Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals (original) (raw)
. Author manuscript; available in PMC: 2013 May 28.
Published in final edited form as: Psychopharmacology (Berl). 2005 Sep 14;180(4):612–623. doi: 10.1007/s00213-005-2205-7
Abstract
Rationale
There is converging evidence for impairments in decision-making in chronic substance users. In the light of findings that substance abuse is associated with disruptions of the functioning of the striato–thalamo–orbitofrontal circuits, it has been suggested that decision-making impairments are linked to frontal lobe dysfunction. We sought to investigate this possibility using functional neuroimaging.
Methods
Decision-making was investigated using the Cambridge Risk Task during H215O PET scans. A specific feature of the Risk Task is the decisional conflict between an unlikely high reward option and a likely low reward option. Four groups, each consisting of 15 participants, were compared: chronic amphetamine users, chronic opiate users, ex-drug users who had been long-term amphetamine/opiate users but are abstinent from all drugs of abuse for at least 1 year and healthy matched controls without a drug-taking history.
Results
During decision-making, control participants showed relatively greater activation in the right dorsolateral prefrontal cortex, whereas participants engaged in current or previous drug use showed relatively greater activation in the left orbitofrontal cortex.
Conclusion
Our results indicate a disturbance in the mediation by the prefrontal cortex of a risky decision-making task associated with amphetamine and opiate abuse. Moreover, this disturbance was observed in a group of former drug users who had been abstinent for at least 1 year.
Keywords: Amphetamine, Opiates, Abstinence, Substance abuse, Decision making, Risk taking, Orbitofrontal, Dorsolateral, Prefrontal, Neuroimaging
Introduction
The behaviour of chronic drug users often seems erratic and ill-judged as exemplified by the sharing of needles (Hahn et al. 2002), the increased frequency of accidents (Alleyne et al. 1991; Pollack et al. 1998) or tendencies to indulge in other risky behaviours such as driving under the influence of drugs (Aitken et al. 2000; Albery et al. 2000; Darke et al. 2004; EMCDDA 1999). Neuropsychological studies have shown impaired decision-making in chronic substance users (Bechara et al. 2001; Clark and Robbins 2002; Grant et al. 2000; Rogers et al. 1999a) which may contribute to this erratic pattern of behaviour. Decision-making is a complex process that involves the integration of emotional information with higher level cognitive processing (Bechara et al. 2000; Damasio 1994). A number of authors have suggested that some drugs of abuse may weaken the ‘rational break’ of cognitive regulatory processes which are necessary for inhibiting motivational impulses (Jentsch and Taylor 1999; Robbins and Everitt 1999; Robinson and Berridge 2001). At present, the neural basis for disrupted decision-making occurring in association with drug abuse is unclear. There is growing evidence that chronic drug use is associated with both structural changes and functional impairments in the frontostriatal systems (Jentsch and Taylor 1999; Robinson and Kolb 1997, 1999; Sklair-Tavron et al. 1996). In addition, reductions in dopamine D2 receptor density in the striatum have been reported in users of opiates (Wang et al. 1997) and methamphetamine (Volkow et al. 2001). In methamphetamine users, reduced D2 binding in the striatum was associated with reduced metabolic activity in the orbitofrontal cortex (OFC) (Volkow et al. 2001).
Previous functional neuroimaging studies have localised abnormal decision-making processes to prefrontal cortical networks including inferior frontal and orbitofrontal gyri as well as the anterior cingulate (Ernst et al. 2002; Rogers et al. 1999b, 2004). Functional magnetic resonance imaging (fMRI) studies of methamphetamine users revealed reduced brain activation in orbitofrontal and dorsolateral prefrontal areas compared with controls when participants were required to make forced two-choice predictions (Paulus et al. 2002, 2003). This task involved guessing and win–stay/lose–shift strategies which appear only indirectly related to risky decision making. Using the Iowa Gambling Task (IGT), Bolla et al. (2003) reported increased cerebral blood flow (CBF) in the OFC coupled with decreased activation in the dorsolateral prefrontal cortex (DLPFC) in cocaine users who were abstinent for 25 days. However, the IGT is a complex task that involves stimulus reinforcement, reversal learning and working memory, as well as decision-making cognition (Clark et al. 2004; Fellows and Farah 2005). In healthy volunteers, the IGT activates virtually the entire prefrontal cortex (Ernst et al. 2002). The Cambridge Risk task was developed in order to isolate the assessment of risk from the learning component that is central to the IGT by requiring participants to make a choice between two mutually exclusive options incorporating a conflict between an unlikely high reward and a likely low reward option in a visually explicit format (see Fig. 1). Since each trial is independent from its predecessor, learning effects are obviated. PET imaging in healthy volunteers has shown that the Risk Task activates predominantly right orbitofrontal areas (Rogers et al. 1999b), whereas manic patients additionally activate the left anterior cingulate (Rubinsztein et al. 2001).
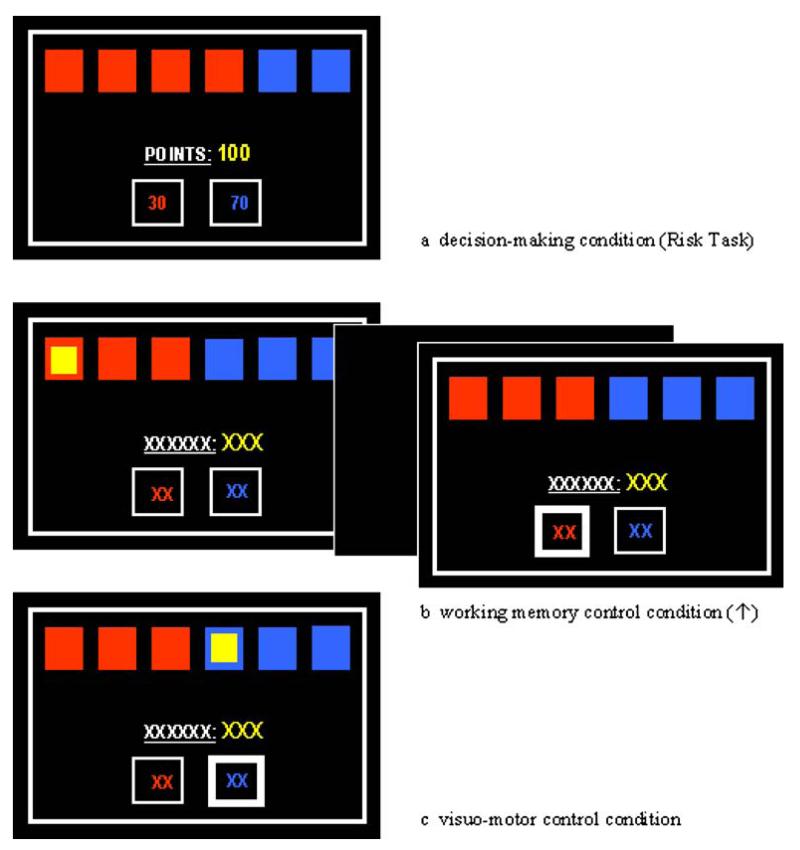
Fig. 1.
Typical displays of the decision-making, working memory and visuomotor condition
The Cambridge Risk Task was used in the current study to explore the neural correlates of decision making in individuals with current and past drug use. For the current drug users, we compared users of two classes of agents, amphetamines and opiates. These drugs have distinct pharmacological characteristics, acting at catecholamine transporters and opioid receptors, respectively (Lingford-Hughes and Nutt 2003; Nestler 2002). Ornstein et al. (2000) previously showed different neuropsychological profiles in chronic users of these two substances. However, to our knowledge, there has been no functional neuroimaging study directly contrasting brain activation in individuals dependent on amphetamines versus opiates. Poor decision making may arise not only under the influence of the drug and as a result of chronic drug exposure (e.g. via neurotoxicity) but it may also occur premorbidly as predisposing cognitive style towards addictive behaviours. A third group of former drug users (abstinent for at least 1 year) was therefore included to control for the effects of recent drug use and to assess recovery of function with prolonged abstinence. We thus investigated the impact of two main factors: current and former drug use and the pharmacological classification of the drug currently used (amphetamines vs opiates) on task-dependent brain activity. We hypothesised that drug users would make more disadvantageous decisions relative to controls with concomitant differences in patterns of brain activation.
Materials and methods
Participants
Sixty participants were recruited for the study which was approved by the Local Research Ethics Committees in Cambridge (LREC 00/405), Huntingdon, Peterborough/Fenland and West Suffolk. Prior to testing, all participants underwent a screening process concerning their history of drug abuse and their general health. Urine samples were analysed for amphetamine, cocaine, morphine, methadone and benzodiazepines. All participants gave informed written consent and received monetary compensation for taking part in the study.
Current substance users were recruited upon referrals of key workers at drug units in Cambridge, Huntingdon, Wisbech and Bury St. Edmunds, UK, as well as through recommendation of participants. Inclusion criteria were dependency on either amphetamines or opiates for the past three years according to DSM-IV (American Psychiatric Association 1994) criteria for substance dependence. Criteria for exclusion were comorbid psychiatric illnesses, psychotropic medication, history of head injury, history of an overdose that required resuscitation and overnight hospitalisation, HIV infection, current pregnancy or breast-feeding. Most of the current substance users were taking other drugs besides their drug of choice (see Table 1). However, a dependence on another drug, apart from nicotine, or a regular alcohol consumption exceeding the recommended units by the UK government (British Medical Association 1995) led to exclusion from the study. Exclusion criteria were applied to all four groups. Seven of 15 amphetamine users were on Dexedrine by prescription from a consultant psychiatrist (mean±SD dose, 34±17.8 mg; dose range, 15–50 mg) but also used street amphetamine. Amphetamine users without a prescription used street amphetamine daily. Urine analysis in the amphetamine group 60 min prior to PET scanning revealed that 14 of 15 samples tested positive for amphetamine; the only sample that was negative for amphetamine was positive for cocaine. Five samples showed additional substances (three morphine, one benzodiazepines, one cocaine). Nine of the 15 opiate users were on a prescription of methadone (mean±SD dose, 38.6±17.8 ml; dose range, 20–80 ml). Six opiate users used only illicit heroin. One methadone-maintained participant also received rabeprazole sodium (20 mg) for treatment of heartburn. No other medication was taken by any participant. Urine analysis revealed that 15 of 15 participants tested positive for morphine or methadone; in addition, eight samples also tested positive for cocaine, four samples tested positive for benzodiazepines and one was positive for amphetamine.
Table 1.
Percentage of participants with current or past regular use of other substances in the amphetamine, opiate, ex-users and control groups
| Controlgroup | Amphetaminegroup | Opiategroup | Ex-druggroup | |
|---|---|---|---|---|
| Amphetamines | ||||
| Never | 100 | 0 | 13 | 13 |
| Past | 0 | 0 | 80 | 87 |
| Current | 0 | 100 | 7 | 0 |
| Opiates | ||||
| Never | 100 | 53 | 0 | 7 |
| Past | 0 | 47 | 0 | 93 |
| Current | 0 | 0 | 100 | 0 |
| Ecstasy | ||||
| Never | 100 | 20 | 40 | 67 |
| Past | 0 | 73 | 60 | 33 |
| Current | 0 | 7 | 0 | 0 |
| Cocaine | ||||
| Never | 100 | 13 | 20 | 7 |
| Past | 0 | 60 | 60 | 93 |
| Current | 0 | 27 | 20 | 0 |
| Benzodiazepines | ||||
| Never | 100 | 73 | 47 | 33 |
| Past | 0 | 27 | 40 | 67 |
| Current | 0 | 0 | 13 | 0 |
| Alcohol | ||||
| Neverabused | 100 | 33 | 60 | 20 |
| Past | 0 | 47 | 33 | 80 |
| Current | 0 | 20 | 7 | 0 |
| Cannabis | ||||
| Never | 100 | 13 | 7 | 7 |
| Past | 0 | 33 | 60 | 93 |
| Current | 0 | 54 | 33 | 0 |
| Tobacco | ||||
| Never | 40 | 7 | 0 | 7 |
| Past | 40 | 0 | 0 | 26 |
| Current | 20 | 93 | 100 | 67 |
Ex-drug users were recruited via Narcotics Anonymous, an international, community-based, nonprofit society of recovering drug users. For inclusion to the study they had to be abstinent from all drugs of abuse (except nicotine) for at least 1 year (mean±SD years of abstinence, 10.2±6.6). Two participants of this group had been ex-stimulant users (dependent on amphetamines and/or cocaine), two had been ex-opiate users and 11 had been dependent on both stimulants and opiates. Urine samples were negative for all substances.
Control participants were recruited via advertisements in the local area and selected to match the substance user groups according to age, premorbid IQ, gender and handedness (Table 2). The inclusion criterion was the absence of a drug-taking history. Five controls smoked tobacco in the past; three were current tobacco smokers. Four of the past/current smokers tried cannabis but never developed a habit. One participant had diabetes that was well controlled on insulin. Urine analyses were negative for all substances.
Table 2.
Mean total scores of descriptive group characteristics
| N | Control group15 | Amphetamine group15 | Opiate group15 | Ex-drug group15 |
|---|---|---|---|---|
| BDI-IIa | 3.5 (3.1) | 14.1 (7.9) | 11.3 (8.9) | 6.3 (5.4) |
| BIS-11b | 60.6 8(8.2) | 71.3 (14.7), _n_=10 | 67.8 (8.7) | 66.4 (9.1) |
| Verbal IQc | 115.7 (6.0) | 111.5 (5.4) | 113.3 (6.2) | 114.9 (7.5) |
| Age (years) | 35.8 (9.0) | 37.6 (9.1) | 37.1 (8.8) | 40.1 (6.4) |
| Gender (M:F) | 9:6 | 9:6 | 13:2 | 10:5 |
| Handedness (R:L) | 13:2 | 14:1 | 15:0 | 13:2 |
| Hepatitis C | 0 | 1 | 3 | 5 |
| Current employment (%) | 100 | 47 | 47 | 73 |
| Years of drug abused | 19.5 (9.4) | 12.0 (9.7) | 11.7 (4.8) | |
| Age of onset | ||||
| Amphetamine use | 16.7 (2.5) | 19.0 (2.6), _n_=13 | 16.8 (2.4), _n_=13 | |
| Opiate use | 25.6 (8.9), _n_=8 | 23.7 (5.9) | 18.6 (3.6), _n_=14 |
Scanning procedure and materials
Scanning took place at the Wolfson Brain Imaging Centre in Cambridge, UK. All participants underwent a T1-weighted MRI scan prior to 12 PET scans performed using a General Electric Advance scanner, which produces 35 image slices at an intrinsic resolution of ~5×5×5 mm. All 12 PET scans were performed on the same day in succession, so that participants spent approximately 120 min in the scanner. Each PET acquisition scan was performed using the slow bolus infusion method of water activation (Raichle et al. 1983), which consisted of the subject receiving through a forearm cannula 300 MBq of [15O]water. Each [15O]water production involved a 100 s accumulation of activity in saline, a 20 s bolus delivery followed by 90 s flushing with saline at a flow rate of 10 ml/min. Each scan provided an image of rCBF (regional Cerebral Blood Flow) integrated over a period of 90 s from the time when the tracer first enters the cerebral circulation. rCBF was measured during separate scans of decision-making and control conditions. The task display was presented on a MicroTouch 20C touch-sensitive screen controlled by a Pentium microcomputer placed in a viewing distance to the participant’s head. Prior to the first scan, the tasks were explained and participants were given one practice example of the decision-making task and of each control component (see Fig. 1). During each scan, participants performed one condition (either decision-making, working memory or simple control condition); thus, each condition was presented four times in a pseudorandom order so that two of the same conditions did not follow each other.
Decision-making was investigated via the modified version of the Cambridge Risk Task first used by Rogers et al. (1999b), which required participants to choose, on each trial, between two alternatives associated with different probabilities of reward and punishment. On each trial, an array of six boxes was presented on the screen, with a proportion of red and blue boxes (3:3, 4:2, 5:1 boxes) that varied from trial to trial, as did the reward values associated with the ratio of boxes (50:50, 60:40, 70:30, 80:20, 90:10 points). Participants were told that the computer had hidden a yellow token at random behind one of the six boxes. They were required to decide if the token was hidden behind a red or blue box. Their decision on each trial was also shaped by a fixed bet associated with each alternative. A larger bet was always associated with the colour in the minority. For example, in a display of two blue boxes and four red boxes, choosing blue may be associated with a 70-point gamble, and choosing red with a 30-point gamble (see Fig. 1). If the participant’s red–blue decision were correct, the participant would win those points, and if the decision were incorrect, those points would be lost. The participant started each task run with 100 points available and indicated their choice on each trial by touching a red or blue square at the bottom of the screen. The probability ratio (relationship between red and blue boxes) and the reward ratio (relationship between gains and losses) varied from trial to trial. After each decision, the computer revealed the hidden token and displayed a message: ‘you win!’ or ‘you lose!’. In contrast to the version used by Rogers et al. (1999b), we did not separate trials into blocks of high-risk ratios (5:1) and moderate-risk ratio (4:2). Instead, we introduced two sepa rate control conditions, which were aimed to further strengthen the advantage of the Risk Task over the IGT, namely, the independence of trials: a working memory condition and a visuomotor condition. The introduction of the working memory condition was also intended to separate risky decision-making from the related (but distinct) wider domain of executive function, which can also be impaired in drug users (Rogers and Robbins 2001). In the working memory condition (see Fig. 1), the screen display was similar to the decision-making condition, except that the ratio of red and blue boxes was 3:3 on every trial, and the bets and points total were replaced by crosses. In an _n_-back procedure, a yellow token was briefly (1.5 ms) displayed behind one box. On the subsequent (n+1) trial, the participant had to indicate the colour of the token by pressing either the red or blue square at the bottom of the screen (i.e. 1 back). In the visuomotor control condition, a token was displayed in a box and the subject had to press the corresponding colour square at the bottom of the screen (i.e. 0 back). No gambling decisions were required and no gains or losses were involved in the working memory and visuomotor control conditions.
Prior to scanning, participants were given two questionnaires, the Barratt Impulsiveness Scale 11 (BIS-11) (Patton et al. 1995) and the Beck Depression Inventory II (BDI-II) (Beck et al. 1996). The former is one of the most widely used questionnaires to assess different aspects of impulsivity in a variety of subject populations. The BDI-II is an established self-rating measure of depression severity.
Statistical PET data analysis
Preprocessing
PET scans were analysed with Statistical Parametric Mapping (SPM2, Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks Inc., Sherborne, MA, USA). First, the 12 scans from each participant were realigned using the first scan as a reference and then normalised to the standard brain template. The normalised images were smoothed with a 16-mm full-width half-maximum (FWHM) isotropic Gaussian kernel. Individual three-dimensional MRI volumes were re-sliced and used as a coreference for the PET data. In order to directly localise anatomical regions with significant changes in CBF between the conditions, the composite stereotaxic MRI and PET volumes were coregistered.
Tasks effects
SPM identifies task-dependent effects using the general linear model. For each participant, three conditions were modelled: the decision-making task and the two conditions designed to provide baseline measurements (i.e. the working memory and the visuomotor control tasks). Because the initial analysis identified little difference between the two baseline tasks, we subsequently combined them as a common baseline task with which to compare the scans acquired during the decision-making task. For each participant, a contrast image was produced through voxelwise comparisons of the decision making with the combined control scans. These contrast images, one from each participant, were taken to a second-level analysis using a one-way analysis of variance (ANOVA) model. That is, we treated intersubject variability as a random effect.
Group analysis
The ANOVA, comprising the four groups (controls, current amphetamine users, current opiate users and ex-drug users) was used to generate an F map (identifying regions in which there was a group×task interaction). Whole-brain analysis was thresholded using the False Discovery Rate (Benjamini and Hochberg 1995; Genovese et al. 2002) in order to obviate the multicomparison problems produced by voxelwise testing. In a further attempt to safeguard against type I error consequent upon multiple comparisons, the F test was confined to regions of a priori interest (OFC, anterior cingulate cortex, caudate/putamen, thalamus, ventral striatum, DLPFC and amygdala) using a masking procedure implemented in Pickatlas (Maldjian et al. 2003). Those regions surviving this threshold were then the subject of post hoc t tests implemented in the Statistical Package for Social Sciences (SPSS). Three orthogonal t tests were carried out (controls vs all current and ex-drug users, current vs ex-drug users, current amphetamine vs current opiate users). A Bonferroni correction was then applied based upon the number of regions of interest. Thus, to summarise, we identified group by task effects based on a set of regions of interest and a whole-brain F map thresholded to protect against type I errors caused by the multiple comparisons.
Statistical behavioural data analysis
Behavioural and questionnaire data were analysed using SPSS version 11 (SPSS Inc.). Proportion data were arcsine transformed and latency data square root transformed prior to analysis to reduce skew (Howell 1997) (data in tables represent untransformed values). After transformation, all data were normally distributed as assessed by the Kolmogorov–Smirnov test. Decision-making performance was analysed with repeated measures ANOVA with proportions of likely options and proportions of risky options as within-subjects variables and groups as between-subjects factor (four levels). Univariate ANOVA was applied on descriptive data and total scores of the questionnaire data, and multivariate analysis of variance (MANOVA) was conducted on the subscales of the BIS. Three orthogonal comparisons were conducted: the three drug user groups compared to controls, current users versus ex-drug users and amphetamine users contrasted with opiate users. The three planned comparisons were carried out via three separate one-way ANOVAs. Pearson correlations, two-tailed with alpha set at .05, were calculated for the groups separately. The behavioural data of one opiate user were lost due to a computer failure. Since the BIS-11 was included into the study only after five amphetamine users had already been scanned, the BIS data for those participants are missing.
Results
Group characteristics
Demographic characteristics and questionnaire scores are displayed in Table 2. The groups did not differ with regard to age, handedness or verbal IQ. Current amphetamine users had been taking drugs longer than current opiate users (_F_2,42=4.24, _p_=.021; _t_42=2.47, _p_=.018). Furthermore, ex-drug users started using opiates at a younger age than current users (_F_2,34=4.30, _p_=.022; _t_34=3.03; _p_=.008). For the age of onset in amphetamine use, there was a trend that current amphetamine users started at a younger age than opiate users (_F_2,38=2.74, _p_=.077; _t_38=2.15, _p_=.038). With regard to the questionnaire data, there was a significant group effect on the BDI (_F_3,56=7.50, p<.001) such that drug users were more depressed than controls (_t_56=3.49, p<.001), and current drug users were more depressed than ex-drug users (_t_56=3.01, _p_=.004). There was no significant difference in depression between current amphetamine and opiate users. Regarding the BIS, there was only a group effect on the attentional impulsiveness subscale (_F_3,51=3.29, _p_=.029; Wilk’s _λ_=71, _p_=.044) such that drug users scored higher than controls (_t_51=2.26, _p_=.028) and amphetamine users scored higher than opiate users (_t_51=2.35, _p_=.023).
Decision-making performance
Performance data for the decision-making and working memory conditions are shown in Table 3. Two amphetamine users performed poorly on the working memory control condition but showed normal performance during decision making. Overall, all four groups had a conservative strategy in decision-making, preferring the likely low-reward option over the unlikely risky one (_F_1,54=115.1, p<.001). Although the amphetamine group appeared less conservative, univariate ANOVA showed that there were no group differences in the proportion of likely option choices (_F_3,55=1.41, _p_=.250). The groups also did not differ in terms of response latencies (_F_3,55=0.63, _p_=.597). None of the demographic or questionnaire variables correlated with the behavioural measures on the Risk Task.
Table 3.
Percentage of choices/answers means (±standard error) of performance on the decision-making task and the working memory control task
| Task | Main measures | Control | Amphetamine | Opiate | Ex-drug |
|---|---|---|---|---|---|
| Decision making | Optimal choices | 87% | 72% | 82% | 79% |
| Overall latency (ms) | 2,768.0 (283.8) | 3,086.4 (367.6) | 2,879.8 (488.0) | 2,478.9 (180.3) | |
| Working memory | Correct answers | 97% | 84% | 97% | 96% |
| Overall latency (ms) | 1,380.4 (87.7) | 2,896.4 (809.5) | 1,585.5 (99.2) | 1,585.6 (281.2) |
Imaging results
Task-related effects within each group
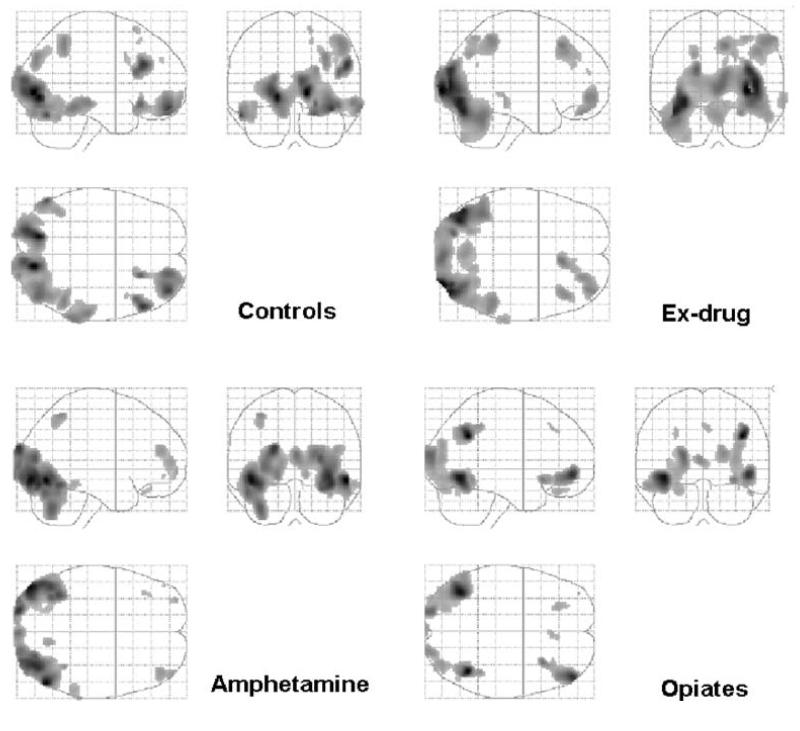
Significance levels were set at p<.001, uncorrected for multiple comparison. Task-related effects within each group are shown in Table 4 and Fig. 2. These are provided to give an initial impression of task-related activation and possible similarities/dissimilarities between the groups. For controls, there was significant right-sided activation in the lateral OFC, superior frontal gyrus, DLPFC, inferior temporal gyrus, inferior parietal gyrus, lingual gyrus, occipital cortex and cerebellum. Amphetamine users showed significant activation in the left hemisphere in the lateral OFC, frontal pole and superior parietal cortex. For the right hemisphere, significant activation was revealed in the DLPFC, inferior temporal lobe, lingual gyrus and occipital cortex. In the opiate-users group, there was significant left-sided activation in the lateral OFC, DLPFC and inferior temporal gyrus. There was significant activation in the right hemisphere in the middle frontal gyrus, inferior parietal gyrus, gyrus fusiformis and cerebellum. Bilaterally, there was significant activation in the frontal lobe and occipital cortex. Ex-drug users showed significant right-sided activation in the frontal pole, DLPFC, middle temporal gyrus, superior and inferior parietal gyrus, cerebellum and bilaterally in the occipital cortex.
Table 4.
Talairach coordinates and corresponding Brodmann’s area (BA) of the task-related within-group effects at p<.001 uncorrected for multiple comparison
| Brain regions | BA | Controls | Amphetamines | Opiates | Ex-drug | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Orbitofrontal cortex | 11 | 38 | 54 | −16 | −24 | 34 | −22 | |||
| Orbitofrontal cortex | 11/47 | −38 | 28 | −24 | ||||||
| Fontal pole | 10 | −32 | 62 | −2 | ||||||
| Frontal pole | 10 | −46 | 50 | −6 | 46 | 50 | −6 | 36 | 52 | −6 |
| Frontal pole | 10 | −38 | 56 | −8 | 36 | 52 | 20 | |||
| Superior frontal gyrus | 8 | 16 | 24 | 50 | ||||||
| Middle frontal gyrus | 8 | 4 | 30 | 42 | ||||||
| Medial frontal gyrus | 8 | 8 | 23 | 46 | ||||||
| Dorsolateral PFC | 9 | 28 | 46 | 32 | 42 | 28 | 42 | |||
| Dorsolateral PFC | 46 | 54 | 28 | 28 | 42 | 46 | 18 | −28 | −68 | 36 |
| Precentral gyrus | 6 | 16 | 20 | 64 | ||||||
| Middle temporal gyrus | 21 | 66 | −34 | −10 | ||||||
| Inferior temporal gyrus | 20 | 60 | −42 | −16 | ||||||
| Inferior temporal gyrus | 37 | 66 | −44 | −16 | −40 | 64 | −8 | |||
| Superior parietal gyrus | 7 | −36 | −54 | 56 | 28 | −64 | 54 | |||
| Inferior parietal gyrus | 40 | 48 | −52 | 50 | 40 | −56 | 34 | 50 | −46 | 52 |
| Lingual gyrus | 18 | 12 | −84 | 0 | 12 | −68 | −6 | |||
| Occipital gyrus | 18 | 20 | −96 | 16 | 30 | −98 | 2 | |||
| Occipital gyrus | 18 | |||||||||
| Occipital gyrus (cuneus) | 18 | −4 | 104 | 10 | ||||||
| Occipital gyrus (cuneus) | 19 | 32 | −80 | 34 | −24 | −70 | 38 | |||
| Occipital gyrus (precuneus) | 19 | −2 | −74 | −24 | ||||||
| Gyrus fusiformis | 19 | 42 | −54 | −10 | ||||||
| Occipital gyrus | 19 | 52 | −70 | −10 | ||||||
| Cerebellum | −54 | −70 | −24 | 48 | −66 | −24 | 34 | −62 | −26 | |
| Cerebellum | 36 | −62 | −20 |
Fig. 2.
Main effects of the decision-making task (subtracting the combined control condition) shown for each of the subject groups separately. For each group ‘glass brain’ maximum intensity projections are shown in saggital, coronal and transverse planes
Task-related effects between groups
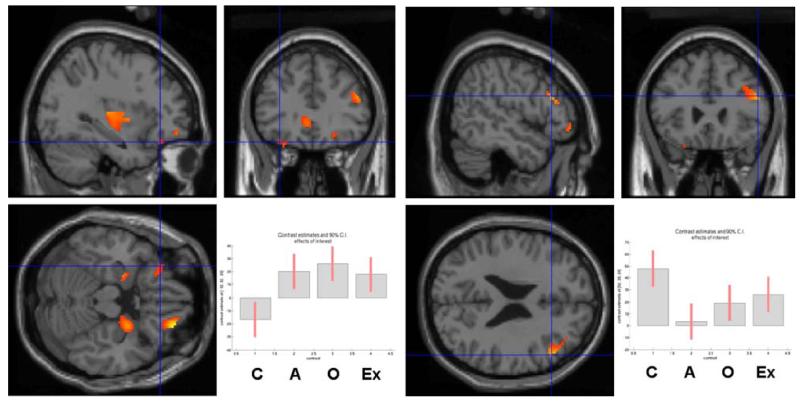
For the between-group comparisons, significance levels were set at p<.05 and masked with the regions of interest (OFC, anterior cingulate cortex, caudate/putamen, thalamus, ventral striatum, DLPFC and amygdala). Three planned comparisons were conducted: controls vs drug users, current vs ex-drug users and current amphetamine versus opiate users. Regions surviving the thresholded F map are shown in Table 5. The planned comparison between controls and drug users (−3, 1, 1, 1) revealed significant differences in activations in the left orbitofrontal, right dorsolateral and left anterior cingulate cortex. Only the differences in activations in the left orbitofrontal and the right DLPFC, but not in the left anterior cingulate, survived correction for multiple comparison (see Table 6 and Fig. 3). The comparison between current and former drug users revealed a significant difference in task-related deactivation in the right putamen, whereas the comparison between current amphetamine vs opiate users showed a significant difference in the left OFC activation, but both effects did not survive multiple comparison correction (Table 6).
Table 5.
Task-related between-group effects uncorrected for multiple comparison
| Region | BA | Talairachcoordinates | z Score | p FDR-corr | ||
|---|---|---|---|---|---|---|
| Right anterior PFC | 10 | 40 | 54 | −6 | 6.03 | <.001 |
| Right OFC | 11 | 28 | 50 | −18 | 5.10 | <.001 |
| Right dorsolateral PFC | 9/46 | 52 | 26 | 24 | 4.48 | <.001 |
| Right putamen | 32 | −6 | 6 | 4.70 | <.001 | |
| Left anterior PFC | 10/11 | −18 | 46 | 12 | 4.51 | <.001 |
| Left anterior cingulate | 24 | −4 | 32 | −2 | 3.74 | .001 |
| Left anterior cingulate | 32 | −4 | 44 | −6 | 3.58 | .002 |
| Left insula | −32 | −20 | 8 | 4.18 | <.001 | |
| Left putamen | −32 | −2 | 2 | 3.98 | .001 | |
| Left amygdala | −22 | −6 | −18 | 4.05 | .001 | |
| Left OFC | 11 | −24 | 30 | −22 | 3.68 | .002 |
| Left OFC | 11 | −32 | 32 | −20 | 3.44 | .003 |
Table 6.
Significant task-related between-group effects at p<.05 in the planned comparisons
| Region | BA | Talairach coordinates | Load | Group × load interaction | ||
|---|---|---|---|---|---|---|
| Controls vs drug users | ||||||
| Lateral OFCa | 11 | −32 | 32 | −20 | ⇓ | Controls ⇓, drug users ⇑ |
| Dorsolateral prefrontal cortexa | 9/46 | 52 | 26 | 24 | ⇑ | Controls ⇑, drug users ⇑ − |
| Anterior cingulateb | 24 | −4 | 32 | −2 | ⇓ | Controls ⇓, drug users ⇓ − |
| Current vs ex-drug users | ||||||
| Putamenb | 32 | −6 | 6 | ⇓ | Current ⇓, ex-drug users ⇓ + | |
| Amphetamine vs opiate users | ||||||
| Lateral OFCb | 11 | −24 | 30 | −22 | ⇑ | Amphetamine ⇑, opiates ⇑ + |
Fig. 3.
Left, the significantly greater (p<.05) task-related activation in the left lateral OFC in drug users (i.e. current and former amphetamine- and opiate-user groups combined) compared with controls is shown in the sagittal, coronal and horizontal planes. Right, the significantly greater (p<.05) task-related activation in the right DLPFC in controls compared with drug users (i.e. current and former amphetamine- and opiate-user groups combined) is shown in the sagittal, coronal and horizontal planes. The plots in the right-hand corners show the mean size of the effect for each region (OFC −32, 32, −20 and DLPFC 52, 26, 24). Differences were also found in the medial temporal cortex; however, this did not fall within our a priori set of regions of interest
In summary, during decision making a double dissociation between orbitofrontal and dorsolateral prefrontal regions was observed. Drug users showed significant task-related activation in the left lateral OFC relative to controls, whereas controls showed greater task-related activation in the right DLPFC relative to drug users.
Correlations
Correlations between task-related activation (decision-making minus combined control conditions) which survived correction for multiple comparison were correlated separately for controls and drug users with measures of impulsivity, mood and risk-taking measures. In controls only, left orbitofrontal activation was negatively correlated with BIS motor impulsiveness scores (Pearson’s _r_=−.59, _p_=.021). However, a high BIS motor score did not influence performance on the Risk Task.
Discussion
Although we hypothesised that drug users would make disadvantageous decisions more frequently than controls, decision-making performance was not significantly deficient in the drug user groups relative to healthy controls. Nevertheless, consistent with our hypothesis for task-related activation, there were significant differences in the left OFC and right DLPFC. Thus, participants with current or previous dependence on either amphetamines or opiates activated the left lateral OFC during risky decision making, while control participants exhibited relative deactivation in the this area, a pattern previously observed in a study of healthy volunteers performing the same task (Rogers et al. 1999b). In the present study, we also found that control participants showed significantly greater activation in the right DLPFC than drug users. A pattern of overactivation in the OFC associated with underactivation in the DLPFC, again in the absence of behavioural differences, has previously been found in abstinent cocaine users on the IGT (Bolla et al. 2003).
There are differing interpretations of how to interpret neuroimaging findings that are not reflected in differences in behavioural task performance (see Wilkinson and Halligan 2004 for a review). We hold the view that differences in brain activation in the absence of behavioural differences are valuable in their own right, as they may indicate underlying cognitive differences which otherwise would not be detectable and also cannot be attributed to nonspecific responses to performance deficits (Fletcher 2004). The Risk Task was adapted from the Cambridge Gamble Task (Rogers et al. 1999a) for neuroimaging purposes. The previous study of the Cambridge Gamble Task in drug users showed that amphetamine users chose the optimal choice in 85% of trials and opiate users in 92%, relative to 95% in controls (Rogers et al. 1999a), while in the present study on the Risk Task all groups made even more disadvantageous choices (see Table 3). However, this difference was not statistically significant in the present study, possibly because of the smaller control group employed. Nonetheless, the absence of significant behavioural differences simplifies the interpretation of the altered pattern of frontal activation in the drug users, which cannot just be attributed to performance factors.
Dissociable brain responses during decision making in drug users and controls
There are several possible explanations for these dissociations in brain activation, for example, compensation due to pathology, use of different strategies and affective style.
Possible compensation for right frontal pathology
One account of the abnormal activation of the OFC in drug users is that there is core pathophysiology in this region. Bolla et al. (2003) suggested that overactivation in the right OFC on the IGT in cocaine users was a compensatory effect for tissue damage in this area. Structural MRI analysis of participants from the study revealed grey matter reductions in the right OFC and right DLPFC (Matochik et al. 2003). In the present data, the increased task-related activation in the left lateral OFC occurred in conjunction with reduced activation in the right DLPFC and a trend towards reduced activation in the right OFC. However, it is also possible that this pathophysiology lies elsewhere and that the overactivation in OFC results from functional compensation. The increased response in the left OFC may plausibly reflect a compensatory response to this apparent underfunctioning. The lateral OFC has been associated with response inhibition (Elliott et al. 2000) as has the DLPFC (Hester and Garavan 2004; Kelly et al. 2004). Behavioural evidence has suggested that some drug users have a hypersensivity to reward, as they favoured the risky high-reward options in the IGT (Bechara et al. 2001, 2002; Clark and Robbins 2002, for review; Grant et al. 2000). Consequently, inhibition of responses to risky high-reward options may be problematic for some drug users. Perhaps this increased activity in the left OFC reflects additional response suppression, enabling drug users to perform as well as non-drug-using controls on this risky decision-making task. This interpretation receives support from findings by Goldstein et al. (2001), who correlated performance on an interference control task (Stroop task) with baseline brain metabolism as measured by PET imaging in substance-de pendent individuals and non-drug-taking controls. They found that in non-drug-taking controls performance on the Stroop task was negatively correlated with activity of the OFC, but in drug users this relationship was reversed. The authors suggest that this may be due to drug-related changes in the neural network in chronic drug users, so that these individuals show greater OFC activation while performing the task successfully.
In addition, the DLPFC activation seen during performance on the IGT by PET imaging in healthy volunteers (Ernst et al. 2002) may not indicate the added complexity of the task but represent an inherent feature of conflict resolution in decision-making (Krawczyk 2002, for review). However, it is somewhat surprising that the volunteers in the study by Rogers et al. (1999b) did not activate the DLPFC but a more inferior part of the right middle frontal gyrus [Brodmann’s area (BA) 10/11]. There are several possible explanations for this, including the use of different control tasks and contrasts, differing numbers of participants and differing demographic characteristics between the two studies. One other consideration is that in contrast to the Rogers et al. (1999b) study, we treated intersubject variability as a random effect, an analytical approach that reduces the likelihood of attributing idiosyncratic effects of individuals to the group as a whole.
Possible different strategies used during decision making
During decision-making, control participants activated the right DLPFC (BA 9/46) to a greater extent than drug users. The right DLPFC has been implicated in maintaining, monitoring and manipulating information in spatial working memory (D’Esposito et al. 1998; Fletcher and Henson 2001; Garavan et al. 2000; Smith and Jonides 1999), and under circumstances of uncertainty it can also play a role in decision-making processes (Krawczyk 2002). However, the design of the Cambridge Risk Task is simple, as decisions are not made by learning or on the basis of prior knowledge, so performance on the Risk Task does not depend on working memory capacity. Nevertheless, it is likely that participants employed different strategies when doing the task. For example, they could focus simply on the likelihood of the outcome or its reward value, or they could attempt to combine this information in a more reflective manner, possibly including the right DLPFC (Zhang et al. 2003). Thus, it could be hypothesised that drug users tended to use an ‘emotional’ strategy involving OFC, whereas controls used a ‘cold’ strategy with DLPFC involvement. The use of an outcome-orientated strategy of drug users also receives support from an fMRI event-related study by O’Doherty et al. (2003), which aimed at dissociating OFC functions in healthy volunteers. They found that the left lateral OFC was activated in response to both rewarding and punishment feedback but only in a condition when participants did not need to change their behavioural responses on the basis of the prior feedback.
Possible differences in affective style
Neuroimaging findings have suggested that the medial part of the OFC responds to rewards, whereas the lateral OFC responds to punishment (Elliott et al. 2000) and to aversive stimuli (Zald and Pardo 1997). This activation of the left lateral OFC in drug users may thus indicate an increased sensitivity to punishment (i.e. losing points). This is in line with the positive correlation reported by Goldstein et al. (2002) between harm avoidance scores and metabolism in the OFC in methamphetamine users. Greater sensitivity to punishment has also been associated with lowered self-control (Segarra et al. 2000), a core characteristic of substance dependence (American Psychiatric Association 1994). Moreover, according to Baker et al. (2004), repeated cycles of withdrawal make regular drug users particularly sensitive to negative affect of any kind, either pharmacologically induced, such as craving or externally provoked, e.g. under time pressure, following negative appraisal or, as in our study, losing points in a computerised game. Baker et al. argue that such increased sensitivity to negative affect leads to further drug use for reducing aversive states. Indeed, it is possible that had our task design involved playing for money instead of points, losing would have become even more aversive to drug users and behavioural differences might have become apparent.
Lack of difference between current and former drug users
The overall comparison between current and ex-drug users showed no significant differences in brain activation. This is an important finding because it indicates that the difference in patterns of brain activation probably did not simply reflect current effects of the drug during scanning. To argue against this view would require the assumption that acute effects of these drugs on brain activation resemble their chronic, long-term effects following extended abstinence, which would appear unlikely. However, in theory, this hypothesis could be tested by directly comparing former and current drug users in the scanner both on and off drugs.
The persistent effect in former drug users (averaging 10 years abstinence) may reflect long-lasting changes in brain function caused by chronic drug use. This interpretation receives some support from a recent [18F]fluorodeoxyglucose PET study by Wang et al. (2004) on abstinent methamphetamine users who had only shown partial brain recovery in the course of drug abstinence. However, alternatively, it may also have reflected a premorbid abnormality that could have been exacerbated by chronic drug use. This view finds support from [11C]raclopride PETstudies demonstrating that non-drug-user responses to methylphenidate depended on D2 receptor density in the striatum (Volkow et al. 1999a,b).
Lack of difference between current amphetamine and current opiate users
That brain activation did not differ significantly between the current amphetamine and opiate users is perhaps surprising, given the different pharmacological characteristics of these two substances. However, both drugs affect the midbrain dopamine system either directly (amphetamines) or indirectly (opiates) (Lingford-Hughes and Nutt 2003; Nestler 2002). Biochemical imaging studies have reported reductions in D2 receptors in individuals addicted to different types of drugs (Volkow et al. 1993 in cocaine users; Volkow et al. 1996 in alcoholics; Volkow et al. 2001 in methamphetamine users; Wang et al. 1997 in opiate users). This abnormality could be related either to a consequence of drug abuse or to a vulnerability towards addictive behaviour in general, or both of these (Volkow et al. 2001). Accordingly, we investigated individuals who have been dependent on either substance for at least 3 years. It is likely that the different pharmacological profiles of amphetamines and opiates would have been reflected in different patterns of brain activation had we administered these substances to drug-naïve volunteers.
General comments and conclusion
Although decision-making was not measurably impaired, we found overactivation in the OFC and underactivation in the DLPFC in currently dependent amphetamine and opiate users as well as in former drug users. In keeping with previous research (Bolla et al. 2003), our findings further show that such functional abnormality is not restricted to a recent abstinence of drug use but is still detectable in former drug users who had been abstinent from all drugs of abuse for an average of 10 years. We have suggested several possibilities to explain our findings, including compensation for pathology, differences in strategy use and affective style, which could be taken individually, or could be the result of a complex interaction between all three. Future studies could consider extending these findings by using fMRI with an event-related design, which may help to disentangle which of the subprocesses of decision making are disrupted in chronic drug users. Ultimately, an important goal must be to define more fully the nature of impaired decision making in chronic drug users and to use this information in designing treatment protocols in order to optimise their recovery and reintegration into society.
Acknowledgements
We would like to thank our volunteers without whom this study would not have been possible, particularly those who aided with recruitment, as well as the key workers Nick Schiller and Marion Martin and members of Narcotics Anonymous. We also thank Dr. Robert Rogers for providing the Risk Task and the staff of the Wolfson Brain Imaging Centre. This work was funded by a Wellcome Trust Programme grant to Profs. T.W. Robbins, B.J. Everitt, B.J. Sahakian and Dr. A.C. Roberts and carried out within the MRC Centre for Behavioural and Clinical Neuroscience. K.D. Ersche received a generous donation for research work on substance abuse from the Fund for Addenbrooke’s and P.C. Fletcher was supported by the Wellcome Trust.
Footnotes
Contributor Information
K. D. Ersche, Department of Psychiatry, School of Clinical Medicine, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK
P. C. Fletcher, Department of Psychiatry, School of Clinical Medicine, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK; MRC Centre for Behavioural and Clinical Neuroscience, University of Cambridge, Cambridge, UK
S. J. G. Lewis, MRC Centre for Behavioural and Clinical Neuroscience, University of Cambridge, Cambridge, UK
L. Clark, MRC Centre for Behavioural and Clinical Neuroscience, University of Cambridge, Cambridge, UK; Department of Experimental Psychology, University of Cambridge, Downing Street, Cambridge, UK
G. Stocks-Gee, Wolfson Brain Imaging Centre, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK
M. London, Brookfields Hospital, Cambridge Drug & Alcohol Service, Mill Road, Cambridge, UK
J. B. Deakin, Department of Psychiatry, School of Clinical Medicine, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK
T. W. Robbins, MRC Centre for Behavioural and Clinical Neuroscience, University of Cambridge, Cambridge, UK; Department of Experimental Psychology, University of Cambridge, Downing Street, Cambridge, UK
B. J. Sahakian, Department of Psychiatry, School of Clinical Medicine, Addenbrooke’s Hospital, University of Cambridge, Cambridge, UK; MRC Centre for Behavioural and Clinical Neuroscience, University of Cambridge, Cambridge, UK
References
- Aitken C, Kerger M, Crofts N. Drivers who use illicit drugs: behaviour and perceived risks. Drugs Educ Prev Policy. 2000;7:39–50. [Google Scholar]
- Albery IP, Strang J, Gossop M, Griffiths P. Illicit drugs and driving: prevalence, beliefs and accident involvement among a cohort of current out-of-treatment drug users. Drug Alcohol Depend. 2000;58:197–204. doi: 10.1016/s0376-8716(99)00101-5. [DOI] [PubMed] [Google Scholar]
- Alleyne BC, Stuart P, Copes R. Alcohol and other drug-use in occupational fatalities. J Occup Environ Med. 1991;33:496–500. [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edn American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate —a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Medical Association . Alcohol: guidelines on sensible drinking. BMA; London: 1995. [Google Scholar]
- Clark L, Robbins TW. Decision-making deficits in drug addiction. Trends Cogn Sci. 2002;6:361–363. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error: emotion, reason, and the human brain. Grosset/Pullman; New York: 1994. [Google Scholar]
- Darke S, Kelly E, Ross J. Drug driving among injecting drug users in Sydney, Australia: prevalence, risk factors and risk perceptions. Addiction. 2004;99:175–185. doi: 10.1046/j.1360-0443.2003.00604.x. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- EMCDDA . Literature review of the relation between drug use, impaired driving and traffic accidents. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); Lisbon: 1999. [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fletcher PC. Functional neuroimaging of psychiatric disorders: exploring hidden behaviour. Psychol Med. 2004;34:577–581. doi: 10.1017/S0033291704002430. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory—insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. NeuroReport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Chang L, Wang GJ, Fowler JS, Depue RA, Gur RC. The orbitofrontal cortexin methamphetamine addiction: involvement in fear. NeuroReport. 2002;13:2253–2257. doi: 10.1097/01.wnr0000044215.09266.bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Hahn JA, Page-Shafer K, Lum PJ, Bourgois P, Stein E, Evans JL, Busch MP, Tobler LH, Phelps B, Moss AR. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002;186:1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical methods for psychology. 4th edn Duxbury Press; London: 1997. [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci Biobehav Rev. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes A, Nutt DJ. Neurobiology of addiction and implications for treatment. Br J Psychiatry. 2003;182:97–100. doi: 10.1192/bjp.182.2.97. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National adult reading test manual. NFER-Nelson; Windsor, UK: 1982. [Google Scholar]
- Nestler EJ. From neurobiology to treatment: progress against addiction. Nat Neurosci. 2002;5:1076–1079. doi: 10.1038/nn945. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Pollack ES, Franklin GM, Fulton-Kehoe D, Chowdhury R. Risk of job-related injury among construction laborers with a diagnosis of substance abuse. J Occup Environ Med. 1998;40:573–577. doi: 10.1097/00043764-199806000-00011. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Martin WRW, Herscovitch P, Mintun MA, Markham J. Brain blood-flow measured with intravenous (H2O)-O-15. 2. Implementation and validation. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JFW, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999a;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999b;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, Robbins TW, Sahakian BJ. Decision-making in mania: a PET study. Brain. 2001;124:2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- Segarra P, Molto J, Torrubia R. Passive avoidance learning in extraverted females. Pers Individ Differ. 2000;29:239–254. [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine-D(2) receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D-2 receptor levels. Am J Psychiatry. 1999a;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D-2 receptors. J Pharmacol Exp Ther. 1999b;291:409–415. [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D-2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D-2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P. Opinion—the relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Leung HC, Johnson MK. Frontal activations associated with accessing and evaluating information in working memory: an fMRI study. Neuroimage. 2003;20:1531–1539. doi: 10.1016/j.neuroimage.2003.07.016. [DOI] [PubMed] [Google Scholar]