account for expression of incubation (see 42). Although CP-AMPARs accumulate in both core and shell subregions of the NAc during incubation [••16,•32,•45], the core appears critical given that intra-core injection of naspm is sufficient to block incubated cocaine craving [••16].
Our results with naspm suggest that blocking CP-AMPAR transmission in the NAc has therapeutic potential in addiction. However, naspm and other CP-AMPAR antagonists are not candidates for drug development, necessitating the identification of alternate strategies for reducing CP-AMPAR transmission and thereby reducing cocaine craving. Since mGluR1 negatively regulates CP-AMPARs in other brain regions (see above), might the same relationship exist in the NAc? As described below, our recent findings indicate that this is indeed the case and identify mGluR1 as a potential therapeutic target for reducing cue-induced relapse in abstinent addicts.
mGluR1 negatively regulates CP-AMPAR function in the NAc
In medium spiny neurons of dorsal striatum and NAc, there is a well-studied form of mGluR-LTD that involves mGluR5, postsynaptic endocannabinoid release, and presynaptic attenuation of glutamatergic transmission via CB1R activation [46,47]. To determine if this would change after incubation of cocaine craving and CP-AMPAR accumulation, we performed patch-clamp recordings in NAc brain slices from saline SA controls and “incubated rats”. We will use the term “incubated rats” to denote rats that underwent extended-access cocaine SA (6 h/day for 10 days) and >30 days of withdrawal, a period sufficient for CP-AMPAR accumulation and robust incubation of cocaine craving [••16,31,48]. As expected from results in drug-naive animals (above), bath application of the nonselective group I mGluR agonist DHPG in slices from saline SA controls elicited synaptic depression in NAc medium spiny neurons that depended on mGluR5 stimulation and presynaptic CB1Rs. However, in NAc synapses from "incubated rats”, this form of synaptic depression was no longer detected. Instead DHPG-induced synaptic depression was mediated through an mGluR1- and PKC-dependent mechanism that reduces CP-AMPAR transmission and simultaneously enhances CI-AMPAR transmission, producing a net effect of decreased AMPAR transmission at negative potentials [••49]. Although the exact mechanism remains to be identified, we speculate that CP-AMPARs are removed from synapses and replaced by CI-AMPARs, similar to the “swap” observed in VTA and cerebellum (see above). Regarding the loss of mGluR5-mediated synaptic depression, it should be noted that there are other reports of decreased mGluR5-LTD in the NAc after cocaine exposure [51,52,53,54], but these studies used different cocaine regimens and withdrawal times, so underlying mechanisms may differ.
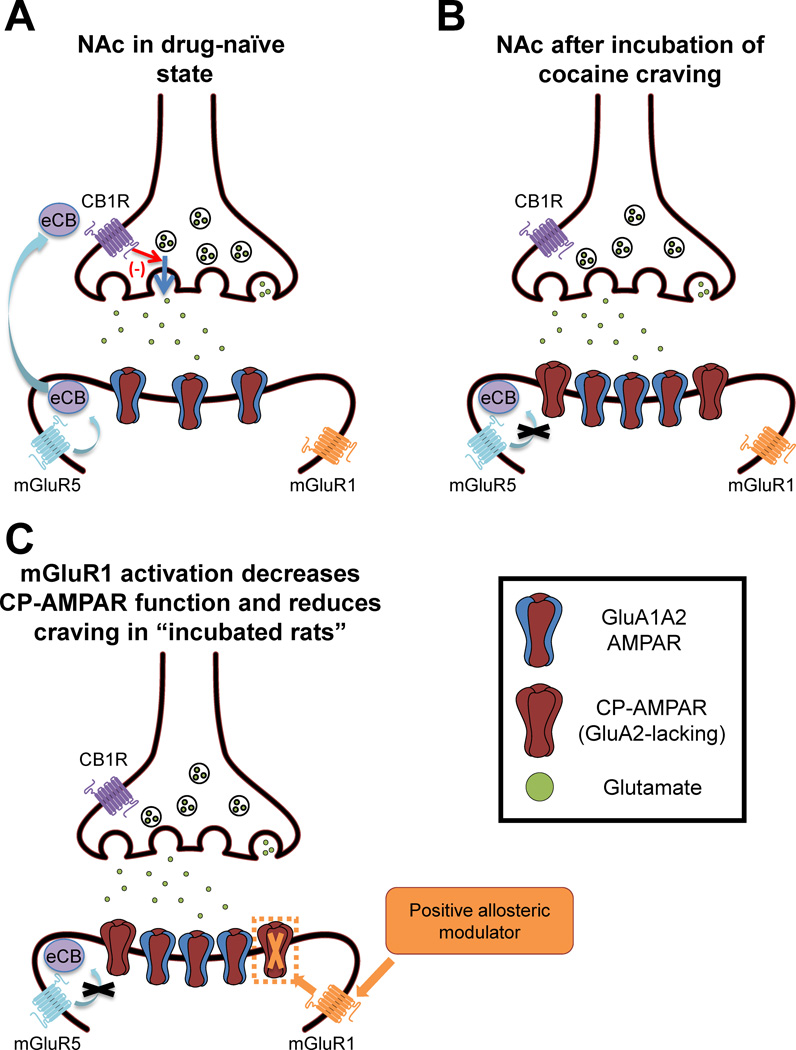
Overall, these results indicate a dramatic shift in the mechanism of group I mGluR-LTD in the NAc after incubation. In saline-exposed rats, group I mGluR-mediated synaptic depression depends on mGluR5 and is expressed presynaptically. After extended-access cocaine SA and prolonged withdrawal, a postsynaptically-expressed form of synaptic depression emerges that relies on mGluR1 and decreased CP-AMPAR transmission (Figure 1). One factor enabling negative modulation of CP-AMPARs by mGluR1 may be physical proximity of the two receptors. Biochemical results indicate that CP-AMPARs in the NAc are loosely associated with the PSD [48], reminiscent of results in hippocampus and cortex [55], and thus may be perisynaptic. This may place them in close proximity to a perisynaptic subpopulation of mGluR1 that has been detected by electron microscopy in the NAc of drug-naïve rats [•7,•8,9], although mGluR5 also exhibits perisynaptic expression yet does not modulate CP-AMPARs.
Figure 1. CP-AMPAR plasticity in the NAc and its reversal by mGluR1 stimulation.
A. In the NAc of drug-naïve animals, group I mGluR activation leads to an mGluR5-dependent, presynaptically-expressed form of synaptic depression mediated by CB1Rs [46,47,••49]. B. After extended-access cocaine self-administration, cue-induced cocaine craving progressively intensifies (“incubates”) over the first two months of withdrawal, remaining higher than “withdrawal day 1” levels for at least 6 months. Mechanisms underlying the expression of incubated craving during the first several weeks of withdrawal may involve brain regions other than the NAc [41,42]. However, after ~4 weeks of withdrawal, CP-AMPARs accumulate in NAc synapses and blocking these receptors in the NAc core prevents the expression of incubated cocaine craving on withdrawal day 45 [••16]. We speculate that the presence of high conductance CP-AMPARs enhances the ability of NAc medium spiny neurons to respond to the glutamate released from cortical and limbic afferents by cocaine-related cues, and thereby enhances cue-induced seeking. Another adaptation observed in the NAc of “incubated rats” is that mGluR5-mediated synaptic depression is disabled (black “X”) [••49]. It should be noted that the situation depicted in this panel is specific to the NAc of rats exposed to extended-access cocaine self-administration and prolonged withdrawal, as adult rats do not exhibit elevated CP-AMPARs after non-contingent cocaine [•45] or limited-access cocaine (A Purgianto et al., abstract in Soc Neurosci Abstr 2012, 669.12), even after prolonged withdrawal. C. Once CP-AMPARs have accumulated in the NAc, group I mGluR activation leads to a postsynaptically-expressed, mGluR1-dependent form of synaptic depression in which CP-AMPAR transmission is eliminated (orange “X”) and CI-AMPAR transmission is enhanced [••49]. We speculate that this occurs as a result of CP-AMPAR removal and CI-AMPAR insertion, as demonstrated in the VTA [28]. The removal of high conductance CP-AMPARs from NAc synapses is expected to decrease the responsiveness of NAc medium spiny neurons to glutamatergic inputs that are activated by cocaine-associated cues. This in turn should reduce NAc output to motor areas and thereby decrease cocaine seeking. eCB, endocannabinoid.
mGluR1 negatively regulates cocaine craving
Given that CP-AMPARs in the NAc mediate the expression of incubated cue-induced cocaine craving [••16], we recently tested the hypothesis that mGluR1 activation, leading to CP-AMPAR removal, would reduce cocaine craving in "incubated rats" [JA Loweth et al., abstract in Soc Neurosci Abstr 2012, 669.14]. Indeed, we found that either intra-NAc or systemic delivery of an mGluR1 positive allosteric modulator (PAM) attenuated incubated cocaine craving - in the case of systemic delivery, we further showed that this was associated with decreased CP-AMPAR function (Figure 1C). This protective effect endures for ~24 hours after a single systemic PAM injection and for several days after repeated PAM injections. Together, these findings identify mGluR1 as a negative regulator of CP-AMPARs in the NAc and point to the potential utility of systemically active mGluR1 PAMs in the treatment of cue-induced cocaine craving and relapse.
Despite the robust effect of mGluR1 activation in NAc medium spiny neurons from “incubated rats”, mGluR1 blockade does not affect DHPG-induced synaptic depression in drug-naïve animals [••49]. The simplest explanation is that no mGluR1 effect is detected because these animals express low synaptic levels of CP-AMPARs (i.e., a “floor effect”). Nevertheless, mGluR1-mediated regulation of CP-AMPAR levels may be “running in the background”. In other words, CP-AMPAR levels in the NAc of drug-naïve animals may be low because endogenous glutamate, acting on mGluR1 receptors in the NAc, is exerting a tonic braking effect on CP-AMPAR synaptic accumulation. Our recent studies suggest that loss of this brake, due to a decrease in mGluR1 surface expression in the NAc during cocaine withdrawal, helps enable CP-AMPAR accumulation in “incubated rats” [JA Loweth et al., abstract in Soc Neurosci Abstr 2012, 669.14].
Concluding remarks
As noted in the Introduction, many studies have found that group I mGluRs contribute to behavioral responses to cocaine and that negative modulation of group I mGluRs may therefore be useful in treating addiction [4,5]. Contrary to this literature, our preliminary studies show that mGluR1 activation is sufficient to block the NAc CP-AMPAR transmission that mediates incubated cue-induced cocaine craving. Thus, after extended-access cocaine SA and prolonged withdrawal (the conditions that lead to incubation and CP-AMPAR accumulation [••16,31]), craving may be more effectively reduced by activating mGluR1 than by blocking group I mGluRs. Indeed, none of the animal studies supporting the “blocking” strategy have tested cocaine seeking after extended-access cocaine SA combined with prolonged withdrawal. Instead, animals were studied after non-contingent cocaine injections, limited-access cocaine self-administration, or extended-access cocaine self-administration followed by a brief withdrawal. None of these three conditions are sufficient to increase CP-AMPAR levels in the NAc of adult rats [•45, A Purgianto et al., abstract in Soc Neurosci Abstr 2012, 669.12].
Therefore, mGluR1 stimulation would not be predicted to reduce cocaine craving under these conditions. On the other hand, mGluR5 function in the NAc is dramatically altered after extended-access cocaine self-administration [••49,56] but also by other types of cocaine regimens [51,52,53,54]. These results indicate that the particular pattern of cocaine exposure will be an important determinant of the outcome of group I mGluR-based therapeutic strategies.
The above discussion presents a specific example of a more general principle – animal models of cocaine addiction differ widely and are therefore associated with different types of plasticity in the reward circuitry. Each model provides insight into different aspects of addiction and is therefore predictive of therapeutic approaches tailored to those aspects. For example, the drug N-acetylcystine normalizes NAc glutamate transmission and behavioral changes observed during the first few weeks of withdrawal from non-contingent or limited-access cocaine self-administration [57], and has shown promise in reducing drug-taking in users who have yet to achieve prolonged abstinence [58,59,60]. In contrast, the incubation model is relevant to heavy cocaine users who undergo a period of forced abstinence, e.g., due to incarceration or hospitalization [61]. Importantly, the cocaine experience required for CP-AMPAR accumulation in rat NAc synapses is not extreme (6 h/day×10 days and ~30 days of withdrawal). Thus, if this adaptation occurs in humans, it may be relatively common in users who undergo forced abstinence as described above. When they return to a world full of drug-related cues, accumulated CP-AMPARs would mediate enhanced cue-induced craving. Our results suggest that mGluR1 PAMs may help maintain abstinence under these conditions. Furthermore, as discussed in detail elsewhere, PAMs of group I mGluRs may ameliorate cognitive deficits associated with cocaine use [5].
Highlights.
- The review focuses on group I mGluR-based strategies for reducing cocaine craving
- Across brain regions, mGluR1 inhibits Ca2+-permeable AMPAR (CP-AMPAR) function
- CP-AMPARs accumulate in nucleus accumbens after specific types of cocaine exposure
- Positive allosteric modulators of mGluR1 may reduce craving after such exposure
- Cocaine history and resulting adaptations determine optimal therapeutic strategy
Acknowledgements
This work was supported by National Institutes of Health grants DA009621 (M.E.W. and K.Y.T.) and postdoctoral National Research Service Award F32 DA030844 (J.A.L.). We thank Craig Werner for assistance with designing the figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(S1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 4.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol. 2010;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. •The first in-depth analysis of the subcellular and subsynaptic localization of group I mGluRs in the nucleus accumbens, revealing that mGluR1 and mGluR5 are expressed throughout this region, often co-localized, and predominantly found in extrasynaptic regions.
- 8.Mitrano DA, Arnold C, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the nucleus accumbens of cocaine-treated rats. Neuroscience. 2008;154:653–666. doi: 10.1016/j.neuroscience.2008.03.049. •This study shows that repeated cocaine exposure in vivo alters mGluR1 but not mGluR5 localization in nucleus accumbens neurons, indicating that alterations in mGluR1-mediated signaling may contribute to cocaine addiction.
- 9.Mitrano DA, Pare J-F, Smith Y. Ultrastructural relationships between cortical, thalamic, and amygdala glutamatergic inputs and group I metabotropic glutamate receptors in the rat accumbens. J Comp Neurol. 2010;518:1315–1329. doi: 10.1002/cne.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. •This paper is the first to report an important role for mGluR5 in the psychomotor-stimulating and reinforcing properties of cocaine, identifying this receptor as a potential therapeutic target in cocaine addiction.
- 11.Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol. 2012;17:287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology. 2006;187:397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- 14.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. ••This study is the first to demonstrate that CP-AMPARs accumulate in the NAc following prolonged withdrawal from extended-access cocaine self-administration, and mediate the expression of enhanced (incubated) cue-induced cocaine craving.
- 17.Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Isaac JTR, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Lee H-K. Ca2+-permeable AMPA receptors in homeostatic synaptic plasticity. Front Mol Neurosci. 2012;5:17. doi: 10.3389/fnmol.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 23.Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol. 2007;36:232–244. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- 24.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 25.Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- 27.Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. ••This study demonstrates that the increased AMPA/NMDA ratio previously reported in VTA DA neurons following a single cocaine injection is due to the incorporation of CP-AMPARs into these synapses. Along with reference 26, it was also the first to identify mGluR1-mediated CP-AMPAR removal as a mechanism that can reverse cocaineinduced plasticity in VTA dopamine neurons.
- 28.Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 29.Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mameli M, Bellone C, Brown MT, Lüscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011;14:414–416. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- 31.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. •This study shows that the level of mGluR1 tone in the VTA determines the duration of cocaine-induced neuroadaptations in the VTA and thereby AMPAR plasticity in the NAc.
- 33.Bellone C, Mameli M, Luscher C. In utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat Neurosci. 2011;14:1439–1446. doi: 10.1038/nn.2930. [DOI] [PubMed] [Google Scholar]
- 34.Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- 35.Liu SJ, Cull-Candy SG. Activity-dependent changes in AMPA receptor properties in cerebellar stellate cells. J Neurosci. 2002;22:3881–3889. doi: 10.1523/JNEUROSCI.22-10-03881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat Neurosci. 2009;12:593–601. doi: 10.1038/nn.2309. ••This study identifies an mGluR1-dependent form of LTD involving CP-AMPAR removal in cerebellar stellate cells, similar to that observed in the VTA, amygdala and NAc.
- 37.Gardner SM, Takamiya K, Xia J, Suh J-G, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Petralia RS, Wang Y-X, Mayat E, Wenthold RJ. Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J Comp Neurol. 1997;385:456–476. doi: 10.1002/(sici)1096-9861(19970901)385:3<456::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Gao C, Wolf ME. Dopamine alters AMPA receptor synaptic expression and subunit composition in dopamine neurons of the ventral tegmental area cultured with prefrontal cortex neurons. J Neurosci. 2007;27:14275–14285. doi: 10.1523/JNEUROSCI.2925-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. ••This study demonstrates that fear conditioning increases CP-AMPAR levels in the lateral amygdala and that erasure of fear memory involves mGluR1-mediated removal of CP-AMPARs.
- 41.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(S1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 44.Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. •This study shows that the type of cocaine exposure greatly influences the nature of cocaine-induced AMPAR plasticity in the NAc and in so doing helps to clarify previous findings and discrepancies in the field.
- 46.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- 48.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng L-J, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. ••This study identifies a form of mGluR1-mediated synaptic depression in the NAc of animals that have undergone prolonged withdrawal from extended-access cocaine self-administration leading to incubation of cocaine craving. This synaptic depression is expressed postsynaptically due to elimination of CP-AMPAR-mediated transmission, similar to a phenomenon reported previously in the VTA, cerebellum, and amygdala.
- 50.Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- 51.Fourgeaud L, Mato S, Bouchet D, Hémar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. Cocaine withdrawal impairse metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31:4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee H-K. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci USA. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 58.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Amen SL, Piacentine LB, Ahmad ME, Li S-J, Mantsch JR, Risinger RC, Baker DA. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36:871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100:801–810. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]