Lysophospholipids and their receptors in the central nervous system (original) (raw)
. Author manuscript; available in PMC: 2014 Jan 1.
Published in final edited form as: Biochim Biophys Acta. 2012 Jul 31;1831(1):20–32. doi: 10.1016/j.bbalip.2012.07.015
Abstract
Lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), two of the best-studied lysophospholipids, are known to influence diverse biological events, including organismal development as well as function and pathogenesis within multiple organ systems. These functional roles are due to a family of at least 11 G protein-coupled receptors (GPCRs), named LPA1–6 and S1P1–5, which are widely distributed throughout the body and that activate multiple effector pathways initiated by a range of heterotrimeric G proteins including Gi/o, G12/13, Gq and Gs, with actual activation dependent on receptor subtypes. In the central nervous system (CNS), a major locus for these signaling pathways, LPA and S1P have been shown to influence myriad responses in neurons and glial cell types through their cognate receptors. These receptor-mediated activities can contribute to disease pathogenesis and have therapeutic relevance to human CNS disorders as demonstrated for multiple sclerosis (MS) and possibly others that include congenital hydrocephalus, ischemic stroke, neurotrauma, neuropsychiatric disorders, developmental disorders, seizures, hearing loss, and Sandhoff disease, based upon the experimental literature. In particular, FTY720 (fingolimod, Gilenya, Novartis Pharma, AG) that becomes an analog of S1P upon phosphorylation, was approved by the FDA in 2010 as a first oral treatment for MS, validating this class of receptors as medicinal targets. This review will provide an overview and update on the biological functions of LPA and S1P signaling in the CNS, with a focus on results from studies using genetic null mutants for LPA and S1P receptors. This article is part of a Special Issue entitled Advances in Lysophospholipid Research.
Keywords: Lysophosphatidic acid, Sphingosine 1-phosphate, G protein-coupled receptor, Central nervous system, CNS disease
1. Introduction
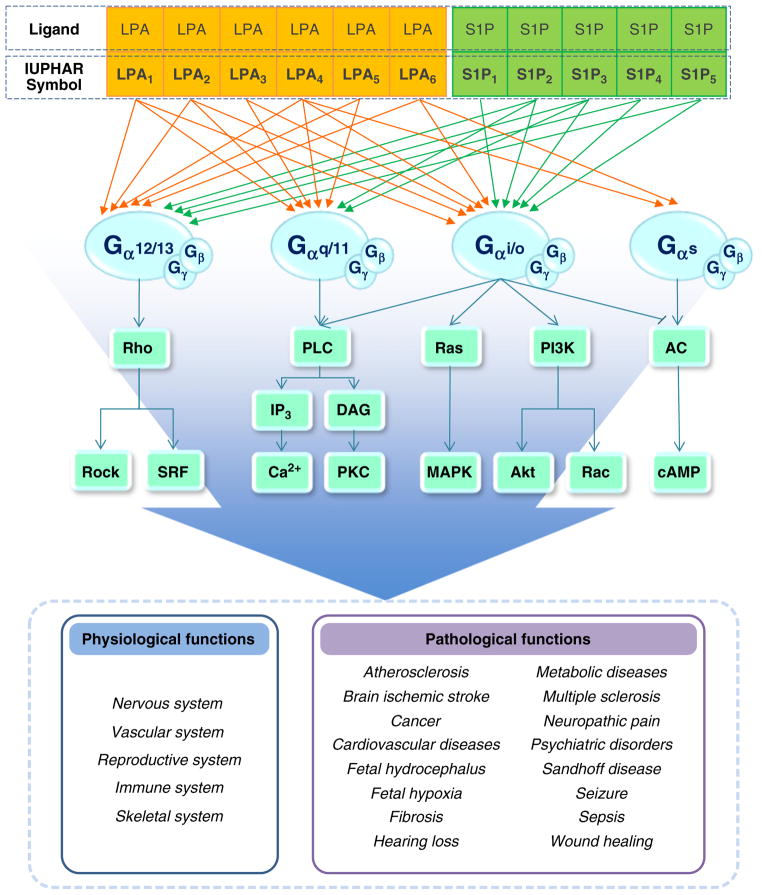
Lysophospholipids (LPs) are phospholipid derivatives originating from cell membranes and two of the best-studied LPs are lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P). These two LPs were previously known as biosynthetic metabolites of cell membrane phospholipids, but they are now regarded as important regulators for diverse biological functions through activation of their specific cognate receptors. At present, there is a family of 11 bona fide G protein-coupled receptors (LPA1–6; S1P1–5) that link into multiple downstream effector pathways (Fig. 1). LPA and S1P receptors are present in various organ systems, which accounts for their diverse biological roles [1–4]. Important LPA and S1P-mediated biological functions were discovered by studies utilizing genetic null mutants for LPA and S1P receptors [1,4,5]. Currently, there are 10 published receptor–null mice for the following LPA or S1P receptors: LPA1, LPA2, LPA3, LPA4, LPA5, S1P1, S1P2, S1P3, S1P4, and S1P5, all of which have been used to uncover valuable receptor-mediated biological functions (summarized in Table 1).
Fig. 1.
LP receptors, effector pathways, and related biological functions. Note: Unlike other LPA receptors, 2-acyl-LPA is preferred for LPA6 as a ligand rather than 1-acyl-LPA. Three more orphan GPCRs have been suggested as new LPA receptors, including GPR87, P2Y10, and GPR35, but more studies are needed to validate these suggested receptors. The effector pathways of LPA6 have not been clearly identified.
Table 1.
Biological functions identified thru studies utilizing LPA or S1P receptor null mutants.
| Receptor | Validated biological functions | |
|---|---|---|
| LPA1 | – Perinatal viability [63] | – Neuropathic pain [17,18,137–140] |
| – Body size, brain mass, suckling behavior, and craniofacial defects [63,72] | – Schizophrenia [14,96,97,142] | |
| – Pulmonary, renal, and dermal fibrosis [151–155] | – Cortical development [65,66,72] | |
| – MEF migration [156] | – Adult hippocampal neurogenesis [70,73] | |
| – Adipogenic activity [157] | – Synaptic functions [14,96,97] | |
| – Vascular injury [158] | – Astrocyte proliferation [101] | |
| – Bone development [159] | – Indirect regulation of neuronal differentiation [99] | |
| – Hypoxia-induced pulmonary remodelinga [160] | – Anxiety, motor alteration, and memory impairments [69–71,73,74] | |
| – NPC signaling [50] | – Fetal hypoxia [15] | |
| – Schwann cell biology [63,161] | – Fetal hydrocephalus [16] | |
| LPA2 | – MEF and NPC signaling [50,68] | – Synaptic function and neuronal hyperexcitability [27] |
| – Tumor formation [162,163] | – Vascular injury [158] | |
| – Indirect effect on neuronal differentiation [99] | – Allergic airway inflammation [164] | |
| LPA3 | – Timing and spacing of implantation [165–167] | – Chemotaxis of immature dendritic cells [170] |
| – Spermatogenesis and germ cell survival [168]b | – Neuropathic pain [139] | |
| – Protease functioning in uterus [169] | – Leukocyte infiltration [171] | |
| – Prostaglandin signaling [166] | ||
| LPA4 | – Embryonic viability [62,172]c | – MEF migration [62] |
| – Vascular development [172] | ||
| LPA5 | – Neuropathic pain [42] | |
| S1P1 | – Vascular development [173,174] | – Myelination-related protein expression [114] |
| – PDGF-induced MEF motility [175] | – EAE [81] | |
| – Pulmonary fibrosis [176] | – FTY720 efficacy on EAE [81] | |
| – Cortical neurogenesis and cell survival [78] | – Cuprizone-induced demyelination [114] | |
| – T or B cell trafficking [141,177,178] | ||
| S1P2 | – Reduced litter size [179] | – Angiogenesis in hypoxia [186] |
| – Vascular dysfunction [180,181] | – Hearing loss and vestibular defects in nulls [23–25] | |
| – Vasoconstriction [182] | – Seizure and offspring death in seizure-prone nulls [26,28]c | |
| – Wound healing in injured liver [183,184] | – Streptozotocin-induced diabetes [187] | |
| – MEF or hepatic myofibroblast biology [179,184,185] | ||
| S1P3 | – Reduced litter size [179] | – Vasodilation by HDL or FTY720 [194–196] |
| – Alveolar epithelial barrier function [188] | – Vascular tone regulation [197] | |
| – MEF signaling [189,190] | – Cardiac fibrosis [198] | |
| – Responses in splenic marginal sinus [191] | – Cardioprotective effect of HDL, S1P, or SPC in ischemic injury [199–201]d | |
| – Myofibroblast differentiation [192] | – PAR-1-mediated sepsis [202] | |
| – Crosstalk with CXCR4 signaling in bone marrow cells [193] | – Sandhoff disease [29] | |
| S1P4 | – Megakaryocyte differentiation [203] | |
| S1P5 | – NK cells trafficking [204–206] |
The CNS is one of the biological systems markedly affected by LPA and S1P signaling. Many subtypes of LPA and S1P receptors are expressed in one or more CNS cell types, and their ligands are also enriched there [1,6,7]. Consequently, LPA and S1P signaling affects CNS cell types such as neuroblasts, neurons, astrocytes, and oligodendrocytes to influence cell survival, proliferation, migration, differentiation, and morphological changes (reviewed in [1,3,8–11]). To date, LPA and/or S1P receptors have been identified as important factors in CNS development, as well as having roles in diseases, including MS [12,13], fetal hypoxia and hydrocephalus [14–16], neuropathic pain [17–19], brain ischemic stroke [20,21], neurotrauma [22], developmental disorders like schizophrenia, as well as hearing loss [23–25], seizures [26–28], and Sandhoff disease [29].
In this review, we will focus on the biological roles of LPA and S1P receptors in different CNS cell types, including neurons, astrocytes, and oligodendrocytes, and discuss their involvement in CNS diseases. Specifically, we discuss in vivo and in vitro data that were obtained from studies using LPA or S1P receptor null mutants. Involved cell types affected in the CNS include not only CNS lineages but also non-CNS lineages such as resident cell types like microglia, which will also be considered.
2. LPA and S1P receptor expression in the CNS
In the past decades, the biological activities of LPA and S1P were mechanistically unclear, with explanations ranging from detergent-like membrane perturbation, second messenger activities, to possible receptors. In 1996, the first LP receptor, LPA1, was identified in the ventricular zone of the embryonic brain [30,31] with subsequent deorphanization based on sequence homology of both new LPA and S1P receptors [7,32–41]. Now, there is a family of 11 LP receptors that are composed of 6 LPA receptors (LPA1–6) and 5 S1P receptors (S1P1–5) (Fig. 1) [2]. In the CNS, many LPA and S1P receptors are present and show varied expression patterns depending on cell type, neuroanatomical location and developmental stage (reviewed in [1,3,7]). This section will focus on the expression of LPA and S1P receptors in the CNS at tissue and cellular levels.
2.1. LPA receptors: brain and spinal cord
LPA1–6 are expressed at varying levels in the CNS during development and/or postnatal life [1,11,41,42]. The temporal changes in expression levels for LPA receptors were characterized in mouse brain using Northern blot and real-time PCR analysis. LPA1, LPA2, and LPA4 are expressed in the developing brain [30,36,43,44], whereas LPA3 is expressed in the postnatal brain [44,45]. LPA1 shows high gene expression in the embryonic ventricular zone (VZ), a major site for neuroprogenitor cell proliferation during prenatal developmental stages, after which it diminishes with the transient VZ, reappearing during postnatal life within oligodendrocytes that may influence myelination [30,43,46], indicating roles for LPA signaling in cortical development. Pathological stimuli can increase expression levels of LPA receptors [22,47,48] (detailed in the “CNS diseases” section).
LPA receptors are involved in a variety of cell biological processes, including proliferation, differentiation, morphological changes, migration, survival/apoptosis, as well as molecular physiological responses that include calcium signaling and electrophysiological changes [1,11,49,50]. In neuroprogenitor cells, a functional locus for LPA signaling, LPA1, LPA2, LPA3, and LPA4 are expressed [50]. In neurons, LPA1 and LPA2 are expressed, but the latter is more abundantly expressed [11,51]. A recent study identified LPA5 expression on sensory and motor neurons in the spinal cord and linked its functional role to pain processing, whereby LPA5 nulls showed a reduced pain phenotype accompanied by downregulation of phosphorylated cAMP response element binding protein (pCREB) signaling in the spinal cord [42]. This unique expression pattern may also involve astrocytes which express LPA1 to LPA5 [11]. Interestingly, LPA1 and LPA2 are upregulated in activated astrocytes following spinal cord injury [47], suggesting that these two receptor subtypes play possible roles in astrogliosis-related pathologies. In microglia, another important glial cell type for CNS pathogenesis, LPA1 and LPA3 are expressed, and the latter is upregulated following the administration of a neuroinflammation-inducing agent [52,53]. In oligodendrocytes, LPA1 and LPA3 are expressed and specifically, the LPA1 expression pattern is correlated with oligodendrocyte differentiation [46,54–56].
2.2. S1P receptors: brain and spinal cord
The CNS is also a major locus for S1P receptors, in which 4 of the 5 S1P receptors are expressed, including S1P1, S1P2, S1P3, and S1P5 [3,4,6]. Among these, S1P1 to S1P3 are widely expressed throughout the body, while S1P5 is expressed at relatively high levels in the CNS [3,6,57,58]. Like the LPA receptors, S1P1 to S1P3 are expressed in the developing brain [3], suggesting a possible role of these receptors in cortical development.
At a cellular level in the CNS, S1P receptors are also involved in a variety of biological processes. All four S1P receptors that are present in the CNS are expressed in neurons, astrocytes, microglia, and oligodendrocytes with varying expression patterns [6]. In astrocytes, 4 S1P receptors can be expressed at varying levels, depending on growth conditions (S1P3>S1P1>S1P2>S1P5) [6]. The astrocyte expression level of S1P5 is very low, but its expression is upregulated upon exposure to growth factors [59]. When activated by pathological stimuli, S1P1 and S1P3 are upregulated in activated astrocytes [60], indicating that these receptor subtypes may be important for pathogenesis. In microglia, the expression level for S1P receptors is dynamically changed upon activation: in activated microglia, S1P1 and S1P3 are downregulated, but S1P2 is upregulated [53]. In oligodendrocytes, the expression pattern of S1P receptors depends on cell maturation [57]. S1P5 is more highly expressed in mature oligodendrocytes than other S1P receptors, but, in contrast, its expression levels are relatively similar to other expressed S1P receptors before maturation.
3. Biological functions of LPA and S1P receptors in CNS cell types
The discovery of the first LPA receptor, LPA1, and the role it plays in nervous system development, has served as a template for LP research in the CNS as well as other tissues. Since then, a variety of specific responses at cellular and tissue levels have been identified, including proliferation, morphological changes, cell migration, differentiation, and survival. This section will discuss biological functions of LPA and S1P receptors in CNS cell types, such as neuroblasts, neurons, astrocytes, and oligodendrocytes, and also in a non-CNS-resident cell type, the microglia.
3.1. Functions in neural progenitor cells (NPCs)
Neural progenitor cells (NPCs) are responsible for neurogenesis, which involves proliferation, morphogenesis, migration, apoptosis and differentiation. LPA and S1P signaling influence a range of neurogenesis-related functions of NPCs. Both signaling lipids are involved in development, but to date, more studies have identified roles for LPA receptors influencing neurogenesis, with comparatively fewer studies also identifying similar S1P receptor effects.
LPA signaling regulates biological responses of NPCs by at least 3 subtypes of LPA receptors, LPA1, LPA2, and LPA4 ([50]; LPA6 expression has not yet been established). As previously noted, the possible role of LPA receptors in CNS development was indicated by a restricted expression pattern of LPA1 in the cortical VZ. Later, LPA2 expression was identified in postmitotic neurons of the embryonic cortex [51,61] and LPA4 in developing brain [38,62]. Neurogenesis-related biological responses relevant to NPCs have been revealed by heterologous expression studies utilizing cell lines where single or multiple LPA receptors (LPA1–LPA5) are expressed (reviewed in [7,11]). Subsequent studies using primary NPCs, neurospheres, and ex vivo cultures have shown that LPA controls cell proliferation and/or differentiation through at least, LPA1 [63–67]. It has become clear that LPA1 acts as an important modulator of cortical development through studies using LPA1-deficient NPCs or whole cortical cultures [63,65,66]. In NPCs from LPA1 nulls, the ability of LPA to induce normal neurogenesis-related responses like migration, morphological changes, proliferation, and differentiation is lost [63,65], strongly suggesting that LPA1 acts as a modulator of neurogenesis. Studies using an organotypic whole cortex, ex vivo culture system, revealed that LPA1 is a crucial factor in LPA-induced survival and differentiation of NPCs (LPA2 was also revealed to be involved in this process) [66]. In addition, LPA has been identified as one of the earliest of neurotransmitter-like stimuli of ionic conductance changes in NPCs, further implicating LPA as a physiological component in cortical development [49,50]. These influences were associated with relatively minor anatomical defects in overall brain development, complicated by differential effects of background strain. LPA1 nulls showed a smaller brain with reduced cortical width and cerebral wall thickness associated with 50% perinatal lethality [63] that limits in vivo examinations to studies of survivors; no obvious neuroanatomical differences were observed in null mutants for LPA2 or LPA4 [62,63,68]. The relatively mild phenotypes may reflect LPA receptor subtype rescue, or, in the case of LPA1 nulls, strain-dependent effects were demonstrated by more pronounced developmental defects in a stable variant of LPA1 null mutants, called the Málaga variant or maLPA1 [69–74]. These null mutants showed increased survival to normal levels compared to the perinatal lethality previously reported for LPA1 null mutants [72]. These maLPA1 null mutants continued to display defective features in cortical development such as reduced cell proliferation and increased apoptosis and also defects in adult neurogenesis including within hippocampal regions [72,73]. With the current data demonstrating the importance of LPA signaling in neurogenesis, more research needs to be done to determine the mechanisms and possible roles of other LPA receptors. In fact, other LPA receptor subtypes, LPA3 and LPA5, are not expressed in NPCs, but they are expressed in postnatal brains and embryonic brains, respectively [37,39,43], and moreover, LPA3 overexpression resulted in morphological changes in NPC cell lines [75].
All five known S1P receptors are expressed in NPCs [76] and S1P1–S1P3 are expressed in the developing brain [61]. In particular, S1P1 is also highly expressed in the VZ, like LPA1 [61]. S1P signaling related to neurogenesis has been demonstrated using cell lines such as PC12 cells, and S1P signaling influences cell proliferation and differentiation (reviewed in [8]). In primary cultures of rat hippocampal NPCs, S1P induced cell proliferation and morphological changes [77]. To date, only the S1P1 null mutants show defects in neurogenesis [78], where cell death was increased and cell proliferation and mitotic cell numbers were reduced in embryos [78]. It may be worth determining how S1P1 regulates neurogenesis by using an ex vivo system like the one used for LPA1/2 [66,79]. Recently, S1P signaling was reported in the migration of transplanted NPCs towards lesion sites of an ischemic brain and injured spinal cord where S1P2 and S1P1 play crucial roles, respectively [76,80] (see “CNS diseases”).
Numerous in vitro and in vivo studies have identified roles for LPA and S1P receptors in CNS development with particular effects on NPCs, particularly in various CNS disorders. Consistent with this view, LPA and S1P ligand levels are increased in several CNS diseases [20,29,76,80–82]. Studies on whether LPA and S1P signaling may be involved in adult neurogenesis after insults will be of interest.
3.2. Functions in neurons
Effects of LPA and S1P signaling have been evaluated in vitro using primary neurons and neuronal cell lines. The effects are largely related to morphological changes involving growth cone collapse and neurite retraction [3]. In addition, both LPA and S1P signaling regulate other neuronal functions involving migration, cell death/survival, synapse formation, and synaptic transmission, as reviewed elsewhere [1,8,9,11]. Here, we will discuss studies using LP receptor-null mutants.
In postmitotic neurons, LPA-induced morphological changes cannot be completely explained by LPA1, LPA2, and LPA3 [51,83]. LPA-induced neurite retraction is accompanied by the formation of F-actin filament retraction fiber caps in postmitotic cortical neurons where two LPA receptors, LPA1 and LPA2, are expressed [51]. The effects were preserved even in LPA1-deficient cortical neurons [51]. Recently, another report showed that LPA1, LPA2, and LPA3 do not appear required to induce morphological changes in retinal neurons in response to LPA [83]. LPA induces neurite retraction and growth cone collapse in retinal neurons, which is preserved in cells lacking LPA1, LPA2, and LPA3 [83]. Therefore, LPA’s effects on morphological changes may be mediated by other LPA receptor subtypes, both known and unknown.
S1P acting through S1P1 likely has similar effects on NPCs based on analyses of receptor and kinase mutants [78] and on studies using systems of gene upregulation by overexpression or gene downregulation by antisense have identified the possible involvement of S1P receptors in neuronal morphological changes [84,85]. Nerve growth factor (NGF) was reported to transactivate S1P receptors, activated via sphingosine kinase 1 (SPHK1), resulting in neurite extension, in which S1P1 overexpression promotes and S1P2 or S1P5 overexpression inhibits [85]. When cellular S1P receptors were downregulated by applying specific antisense probes, similar results were obtained. S1P1 downregulation reversed NGF-S1P-induced neurite extension [85], but S1P2 downregulation enhanced the extension [84]. Roles for other receptor subtypes are still unknown, however the effects of sphingosine kinase mutants that disrupt normal ligand concentrations suggest that other relevant neuronal abnormalities may exist in S1P receptor genetic null mice that have not yet been fully assessed.
Another function modulated by LP receptors is the regulation of synaptic activity [27,86–88]. Regulation of synaptic activity, along with neuronal survival/death and neurogenesis, is likely an important issue related to memory impairments and psychiatric dysfunctions. Both LPA and S1P signaling are implicated as novel factors that regulate these responses. Regarding neuronal survival/death, both signaling pathways also seem to exert dual actions – neurotoxic and neuroprotective – depending on the applied concentrations or experimental conditions (reviewed in [8,9]). However, it is clear that LPA1 is normally a survival factor for neurons because apoptotic cell death occurs in embryonic and postnatal LPA1-null mouse brains [72]. In addition to neuronal survival/death, LPA and/or S1P signaling are linked to neuronal excitability [26,89–93], synapse formation [86,87], and synaptic transmission [14,94–96]. To date, a few pieces of this puzzle have been directly verified, but as yet unidentified receptor subtypes may mediate these responses. For example, some functional defects have been reported using genetic null mutants: in neurons lacking LPA1, synaptic dysregulation occurs [97] along with a decreased release of neurotransmitters [14,96]; in neurons lacking LPA2, LPA failed to evoke hyperexcitability [27], and more-over, LPA2 deletion normalized seizure-like overexcitability by genetic deletion of plasticity related gene-1 [27]; and in cells lacking S1P2, abnormal hyperexcitability with age-dependent seizures can also occur [26].
3.3. Functions in astrocytes
Astrocytes, the most abundant glial cell type in the CNS, regulate many biological as well as pathological processes that are epitomized by astrogliosis. Many in vitro effects of LPA and S1P signaling have been related to astrogliosis to affect proliferation, migration, morphological changes, and activation of related intracellular signaling [1,9,10,98–100]. So far, at least, 3 receptor subtypes, LPA1, S1P1, and S1P3, have been shown to influence astrogliosis, however other receptor subtypes expressed by astrocytes need to be functionally examined. The cell proliferative effect of LPA was absent in astrocytes lacking LPA1 [101]. S1P1 and S1P3 have been shown to be involved in astrogliosis in different disease models: S1P1 plays a role in astrogliosis in multiple sclerosis animal models [81], while S1P3 has roles in a Sandhoff disease model [29] or lysolecithin-induced demyelination [102]. Interestingly, functional discrepancy was observed in comparing astrogliosis that was associated with demyelination and CNS damage in an MS model vs. enhanced remyelination in a lysolecithin-induced demyelination model [81,103], suggesting the existence of contextual variables that dictate whether astrogliosis is associated with damage or repair. Similarly, in chronic disease conditions, S1P-mediated astrogliosis seems to be linked to neurotoxicity as in the above disease conditions [29,81], while in acute conditions, it seems to provide neuroprotective effects [102]. In addition to astrogliosis regulation, S1P1 in this cell type is also important for FTY720 activity: FTY720 (fingolimod) is a new oral medication for MS, which may act through both immunological and non-immunological mechanisms [12,13,81,104–107].
Neuronal differentiation is another prominent function of astrocytes related to LPA signaling. LPA-primed astrocytes secrete soluble factors to increase neuronal differentiation [99,100]. To date, we do not know what soluble factors are released from LPA-primed astrocytes to induce the differentiation, but growth may be involved in this response. In fact, this neuronal differentiation was reversed by blocking the epidermal growth factor receptor [100] and LPA was reported to produce growth factor expression in astrocytes [108]. S1P is also known to produce growth factor expression in astrocytes [109–111]. In this regard, whether S1P-primed astrocytes exert a similar effect on neuronal differentiation remains to be determined. Astrocyte–neuron communication is important for CNS development and post-disease regeneration. Therefore, it is possible that LPA and S1P receptors in astrocytes are novel factors that indirectly regulate regeneration through aspects of neuronal differentiation.
3.4. Functions in oligodendrocytes
Oligodendrocytes, another type of glia in the CNS, play a major role in myelination. Their functions are also important for CNS development as well as repair after injuries, particularly remyelination that is composed of a series of events like proliferation, migration, and differentiation of oligodendrocyte progenitor cells. Possible roles for LPA and S1P signaling were suggested by the restricted expression pattern of LPA and S1P receptors in oligodendrocytes. LPA1 expression changes with oligodendrocyte maturation [46,54,55] while S1P5 expression is highest in oligodendrocytes [6,57,58]. Many in vitro studies have reported that LPA or S1P influences cellular responses that are also varied, depending on oligodendrocyte maturation (reviewed in [8,9,11]). Notably, a study using primary oligodendrocytes cultured from S1P5 nulls clearly showed the importance of this receptor subtype as a survival factor in mature cells [112]. However, there is no obvious myelination defect in LPA1 nulls [63] or S1P5 nulls [112], which may reflect functional redundancy between LPA1 and S1P5 signaling and/or possible involvement of other receptor subtypes considering that many LPA and S1P signaling pathways share downstream effector pathways [4,113]. In fact, S1P1 conditional nulls that lack S1P1 in oligodendrocytic cell lineages have a subtle decrease in expression of myelination-related proteins and subtly thinner myelin in the corpus callosum of nulls [114]. These conditional nulls were more susceptible to cuprizone, a reagent used in a CNS demyelination model [114], indicating that oligodendrocytic S1P1 is a possible factor in myelination.
Effects of S1P signaling on myelination in oligodendrocytes have been assessed in view of studies using FTY720 that also targets S1P5, a major S1P receptor subtype in oligodendrocytes. Although there is no direct in vivo evidence that the therapeutic effect of FTY720 on MS is via S1P5, many in vitro studies have revealed that FTY720 influences remyelination-relevant biological responses of oligodendrocyte cell lineages, including survival, proliferation, migration, and differentiation (reviewed in [10]). Of note, a study using organotypic cultures identified that demyelinated slice cultures produced by lysolecithin exposure showed improved remyelination with FTY720 exposure [102]. FTY720 reduces clinical symptoms in an animal model of MS accompanied by enhanced remyelination [81] or reduced demyelination [115]. In the cuprizone model, FTY720 reduces demyelination via an unidentified receptor subtype [114]. To evaluate the direct action of FTY720 on remyelination in disease models, S1P receptor conditional nulls targeting implicated S1P receptor subtypes in oligodendrocyte and related cell lineages would be of interest.
3.5. Functions in microglia
Microglia are categorized as a non-neural cell type, but are CNS resident cells. Upon activation, microglia respond to mediate neuroinflammatory processes. Microglia can express a range of LP receptors including LPA1, LPA2, LPA3, S1P1, S1P2, S1P3, and S1P5 [52,53,116–118]. LPA signaling was reported to regulate proliferation, membrane ruffling and hyperpolarization, metabolic changes, migration, chemokinesis, and growth factor upregulation [52,116,117,119,120]. S1P or its biosynthetic enzymes were reported to regulate membrane hyperpolarization and production of neurotoxic molecules such as tumor necrosis factor-α, interleukin-1β, and nitric oxide [53,116,121,122]. LPA3 is upregulated in immunostimulated microglia [53], while de novo synthesis of LPA in activated microglia could be one of the factors for the pathogenesis of neuropathic pain [123], and demyelinating agent-induced microglial activation was attenuated by antagonizing S1P1 and S1P5 [102]. Furthermore, increased microglial activation in EAE spinal cords was reduced by FTY720 and S1P1 deletion in CNS cell lineages [81]. The latter indicates that indirect modulation of S1P1 can control microglial activation. The precise roles of these receptors in microglial activities remain to be determined.
4. LPA and S1P receptors as promising therapeutic targets in CNS diseases
The biological functions of LPA and S1P signaling on CNS cell types have led to translational research to identify medically relevant roles for LP receptors. Genetic nulls, combined with pharmacological LP receptor modulators, have been utilized to validate therapeutic functions in CNS diseases. The clearest success of this research is FTY720, an S1P receptor modulator approved by the FDA in 2010 as a first oral MS therapy [12,13]. This success has provided proof-of-concept for other therapeutic agents targeting LP receptors in CNS diseases that include ischemic stroke, neurotrauma, psychiatric diseases, seizures, hearing loss, and Sandhoff disease, and for the treatment of developmental disorders including fetal hypoxia and hydrocephalus that can also contribute to psychiatric disorders. In particular, recent data implicating LPA signaling in fetal (congenital) hydrocephalus suggests a novel, medicinal therapy through targeting LPA receptors (discussed below). This section will discuss CNS diseases that may be mechanistically and/or therapeutically accessed through LPA and S1P signaling (summarized in Table 2).
Table 2.
Identified functions of LPA or S1P receptors in CNS diseases.
| Disease | Receptor | Validated functions |
|---|---|---|
| Multiple sclerosis | S1P1 | – Attenuation of EAE development and pathogenesis like astrogliosis in nulls [81] |
| S1P1 | – Loss of FTY720 efficacy in nulls [81] | |
| S1P1 | – Blockade of the increase in S1P levels in EAE spinal cord in nulls [81] | |
| – Upregulation of S1P1 and S1P3 on reactive astrocytes of MS patients [60] | ||
| – Upregulation of S1P3 and S1P4 and downregulation of S1P1 and S1P5 in EAE, and reversal of these changes by FTY720 [129] | ||
| S1P1 | – Downregulation of myelination-related proteins expression in nulls [114] | |
| S1P5 | – FTY720-enhnaced remyelination [102] | |
| S1P1 | – Increased susceptibility to cuprizone-induced demyelination in nulls [114] | |
| Neuropathic pain | LPA1 | – Reduced responses to pain via a likely peripheral mechanism [1,17,18,137,138,140] |
| LPA3 | – Reduced LPA levels relevant to pain [139] | |
| LPA5 | – Reduced responses to pain through a distinct, CNS mechanism [19] | |
| Ischemic stroke | LPA1 | – Increased LPA or S1P levels in patients or animal models [20,76,82] |
| – Upregulation or no changes of S1P producing protein in the ischemic brains [130–132] | ||
| – Upregulation of LPA1 and LPA2 by retinal ischemic injury [48] | ||
| – Decreased retinal ganglion cell death by hypoxia [133] | ||
| – Protective effect of FTY720 against transient or permanent ischemic injury [21,118,132,134] | ||
| S1P2 | – Enhanced NPC migration into ischemic lesion sites by blocking S1P2 [76] | |
| Neurotrauma | ||
| Traumatic brain injury | – Upregulation of LPA2 and appearance of LPA1 on reactive astrocytes of patients [22] | |
| – Upregulation of LPA2 or LPA3 on reactive astrocytes or neurons after the injury [47] | ||
| Spinal cord injury | – Upregulation of LPA1 and LPA2 on reactive astrocytes and LPA3 on neurons after the injury [47] | |
| – Increased S1P levels in injured spinal cords [80] | ||
| S1P1 | – NPC migration into lesion sites of spinal cord injury via S1P1 [80] | |
| Neuropsychiatric diseases | ||
| Schizophrenia | LPA1 | – Prepulse inhibition, 5-HT synthesis alteration, and craniofacial dysmorphism in nulls [14] |
| LPA1 | – Schizophrenia-related neurochemical and biochemical changes in nulls [96,97,142] | |
| – LPA1 downregulation in patients [143] | ||
| Behavioral dysfunctions | LPA1 | – Anxiety, motor alterations, and memory impairments in nulls [71,73,74,144] |
| S1P2 | – Anxiety and spatial memory impairments in seizure-prone nulls [28] | |
| LPA1 | – Attenuation of cocaine-induced locomotor activity in nulls [69] | |
| LPA1 | – Enhanced defects of hippocampal neurogenesis in nulls under chronic stress conditions [70] | |
| Alzheimer’s disease | – Upregulation of LPA or S1P producing proteins in patients [145,146] | |
| – Direct or indirect S1P exposure reduces BACE1 activity [145,147] | ||
| – Decreased S1P levels in patients [148] | ||
| – Interconnection between Aβ and S1P producing protein [149] | ||
| – LPA reduces neuronal death by Aβ [150] | ||
| Developmental disorders | ||
| Fetal hypoxia | LPA1 | – Attenuation of hypoxia-induced cortical disorganization in nulls [15] |
| Fetal hydrocephalus | – Development of fetal hydrocephalus model by injecting LPA into embryo [16] | |
| LPA1 | – Attenuation of LPA-induced fetal hydrocephalus features in nulls [16] | |
| Others | ||
| Hearing loss | S1P2 | – Neurodegenerative hearing loss and vestibular defects in nulls [23–25] |
| Seizure | S1P2 | – Seizure-like behaviors and hyperexcitability in nulls [26] |
| S1P2 | – Offspring death in nulls along with astrogliosis in the hippocampus and neighboring cortex, anxiety, and spatial memory impairments [28] | |
| LPA1,2/ S1P2 | – LPA- or S1P-evoked neuronal hyperexcitability [26,27,89–93] | |
| LPA2 | – Attenuation of PRG-1 deficiency-mediated seizure-like symptoms in nulls [27] | |
| Sandhoff disease | – Increased S1P levels and upregulation of S1P2 and S1P3 [29] | |
| S1P3 | – Delayed disease progression in nulls for S1P3 or S1P producing protein [29] | |
| S1P3 | – Reduced astrogliosis in nulls [29] |
4.1. Multiple sclerosis (MS)
MS is an autoimmune and neurodegenerative disorder of the CNS, representing the most common demylinating disease of young adults and which includes pathological hallmarks of immune cell infiltration into the CNS, astrogliosis, axonal damage, and demyelination [124,125]. While the underlying cause of MS is still unclear, both immunological and CNS components are essential to MS pathogenesis and progression.
The link between MS and LP receptors came through the discovery of FTY720 (fingolimod) that is a non-selective S1P receptor modulator that binds to 4 of the 5 S1P receptor subtypes (S1P1,3,4,5) [126,127]. Fingolimod (Gilenya) is currently approved to treat relapsing forms of MS in the United States, Europe, United Kingdom, Russia, Mexico, Brazil, Japan and Australia, as well as other markets [12]. It is believed that this drug exerts its therapeutic effect via targeting S1P1 in T-lymphocytes, resulting in the redistribution of lymphocytes to secondary lymphoid organs and a concomitant reduction of lymphocytes in peripheral blood to reduce entry of pathogenic T-cells into the CNS (reviewed in [13,107,128]). However, recent work in animal models of MS (using experimental autoimmune encephalomyelitis: EAE) revealed that FTY720’s mechanism of action also involves direct CNS actions wherein it targets S1P1 in astrocytes [81]. Disease symptoms in conditional nulls lacking S1P1 in CNS cell lineages and particularly astrocyte lineages were not reduced by FTY720 administration despite the maintenance of its effects on the reduction of peripheral lymphocyte levels. This result indicates that the CNS, particularly astrocytes, may be a new locus of FTY720 efficacy in MS [81]. In addition, disease signs were lower in astrocyte conditional nulls than wild type mice challenged with EAE, indicating that the S1P1 in the CNS, and more specifically the astrocyte population, may be an important factor for in MS [81]. It is of note that neuronal S1P1 was not involved in either MS pathogenesis or FTY720 efficacy [81].
What we now know is probably just the tip of the iceberg and many questions remain to be answered regarding S1P1 loss from oligodendrocytes, microglia, and/or other LP receptors which may reduce MS signs and symptoms and alter FTY720 efficacy. In oligodendrocytes, an important cell type for myelination, two important targets of FTY720, S1P1 and S1P5, are quantitatively highly expressed, so S1P signaling may be involved in demyelination as well as remyelination, all of which could be related to MS pathogenesis and FTY720 efficacy. In fact, S1P5 may be important for FTY720-enhanced remyelination in lysolecithin-induced demyelination [102]. To verify these possibilities, studies need to be pursued using conditional nulls for S1P1 in oligodendrocytes, S1P5 nulls, or combined null mutants. In microglia, the other important cell type for neuroinflammation, S1P1 could be a possible target for assessment. During EAE, microglia are activated, which is histologically reduced by genetic deletion of S1P1 in the CNS or by FTY720 exposure [81], suggesting that microglial S1P1 is involved in MS pathogenesis and FTY720 activity. It cannot be excluded that other LP receptors may also be involved in MS or FTY720 efficacy because some of the reported LP receptors regulate biological events that are related to MS pathogenesis or recovery. Considering that FTY720 targets other S1P receptors – S1P3, S1P4, and S1P5 – it would not be surprising to uncover other receptor-mediated activities relevant to MS, including effects on the endothelium and blood–brain barrier. Moreover, it has been shown that expression levels of each S1P receptor are altered during MS and by FTY720 exposure. In human MS brain lesion sites, S1P1 and S1P3 are upregulated in reactive astrocytes [60]. In rat EAE spinal cords, S1P1 and S1P5 are downregulated while S1P3 and S1P4 are upregulated, and FTY720 administration restores these changes [129]. While some reports provide conflicting results, these studies nonetheless implicate other S1P receptors as possible targets for FTY720. The precise in vivo mechanisms of FTY720 efficacy need to be clarified, especially regarding aspects of inflammation, regeneration, and remyelination, all of which are influenced by FTY720 administration in EAE [81]. FTY720 did not prevent neuronal death in a model of optic neuritis as compared to protection seen in EAE models, suggesting response variability as a function of model and possibly neuronal subtypes [115]. In addition, S1P levels are elevated during MS, and can be reduced by S1P1 deletion in CNS cell lineages, suggesting other levels of control that may be accessed through S1P receptor modulation [81].
4.2. Ischemic stroke
Ischemic stroke is caused by interruption of the blood supply to the brain that results in damage through excitotoxicity and neuroinflammation; both LPA and S1P levels are increased in ischemic stroke, showing elevated LPA levels in plasma and local S1P levels in the infarct area [20,76,82]. Along with elevated ligand levels, SPHK2, an enzyme that produces S1P, is upregulated in ischemic brains [130,131]. However, another study showed no changes in SPHK1/2 in transient ischemic stroke, but instead revealed SPHK2 as a protective factor, through the use of genetic null mutants [132]. In addition to the elevated LP ligand levels, it has been reported that two LPA receptor subtypes, LPA1 and LPA2, are also upregulated during retinal ischemia–reperfusion injury [48]. Among these, one study suggested the importance of LPA1 in a similar system where the blockade of LPA1 functions by shRNA expression reduced hypoxia-induced retinal ganglion cell death [133]. Distinct mechanisms involving LPA1 potentiation that promotes fetal CNS damage have also been reported [15]. Combined, these results implicate the involvement of LP signaling in brain ischemic injury, with responses that appear to be neurotoxic or neuroprotective. FTY720 exposure has been reported to reduce brain damage in model systems, such as infarct size, neurological score, and neuronal death, which is induced by transient and permanent ischemic injury [21,118,132,134]. The efficacy may come from an indirect action through reduced inflammatory reactions as well as direct CNS effects. FTY720 administration reduces T cell or neutrophil infiltration into the CNS and microglial activation [21,118,134]. S1P2, which is not a target of FTY720, has also been associated with brain ischemic injury through a different mechanism — the blockade of S1P2 by an antagonist or shRNA expression enhanced migration of transplanted neural stem/ progenitor cells into the lesion sites where S1P levels are elevated [76]. Considering that LP signaling induces neuroinflammatory responses like astrogliosis, which as noted previously can have opposing roles, the contradictory LP associations observed in stroke likely reflect combinations of altered ligand levels and/or receptor-based changes that, combined with cellular effects of astrogliosis or microglia, produce a more complex picture of LP signaling that is nonetheless of increasing relevance to stroke.
4.3. Neurotrauma
Neurotrauma is used here to refer to traumatic brain injury and spinal cord injury where both neurons and glia play key roles in pathogenesis as well as recovery. LPA and S1P signaling have recently become possible targets to manage traumatic injuries. Temporal changes of LPA receptor expression following neurotrauma in rodents, as well as humans has been reported [22,47]. In human postmortem trauma brain tissues, LPA2 is upregulated compared to normal brains and has been reported to be expressed in ependymal cells lining cerebral ventricles around the corpus callosum, while expression levels of the other receptors examined (LPA1 and LPA3) are not changed [22]. LPA1 is expressed in injured tissues and possibly in reactive astrocytes in lesion sites [22]. In rodents, temporal expression changes for 3 LPA receptor subtypes (LPA1–LPA3) have been reported in two different injury models, traumatic brain injury and spinal cord injury [47]. In both injury models, LPA2 and LPA3 expression is upregulated in reactive astrocytes and motor neurons, respectively, but LPA1 upregulation is only observed in reactive astrocytes following spinal cord injury. These observations may be relevant to the pathogenesis of neuropathic pain that can involve activation of astrocytes in the spinal cord [135,136]. More importantly, a series of studies have identified LPA1 signaling as an initiator of neuropathic pain in a pain model (partial sciatic nerve ligation (PSNL)) [17,18,88,137–140]. Direct linkage between LPA1 in spinal cord astrocytes and induction of neuropathic pain remains to be explored. LP signaling pathways in spinal cord and the pathogenesis of neuropathic pain have received further recent support [42]. LPA5 null mutants were protected from neuropathic pain, which is associated with the downregulation of phosphorylated cAMP response element binding proteins in dorsal horn neurons where LPA5 is expression is prominent [42].
An experimental therapeutic approach to neurotrauma is transplanting neural stem/progenitor cells. S1P signaling in this context may promote migration to the injury site. Since S1P is locally produced in injury sites, transplanted cells could migrate to the injury site through an S1P gradient mechanism as observed with S1P1-mediated lymphocyte trafficking [80,141], while S1P2 may have an inhibitory effect on migration based on studies of stroke models [76] (see “Ischemic stroke”). These results implicate S1P signaling as a possible therapeutic avenue in neurotrauma.
4.4. Neuropsychiatric diseases including Alzheimer’s disease (AD)
Neurobiological functions of LPA and S1P signaling related to cell survival, neurogenesis, differentiation and synaptic activities may be relevant to neuropsychiatric disorders, particularly those associated with developmental defects, CNS injuries, and inflammation. To date, in vivo and in vitro studies have indicated that LPA and S1P signaling can play multiple roles in relevant to CNS disorders, based upon analyses of model systems for psychiatric disorders that include schizophrenia, anxiety, memory impairment, and neurological disorders like Alzheimer’s disease (AD). In particular, LPA1 has been associated with these disorders, while other LPA and S1P receptor subtypes may also have disease relevance.
LPA1 null mutants were reported to exhibit schizophrenia-like defects involving deficits in pre-pulse inhibition, 5-HT synthesis alterations, and craniofacial dysmorphism [14]. Additional studies have reported that LPA1 null mutants share phenotypes consistent with schizophrenia including neurochemical [96,142] and biochemical changes [97]. Tracking with these observations, a microarray study, along with real-time PCR analysis, revealed that the LPAR1 gene is downregulated in blood lymphocytes of schizophrenia patients [143]. LPA1 and other aspects of LPA signaling may therefore provide new insights into this complex disorder.
Behavioral studies have indicated that LPA1 deficiency is also linked to anxiety, memory impairment, and motor abnormalities [70,71,73,74]. A recent report also showed that cocaine-induced conditioned locomotor response is attenuated in maLPA1, a variant derived from the original LPA1-null mice [69]. LPA infusion into the hippocampus improves spatial memory [144], which seems to be mainly mediated by LPA1 [70,71,74], and LPA signaling influences neurogenesis in the hippocampus via LPA1 [70,73]. The maLPA1 nulls show defects in a series of neurogenestic events in newborn hippocampal neurons under normal conditions, as well as after enrichment and exercise [73]. Further studies indicate that growth factors such as brain-derived neurotrophic factors and insulin growth factor 1 may represent some of the downstream effectors induced after initiating LPA1 signaling during neurogenesis [73]. Under chronic stress conditions, defects in hippocampal neurogenesis are much higher in maLPA1 than wild type mice [70]. Consistent with these defects in hippocampal neurogenesis, memory impairment occurs in mice lacking LPA1 [70,71,73]. Contrasting with LPA, little is known about the regulatory function of S1P signaling on the alterations that are seen in LPA1 nulls, even though S1P signaling is involved in neurogenesis and hyperexcitability via, at least, S1P1 and S1P2 [26,77,78]. One recent study showed anxiety and spatial memory impairments in seizure-prone S1P2 null mutants [28], a phenotype that shows background strain dependence. Overall, these results support roles for LP signaling in neuropsychiatric disorders.
There is no direct evidence of LPA or S1P signaling involvement in the pathogenesis of AD. However, a variety of correlative signals have been observed in AD patients and relevant cellular systems. For example, two enzymes partially responsible for LPA or S1P production, autotaxin or SPHK2, respectively, have been reported to be upregulated in AD brain samples [145,146], while S1P exposure reduces the activity of the β-site APP cleaving enzyme-1 (BACE1) that causes abnormal amyloid β (Aβ) accumulation, a characteristic feature of AD [145,147]. It has also been reported that S1P levels are reduced in AD patients [148] and that Aβ downregulates another S1P synthetic enzyme, SPHK1, while overexpression of SPHK1 reduces Aβ-induced death [149]. In addition, it was also reported that LPA promotes neuronal survival upon Aβ exposure [150]. To clarify the neuroprotective or neurotoxic effects of LPA and S1P signaling in AD, additional studies are needed. Clarifying the role of LP signaling in AD could extend these signaling mechanisms to other aspects of CNS dysfunction.
4.5. Developmental disorders
Hypoxic/ischemic injury is common in preterm infants where the resulting neurological defects can manifest as neurological and psychiatric disorders. A recent study identified LPA signaling as a requisite element in a variety of sequelae induced by fetal hypoxia within the developing CNS. Ex vivo and in vivo results showed that fetal hypoxia causes cortical disorganization amongst NPCs through N-cadherin disruption, displacement of mitotic NPCs, and impairment of neuronal migration, in which LPA1 has a pivotal role along with its effector pathways, Gi and Ras [15]. These data also implicate LPA1 as a possible therapeutic target for ischemic stroke, so it will be interesting to determine whether brain damage by ischemic stroke is reduced by either pharmacological or genetic inhibition of LPA1 signaling.
Another recent study also identified new aspects of LPA1 signaling in fetal hydrocephalus [16] that has been epidemiologically associated with psychiatric diseases, such as schizophrenia and autism. Fetal hydrocephalus is the most common neurological disorder of newborns and young children. It is characterized by abnormal accumulation of cerebrospinal fluid in the cortex, an enlarged head, and neurological dysfunction where prenatal bleeding was thought to be a responsible factor, and currently lack medical therapies. This study generated a new animal model of hydrocephalus that is achieved by injecting blood components or LPA into the fetal brain of embryos to over-activate LPA receptors, followed by birth and postnatal development. Data in this study showed that blood-borne LPA induces fetal hydrocephalus via LPA1, a result that ties post-hemorrhagic clinical events previously associated with fetal hydrocephalus to LPA receptor activities and raising the possibility of medically relevant therapies that target LPA receptors towards the prevention and amelioration of fetal hydrocephalus [16]. This study underscores not only the elucidation of a new role for LPA1 signaling, but also the development of new animal model to study fetal hydrocephalus.
4.6. Others
LPA and S1P signaling have also been identified as regulators of pathogenesis for other CNS disorders associated with hearing loss, seizures, and Sandhoff disease. Three independent groups have reported hearing loss in S1P2 null mutants [23–25]. This defect results from degeneration of vestibular and cochlear hair cells in the absence of S1P2 signaling. Further studies are needed to clarify the precise mechanisms, but this functional role may be useful to manage degenerative hearing loss. S1P2 null mutants backcrossed onto C57BL/6 background were reported to have seizure-like behaviors and hyperexcitability [26,28]. S1P2 null mutants this pure background can experience episodic running, freezing, and tonic–chronic convulsion, with almost half of the null mutants die from the seizure [28]. Interestingly, postnatal lethality is markedly increased in pups from S1P2 null mutant dams probably due to impaired maternal nurturing [28]. Therefore, it would be interesting to establish links between psychiatric dysfunction and impaired maternal nurturing. The possible involvement of LPA2 signaling has also been implicated based on its role in hyperexcitability [27], however there is currently no direct evidence linking LPA2 or other LPA receptors to seizures.
Sandhoff disease is a genetic lipid storage disorder where lysosomal β-hexosaminidase A is deficient, thereby blocking ganglioside degradation, resulting in an accumulation of abnormal lipid metabolites. This accumulation occurs in neurons, which triggers neurodegeneration and astrogliosis. Sandhoff disease is clinically similar to Tay–Sachs disease, another genetic lipid storage disorder where lysosomal β-hexosaminidase A malfunctions. In a study using a mouse model of Sandhoff disease, Hexb nulls, S1P signaling was revealed to be important in the pathogenesis of this disease, especially at terminal stages [29] when S1P levels and relevant receptors, S1P2 and S1P3, are increased, and genetic deletion of S1P3 or SPHK1 slows the progress of the disease by inhibiting astrogliosis [29]. Thus, both S1P1, as identified in studies of MS, and S1P3, appear to contribute to distinct initiating stimuli that promote astrogliosis, raising the possibility of more general effects of S1P-mediated astrogliosis in the pathology of other CNS disorders.
5. Conclusions
Since the discovery of the first LP receptor, LPA1, 11 receptors are now included in this family of 6 LPA and 5 S1P receptors. These bonafide GPCRs are widely and diversely expressed throughout the body and during development, to influence myriad biological as well as pathological processes. A growing number of studies using genetic null mutants for these LP receptor subtypes (LPA1, LPA2, LPA3, LPA4, LPA5, S1P1, S1P2, S1P3, S1P4, and S1P5) have clarified specific functions mediated by each, singly and in combination with other receptor subtypes. Recent data also indicate that LPA and S1P receptors are regulators of diverse biological functions in the CNS, a major locus for LPA and S1P receptors. We now know that LPA and S1P receptors are tractable therapeutic targets for the development of small molecule agents in human CNS diseases, through the success of fingolimod as an oral treatment for MS. However, work to reveal the functions of LPA and S1P signaling in the CNS is still in its nascent stage. Using genetic and pharmacological approaches, more functions await discovery, towards a better understanding of the mechanistic and therapeutic roles of LP signaling in CNS disorders.
Acknowledgments
We thank Danielle Letourneau for editorial assistance and Sook-Hyun Yoon for figure editing. We apologize for any oversights. This work was supported by the National Institutes of Health (NS048478 and DA019674) to JC, and the Korean National Research Foundation (201030003058) and a Novartis Postdoctoral Fellowship (Novartis Pharma, AG) to JWC.
Footnotes
☆
This article is part of a Special Issue entitled Advances in Lysophospholipid Research.
References
- 1.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 2.Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 4.Mutoh T, Rivera R, Chun J. Insights into the pharmacological relevance of lysophospholipid receptors. Br J Pharmacol. 2012;165:829–844. doi: 10.1111/j.1476-5381.2011.01622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: an update. Biochim Biophys Acta. 2008;1781:531–539. doi: 10.1016/j.bbalip.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, Reuschel R, Beerli C, Schwartz M, Billich A. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima N, Ishii I, Contos JJ, Weiner JA, Chun J. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001;41:507–534. doi: 10.1146/annurev.pharmtox.41.1.507. [DOI] [PubMed] [Google Scholar]
- 8.Dev KK, Mullershausen F, Mattes H, Kuhn RR, Bilbe G, Hoyer D, Mir A. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther. 2008;117:77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets. 2007;8:155–167. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi K, Chun J. Roles for lysophospholipid S1P receptors in multiple sclerosis. Crit Rev Biochem Mol Biol. 2011;46:2–10. doi: 10.3109/10409238.2010.522975. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol. 2009;9:15–23. doi: 10.1016/j.coph.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 13.Chun J, Brinkmann V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya) Discov Med. 2011;12:213–228. [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison SM, Reavill C, Brown G, Brown JT, Cluderay JE, Crook B, Davies CH, Dawson LA, Grau E, Heidbreder C, Hemmati P, Hervieu G, Howarth A, Hughes ZA, Hunter AJ, Latcham J, Pickering S, Pugh P, Rogers DC, Shilliam CS, Maycox PR. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol Cell Neurosci. 2003;24:1170–1179. doi: 10.1016/j.mcn.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Herr KJ, Herr DR, Lee CW, Noguchi K, Chun J. Stereotyped fetal brain disorganization is induced by hypoxia and requires lysophosphatidic acid receptor 1 (LPA1) signaling. Proc Natl Acad Sci U S A. 2011;108:15444–15449. doi: 10.1073/pnas.1106129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med. 2011;3:99ra87. doi: 10.1126/scitranslmed.3002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- 18.Nagai J, Uchida H, Matsushita Y, Yano R, Ueda M, Niwa M, Aoki J, Chun J, Ueda H. Autotaxin and lysophosphatidic acid1 receptor-mediated demyelination of dorsal root fibers by sciatic nerve injury and intrathecal lysophosphatidylcholine. Mol Pain. 2010;6:78. doi: 10.1186/1744-8069-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin ME, Rivera RR, Chun J. Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J Biol Chem. 2012;287:17608–17617. doi: 10.1074/jbc.M111.330183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li ZG, Yu ZC, Wang DZ, Ju WP, Zhan X, Wu QZ, Wu XJ, Cong HM, Man HH. Influence of acetylsalicylate on plasma lysophosphatidic acid level in patients with ischemic cerebral vascular diseases. Neurol Res. 2008;30:366–369. doi: 10.1179/174313208X300369. [DOI] [PubMed] [Google Scholar]
- 21.Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, Rami A, Huwiler A, Pfeilschifter J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem Biophys Res Commun. 2009;389:251–256. doi: 10.1016/j.bbrc.2009.08.142. [DOI] [PubMed] [Google Scholar]
- 22.Frugier T, Crombie D, Conquest A, Tjhong F, Taylor C, Kulkarni T, McLean C, Pebay A. Modulation of LPA receptor expression in the human brain following neurotrauma. Cell Mol Neurobiol. 2011;31:569–577. doi: 10.1007/s10571-011-9650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLennan AJ, Benner SJ, Andringa A, Chaves AH, Rosing JL, Vesey R, Karpman AM, Cronier SA, Lee N, Erway LC, Miller ML. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res. 2006;220:38–48. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Herr DR, Grillet N, Schwander M, Rivera R, Muller U, Chun J. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J Neurosci. 2007;27:1474–1478. doi: 10.1523/JNEUROSCI.4245-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, Proia RL. Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- 26.MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR, Anderson KJ, Roper SN, Lee N. An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. Eur J Neurosci. 2001;14:203–209. doi: 10.1046/j.0953-816x.2001.01634.x. [DOI] [PubMed] [Google Scholar]
- 27.Trimbuch T, Beed P, Vogt J, Schuchmann S, Maier N, Kintscher M, Breustedt J, Schuelke M, Streu N, Kieselmann O, Brunk I, Laube G, Strauss U, Battefeld A, Wende H, Birchmeier C, Wiese S, Sendtner M, Kawabe H, Kishimoto-Suga M, Brose N, Baumgart J, Geist B, Aoki J, Savaskan NE, Brauer AU, Chun J, Ninnemann O, Schmitz D, Nitsch R. Synaptic PRG-1 modulates excitatory transmission via lipid phosphate-mediated signaling. Cell. 2009;138:1222–1235. doi: 10.1016/j.cell.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akahoshi N, Ishizaki Y, Yasuda H, Murashima YL, Shinba T, Goto K, Himi T, Chun J, Ishii I. Frequent spontaneous seizures followed by spatial working memory/anxiety deficits in mice lacking sphingosine 1-phosphate receptor 2. Epilepsy Behav. 2011;22:659–665. doi: 10.1016/j.yebeh.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet. 2008;17:2257–2264. doi: 10.1093/hmg/ddn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hecht JH, Weiner JA, Post SR, Chun J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun J. How the lysophospholipid got its receptor. The Scientist. 2007;21:48–54. [Google Scholar]
- 32.Lee M, Van Brocklyn J, Thangada S, Liu C, Hand A, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 33.Zondag GC, Postma FR, Etten IV, Verlaan I, Moolenaar WH. Sphingosine 1-phosphate signalling through the G-protein-coupled receptor Edg-1. Biochem J. 1998;330:605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- 35.Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem. 1999;274:27776–27785. doi: 10.1074/jbc.274.39.27776. [DOI] [PubMed] [Google Scholar]
- 36.Contos JJ, Chun J. Genomic characterization of the lysophosphatidic acid receptor gene, lp(A2)/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics. 2000;64:155–169. doi: 10.1006/geno.2000.6122. [DOI] [PubMed] [Google Scholar]
- 37.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 38.Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem. 2007;282:4310–4317. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- 39.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for Lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 41.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 42.Lin ME, Rivera RR, Chun J. Targeted deletion of LPA5 identifies novel roles for LPA signaling in the development of neuropathic pain. J Biol Chem. 2012;287:17608–17617. doi: 10.1074/jbc.M111.330183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 44.Ohuchi H, Hamada A, Matsuda H, Takagi A, Tanaka M, Aoki J, Arai H, Noji S. Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev Dyn. 2008;237:3280–3294. doi: 10.1002/dvdy.21736. [DOI] [PubMed] [Google Scholar]
- 45.Contos JJ, Chun J. The mouse lp(A3)/Edg7 lysophosphatidic acid receptor gene: genomic structure, chromosomal localization, and expression pattern. Gene. 2001;267:243–253. doi: 10.1016/s0378-1119(01)00410-3. [DOI] [PubMed] [Google Scholar]
- 46.Weiner JA, Hecht JH, Chun J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J Comp Neurol. 1998;398:587–598. [PubMed] [Google Scholar]
- 47.Goldshmit Y, Munro K, Leong SY, Pebay A, Turnley AM. LPA receptor expression in the central nervous system in health and following injury. Cell Tissue Res. 2010;341:23–32. doi: 10.1007/s00441-010-0977-5. [DOI] [PubMed] [Google Scholar]
- 48.Savitz SI, Dhallu MS, Malhotra S, Mammis A, Ocava LC, Rosenbaum PS, Rosenbaum DM. EDG receptors as a potential therapeutic target in retinal ischemia–reperfusion injury. Brain Res. 2006;1118:168–175. doi: 10.1016/j.brainres.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 49.Dubin AE, Bahnson T, Weiner JA, Fukushima N, Chun J. Lysophosphatidic acid stimulates neurotransmitter-like conductance changes that precede GABA and L-glutamate in early, presumptive cortical neuroblasts. J Neurosci. 1999;19:1371–1381. doi: 10.1523/JNEUROSCI.19-04-01371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubin AE, Herr DR, Chun J. Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis. J Neurosci. 2010;30:7300–7309. doi: 10.1523/JNEUROSCI.6151-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukushima N, Weiner JA, Kaushal D, Contos JJ, Rehen SK, Kingsbury MA, Kim KY, Chun J. Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol Cell Neurosci. 2002;20:271–282. doi: 10.1006/mcne.2002.1123. [DOI] [PubMed] [Google Scholar]
- 52.Moller T, Contos JJ, Musante DB, Chun J, Ransom BR. Expression and function of lysophosphatidic acid receptors in cultured rodent microglial cells. J Biol Chem. 2001;276:25946–25952. doi: 10.1074/jbc.M102691200. [DOI] [PubMed] [Google Scholar]
- 53.Tham CS, Lin FF, Rao TS, Yu N, Webb M. Microglial activation state and lysophospholipid acid receptor expression. Int J Dev Neurosci. 2003;21:431–443. doi: 10.1016/j.ijdevneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Allard J, Barron S, Diaz J, Lubetzki C, Zalc B, Schwartz JC, Sokoloff P. A rat G protein-coupled receptor selectively expressed in myelin-forming cells. Eur J Neurosci. 1998;10:1045–1053. doi: 10.1046/j.1460-9568.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- 55.Cervera P, Tirard M, Barron S, Allard J, Trottier S, Lacombe J, Daumas-Duport C, Sokoloff P. Immunohistological localization of the myelinating cell-specific receptor LP(A1) Glia. 2002;38:126–136. doi: 10.1002/glia.10054. [DOI] [PubMed] [Google Scholar]
- 56.Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]
- 57.Miron VE, Hall JA, Kennedy TE, Soliven B, Antel JP. Cyclical and dose-dependent responses of adult human mature oligodendrocytes to fingolimod. Am J Pathol. 2008;173:1143–1152. doi: 10.2353/ajpath.2008.080478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, Okada M, Yamaguchi T. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116:1053–1062. doi: 10.1016/s0306-4522(02)00791-1. [DOI] [PubMed] [Google Scholar]
- 59.Rao TS, Lariosa-Willingham KD, Lin FF, Yu N, Tham CS, Chun J, Webb M. Growth factor pre-treatment differentially regulates phosphoinositide turnover downstream of lysophospholipid receptor and metabotropic glutamate receptors in cultured rat cerebrocortical astrocytes. Int J Dev Neurosci. 2004;22:131–135. doi: 10.1016/j.ijdevneu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, Lakeman K, Dijkstra CD, Van Der Valk P, Reijerkerk A, Alewijnse AE, Peters SL, De Vries HE. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58:1465–1476. doi: 10.1002/glia.21021. [DOI] [PubMed] [Google Scholar]
- 61.McGiffert C, Contos JJ, Friedman B, Chun J. Embryonic brain expression analysis of lysophospholipid receptor genes suggests roles for s1p(1) in neurogenesis and s1p(1–3) in angiogenesis. FEBS Lett. 2002;531:103–108. doi: 10.1016/s0014-5793(02)03404-x. [DOI] [PubMed] [Google Scholar]
- 62.Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, Windle JJ, Chen CK, Fang X. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell. 2008;19:5435–5445. doi: 10.1091/mbc.E08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukushima N, Kimura Y, Chun J. A single receptor encoded by vzg-1/lpA1/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc Natl Acad Sci U S A. 1998;95:6151–6156. doi: 10.1073/pnas.95.11.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukushima N, Shano S, Moriyama R, Chun J. Lysophosphatidic acid stimulates neuronal differentiation of cortical neuroblasts through the LPA1-G(i/o) pathway. Neurochem Int. 2007;50:302–307. doi: 10.1016/j.neuint.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6:1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- 67.Svetlov SI, Ignatova TN, Wang KK, Hayes RL, English D, Kukekov VG. Lysophosphatidic acid induces clonal generation of mouse neurospheres via proliferation of Sca-1- and AC133-positive neural progenitors. Stem Cells Dev. 2004;13:685–693. doi: 10.1089/scd.2004.13.685. [DOI] [PubMed] [Google Scholar]
- 68.Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 2002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blanco E, Bilbao A, Luque-Rojas MJ, Palomino A, Bermudez-Silva FJ, Suarez J, Santin LJ, Estivill-Torrus G, Gutierrez A, Campos-Sandoval JA, Alonso-Carrion FJ, Marquez J, de Fonseca FR. Attenuation of cocaine-induced conditioned locomotion is associated with altered expression of hippocampal glutamate receptors in mice lacking LPA1 receptors. Psychopharmacology (Berl) 2012;220:27–42. doi: 10.1007/s00213-011-2446-6. [DOI] [PubMed] [Google Scholar]
- 70.Castilla-Ortega E, Hoyo-Becerra C, Pedraza C, Chun J, Rodriguez De Fonseca F, Estivill-Torrus G, Santin LJ. Aggravation of chronic stress effects on hippocampal neurogenesis and spatial memory in LPA receptor knockout mice. PLoS One. 2011;6:e25522. doi: 10.1371/journal.pone.0025522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castilla-Ortega E, Sanchez-Lopez J, Hoyo-Becerra C, Matas-Rico E, Zambrana-Infantes E, Chun J, De Fonseca FR, Pedraza C, Estivill-Torrus G, Santin LJ. Exploratory, anxiety and spatial memory impairments are dissociated in mice lacking the LPA1 receptor. Neurobiol Learn Mem. 2010;94:73–82. doi: 10.1016/j.nlm.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Estivill-Torrus G, Llebrez-Zayas P, Matas-Rico E, Santin L, Pedraza C, De Diego I, Del Arco I, Fernandez-Llebrez P, Chun J, De Fonseca FR. Absence of LPA1 signaling results in defective cortical development. Cereb Cortex. 2008;18:938–950. doi: 10.1093/cercor/bhm132. [DOI] [PubMed] [Google Scholar]
- 73.Matas-Rico E, Garcia-Diaz B, Llebrez-Zayas P, Lopez-Barroso D, Santin L, Pedraza C, Smith-Fernandez A, Fernandez-Llebrez P, Tellez T, Redondo M, Chun J, De Fonseca FR, Estivill-Torrus G. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol Cell Neurosci. 2008;39:342–355. doi: 10.1016/j.mcn.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santin LJ, Bilbao A, Pedraza C, Matas-Rico E, Lopez-Barroso D, Castilla-Ortega E, Sanchez-Lopez J, Riquelme R, Varela-Nieto I, de la Villa P, Suardiaz M, Chun J, De Fonseca FR, Estivill-Torrus G. Behavioral phenotype of maLPA1-null mice: increased anxiety-like behavior and spatial memory deficits. Genes Brain Behav. 2009;8:772–784. doi: 10.1111/j.1601-183X.2009.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishii I, Contos JJ, Fukushima N, Chun J. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol Pharmacol. 2000;58:895–902. doi: 10.1124/mol.58.5.895. [DOI] [PubMed] [Google Scholar]
- 76.Kimura A, Ohmori T, Kashiwakura Y, Ohkawa R, Madoiwa S, Mimuro J, Shimazaki K, Hoshino Y, Yatomi Y, Sakata Y. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411–3417. doi: 10.1161/STROKEAHA.108.514612. [DOI] [PubMed] [Google Scholar]
- 77.Harada J, Foley M, Moskowitz MA, Waeber C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem. 2004;88:1026–1039. doi: 10.1046/j.1471-4159.2003.02219.x. [DOI] [PubMed] [Google Scholar]
- 78.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rehen SK, Kingsbury MA, Almeida BS, Herr DR, Peterson S, Chun J. A new method of embryonic culture for assessing global changes in brain organization. J Neurosci Methods. 2006;158:100–108. doi: 10.1016/j.jneumeth.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 80.Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, Kobayashi E, Hoshino Y, Yatomi Y, Sakata Y. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells. 2007;25:115–124. doi: 10.1634/stemcells.2006-0223. [DOI] [PubMed] [Google Scholar]
- 81.Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A. 2011;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Birgbauer E, Chun J. Lysophospholipid receptors LPA1–3 are not required for the inhibitory effects of LPA on mouse retinal growth cones. Eye Brain. 2010;2:1–13. doi: 10.2147/EB.S7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.MacLennan AJ, Devlin BK, Marks L, Gaskin AA, Neitzel KL, Lee N. Antisense studies in PC12 cells suggest a role for H218, a sphingosine 1-phosphate receptor, in growth-factor-induced cell–cell interaction and neurite outgrowth. Dev Neurosci. 2000;22:283–295. doi: 10.1159/000017452. [DOI] [PubMed] [Google Scholar]
- 85.Toman RE, Payne SG, Watterson KR, Maceyka M, Lee NH, Milstien S, Bigbee JW, Spiegel S. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J Cell Biol. 2004;166:381–392. doi: 10.1083/jcb.200402016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanno T, Nishizaki T, Proia RL, Kajimoto T, Jahangeer S, Okada T, Nakamura S. Regulation of synaptic strength by sphingosine 1-phosphate in the hippocampus. Neuroscience. 2010;171:973–980. doi: 10.1016/j.neuroscience.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 87.Pilpel Y, Segal M. The role of LPA1 in formation of synapses among cultured hippocampal neurons. J Neurochem. 2006;97:1379–1392. doi: 10.1111/j.1471-4159.2006.03825.x. [DOI] [PubMed] [Google Scholar]
- 88.Ueda H. Lysophosphatidic acid as the initiator of neuropathic pain. Biol Pharm Bull. 2011;34:1154–1158. doi: 10.1248/bpb.34.1154. [DOI] [PubMed] [Google Scholar]
- 89.Chi XX, Nicol GD. The sphingosine 1-phosphate receptor, S1PR, plays a prominent but not exclusive role in enhancing the excitability of sensory neurons. J Neurophysiol. 2010;104:2741–2748. doi: 10.1152/jn.00709.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coste O, Brenneis C, Linke B, Pierre S, Maeurer C, Becker W, Schmidt H, Gao W, Geisslinger G, Scholich K. Sphingosine 1-phosphate modulates spinal nociceptive processing. J Biol Chem. 2008;283:32442–32451. doi: 10.1074/jbc.M806410200. [DOI] [PubMed] [Google Scholar]
- 91.Elmes SJ, Millns PJ, Smart D, Kendall DA, Chapman V. Evidence for biological effects of exogenous LPA on rat primary afferent and spinal cord neurons. Brain Res. 2004;1022:205–213. doi: 10.1016/j.brainres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 92.Norman E, Cutler RG, Flannery R, Wang Y, Mattson MP. Plasma membrane sphingomyelin hydrolysis increases hippocampal neuron excitability by sphingosine-1-phosphate mediated mechanisms. J Neurochem. 2010;114:430–439. doi: 10.1111/j.1471-4159.2010.06779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sim-Selley LJ, Goforth PB, Mba MU, Macdonald TL, Lynch KR, Milstien S, Spiegel S, Satin LS, Welch SP, Selley DE. Sphingosine-1-phosphate receptors mediate neuromodulatory functions in the CNS. J Neurochem. 2009;110:1191–1202. doi: 10.1111/j.1471-4159.2009.06202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol. 2007;27:3429–3440. doi: 10.1128/MCB.01465-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanno T, Nishizaki T. Endogenous sphingosine 1-phosphate regulates spontaneous glutamate release from mossy fiber terminals via S1P(3) receptors. Life Sci. 2011;89:137–140. doi: 10.1016/j.lfs.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 96.Roberts C, Winter P, Shilliam CS, Hughes ZA, Langmead C, Maycox PR, Dawson LA. Neurochemical changes in LPA1 receptor deficient mice—a putative model of schizophrenia. Neurochem Res. 2005;30:371–377. doi: 10.1007/s11064-005-2611-6. [DOI] [PubMed] [Google Scholar]
- 97.Musazzi L, Di Daniel E, Maycox P, Racagni G, Popoli M. Abnormalities in alpha/beta-CaMKII and related mechanisms suggest synaptic dysfunction in hippocampus of LPA1 receptor knockout mice. Int J Neuropsychopharmacol. 2011;14:941–953. doi: 10.1017/S1461145710001240. [DOI] [PubMed] [Google Scholar]
- 98.Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, Traynelis SF, Hepler JR. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol. 2003;64:1199–1209. doi: 10.1124/mol.64.5.1199. [DOI] [PubMed] [Google Scholar]
- 99.Spohr TCSe, Choi JW, Gardell SE, Herr DR, Rehen SK, Gomes FC, Chun J. Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation. J Biol Chem. 2008;283:7470–7479. doi: 10.1074/jbc.M707758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spohr TCSe, Dezonne RS, Rehen SK, Gomes FC. Astrocytes treated by lysophosphatidic acid induce axonal outgrowth of cortical progenitors through extracellular matrix protein and epidermal growth factor signaling pathway. J Neurochem. 2011;119:113–123. doi: 10.1111/j.1471-4159.2011.07421.x. [DOI] [PubMed] [Google Scholar]
- 101.Shano S, Moriyama R, Chun J, Fukushima N. Lysophosphatidic acid stimulates astrocyte proliferation through LPA(1) Neurochem Int. 2008;52:216–220. doi: 10.1016/j.neuint.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol. 2010;176:2682–2694. doi: 10.2353/ajpath.2010.091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- 104.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 105.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 106.Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, Pelletier J, Stites T, Wu S, Holdbrook F, Zhang-Auberson L, Francis G, Cohen JA. Comparison of fingolimod with interferon beta-1a in relapsing–remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10:520–529. doi: 10.1016/S1474-4422(11)70099-0. [DOI] [PubMed] [Google Scholar]
- 107.Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- 108.Tabuchi S, Kume K, Aihara M, Shimizu T. Expression of lysophosphatidic acid receptor in rat astrocytes: mitogenic effect and expression of neurotrophic genes. Neurochem Res. 2000;25:573–582. doi: 10.1023/a:1007542532395. [DOI] [PubMed] [Google Scholar]
- 109.Malchinkhuu E, Sato K, Muraki T, Ishikawa K, Kuwabara A, Okajima F. Assessment of the role of sphingosine 1-phosphate and its receptors in high-density lipoprotein-induced stimulation of astroglial cell function. Biochem J. 2003;370:817–827. doi: 10.1042/BJ20020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sato K, Ishikawa K, Ui M, Okajima F. Sphingosine 1-phosphate induces expression of early growth response-1 and fibroblast growth factor-2 through mechanism involving extracellular signal-regulated kinase in astroglial cells. Brain Res Mol Brain Res. 1999;74:182–189. doi: 10.1016/s0169-328x(99)00279-x. [DOI] [PubMed] [Google Scholar]
- 111.Yamagata K, Tagami M, Torii Y, Takenaga F, Tsumagari S, Itoh S, Yamori Y, Nara Y. Sphingosine 1-phosphate induces the production of glial cell line-derived neurotrophic factor and cellular proliferation in astrocytes. Glia. 2003;41:199–206. doi: 10.1002/glia.10180. [DOI] [PubMed] [Google Scholar]
- 112.Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, Walsh FS, Pangalos MN, Arimura N, Kaibuchi K, Zalc B, Lubetzki C. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 114.Kim HJ, Miron VE, Dukala D, Proia RL, Ludwin SK, Traka M, Antel JP, Soliven B. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J. 2011;25:1509–1518. doi: 10.1096/fj.10-173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rau CR, Hein K, Sattler MB, Kretzschmar B, Hillgruber C, McRae BL, Diem R, Bahr M. Anti-inflammatory effects of FTY720 do not prevent neuronal cell loss in a rat model of optic neuritis. Am J Pathol. 2011;178:1770–1781. doi: 10.1016/j.ajpath.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schilling T, Repp H, Richter H, Koschinski A, Heinemann U, Dreyer F, Eder C. Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1 Ca2+-dependent K+ channels. Neuroscience. 2002;109:827–835. doi: 10.1016/s0306-4522(01)00534-6. [DOI] [PubMed] [Google Scholar]
- 117.Bernhart E, Kollroser M, Rechberger G, Reicher H, Heinemann A, Schratl P, Hallstrom S, Wintersperger A, Nusshold C, DeVaney T, Zorn-Pauly K, Malli R, Graier W, Malle E, Sattler W. Lysophosphatidic acid receptor activation affects the C13NJ microglia cell line proteome leading to alterations in glycolysis, motility, and cytoskeletal architecture. Proteomics. 2010;10:141–158. doi: 10.1002/pmic.200900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V, Liao JK, Lo EH, Waeber C. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujita R, Ma Y, Ueda H. Lysophosphatidic acid-induced membrane ruffling and brain-derived neurotrophic factor gene expression are mediated by ATP release in primary microglia. J Neurochem. 2008;107:152–160. doi: 10.1111/j.1471-4159.2008.05599.x. [DOI] [PubMed] [Google Scholar]
- 120.Schilling T, Stock C, Schwab A, Eder C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur J Neurosci. 2004;19:1469–1474. doi: 10.1111/j.1460-9568.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- 121.Lin H, Baby N, Lu J, Kaur C, Zhang C, Xu J, Ling EA, Dheen ST. Expression of sphingosine kinase 1 in amoeboid microglial cells in the corpus callosum of postnatal rats. J Neuroinflammation. 2011;8:13. doi: 10.1186/1742-2094-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nayak D, Huo Y, Kwang WX, Pushparaj PN, Kumar SD, Ling EA, Dheen ST. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience. 2010;166:132–144. doi: 10.1016/j.neuroscience.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 123.Ma L, Nagai J, Ueda H. Microglial activation mediates de novo lysophosphatidic acid production in a model of neuropathic pain. J Neurochem. 2010;115:643–653. doi: 10.1111/j.1471-4159.2010.06955.x. [DOI] [PubMed] [Google Scholar]
- 124.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 125.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 126.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 127.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 128.Lee CW, Choi JW, Chun J. Neurological S1P signaling as an emerging mechanism of action of oral FTY720 (fingolimod) in multiple sclerosis. Arch Pharm Res. 2010;33:1567–1574. doi: 10.1007/s12272-010-1008-5. [DOI] [PubMed] [Google Scholar]
- 129.Foster CA, Mechtcheriakova D, Storch MK, Balatoni B, Howard LM, Bornancin F, Wlachos A, Sobanov J, Kinnunen A, Baumruker T. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood–brain-barrier damage. Brain Pathol. 2009;19:254–266. doi: 10.1111/j.1750-3639.2008.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blondeau N, Lai Y, Tyndall S, Popolo M, Topalkara K, Pru JK, Zhang L, Kim H, Liao JK, Ding K, Waeber C. Distribution of sphingosine kinase activity and mRNA in rodent brain. J Neurochem. 2007;103:509–517. doi: 10.1111/j.1471-4159.2007.04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wacker BK, Park TS, Gidday JM. Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke. 2009;40:3342–3348. doi: 10.1161/STROKEAHA.109.560714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pfeilschifter W, Czech-Zechmeister B, Sujak M, Mirceska A, Koch A, Rami A, Steinmetz H, Foerch C, Huwiler A, Pfeilschifter J. Activation of sphingosine kinase 2 is an endogenous protective mechanism in cerebral ischemia. Biochem Biophys Res Commun. 2011;413:212–217. doi: 10.1016/j.bbrc.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 133.Yang C, Lafleur J, Mwaikambo BR, Zhu T, Gagnon C, Chemtob S, Di Polo A, Hardy P. The role of lysophosphatidic acid receptor (LPA1) in the oxygen-induced retinal ganglion cell degeneration. Invest Ophthalmol Vis Sci. 2009;50:1290–1298. doi: 10.1167/iovs.08-1920. [DOI] [PubMed] [Google Scholar]
- 134.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 135.Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- 136.Frugier T, Morganti-Kossmann MC, O’Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010;27:497–507. doi: 10.1089/neu.2009.1120. [DOI] [PubMed] [Google Scholar]
- 137.Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 2008;152:296–298. doi: 10.1016/j.neuroscience.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 138.Inoue M, Yamaguchi A, Kawakami M, Chun J, Ueda H. Loss of spinal substance P pain transmission under the condition of LPA1 receptor-mediated neuropathic pain. Mol Pain. 2006;2:25. doi: 10.1186/1744-8069-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma L, Uchida H, Nagai J, Inoue M, Chun J, Aoki J, Ueda H. Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain. Mol Pain. 2009;5:64. doi: 10.1186/1744-8069-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xie W, Matsumoto M, Chun J, Ueda H. Involvement of LPA1 receptor signaling in the reorganization of spinal input through Abeta-fibers in mice with partial sciatic nerve injury. Mol Pain. 2008;4:46. doi: 10.1186/1744-8069-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 142.Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bowden NA, Weidenhofer J, Scott RJ, Schall U, Todd J, Michie PT, Tooney PA. Preliminary investigation of gene expression profiles in peripheral blood lymphocytes in schizophrenia. Schizophr Res. 2006;82:175–183. doi: 10.1016/j.schres.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 144.Dash PK, Orsi SA, Moody M, Moore AN. A role for hippocampal Rho-ROCK pathway in long-term spatial memory. Biochem Biophys Res Commun. 2004;322:893–898. doi: 10.1016/j.bbrc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 145.Takasugi N, Sasaki T, Suzuki K, Osawa S, Isshiki H, Hori Y, Shimada N, Higo T, Yokoshima S, Fukuyama T, Lee VM, Trojanowski JQ, Tomita T, Iwatsubo T. BACE1 activity is modulated by cell-associated sphingosine-1-phosphate. J Neurosci. 2011;31:6850–6857. doi: 10.1523/JNEUROSCI.6467-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Umemura K, Yamashita N, Yu X, Arima K, Asada T, Makifuchi T, Murayama S, Saito Y, Kanamaru K, Goto Y, Kohsaka S, Kanazawa I, Kimura H. Autotaxin expression is enhanced in frontal cortex of Alzheimer-type dementia patients. Neurosci Lett. 2006;400:97–100. doi: 10.1016/j.neulet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 147.Yi H, Lee SJ, Lee J, Myung CS, Park WK, Lim HJ, Lee GH, Kong JY, Cho H. Sphingosylphosphorylcholine attenuated beta-amyloid production by reducing BACE1 expression and catalysis in PC12 cells. Neurochem Res. 2011;36:2083–2090. doi: 10.1007/s11064-011-0532-0. [DOI] [PubMed] [Google Scholar]
- 148.He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging. 2010;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gomez-Brouchet A, Pchejetski D, Brizuela L, Garcia V, Altie MF, Maddelein ML, Delisle MB, Cuvillier O. Critical role for sphingosine kinase-1 in regulating survival of neuroblastoma cells exposed to amyloid-beta peptide. Mol Pharmacol. 2007;72:341–349. doi: 10.1124/mol.106.033738. [DOI] [PubMed] [Google Scholar]
- 150.Zheng ZQ, Fang XJ, Zhang Y, Qiao JT. Neuroprotective effect of lysophosphatidic acid on AbetaP31–35-induced apoptosis in cultured cortical neurons. Sheng Li Xue Bao. 2005;57:289–294. [PubMed] [Google Scholar]
- 151.Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Funke M, Zhao Z, Xu Y, Chun J, Tager AM. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am J Respir Cell Mol Biol. 2012;46:355–364. doi: 10.1165/rcmb.2010-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, Calise D, Chun J, Bascands JL, Saulnier-Blache JS, Schanstra JP. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007;18:3110–3118. doi: 10.1681/ASN.2007020196. [DOI] [PubMed] [Google Scholar]
- 154.Tager AM, Lacamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA(1) links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 155.Zhao J, He D, Su Y, Berdyshev E, Chun J, Natarajan V, Zhao Y. Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. Am J Physiol Lung Cell Mol Physiol. 2011;301:L547–L556. doi: 10.1152/ajplung.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, Ohta H, Yamori T, Watanabe M, Chun J, Arai H. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem. 2004;279:17634–17639. doi: 10.1074/jbc.M313927200. [DOI] [PubMed] [Google Scholar]
- 157.Simon MF, Daviaud D, Pradere JP, Gres S, Guigne C, Wabitsch M, Chun J, Valet P, Saulnier-Blache JS. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J Biol Chem. 2005;280:14656–14662. doi: 10.1074/jbc.M412585200. [DOI] [PubMed] [Google Scholar]
- 158.Panchatcharam M, Miriyala S, Yang F, Rojas M, End C, Vallant C, Dong A, Lynch K, Chun J, Morris AJ, Smyth SS. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ Res. 2008;103:662–670. doi: 10.1161/CIRCRESAHA.108.180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gennero I, Laurencin-Dalicieux S, Conte-Auriol F, Briand-Mesange F, Laurencin D, Rue J, Beton N, Malet N, Mus M, Tokumura A, Bourin P, Vico L, Brunel G, Oreffo RO, Chun J, Salles JP. Absence of the lysophosphatidic acid receptor LPA1 results in abnormal bone development and decreased bone mass. Bone. 2011;49:395–403. doi: 10.1016/j.bone.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cheng HY, Dong A, Panchatcharam M, Mueller P, Yang F, Li Z, Mills G, Chun J, Morris AJ, Smyth SS. Lysophosphatidic acid signaling protects pulmonary vasculature from hypoxia-induced remodeling. Arterioscler Thromb Vasc Biol. 2012;32:24–32. doi: 10.1161/ATVBAHA.111.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Weiner JA, Fukushima N, Contos JJ, Scherer SS, Chun J. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci. 2001;21:7069–7078. doi: 10.1523/JNEUROSCI.21-18-07069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lin S, Lee SJ, Shim H, Chun J, Yun CC. The absence of LPA receptor 2 reduces the tumorigenesis by ApcMin mutation in the intestine. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1128–G1138. doi: 10.1152/ajpgi.00321.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, Chun J, Yun CC. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology. 2009;136:1711–1720. doi: 10.1053/j.gastro.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao Y, Tong J, He D, Pendyala S, Evgeny B, Chun J, Sperling AI, Natarajan V. Role of lysophosphatidic acid receptor LPA2 in the development of allergic airway inflammation in a murine model of asthma. Respir Res. 2009;10:114. doi: 10.1186/1465-9921-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, Kanai M, Ye X, Chun J, Matsuki N, Suzuki H, Shibasaki M, Arai H. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol Reprod. 2007;77:954–959. doi: 10.1095/biolreprod.107.060293. [DOI] [PubMed] [Google Scholar]
- 166.Ye X, Diao H, Chun J. 11-Deoxy prostaglandin F(2alpha), a thromboxane A2 receptor agonist, partially alleviates embryo crowding in Lpar3((−/−)) females. Fertil Steril. 2012;97:757–763. doi: 10.1016/j.fertnstert.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ye X, Skinner MK, Kennedy G, Chun J. Age-dependent loss of sperm production in mice via impaired lysophosphatidic acid signaling. Biol Reprod. 2008;79:328–336. doi: 10.1095/biolreprod.108.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Diao H, Aplin JD, Xiao S, Chun J, Li Z, Chen S, Ye X. Altered spatiotemporal expression of collagen types I, III, IV, and VI in Lpar3-deficient peri-implantation mouse uterus. Biol Reprod. 2011;84:255–265. doi: 10.1095/biolreprod.110.086942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chan LC, Peters W, Xu Y, Chun J, Farese RV, Jr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA) J Leukoc Biol. 2007;82:1193–1200. doi: 10.1189/jlb.0407221. [DOI] [PubMed] [Google Scholar]
- 171.Zhao C, Sardella A, Chun J, Poubelle PE, Fernandes MJ, Bourgoin SG. TNF-alpha promotes LPA1- and LPA3-mediated recruitment of leukocytes in vivo through CXCR2 ligand chemokines. J Lipid Res. 2011;52:1307–1318. doi: 10.1194/jlr.M008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sumida H, Noguchi K, Kihara Y, Abe M, Yanagida K, Hamano F, Sato S, Tamaki K, Morishita Y, Kano MR, Iwata C, Miyazono K, Sakimura K, Shimizu T, Ishii S. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116:5060–5070. doi: 10.1182/blood-2010-03-272443. [DOI] [PubMed] [Google Scholar]
- 173.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- 174.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 176.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol. 2010;43:662–673. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 178.Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL, Cyster JG. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med. 2006;203:2683–2690. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 180.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 181.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R440–R446. doi: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 182.Szczepaniak WS, Pitt BR, McVerry BJ. S1P2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. Am J Physiol Lung Cell Mol Physiol. 2010;299:L137–L145. doi: 10.1152/ajplung.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ikeda H, Watanabe N, Ishii I, Shimosawa T, Kume Y, Tomiya T, Inoue Y, Nishikawa T, Ohtomo N, Tanoue Y, Iitsuka S, Fujita R, Omata M, Chun J, Yatomi Y. Sphingosine 1-phosphate regulates regeneration and fibrosis after liver injury via sphingosine 1-phosphate receptor 2. J Lipid Res. 2009;50:556–564. doi: 10.1194/jlr.M800496-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Serriere-Lanneau V, Teixeira-Clerc F, Li L, Schippers M, de Wries W, Julien B, Tran-Van-Nhieu J, Manin S, Poelstra K, Chun J, Carpentier S, Levade T, Mallat A, Lotersztajn S. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 2007;21:2005–2013. doi: 10.1096/fj.06-6889com. [DOI] [PubMed] [Google Scholar]
- 185.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Imasawa T, Koike K, Ishii I, Chun J, Yatomi Y. Blockade of sphingosine 1-phosphate receptor 2 signaling attenuates streptozotocin-induced apoptosis of pancreatic beta-cells. Biochem Biophys Res Commun. 2010;392:207–211. doi: 10.1016/j.bbrc.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci U S A. 2005;102:9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 189.Baudhuin LM, Jiang Y, Zaslavsky A, Ishii I, Chun J, Xu Y. S1P3-mediated Akt activation and cross-talk with platelet-derived growth factor receptor (PDGFR) FASEB J. 2004;18:341–343. doi: 10.1096/fj.03-0302fje. [DOI] [PubMed] [Google Scholar]
- 190.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, Kingsbury MA, Zhang G, Brown JH, Chun J. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 191.Girkontaite I, Sakk V, Wagner M, Borggrefe T, Tedford K, Chun J, Fischer KD. The sphingosine-1-phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM-1+ endothelial cells in splenic marginal sinus organization. J Exp Med. 2004;200:1491–1501. doi: 10.1084/jem.20041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Keller CD, Rivera Gil P, Tolle M, van der Giet M, Chun J, Radeke HH, Schafer-Korting M, Kleuser B. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and Smad3 signaling. Am J Pathol. 2007;170:281–292. doi: 10.2353/ajpath.2007.060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, Spyridopoulos I, Chun J, Brinkmann V, Keul P, Levkau B, Zeiher AM, Dimmeler S, Haendeler J. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–282. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- 194.Levkau B, Hermann S, Theilmeier G, van der Giet M, Chun J, Schober O, Schafers M. High-density lipoprotein stimulates myocardial perfusion in vivo. Circulation. 2004;110:3355–3359. doi: 10.1161/01.CIR.0000147827.43912.AE. [DOI] [PubMed] [Google Scholar]
- 195.Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tolle M, Levkau B, Keul P, Brinkmann V, Giebing G, Schonfelder G, Schafers M, von Wnuck Lipinski K, Jankowski J, Jankowski V, Chun J, Zidek W, Van der Giet M. Immunomodulator FTY720 induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ Res. 2005;96:913–920. doi: 10.1161/01.RES.0000164321.91452.00. [DOI] [PubMed] [Google Scholar]
- 197.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol. 2008;153:140–147. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Takuwa N, Ohkura S, Takashima S, Ohtani K, Okamoto Y, Tanaka T, Hirano K, Usui S, Wang F, Du W, Yoshioka K, Banno Y, Sasaki M, Ichi I, Okamura M, Sugimoto N, Mizugishi K, Nakanuma Y, Ishii I, Takamura M, Kaneko S, Kojo S, Satouchi K, Mitumori K, Chun J, Takuwa Y. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc Res. 2010;85:484–493. doi: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Herzog C, Schmitz M, Levkau B, Herrgott I, Mersmann J, Larmann J, Johanning K, Winterhalter M, Chun J, Muller FU, Echtermeyer F, Hildebrand R, Theilmeier G. Intravenous sphingosylphosphorylcholine protects ischemic and postischemic myocardial tissue in a mouse model of myocardial ischemia/reperfusion injury. Mediators Inflamm. 2010;2010:425191. doi: 10.1155/2010/425191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 201.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 202.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1–S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 203.Golfier S, Kondo S, Schulze T, Takeuchi T, Vassileva G, Achtman AH, Graler MH, Abbondanzo SJ, Wiekowski M, Kremmer E, Endo Y, Lira SA, Bacon KB, Lipp M. Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine-1-phosphate receptor S1P4. FASEB J. 2010;24:4701–4710. doi: 10.1096/fj.09-141473. [DOI] [PubMed] [Google Scholar]
- 204.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, Xu Y, Roots CM, Beilke JN, Banerjee A, Reiner SL, Miller SA, Weinmann AS, Goodnow CC, Lanier LL, Cyster JG, Chun J. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mayol K, Biajoux V, Marvel J, Balabanian K, Walzer T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood. 2011;118:4863–4871. doi: 10.1182/blood-2011-06-362574. [DOI] [PubMed] [Google Scholar]
- 206.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]