Active Akt and Functional p53 Modulate Apoptosis in Abelson Virus-Transformed Pre-B Cells (original) (raw)
Abstract
Suppression of apoptosis is an important feature of the Abelson murine leukemia virus (Ab-MLV) transformation process. During multistep transformation, Ab-MLV-infected pre-B cells undergo p53-dependent apoptosis during the crisis phase of transformation. Even once cells are fully transformed, an active v-Abl protein tyrosine kinase is required to suppress apoptosis because cells transformed by temperature-sensitive (ts) kinase mutants undergo rapid apoptosis after a shift to the nonpermissive temperature. However, inactivation of the v-Abl protein by a temperature shift interrupts signals transmitted via multiple pathways, making it difficult to identify those that are critically important for the suppression of apoptosis. To begin to dissect these pathways, we tested the ability of an SH2 domain Ab-MLV mutant, P120/R273K, to rescue aspects of the ts phenotype of pre-B cells transformed by the conditional kinase domain mutant. The P120/R273K mutant suppressed apoptosis at the nonpermissive temperature, a phenotype correlated with its ability to activate Akt. Apoptosis also was suppressed at the nonpermissive temperature by constitutively active Akt and in _p53_-null pre-B cells transformed with the ts kinase domain mutant. These data indicate that an intact Src homology 2 (SH2) domain is not critical for apoptosis suppression and suggest that signals transmitted through Akt and p53 play an important role in the response.
Abelson murine leukemia virus (Ab-MLV) induces pre-B-cell lymphoma in mice and transforms primary pre-B cells, some factor-dependent hematopoietic cell lines, and several immortalized fibroblastoid cell lines in vitro (reviewed in reference 37). The protein tyrosine kinase activity of the v-Abl protein, the single product of Ab-MLV, is required for transformation. This activity stimulates multiple downstream signaling pathways, including those involving Ras, phosphatidylinositol 3-kinase (PI3-K), Raf, Myc, and Akt, all of which are required for transformation (40, 41, 43-45, 51, 54). In addition, several forms of abl oncogenes upregulate the expression of antiapoptotic Bcl-2 family members (1, 2, 4, 39, 46, 53). As a consequence, growth is stimulated and apoptosis is suppressed (5, 8, 9). However, the way in which the different signaling pathways are coordinated to give rise to transformed cells is not fully understood.
Although v-Abl transmits potent signals that affect apoptosis and growth, the cellular response to these oncogenic signals also influences transformation. These cellular signals involve the p19Arf-p53 pathway (reviewed in reference 42). Ab-MLV-infected pre-B cells must overcome the effects of this pathway in order to become fully transformed cell lines with malignant potential (32, 47, 48), and the transformation defects of some Ab-MLV mutants are reduced when _p53_-null cells are used as a target population (49, 55). Many transformants acquire p53 mutations as they become established (21, 32, 47), while others down-modulate the expression of the p19Arf protein, a molecule that can activate p53 through effects on Mdm2 (42). This latter type of transformant retains functional p53 protein and responds to gamma irradiation by undergoing rapid apoptosis and upregulating the expression of the p53-responsive gene p21Cip1 (47). Thus, in these cells, under normal conditions, p53 no longer senses the oncogenic signals transmitted by v-Abl. Down-regulation of p19Arf expression appears to be important for this event because ectopic expression of the protein induces apoptosis (32).
The complex relationship between growth-stimulatory and survival signals delivered by v-Abl and growth-suppressive and proapoptotic signals emanating from the cell is exemplified by the phenotype of the P120/R273K mutant. This mutant expresses a v-Abl protein in which the FLVRES pocket in the SH2 domain has been altered and fails to activate the mitogen-activated protein (MAP) kinase pathway and to stimulate normal pre-B-cell growth at 39.5°C (24). However, P120/R273K is able to transform bone marrow cells from _p53_-null mice at the high temperature (49), suggesting that P120/R273K is a useful tool for probing the way in which v-Abl interacts with the p53 pathway. To this end, we have taken advantage of this mutant and the temperature-sensitive (ts) 7C411 cell complementation assay (14), an assay that monitors the ability of Ab-MLV strains or other oncogenes to reverse the growth suppression and apoptosis characteristics of pre-B cells transformed with ts kinase domain mutants (5, 16). When P120/R273K was expressed in 7C411 cells, apoptosis at the nonpermissive temperature was suppressed, a feature that was correlated with the expression of activated Akt and the presence of wild-type p53. Consistent with a role for p53 in the rapid apoptosis that follows v-Abl inactivation, _p53_-null cells expressing a ts kinase domain mutant did not grow at the nonpermissive temperature but did display extended survival. These data suggest that functional p53 is important for mediating apoptosis in fully transformed pre-B cells and that v-Abl-mediated activation of the Akt pathway plays an important role in the response.
MATERIALS AND METHODS
Cells and virus preparation.
The ts Ab-MLV-transformed pre-B-cell line 7C411 was derived by infecting bone marrow cells with ts Ab-MLV strain P70/H590 (16) and was grown routinely in RPMI 1640 medium supplemented with 10% fetal calf serum (Sigma), 50 μM 2-mercaptoethanol, and 2 μM l-glutamine. The cells were maintained at 34°C, the permissive temperature for the ts Ab-MLV mutants; the nonpermissive temperature used in all experiments was 39.5°C. 293T cells (13) were maintained in Dulbecco's modified Eagle's medium (Life Technologies) supplemented with 10% fetal calf serum; transient transfection of these cells was used to prepare virus stocks as described previously (24). Bone marrow cells from _p53_-null mice were infected with ts mutant P120/G+H (16) in the presence of 4 μg of Polybrene/ml and plated at 2 × 106 nucleated cells per ml in 35-mm tissue culture plates at 34°C. Rapidly growing pre-B cells were harvested from the culture about 2 weeks later and maintained at 34°C. To obtain derivatives of 7C411 cells expressing wild-type or mutant Ab-MLV or other oncogenes, 106 cells were infected with retroviruses expressing the gene of interest and a drug resistance gene in the presence of 4 μg of Polybrene/ml for 4 h. In some experiments, 2 × 107 cells were electroporated (300 V, 960 μF) by using a Bio-Rad gene pulser in the presence of 20 μg of plasmid DNA expressing the gene under study. The cells then were plated in standard growth medium; 24 h later, 1 mg of G418/ml or 1.5 μg of puromycin/ml was added to the medium, and the cells were plated in 96-well plates. Populations of G418-resistant or puromycin-resistant cells were isolated 7 to 14 days later.
Plasmids and viral mutant construction.
All of the Ab-MLV strains were prepared from Ab-MLV strain P120 originally contained in vector pUC120 (15). P120/D484N expresses a kinase-negative v-Abl protein in which aspartic acid 484 in the ATP binding region has been replaced by an asparagine; this protein lacks kinase activity (33). P120/Y514F expresses a v-Abl protein in which tyrosine 514, the major autophosphorylation site (36), has been replaced by a phenylalanine. P120/R273K expresses a v-Abl protein in which arginine 273 in the conserved FLVRES motif has been replaced by a lysine (24). These sequences were expressed in retroviral vector pSRα MSVtkneo (29). The Ab-MLV P120G+H mutant expresses a v-Abl protein in which lysine 536 has been replaced by a glycine residue and tyrosine 590 has been replaced by a histidine residue (16); this mutant was expressed in vector pMIG (19, 50). The fragments containing the Ha-Ras and Myr-Akt coding sequences were removed from pSRα MSVtkneo-Ha-Ras (41) and pSRα MSVtkneo-Myr-Akt (17), respectively, by using _Eco_RI and were cloned into retroviral vector pBabe-puro (27), which contains a puromycin resistance gene. Ras(12V, 35S) (52), Ras(12V, 37G) (52), and Ras(12V, 40C) (22) were expressed in either pBabe-puro or pMSCV-puro (19) and introduced into 7C411 cells by electroporation.
Protein analysis.
Cell lysates were prepared exactly as described previously (6). Briefly, the cells were washed twice with phosphate-buffered saline and treated with lysis buffer (10 mM Tris [pH 7.4], 1% sodium dodecyl sulfate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride). The lysates were boiled immediately and sheared through a 25-gauge needle. In some cases, the cells were treated with wortmannin at a final concentration of 50 nM for 40 min before the samples were lysed. The amount of proteins was quantitated by using a bicinchoninic acid protein assay kit (Pierce) according to the manufacturer's instructions, and 50 μg of each sample was fractionated through a sodium dodecyl sulfate-polyacrylamide gel. The proteins were transferred to polyvinylidene difluoride membranes (Millipore) and then subjected to Western blotting with anti-Gag/v-Abl (H548) (7), anti-Akt1 PH domain (Upstate Biotechnology), anti-phospho-Akt serine 473 (Cell Signaling Technology), antiphosphotyrosine (Upstate Biotechnology), anti-p53 (Calbiochem), anti-Bcl-x (Santa Cruz), anti-Bcl-2 (Santa Cruz), anti-p19Arf (Novus), anti-Ras (Transduction Laboratories), antihemagglutinin (BABCO), and anti-β-actin (Sigma) antibodies. The proteins were visualized by using a chemiluminescence kit (Tropix).
Proliferation and apoptosis assays.
Cells were seeded at 106 cells/ml, maintained at 34 or 39.5°C, and counted by using a hemocytometer; viability was determined by trypan blue exclusion. Duplicate samples were counted at each time point, and each experiment was repeated three or more times. For cell cycle analysis, cells were suspended in 1.12% sodium citrate containing 2 mg of RNase A/ml and then mixed with an equal volume of 0.2% Triton X-100- 0.1% sodium citrate containing 100 μg of propidium iodide/ml (5). Samples were analyzed immediately by using a FACScan instrument (Becton Dickinson) and ModFit Software (Verity Software). To monitor apoptosis, cells were stained with propidium iodide or merocyanin 540, a dye that specifically stains apoptotic cells (35), and analyzed by flow cytometry; comparable results were obtained with both methods.
RESULTS
The expression of P120/R273K supports survival at the nonpermissive temperature.
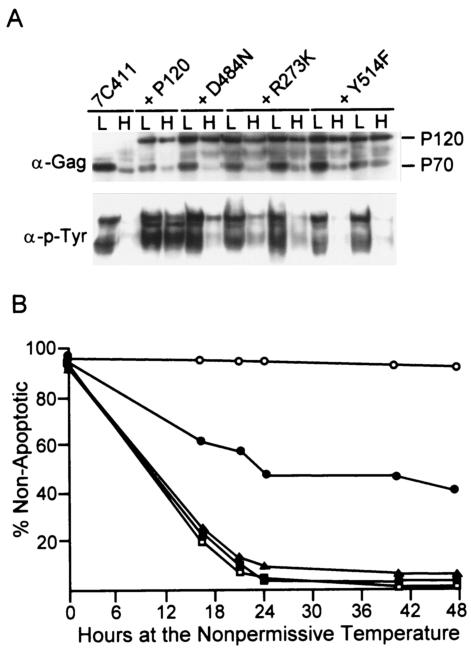
The P120/R273K mutant is highly compromised in its ability to transform pre-B cells from wild-type mice at 39.5°C and does not activate the MAP kinase pathway or stimulate growth when expressed in the ts 7C411 cell line at 39.5°C (24). To examine the effect of P120/R273K expression on the apoptotic response characteristics of pre-B cells transformed with a ts kinase domain mutant (5, 16), 7C411 cells were superinfected with P120/R273K or wild-type P120. Two other Ab-MLV mutants that do not transform pre-B cells at any temperature (24, 33; unpublished data)—P120/D484N, a kinase-negative strain, and P120/Y514F, a mutant that expresses a v-Abl protein in which a tyrosine that is strongly autophosphorylated (36) has been replaced by a phenylalanine—were used as additional controls. A minimum of five independent populations expressing the different viruses were selected at the permissive temperature by using the drug resistance marker present in the superinfecting virus. All of the samples, including the representatives shown (Fig. 1A), expressed v-Abl proteins of the expected sizes at both the permissive and the nonpermissive temperatures and grew equally well at the permissive temperature (data not shown). Immunofluorescence staining with an antibody that reacts with carboxyl-terminal determinants present in the P120 proteins but not in the P70 protein revealed that greater than 90% of the cells in these populations expressed the protein encoded by the superinfecting virus (data not shown). Despite this finding, all of the populations except those expressing P120 displayed reduced levels of tyrosine phosphorylation at the nonpermissive temperature (Fig. 1A).
FIG. 1.
P120/R273K suppresses apoptosis. 7C411 pre-B cells transformed with ts mutant P70/H590 (16) were superinfected with wild-type strain P120 or the P120/D484N, P120/R273K, or P120/Y514F mutant at the permissive temperature. Infected populations were selected with G418. (A) Lysates were prepared from cells maintained at the permissive temperature (L) or shifted to the nonpermissive temperature (H) for 20 h and analyzed by Western blotting with anti-Gag/v-Abl antibodies or antiphosphotyrosine antibodies. (B) 7C411 cells and superinfected populations were shifted to the nonpermissive temperature. Cell number and viability were monitored by counting trypan blue-stained cells with a hemocytometer over a 48-h period. The data shown are the average of results obtained from three independently derived cell populations analyzed in triplicate in two independent experiments. The values obtained varied by less than 10%. Symbols: ▴, 7C411 cells; □, 7C411 cells expressing P120/Y514F; ▪, 7C411 cells expressing P120/D484N; •, 7C411 cells expressing P120/R273K; ○, 7C411 cells expressing P120.
To assess the ability of mutants to rescue the apoptotic phenotype characteristics of cells transformed by ts kinase domain mutants (5), superinfected 7C411 cell populations were shifted to the nonpermissive temperature and monitored for survival (Fig. 1B). As expected (14), 7C411 cells expressing P120 maintained high viability at 39.5°C; those expressing kinase-inactive P120/D484N or P120/Y514F were similar to parental 7C411 cells and underwent rapid apoptosis at this temperature. In contrast, the apoptotic response was blunted in cells expressing P120/R273K; between 40 and 45% of the cells remained viable throughout the 2-day experiment. Although many of these cells survived, consistent with earlier experiments (14, 24), only cells superinfected with wild-type virus proliferated at the nonpermissive temperature (data not shown). Thus, the expression of P120/R273K suppresses the apoptotic response characteristic of cells transformed by ts kinase domain mutants at the nonpermissive temperature. These data indicate that even though P120/R273K cannot transform pre-B cells at 39.5°C (49), it transmits signals that are sufficient to suppress apoptosis.
Ras can suppress apoptosis at the nonpermissive temperature.
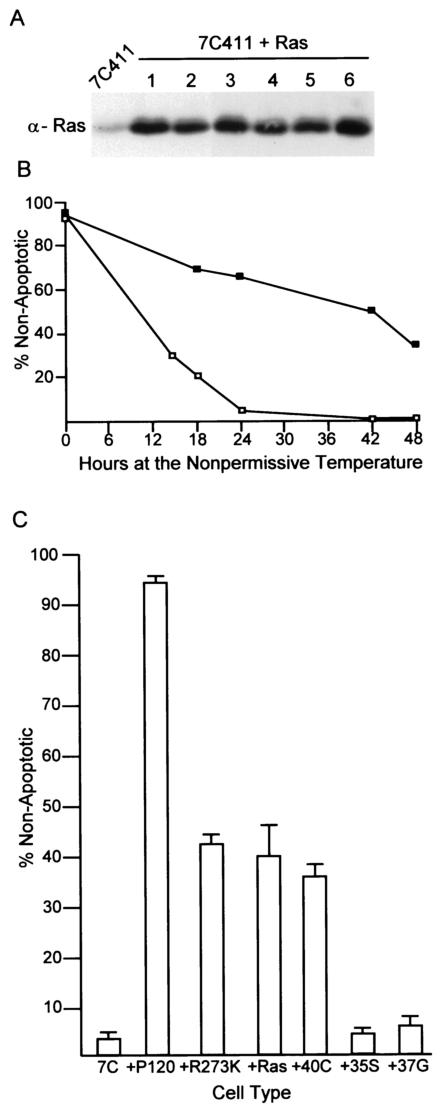
The Ras pathway is an important signaling pathway stimulated by v-Abl (41, 56). An earlier study (14) revealed that the expression of Ras was not sufficient to restore the growth of ts transformants at the nonpermissive temperature. However, Ras-mediated effects on cell survival were not investigated. To explore this avenue, populations of 7C411 cells expressing Ha-Ras were derived at the permissive temperature, and their survival following a shift to the nonpermissive temperature was analyzed (Fig. 2A and B). Like populations expressing P120/R273K, cells expressing Ras displayed enhanced survival at the nonpermissive temperature. Consistent with earlier experiments (14), the cells did not proliferate and accumulated in the G1 phase of the cell cycle (Table 1).
FIG. 2.
Ras suppresses apoptosis. 7C411 cells were infected with a retrovirus expressing Ha-Ras and plated at the permissive temperature in the presence of G418. (A) The expression of Ras was confirmed by Western analysis of whole-cell lysates with an anti-Ras antibody. (B) The cells were shifted to the nonpermissive temperature and monitored for survival over a 48-h period. The data shown are the average of results obtained with three independently derived clones analyzed in triplicate in two independent experiments. The values obtained varied by less than 10%. Symbols: □, 7C411 cells; ▪, 7C411 cells expressing Ras. (C) 7C411 (7C) cells were transfected with plasmids expressing the Ras effector mutants Ras(12V, 40C), Ras(12V, 35S), and Ras(12V, 37G) (22, 52). The cells were maintained at the permissive temperature in the presence of G418, and populationsconfirmed by Western analysis to express the Ras effector mutants were shifted to the nonpermissive temperature for 24 h. Survival was analyzed by flow cytometric analysis of merocyanin 540-stained cells. Five different populations of each type were examined; error bars indicate standard deviations.
TABLE 1.
7C411 cells expressing Ras or activated Akt undergo G1 arrest at 39.5°Ca
| Cell line and protein | Temp (°C) | % of cells in: | ||
|---|---|---|---|---|
| G1 | S | G2/M | ||
| 7C411 | 34 | 43 | 43 | 15 |
| 39.5 | 84 | 9 | 6 | |
| 7C411 + P120 | 34 | 50 | 35 | 15 |
| 39.5 | 61 | 34 | 5 | |
| 7C411 + P120/R273K | 34 | 44 | 39 | 17 |
| 39.5 | 83 | 10 | 6 | |
| 7C411 + Ras | 34 | 40 | 54 | 6 |
| 39.5 | 92 | 8 | <1 | |
| 7C411 + Myr-Akt | 34 | NT | NT | NT |
| 39.5 | 89 | 9 | 2 |
Signals from Ras to PI3-K stimulate survival.
Ras can stimulate multiple downstream signaling elements. Partial loss-of-function mutants of Ras—including Ras(12V, 35S), which activates the Raf/MAP kinase pathways (2), Ras(12V, 37G), which interacts with Ral-GDS (2), and Ras(12V, 40C), which activates PI3-K (22)—can be used to distinguish the effects of the different Ras effector pathways (26). A panel of 7C411 cells expressing each of these forms of Ras was isolated and tested for survival at the nonpermissive temperature. Like 7C411 cells expressing wild-type Ras, those expressing Ras(12V, 40C), which activates the PI3-K pathway, displayed extended survival at the nonpermissive temperature (Fig. 2C). The expression of either Ras(12V, 35S) or Ras(12V, 37G) had no effect on survival and, as expected, none of the cells expressing any of the Ras forms proliferated at the nonpermissive temperature (data not shown). These data demonstrate that signals transmitted from Ras via the MAP kinase or Ral pathway alone are not sufficient to sustain the viability of cells, while those transmitted via the PI3-K pathway can extend cell survival.
Akt remains active in cells expressing P120/R273K and can suppress apoptosis.
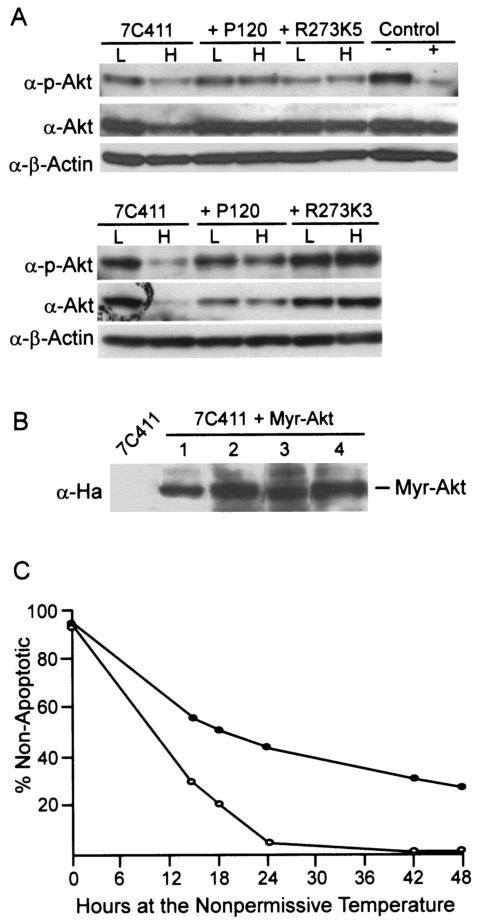
Akt is a key intermediate downstream of Ras and the PI3-K pathway that is important in mediating the survival of cells transformed by several forms of abl oncogenes (25, 38, 43). To determine whether active Akt correlates with cell survival at the nonpermissive temperature, parental 7C411 cells and populations expressing P120 or P120/R273K were maintained at the permissive temperature or shifted to the nonpermissive temperature for 30 h. Western blot analysis revealed that all of the samples prepared at the permissive temperature expressed phosphorylated Akt (Fig. 3A), a modification that correlates with enzyme activity (23). When 7C411 cells were maintained at the nonpermissive temperature, the level of Akt decreased and less phosphorylated Akt was recovered. In contrast, cells expressing P120 and P120/R273K expressed similar levels of Akt at both temperatures, and the amount of phosphorylated Akt was unaffected by incubation at 39.5°C. These data demonstrate that the P120/R273K mutant retains the ability to signal to Akt at the nonpermissive temperature and suggest that Akt may be required for the extended survival observed in cells expressing this mutant.
FIG. 3.
Akt remains active in cells expressing R273K and can enhance survival. (A) Whole-cell lysates were prepared from parental ts cells and superinfected populations maintained at the permissive temperature (L) or at the nonpermissive temperature (H) for 30 h. Western blots were probed with anti-phospho-Akt or anti-Akt antibodies. P120/R273K5 and P120/R273K3 are independently derived populations of superinfected cells. Lysates from 7C411 cells that were treated with 50 nM wortmannin (+) or mock treated (−) for 40 min prior to lysis are shown in the control lanes. (B) 7C411 cells were superinfected with a retrovirus expressing Myr-Ha-Akt, selected with puromycin, and analyzed for expression of the introduced Akt by probing of a Western blot with an antihemagglutinin antibody. (C) 7C411 cells and populations expressing Myr-Akt were shifted to the nonpermissive temperature and monitored for survival over a 48-h period. The data shown are the average of results obtained with three independently derived populations analyzed in triplicate in two independent experiments. The values obtained varied by less than 10%. Symbols: ○, 7C411; •, 7C411 expressing Myr-Akt.
If the expression of activated Akt plays a critical role in suppressing the apoptotic response in Ab-MLV-transformed cells, 7C411 cells expressing a constitutively active form of the protein should be phenotypically similar to 7C411 cells expressing P120/R273K. To test this idea, 7C411 cells were infected with a retroviral vector expressing a constitutively active, myristoylated form of Akt (17), and populations expressing this protein were selected at the permissive temperature (Fig. 3B). When these cells were shifted to the nonpermissive temperature, 30 to 40% of the cells survived during the 48-h experiment (Fig. 3C). However, consistent with the phenotype of R273K-expressing cells (24), the surviving cells were arrested in the G1 phase of the cell cycle (Table 1).
Bcl-2 and Bcl-x levels show a modest decrease or no decrease at the nonpermissive temperature.
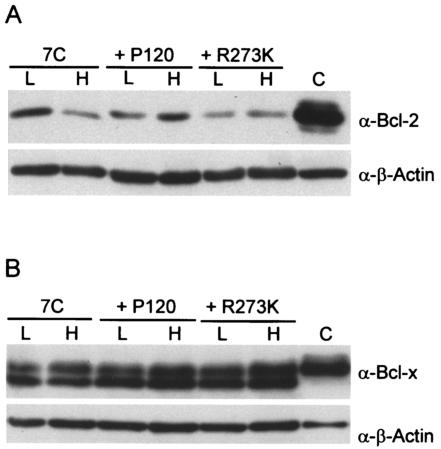
The ability of Akt to foster the survival of ts transformants suggests that Akt is a critical intermediate in the v-Abl survival pathway. Akt can affect survival mediated by Bcl-2 family members (3, 11, 12), and the expression of several forms of abl oncogenes increases the levels of antiapoptotic members of the Bcl-2 family (1, 2, 4, 39, 46, 53). In addition, the overexpression of either Bcl-2 or Bcl-x enhances the survival of v-Abl-transformed cells when the kinase is inactivated (6, 28). To determine whether changes in Bcl-2 and Bcl-x levels correlated with cell survival, cell lysates were examined by Western blotting. Bcl-2 levels decreased severalfold when 7C411 cells were incubated at the nonpermissive temperature but did not decrease in 7C411 cells expressing either P120 or P120/R273K (Fig. 4A). These data could indicate that this decrease in Bcl-2 levels contributes to apoptosis in 7C411 cells. However, the levels of Bcl-2 recovered in 7C411 cells at the nonpermissive temperature are similar to those observed in 7C411 cells expressing P120/R273K at 39.5°C, suggesting that this decrease may not be functionally important in the apoptotic response. Bcl-x levels were similar at both the permissive and the nonpermissive temperatures (Fig. 4B), indicating that the loss of v-Abl function does not affect the expression of this protein, at least during the time period examined. These data suggest that changes in these Bcl-2 family proteins probably do not play a dominant role in determining cell survival at the nonpermissive temperature.
FIG. 4.
The levels of Bcl-2 and Bcl-x do not change when cells are shifted to the nonpermissive temperature. Whole-cell lysates were prepared from 7C411 cells and 7C411 cells expressing either P120 or P120/R273K and maintained at the permissive temperature (L) or incubated at the nonpermissive temperature (H) for 20 h. Lysates were analyzed by Western blotting with anti-Bcl-2 (A) and anti-Bcl-x (B) antibodies. Lanes C contain a lysate from Bal17, a pre-B-cell line known to express readily detectable Bcl-2 (A), and a lysate from 293T cells transfected with human Bcl-x (B).
7C411 cells retain wild-type p53.
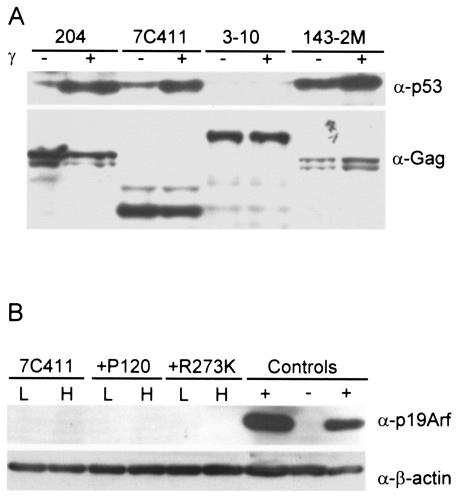
Another target of Akt is the p53 regulatory protein Mdm2; phosphorylation of Mdm2 by Akt increases the ability of Mdm2 to target p53 for degradation, thereby interfering with p53 activity (18). About half of all pre-B cells fully transformed by Ab-MLV express wild-type p53 (47). To determine whether 7C411 cells retain wild-type p53, the response of the cells to gamma irradiation was examined. Ab-MLV-transformed cells that retain wild-type p53 respond to gamma irradiation by undergoing rapid apoptosis, an event that is characterized by the stabilization of p53 (47). When 7C411 cells were irradiated, >90% of the treated cells underwent rapid apoptosis. In addition, steady-state levels of p53 increased markedly in the cells following irradiation (Fig. 5A). These data indicate that 7C411 cells retain a functional, wild-type p53 protein.
FIG. 5.
7C411 cells retain wild-type p53. (A) 7C411 cells were treated with 1,100 rads of gamma irradiation or mock treated and lysed 4 h later. Lysates were analyzed by Western blotting with an anti-p53 antibody and an anti-Gag/v-Abl antibody. Control samples included 204, an Ab-MLV-transformed cell line that expresses wild-type p53 (47); 3-10, an Ab-MLV-transformed cell line prepared from _p53_-null mice; and 143-2M, an Ab-MLV-transformed cell line that expresses mutant p53 (47). The 204 and 143-2M cell lines were transformed with Ab-MLV P120, the _p53_-null cell line was transformed with Ab-MLV P160, and the 7C411 cell line was transformed with Ab-MLV P70/H590. (B) Lysates from 7C411 cells and populations expressing either P120 or P120/R273K were incubated at the permissive temperature (L) or shifted to the nonpermissive temperature (H) for 30 h. Lysates were analyzed by Western blotting with anti-p19Arf and anti-β-actin antibodies. +, lysates from two cell lines that express abundant p19Arf; −, lysate from an Ink4a/_Arf_-null transformant (32).
Although Akt activity influences p53 activity (18), the p19Arf protein is known to be a critical intermediate in regulating p53 responses during Ab-MLV transformation (32). Fully transformed cell lines that retain wild-type p53 usually have down-modulated endogenous p19Arf expression, but because the reexpression of p19Arf is sufficient to induce apoptosis in transformants expressing wild-type p53 (32), we examined the possibility that the inactivation of v-Abl in 7C411 cells allows the reexpression of endogenous p19Arf. However, Western blotting of cell lysates prepared from these cells and populations expressing P120 or P120/R273K with anti-p19Arf antibodies demonstrated that p19Arf was not detected in any of these cells at either the permissive or the nonpermissive temperature (Fig. 5B). Thus, p19Arf does not play a role in the apoptotic response characteristics of cells in which the v-Abl kinase has been inactivated by a temperature shift.
_p53_-null cells transformed by a ts kinase domain mutant do not undergo rapid apoptosis at the nonpermissive temperature.
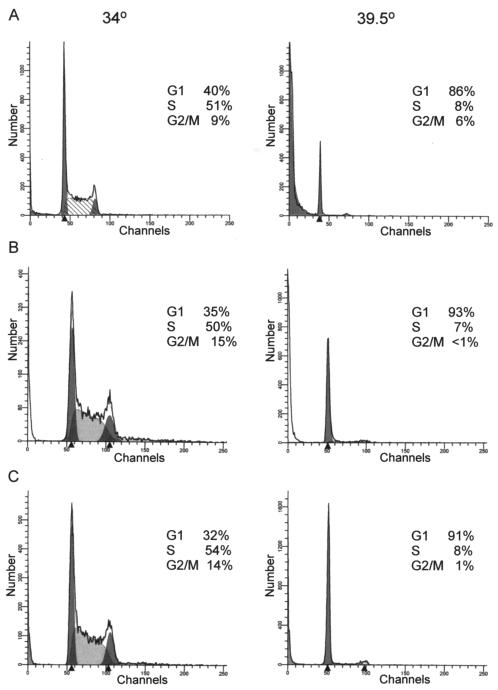
Several observations suggest that p53 function could be important for rapid apoptosis when ts cells are shifted to the nonpermissive temperature. To test this possibility directly, ts cell lines were prepared from _p53_-null mice with the ts Ab-MLV mutant P120/G+H (16) and maintained at 34°C. The P120/G+H mutant was used because it transforms pre-B cells more readily than P70/H590, the mutant used to generate 7C411 cells; cells transformed by both viruses are similar (16). Cells then were shifted to 39.5°C, and growth and survival were examined. Cell cycle analysis revealed that all five _p53_-null cell lines transformed with the ts virus, including the representatives shown (Fig. 6), underwent G1 arrest at the nonpermissive temperature but displayed lower levels of apoptosis than ts transformants that retained functional p53 (Table 2). These data suggest that functional p53 contributes to apoptosis in ts transformants. When considered in the context of the results of experiments examining Akt and p19Arf, the data also indicate that p53 is activated in an Arf-independent but Akt-dependent fashion in ts cells maintained at the nonpermissive temperature.
FIG. 6.
ts transformants from _p53_-null mice undergo G1 arrest at the nonpermissive temperature. 7C411 cells (A) or two independently derived transformed cell lines derived by infecting _p53_-null bone marrow with ts kinase mutant P120/G+H (B and C) were maintained at the permissive temperature (34°C) or shifted to the nonpermissive temperature (39.5°C) for 21 h. The cells were stained with propidium iodide and analyzed by flow cytometry. The results shown are representative of analyses of at least eight independently derived ts transformants for wild-type mice and five independently derived _p53_-null ts cell lines.
TABLE 2.
ts transformants from _p53_-null mice display extended survival at 39.5°Ca
| Cell line | Virus | p53 status | Temp (°C) | % Apoptotic |
|---|---|---|---|---|
| U2-1 | Wild type | Null | 34 | 10 |
| 39.5 | 9 | |||
| 7C411 | ts | Wild type | 34 | 10 |
| 39.5 | 94 | |||
| U8-1 | ts | Null | 34 | 14 |
| 39.5 | 44 | |||
| U35-1 | ts | Null | 34 | 7 |
| 39.5 | 37 | |||
| U35-2 | ts | Null | 34 | 8 |
| 39.5 | 32 |
DISCUSSION
Our study reveals that functional p53 contributes to apoptosis when the v-Abl protein is inactivated in cells transformed by a ts kinase domain mutant. These data suggest that v-Abl suppresses the function of wild-type p53 in transformants that become fully established and retain wild-type p53, expanding the ways in which p53 affects Ab-MLV-transformed cells. Depending on the stage of the transformation process, different pathways play dominant roles in the p53 response. Growth signals passing through the MAP kinase pathway and c-Myc appear to be important for the p53-mediated effects that have an impact on primary transformation (49, 55), and the p19Arf regulatory pathway plays a central role during the crisis phase (32, 48). In fully transformed cells, sustained signaling via the PI3-K/Akt pathway is sufficient to suppress apoptosis. These effects likely reflect the ability of Akt to regulate p53 through effects on Mdm2 (18). Alternatively, several pathways may be involved in apoptosis suppression, including some that may function in a p53-independent fashion. Nonetheless, unmasking the effects of p53 in fully transformed cells suggests that interfering with pathways that suppress p53 function at any stage of the transformation process will have a significant impact on tumorigenesis.
The ts complementation assay can be used to reveal functions retained by forms of Abl under conditions where the mutants cannot induce transformation and allows a comparison of different mutants and other oncogenes in a uniform genetic background. The effects of P120/R273K on survival revealed by this assay illustrate this point. P120/R273K does not transform normal pre-B cells at 39.5°C and fails to activate the MAP kinase pathway at this temperature (24, 49); the latter pathway is required for the growth of transformed pre-B cells but is not essential for their survival (unpublished data). However, signals from the mutant are sufficient to sustain Akt activation and reveal a survival phenotype that is not evident in primary transformation assays. Consistent with these findings, activated Akt is not sufficient to transform pre-B cells (unpublished data), even though signals transmitted via Akt participate in both stimulating growth and blocking apoptosis in a variety of systems (reviewed in references 10 and 34). Indeed, the requirement for Akt activation by other forms of abl oncogenes (43-45) may reflect the ability of active Akt to suppress apoptosis.
Several investigators have shown that active forms of Abl increase the expression of different antiapoptotic Bcl-2 family members in interleukin 3 (IL-3)- or IL-7-dependent cells (1, 2, 4, 39, 46, 53). In these instances, activated Abl replaces signals normally provided by the cytokine, and these pathways may not be identical to those used for the transformation of primary pre-B cells. Consistent with this idea, IL-3 does not support the survival of ts transformants at the nonpermissive temperature (unpublished data). Nonetheless, modest decreases in the levels of Bcl-2 were observed when 7C411 cells were shifted to the nonpermissive temperature, and the overexpression of Bcl-2 or Bcl-x in transformants following v-Abl inactivation does suppress apoptosis (5, 28). Because apoptosis suppression is not complete in ts cells expressing P120/R273K or in those expressing Ras or Akt, some circuits that normally contribute to cell survival likely either are missing or are incompletely activated under these circumstances, and some of the missing signals may have an impact on Bcl-2 family member function.
The analysis of P120/R273K revealed that a fully functional SH2 domain is not required for v-Abl-mediated signaling to Akt. However, the pathway by which Akt activation is sustained in cells expressing the mutant is not fully clear. P120/R273K may signal to Akt via Ras. Ras can stimulate PI3-K and Akt, and experiments with Ras(12V, 40C) revealed that a signal delivered in this fashion is sufficient to promote survival. However, P120/R273K transmits reduced signals to Ras and does not promote the phosphorylation of Erk at the nonpermissive temperature (24). Nonetheless, reduced signaling to Ras may be sufficient to stimulate PI3-K-mediated signaling. Alternatively, P120/R273K may not affect survival via this circuit and may signal directly through PI3-K or through effects mediated via protein kinase C family members (4, 30). Because both activated Akt and the Ras alleles used here were expressed at levels higher than those normally present in cells, it is also possible that molecules not normally targeted by these proteins can be activated in cells. Additional analyses with the ts complementation assay should help to uncover the pathway(s) involved.
The expression of the Y514F mutant does not affect the growth or survival of 7C411 cells at the nonpermissive temperature. Although this mutant transforms NIH 3T3 cells, it does not transform pre-B cells (unpublished data). Thus, mutations affecting this autophosphorylation site dramatically reduce the ability of the protein to transmit downstream signals. Bcr/Abl alleles containing the analogous mutation are deficient in fibroblast transformation but restore factor-independent growth of IL-3-dependent hematopoietic cells (8, 31). These data may reflect inherent differences in the pathways used by Bcr/Abl and v-Abl or differences in the transformation of primary pre-B cells and a factor-dependent cell line. These differences also may explain the ability of Bcr/Abl alleles lacking SH2 function to replace signals normally provided by IL-3 in factor-dependent hematopoietic cells (8, 20).
The expression of activated Akt or P120/R273K or a loss of p53 failed to rescue all of the ts transformants from apoptosis, suggesting that these are not the only pathways that are important for cell survival. Such pathways may intersect with those studied here or may function independently. Rescue experiments in which additional intermediates are introduced into partially protected cells might reveal additive effects and help to determine whether the apoptosis suppression occurs in a linear fashion. In addition, neither Akt nor P120/R273K stimulated growth at 39.5°C, and _p53_-null transformants did not grow in the absence of an active v-Abl protein. Thus, none of these signals can replace those provided by v-Abl. The expression of c-Myc, another critical intermediate in the v-Abl transformation pathway (40), is not maintained at the nonpermissive temperature under circumstances where the cells survive but do not grow (24), suggesting that this molecule plays a more critical role in growth than in survival. However, like that of the other proteins discussed here, the expression of c-Myc in the ts 7C411 cell line does not restore growth at the nonpermissive temperature (14). When considered as a whole, these data suggest that the activation of multiple pathways, including those that play a dominant role in stimulating growth and others that play a dominant role in suppressing apoptosis, is required for Ab-MLV-mediated transformation.
Acknowledgments
We thank Henry Wortis and Allen Parmelee for assistance with flow cytometry and Charles Sawyers, Philip Tsichlis, and Larry Feig for several of the reagents used in this study.
I.U. was supported by a fellowship from the Leukemia and Lymphoma Society. This work was supported by grant CA 33771 from the National Cancer Institute.
REFERENCES
- 1.Amarante-Mendes, G. P., A. J. McGahon, W. K. Nishioka, D. E. Afar, O. N. Witte, and D. R. Green. 1998. Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-xL. Oncogene 16**:**1383-1390. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, A., and P. Rothman. 1998. IL-7 reconstitutes multiple aspects of v-Abl-mediated signaling. J. Immunol. 161**:**4611-4617. [PubMed] [Google Scholar]
- 3.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96**:**857-868. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Q., J. Turner, A. J. Watson, and C. Dive. 1997. v-Abl protein tyrosine kinase (PTK) mediated suppression of apoptosis is associated with the up-regulation of Bcl-xL. Oncogene 15**:**2249-2254. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Y. Y., and N. Rosenberg. 1992. Lymphoid cells transformed by Abelson virus require the v-abl protein tyrosine kinase only during early G1. Proc. Natl. Acad. Sci. USA 89**:**6683-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y.-Y., L. C. Wang, M. S. Huang, and N. Rosenberg. 1994. An active v-abl protein tyrosine kinase blocks immunoglobulin light-chain gene rearrangement. Genes Dev. 8**:**688-697. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro, B., K. Wehrly, M. Cloyd, W. Britt, J. Portis, J. Collins, and J. Nishio. 1981. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology 112**:**131-144. [DOI] [PubMed] [Google Scholar]
- 8.Cortez, D., L. Kadlec, and A. M. Pendergast. 1995. Structural and signaling requirements for BCR-ABL-mediated transformation and inhibition of apoptosis. Mol. Cell. Biol. 15**:**5531-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez, D., G. Reuther, and A. M. Pendergast. 1997. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene 15**:**2333-2342. [DOI] [PubMed] [Google Scholar]
- 10.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13**:**2905-2927. [DOI] [PubMed] [Google Scholar]
- 11.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91**:**231-241. [DOI] [PubMed] [Google Scholar]
- 12.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin 3-induced phosphorylation of BAD through the protein kinase Akt. Science 278**:**687-689. [DOI] [PubMed] [Google Scholar]
- 13.DuBridge, R. B., P. Tang, H. C. Hsai, P.-M. Leong, J. H. Miller, and M. P. Calos. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 7**:**379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman, A., and N. Rosenberg. 1990. bcr/abl and src but not myc and ras replace v-abl in lymphoid transformation. Mol. Cell. Biol. 10**:**4365-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman, A., and N. Rosenberg. 1987. Isolation of temperature-sensitive Abelson virus mutants by site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 84**:**8021-8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelman, A., and N. Rosenberg. 1990. Temperature-sensitive mutants of Abelson murine leukemia virus deficient in protein tyrosine kinase activity. J. Virol. 64**:**4242-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franke, T. F., S. I. Yang, T. O. Chan, K. Datta, A. Kazlauskas, D. K. Morrison, D. R. Kaplan, and P. N. Tsichlis. 1995. The protein kinase encoded by Akt proto-oncogene is a target of the PDGF activated PI 3-kinase. Cell 81**:**727-736. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb, T. M., J. F. M. Leal, R. Seger, Y. Taya, and M. Oren. 2002. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene 21**:**1299-1303. [DOI] [PubMed] [Google Scholar]
- 19.Hawley, R. G., F. H. Lieu, A. Z. Fong, and T. S. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1**:**136-138. [PubMed] [Google Scholar]
- 20.Ilaria, R. L. J., and R. A. Van Etten. 1995. The SH2 domain of P210BCR/ABL is not required for the transformation of hematopoietic factor-dependent cells. Blood 86**:**3897-3904. [PubMed] [Google Scholar]
- 21.Jenab-Wolcott, J., D. Rodriguez-Correa, A. Reitmair, T. Mak, and N. Rosenberg. 2000. The absence of Msh2 alters Abelson virus pre-B-cell transformation by influencing p53 mutation. Mol. Cell. Biol. 20**:**8373-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joneson, T., M. A. White, M. H. Wigler, and D. Bar-Sagi. 1996. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science 271**:**810-812. [DOI] [PubMed] [Google Scholar]
- 23.Kohn, A. D., F. Takeuchi, and R. A. Roth. 1996. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J. Biol. Chem. 271**:**21920-21926. [DOI] [PubMed] [Google Scholar]
- 24.Mainville, C., K. Parmar, I. Unnikrishnan, G. D. Raffel, L. Gong, and N. Rosenberg. 2001. Alteration of the FLVRES motif in the v-Abl SH2 domain confers a temperature-sensitive transformation phenotype. J. Virol. 75**:**1816-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majewski, M., M. Nieborowska-Skorska, P. Salomoni, A. Slupianek, K. Reiss, R. Trotta, B. Calabretta, and T. Skorski. 1999. Activation of mitochondrial Raf-1 is involved in the antiapoptotic effects of Akt. Cancer Res. 59**:**2815-2819. [PubMed] [Google Scholar]
- 26.Marshall, C. 1996. Ras effectors. Curr. Opin. Cell Biol. 8**:**197-204. [DOI] [PubMed] [Google Scholar]
- 27.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18**:**3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muljo, S. A., and M. S. Schlissel. 2003. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat. Immunol. 4**:**31-37. [DOI] [PubMed] [Google Scholar]
- 29.Muller, A. J., J. C. Young, A. M. Pendergast, M. Pondel, N. R. Landau, D. R. Littman, and O. N. Witte. 1991. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol. Cell. Biol. 11**:**1785-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen, P. J., P. Musk, C. A. Evans, and A. D. Whetton. 1993. Cellular signaling events elicated by v-abl associated with growth factor independence in an interluekin-3-dependent cell line. J. Biol. Chem. 268**:**15696-15703. [PubMed] [Google Scholar]
- 31.Pendergast, A. M., M. L. Gishizky, M. H. Havlik, and O. N. Witte. 1993. SH1 domain autophosphorylation of P210 BCR/ABL is required for transformation but not growth factor independence. Mol. Cell. Biol. 13**:**1728-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radfar, A., I. Unnikrishnan, H.-W. Lee, R. A. DePinho, and N. Rosenberg. 1998. p19Arf induces p53-dependent apoptosis during Abelson virus-mediated pre-B cell transformation. Proc. Natl. Acad. Sci. USA 95**:**13194-13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raffel, G. D., K. Parmar, and N. Rosenberg. 1996. In vivo association of v-Abl with Shc mediated by a non-phosphotyrosine-dependent SH2 interaction. J. Biol. Chem. 271**:**4640-4645. [DOI] [PubMed] [Google Scholar]
- 34.Rameh, L. E., and L. C. Cantley. 1999. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274**:**8347-8350. [DOI] [PubMed] [Google Scholar]
- 35.Reid, S., R. Cross, and E. Snow. 1996. Combined Hoechst 33342 and merocyanin 540 staining to examine murine B cell cycle stage, viability and apoptosis. J. Immunol. Methods 192**:**43-54. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds, F. H., Jr., S. Oroszlan, and J. R. Stephenson. 1982. Abelson murine leukemia virus P120: identification and characterization of tyrosine phosphorylation sites. J. Virol. 44**:**1097-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg, N., and O. N. Witte. 1988. The viral and cellular forms of the Abelson (abl) oncogene. Adv. Virus Res. 35**:**39-81. [DOI] [PubMed] [Google Scholar]
- 38.Salomoni, P., M. A. Wasik, R. F. Riedel, K. Reiss, J. K. Choi, T. Skorski, and B. Calabretta. 1998. Expression of constitutively active Raf-1 in the mitochondria restores antiapoptotic and leukemogenic potential of a transformation-deficient BCR/ABL mutant. J. Exp. Med. 187**:**1995-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Garcia, I., and G. Grutz. 1995. Tumorigenic activity of the BCR-ABL oncogenes is mediated by BCL2. Proc. Natl. Acad. Sci. USA 92**:**5287-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawyers, C. L., W. Callahan, and O. N. Witte. 1992. Dominant negative myc blocks transformation by abl oncogenes. Cell 70**:**901-910. [DOI] [PubMed] [Google Scholar]
- 41.Sawyers, C. L., J. McLaughlin, and O. N. Witte. 1995. Genetic requirement for ras in the transformation of fibroblasts and hematopoietic cells by the bcr-abl oncogene. J. Exp. Med. 181**:**307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherr, C. J. 2001. The Ink4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2**:**731-737. [DOI] [PubMed] [Google Scholar]
- 43.Skorski, T., A. Bellacosa, M. Nieborowska-Skorska, M. Majewski, R. Martinez, J. K. Choi, R. Trotta, P. Wlodarski, D. Perrotti, T. O. Chan, M. A. Wasik, P. N. Tsichlis, and B. Calabretta. 1997. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3K/Akt-dependent pathway. EMBO J. 16**:**6151-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skorski, T., P. Kanakaraj, M. R. Neiborowska-Skorska, M. Z., S. C. Wen, G. Zon, A. M. Gerwitz, P. Perussia, and B. Calabretta. 1995. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for growth of Philadelphia chromosome-positive cells. Blood 2**:**726-736. [PubMed] [Google Scholar]
- 45.Skorski, T., M. Nieborowska-Skorska, C. Szcylik, P. Kanakaraj, D. Perrotti, G. Zon, A. Gerwirtz, B. Perussia, and B. Calabretta. 1995. c-Raf-1 serine/threonine kinase is required in BCR/ABL-dependent and normal hamatopoiesis. Cancer Res. 55**:**2275-2278. [PubMed] [Google Scholar]
- 46.Tang, X., C. P. Downes, A. D. Whetton, and P. J. Owen-Lych. 2000. Role of phosphatidylinositol 3-kinase and specific protein kinase B isoforms in the suppression of apoptosis mediated by the Abelson protein-tyrosine kinase. J. Biol. Chem. 275**:**13142-13148. [DOI] [PubMed] [Google Scholar]
- 47.Thome, K., A. Radfar, and N. Rosenberg. 1997. Mutation of Tp53 contributes to the malignant phenotype of Abelson virus-transformed lymphoid cells. J. Virol. 77**:**8149-8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unnikrishnan, I., A. Radfar, J. Jenab-Wolcott, and N. Rosenberg. 1999. p53 mediates apoptotic crisis in primary Abelson murine leukemia virus-transformed pre-B cells. Mol. Cell. Biol. 19**:**4825-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unnikrishnan, I., and N. Rosenberg. 2003. Absence of p53 complements defects in Abelson murine leukemia virus signaling. J. Virol. 77**:**6208-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Parijs, L., Y. Refaeli, J. D. Lord, B. H. Nelson, A. K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation induced cell death. Immunity 11**:**281-288. [DOI] [PubMed] [Google Scholar]
- 51.Weissinger, E. M., G. Eissner, C. Grammer, A. Fackler, B. Haefner, L. S. Yoon, K. S. Lu, A. Bazarov, J. M. Sedivy, H. Mischak, and W. Kolch. 1997. Inhibition of the Raf-1 kinase by cyclin AMP agonists causes apoptosis of v-_abl_-transformed cells. Mol. Cell. Biol. 17**:**3229-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White, Q. P., C. Nicolette, A. Minden, A. Polverino, L. Vanaelst, M. Karin, and M. H. Wigler. 1995. Multiple ras functions can contribute to mammalian-cell transformation. Cell 80**:**533-541. [DOI] [PubMed] [Google Scholar]
- 53.Zhu, J., P. M. Nabissa, B. Hoffman, D. A. Liebermann, and S. K. Shore. 1996. Activated abl oncogenes and apoptosis: differing response of transformed myeloid progenitor cells. Blood 87**:**4368-4375. [PubMed] [Google Scholar]
- 54.Zou, X., and K. Calame. 1999. Signaling pathways activated by oncogenic forms of Abl tyrosine kinase. J. Biol. Chem. 274**:**18141-18144. [DOI] [PubMed] [Google Scholar]
- 55.Zou, X., F. Cong, M. Coutts, G. Cattoretti, S. P. Goff, and K. Calame. 2000. p53 deficiency increases transformation by v-Abl and rescues the ability of a C-terminally truncated v-Abl mutant to induce pre-B lymphoma in vivo. Mol. Cell. Biol. 20**:**628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou, X., S. Rudchenko, K.-K. Wong, and K. Calame. 1997. Induction of c-myc transcription by the v-Abl tyrosine kinase requires Ras, Raf1, and cyclin-dependent kinases. Genes Dev. 11**:**654-662. [DOI] [PubMed] [Google Scholar]