Effects of vildagliptin versus sitagliptin, on cardiac function, heart rate variability and mitochondrial function in obese insulin-resistant rats (original) (raw)
Abstract
Background and Purpose
Long-term high-fat diet (HFD) consumption has been shown to cause insulin resistance, which is characterized by hyperinsulinaemia with metabolic inflexibility. Insulin resistance is associated with cardiac sympathovagal imbalance, cardiac dysfunction and cardiac mitochondrial dysfunction. Dipeptidyl peptidase-4 (DPP-4) inhibitors, vildagliptin and sitagliptin, are oral anti-diabetic drugs often prescribed in patients with cardiovascular disease. Therefore, in this study, we sought to determine the effects of vildagliptin and sitagliptin in a murine model of insulin resistance.
Experimental Approach
Male Wistar rats weighing 180–200 g, were fed either a normal diet (20% energy from fat) or a HFD (59% energy from fat) for 12 weeks. These rats were then divided into three subgroups to receive vildagliptin (3 mg·kg−1·day−1), sitagliptin (30 mg·kg−1·day−1) or vehicle for another 21 days. Metabolic parameters, oxidative stress, heart rate variability (HRV), cardiac function and cardiac mitochondrial function were determined.
Key Results
Rats that received HFD developed insulin resistance characterized by increased body weight, plasma insulin, total cholesterol and oxidative stress levels along with a decreased high-density lipoprotein (HDL) level. Moreover, cardiac dysfunction, depressed HRV, cardiac mitochondrial dysfunction and cardiac mitochondrial morphology changes were observed in HFD rats. Both vildagliptin and sitagliptin decreased plasma insulin, total cholesterol and oxidative stress as well as increased HDL level. Furthermore, vildagliptin and sitagliptin attenuated cardiac dysfunction, prevented cardiac mitochondrial dysfunction and completely restored HRV.
Conclusions and Implications
Both vildagliptin and sitagliptin share similar efficacy in cardioprotection in obese insulin-resistant rats.
Keywords: sitagliptin, vildagliptin, high-fat diet, insulin resistance, cardiac function
Introduction
Long-term high-fat diet (HFD) consumption is known to cause insulin resistance (Pratchayasakul et al., 2011; Pipatpiboon et al., 2012). Insulin resistance is a risk factor for developing cardiac sympathovagal dysregulation (Pongchaidecha et al., 2009), cardiac dysfunction (Ouwens et al., 2005) and cardiac mitochondrial dysfunction (Dong et al., 2007). Dipeptidyl peptidase-4 (DPP-4) inhibitors, including vildagliptin and sitagliptin, are oral anti-diabetic drugs that inhibit the DPP-4 enzyme, resulting in a prolonged action of the glucagon-like peptide-1 (GLP-1) hormone. GLP-1 is an incretin hormone secreted from intestinal L-cells. Several studies have reported that GLP-1 possesses beneficial effects resulting in decreased plasma glucose levels and improved cardiac function in clinical studies and animal models (Buse et al., 2004; Bose et al., 2005; Poornima et al., 2008). Moreover, DPP-4 inhibitors show a beneficial effect on metabolic parameters and the heart (Ahren et al., 2004; Lenski et al., 2011; Apaijai et al., 2012). Clinical and animal studies have reported that vildagliptin and sitagliptin can increase plasma insulin and decrease glucose levels in type 2 diabetes models (Mari et al., 2005; Tremblay et al., 2011). Moreover, vildagliptin shows a cardioprotective effect in the hearts of swine (Chinda et al., 2012) and insulin-resistant rats (Apaijai et al., 2012) and sitagliptin prevents cardiac fibrosis in diabetic mice (Lenski et al., 2011). Although the effects of the two DPP-4 inhibitors, vildagliptin and sitagliptin, have been studied, their comparative effects on metabolic parameters as well as cardiac function and mitochondrial function of HFD-induced insulin-resistant rats are still unclear.
This study aimed to determine the effects of vildagliptin and sitagliptin on metabolic parameters, oxidative stress levels, heart rate (HR) variability (HRV), cardiac function, cardiac mitochondrial function and cardiac mitochondrial morphology in long-term HFD consumption induced insulin-resistant rats. We hypothesized that vildagliptin and sitagliptin would improve metabolic parameters and prevent an increase in oxidative stress levels, preserve HRV and cardiac function, and cardiac mitochondrial function in HFD-induced insulin-resistant rats.
Methods
Animals and diet
All experiment protocols in this study were approved by the Faculty of Medicine, Chiang Mai University Institutional Animal Care and Use Committee, in compliance with NIH guidelines, and in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). Thirty-six male Wistar rats, weighing 180–200 g, were obtained from the National Animal Center, Salaya Campus, Mahidol University, Thailand. Rats were housed in a 12 h light/dark cycle with controlled temperature (25°C). All rats were allowed to acclimate for 7 days and then divided into either the normal-diet (ND) group, fed the standard laboratory pelleted diet containing 20% energy from fat or HFD group fed a diet containing 59% energy from fat. The rats were fed with their respective diets for 12 weeks (Pratchayasakul et al., 2011; Apaijai et al., 2012; Pipatpiboon et al., 2012). Each diet group was further divided into three treatment groups (n = 6/group) consisting of vildagliptin 3 mg kg−1·day−1 (Gulvus, Novartis, Bangkok, Thailand; Burkey et al., 2005; Apaijai et al., 2012), sitagliptin 30 mg kg−1·day−1 (Januvia, MSD, Bangkok, Thailand; Chen et al., 2011) and vehicle (0.9% normal saline solution in an equal volume). These concentrations were chosen as they were shown previously to be effective in improving insulin sensitivity (Burkey et al., 2005; Chen et al., 2011). Rats were fed via gavage feeding for 21 days. Body weight and food intake were recorded weekly. Blood samples were drawn from the tail vein at week 0, week 12 and upon completion of treatment. The plasma was separated and kept frozen at −85°C until use. HRV was determined at the baseline (week 0), week 4, week 8, week 12 and post-treatment. Cardiac function parameters were determined using the pressure-volume catheter (Scisence Inc., ON, Canada; Apaijai et al., 2012). After the cardiac function study was finished, the heart was rapidly removed and the left ventricular tissue was used to determine cardiac mitochondrial function and cardiac malondialdehyde (MDA) levels (Apaijai et al., 2012).
Determination of metabolic parameters
Plasma glucose and total cholesterol levels were determined using a commercial colorimetric assay kit (Biotech, Bangkok, Thailand) (Pipatpiboon et al., 2012). Plasma high-density lipoprotein (HDL) and low-density lipoprotein (LDL)/very low-density lipoprotein (VLDL) levels were determined using a commercial colorimetric assay kit (Biovision, Milpitas, CA, USA; Singh et al., 2008). Plasma insulin levels were determined using the sandwich ELISA kit (LINCO Research, St. Charles, MO, USA; Pratchayasakul et al., 2011; Pipatpiboon et al., 2012). The homeostasis model assessment (HOMA) index, a mathematical model, was used to assess insulin resistance. The HOMA index is calculated from fasting plasma glucose levels and fasting plasma insulin concentration. A higher HOMA index indicates a higher degree of insulin resistance (Pratchayasakul et al., 2011; Pipatpiboon et al., 2012).
Determination of plasma and cardiac MDA levels
Plasma and cardiac MDA levels were determined using a HPLC-based assay (Thermo Scientific, Bangkok, Thailand; Apaijai et al., 2012). Plasma and cardiac MDA were mixed with H3PO4 and thiobarbituric acid (TBA) to produce TBA reactive substances (TBARS). The plasma and cardiac TBARS concentration were determined directly from a standard curve and reported as equivalent to the MDA concentration (Apaijai et al., 2012).
Determination of HRV
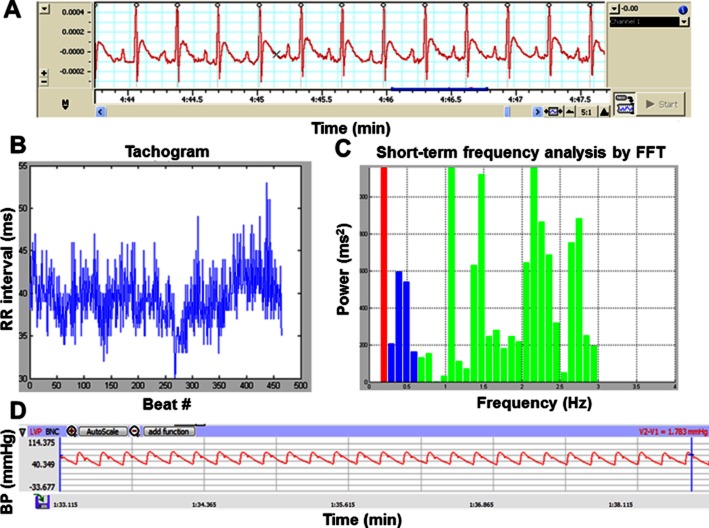
Lead II ECG was recorded in all conscious rats using PowerLab (ADInstruments, Colorado Springs, CO, USA) equipped with the Chart 5.0 program. ECG was recorded for 20 min in each rat. The stable ECG trace and the relationship between the RR interval and the beat numbers (Tachogram) are shown in Figure 1A and 1, respectively. Power spectra of RR interval variability (Figure 1C) were obtained using fast Fourier transform (FFT) algorithm (Chattipakorn et al., 2007; Pongchaidecha et al., 2009; Apaijai et al., 2012; Kumfu et al., 2012). Three major oscillatory components were detected as high-frequency (HF; 0.6–3 Hz) band, low-frequency (LF; 0.2–0.6 Hz) band and very low-frequency (VLF; below 0.2 Hz) band. Each spectral component was calculated as integrals under the respective part of the power spectral density function and was presented in the absolute unit (ms2). To minimize the effect of changes in total power on the LF and HF bands, LF and HF were expressed as normalized units by dividing it by the total power minus VLF (Chattipakorn et al., 2007; Incharoen et al., 2007). LF (0.2–0.6 Hz) and HF (0.6–3 Hz) ratios were analysed using MATLAB program (Pongchaidecha et al., 2009; Apaijai et al., 2012). LF/HF ratio is considered as an index of sympathovagal balance (Chattipakorn et al., 2007; Kumfu et al., 2012). Increased LF/HF ratio indicated depressed HRV (Apaijai et al., 2012).
Figure 1.
Representative figure of stable ECG trace (A), The RR interval and the beat numbers (Tachogram) (B), Power spectra of RR interval variability (C), and stable BP trace (D). The different colours in panel C represent the different frequency intervals for VLF, LF and HF for HRV analysis.
Cardiac function
Rats were anaesthetized using Zoletil (50 mg·kg−1; Virbac Laboratories, Carros, France) and Xylazine (0.15 mg·kg−1, Laboratorios Calier, Barcelona, Spain) injected intramuscularly. Tracheostomy was performed and rats were ventilated with room air. The right carotid artery was identified and a pressure–volume (P–V) catheter was inserted. Systolic BP (SBP) and diastolic blood BP (DBP) were determined from the carotid artery during the P–V loop measurement (Figure 1D). The catheter was then advanced into the left ventricle. Rats were allowed to stabilize for 5 min and then data were recorded for 20 min. The cardiac function parameters, including heart rate (HR), end-systolic and diastolic pressure (ESP and EDP), maximum and minimum dP/dt (±dP/dt), stroke work (SW) and stroke volume (SV), were determined using an analytical program (Labscribe, Dover, NH, USA) (Apaijai et al., 2012; Kumfu et al., 2012).
Cardiac mitochondrial isolation and determination of mitochondrial function
Cardiac mitochondrial isolation was performed as previously described (Thummasorn et al., 2011; Chinda et al., 2012). The heart of each rat was perfused with normal saline solution and rapidly removed. The left ventricular tissue was minced and homogenized in ice-cold buffer containing 300 mM sucrose, 5 mM N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic sodium salt, and 0.2 mM EGTA. The homogenate was centrifuged at 800 g for 5 min. The supernatant was then collected and centrifuged at 8800 g for 5 min. The pellet was resuspended in respiration buffer containing 50 mM sucrose, 100 mM KCl, 10 mM HEPES and 5 mM KH2PO4. In the present study, cardiac mitochondrial function, detected as cardiac mitochondrial reactive oxygen species (ROS) production, cardiac mitochondrial membrane potential change and cardiac mitochondrial swelling was determined.
Cardiac mitochondrial ROS production was determined by incubating cardiac mitochondria with 2-μM 2′,7′-dichlorfluorescein-diacetate dye at 25°C for 20 min. ROS production was detected using a fluorescent microplate reader (BioTek Instruments, Winooski, VT, USA). The dye was excited at _λ_ex 485 nm and detected at _λ_em 530 nm (Thummasorn et al., 2011; Apaijai et al., 2012; Chinda et al., 2012).
Cardiac mitochondrial membrane potential change was determined by incubating cardiac mitochondria with 5-μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra ethylbenzimidazolcarbocyanine iodide (JC-1) dye at 37°C for 30 min. Cardiac mitochondrial membrane potential changes were detected using a fluorescent microplate reader. JC-1 monomer form (green) fluorescence was excited at _λ_ex 485 nm and detected at _λ_em 590 nm. JC-1 aggregate form (red) fluorescence was excited at _λ_ex 485 nm and detected at _λ_em 530 nm. A decrease in the red/green fluorescence intensity ratio was considered an indicator of cardiac mitochondrial membrane depolarization (Thummasorn et al., 2011; Apaijai et al., 2012; Chinda et al., 2012).
Cardiac mitochondrial swelling was determined after incubation in respiration buffer. The absorbance was measured using a spectrophotometer. A decrease in absorbance was considered an indicator of cardiac mitochondrial swelling (Thummasorn et al., 2011; Apaijai et al., 2012; Chinda et al., 2012).
Cardiac mitochondrial morphology determination
Cardiac mitochondria were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, post fixed in 1% cacodylate-buffer osmium tetroxide for 2 h at room temperature, and dehydrated in a graded series of ethanol. Then, cardiac mitochondria were embedded in Epon-Aradite. Ultrathin sections were cut with a diamond knife and stained with uranyl acetate and lead citrate. Cardiac mitochondrial morphology was observed with a transmission electron microscope (Thummasorn et al., 2011).
Statistical analysis
Data were presented as mean ± SE. One way anova followed by Fisher's least significant difference post hoc was used to test the difference among the groups. P < 0.05 was considered statistically significant.
Results
Metabolic parameters
We found that the body weight, food intake, plasma insulin, glucose, total cholesterol and MDA levels, did not vary between ND and HFD rats at the baseline (Table 1). After 12 weeks of HFD consumption, body weight, plasma insulin, HOMA index, total cholesterol and plasma MDA levels were significantly increased compared with ND rats (Table 1). We found that plasma insulin, total cholesterol, HDL levels and HOMA index were significantly improved in HFD rats treated with vildagliptin and sitagliptin. Plasma and cardiac MDA levels were also improved in HFD rats treated with vildagliptin and sitagliptin. However, body weight, visceral fat weight and plasma glucose level were unaffected by both drugs (Table 2). In ND rats, no difference was found among the vildagliptin-, sitagliptin- and vehicle-treated groups.
Table 1.
Effects of vildagliptin and sitagliptin on metabolic parameters and oxidative stress levels in ND and HFD rats
| Metabolic parameters | Baseline | Week 12 | ||
|---|---|---|---|---|
| ND | HFD | ND | HFD | |
| Body weight (g) | 209 ± 9 | 208 ± 9 | 465 ± 6* | 565 ± 13*† |
| Food intake (g·day−1) | 20 ± 1 | 19 ± 5 | 23 ± 1 | 28 ± 3 |
| Plasma insulin (ng·mL−1) | 2.0 ± 0.4 | 2 ± 0.8 | 2 ± 0.3 | 4 ± 0.4*† |
| Plasma glucose (mg·dL−1) | 136 ± 8 | 138 ± 8 | 141 ± 8 | 144 ± 3 |
| HOMA index | 17 ± 1 | 17 ± 2 | 18 ± 3 | 27 ± 4*† |
| Plasma total cholesterol (mg·dL−1) | 83 ± 5 | 83 ± 8 | 83 ± 9 | 152 ± 7*† |
| Plasma MDA (μmol·mL−1) | 2 ± 0.2 | 2 ± 0.1 | 3 ± 0.4 | 6 ± 0.1*† |
Table 2.
Effects of vildagliptin and sitagliptin on metabolic parameters and oxidative stress levels in ND and HFD rats treated with vehicle, vildagliptin and sitagliptin
| Metabolic parameters | NDV | NDVil | NDSi | HFDV | HFDVil | HFDSi |
|---|---|---|---|---|---|---|
| Body weight (g) | 434 ± 5 | 442 ± 12 | 450 ± 9 | 588 ± 12* | 563 ± 2* | 577 ± 19* |
| Food intake (g) | 21 ± 1 | 22 ± 2 | 21 ± 2 | 24 ± 2 | 23 ± 2 | 23 ± 2 |
| Visceral fat (g) | 23 ± 2 | 24 ± 2 | 23 ± 3 | 56 ± 3* | 50 ± 5* | 50 ± 4* |
| Plasma insulin (ng·mL−1) | 2.5 ± 0.4 | 2.3 ± 0.4 | 2.7 ± 0.4 | 3.7 ± 0.4* | 2.4 ± 0.5† | 2.8 ± 0.6† |
| Plasma glucose (mg·dL−1) | 144 ± 5 | 146 ± 8 | 146 ± 7 | 149 ± 10 | 146 ± 9 | 144 ± 8 |
| HOMA index | 18.2 ± 3.7 | 19.7 ± 4.1 | 19.2 ± 5.9 | 27.8 ± 5.5* | 17.4 ± 5.2† | 18.4 ± 6.1† |
| Plasma total cholesterol (mg·dL−1) | 86 ± 7 | 84 ± 5 | 87 ± 6 | 167 ± 7* | 104 ± 7† | 104 ± 4† |
| HDL cholesterol (mg·dL−1) | 0.9 ± 0.1 | 1.1 ± 0.0 | 1.4 ± 0.2 | 0.6 ± 0.1* | 1.3 ± 0.4† | 1.2 ± 0.0† |
| LDL/VLDL cholesterol (mg·dL−1) | 0.9 ± 0.3 | 0.8 ± 0.0 | 0.8 ± 0.1 | 1.0 ± 0.3 | 0.5 ± 0.1 | 0.6 ± 0.2 |
| Plasma MDA (μmol·mL−1) | 2.4 ± 0.1 | 2.7 ± 0.2 | 2.4 ± 0.1 | 7.07 ± 0.1* | 6.4 ± 0.2*† | 6.2 ± 0.3*† |
| Cardiac MDA (μmol·mg−1 protein) | 5.4 ± 1.8 | 5.5 ± 2.4 | 5.9 ± 1.3 | 10.5 ± 2.6* | 7.9 ± 1.6*† | 7.9 ± 1.2*† |
HRV
At baseline, the LF/HF ratio did not differ between the ND and HFD groups (Figure 2A). We found that the LF/HF ratio was increased in week 8 of HFD consumption and markedly increased in week 12 (0.19 ± 0.02 at baseline, 0.26 ± 0.03 at week 8 and 0.33 ± 0.01 at week 12) (Figure 2A). After 21 days of treatment, LF/HF ratio was increased in HFD group treated with vehicle (HFDV) rats [HFDV 0.37 ± 0.02, P < 0.05 versus ND group treated with vehicle (NDV) ]. Both vildagliptin and sitagliptin returned the LF/HF ratio to the normal level [HFD group treated with vildagliptin (HFDVil) 0.19 ± 0.01, HFD group treated with sitagliptin (HFDSi) 0.22 ± 0.03, P < 0.05 versus HFDV] (Figure 2B).
Figure 2.
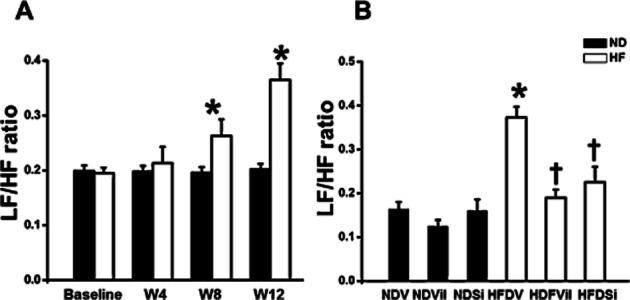
LF/HF ratio in ND and HFD rats (A). The LF/HF ratio significantly increased in weeks 8 and 12 of HFD consumption, in comparison with the baseline. *P < 0.05 versus baseline. LF/HF ratio in ND and HFD rats treated with vehicle, vildagliptin, and sitagliptin (B). In HFD rats, vildagliptin and sitagliptin restored the LF/HF ratio, in comparison with the vehicle. *P < 0.05 versus NDV, †P < 0.05 versus HFDV.
Cardiac function parameters
In the ND groups, cardiac function parameters did not differ among the three treatment groups (Table 3). In the HFD groups treated with vehicle, cardiac dysfunction was observed as shown by an increase in HR, EDP and −dP/dt, and a decrease in ESP, +dP/dt and SV. We found that ESP, EDP, +dP/dt, −dP/dt and SV were significantly improved in HFD rats treated with vildagliptin and sitagliptin. However, treatment with vildagliptin, but not sitagliptin, eliminated pathophysiologic elevation of HR compared with the mean HR of the group treated with vehicle only (Table 3). Moreover, we found that SBP, DBP and SW did not differ among treatment groups.
Table 3.
Effects of vildagliptin and sitagliptin on cardiac function in ND and HFD rats treated with vehicle, vildagliptin and sitagliptin
| Cardiac function | NDV | NDVil | NDSi | HFDV | HFDVil | HFDSi |
|---|---|---|---|---|---|---|
| HR (bpm) | 313 ± 26 | 322 ± 32 | 344 ± 0.3 | 412 ± 14* | 334 ± 26† | 378 ± 27*† |
| SBP (mmHg) | 134 ± 1 | 132 ± 1 | 132 ± 3 | 130 ± 2 | 136 ± 3 | 134 ± 3 |
| DBP (mmHg) | 107 ± 1 | 107 ± 1 | 108 ± 2 | 111 ± 1 | 109 ± 2 | 110 ± 3 |
| ESP (mmHg) | 134 ± 6 | 137 ± 5 | 137 ± 3 | 116 ± 14* | 136 ± 13† | 143 ± 21† |
| EDP (mmHg) | 18 ± 2 | 17 ± 1 | 17 ± 1 | 38 ± 2* | 23 ± 4† | 25 ± 2† |
| +dP/dt (mmHg·s−1) | 8 804 ± 385 | 8 763 ± 230 | 8 532 ± 360 | 6 787 ± 284* | 8 072 ± 234*† | 7 595 ± 382*† |
| −dP/dt (mmHg·s−1) | −5 309 ± 279 | −5 753 ± 100 | −5 011 ± 185 | −3 923 ± 394* | −5 566 ± 858† | −4 701 ± 687† |
| SW (mmHg·mL−1) | 10 466 ± 95 | 10 288 ± 75 | 10 456 ± 73 | 10 406 ± 80 | 10 330 ± 62 | 10 263 ± 66 |
| SV (μL·g−1) | 1.02 ± 0.01 | 1.03 ± 0.06 | 0.99 ± 0.03 | 0.77 ± 0.04* | 0.93 ± 0.01† | 0.96 ± 0.03† |
Cardiac mitochondrial function and morphology
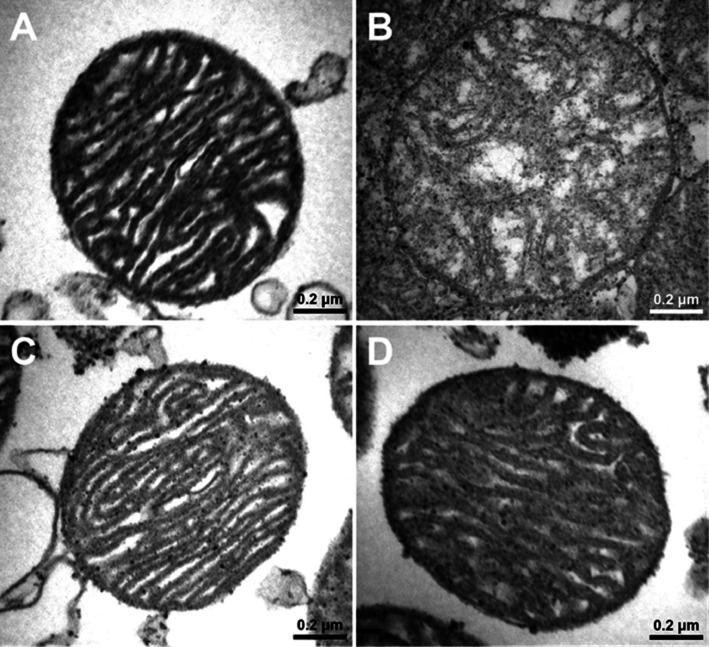
In the ND group, cardiac mitochondrial ROS production [NDV 71 ± 4 au, ND group treated with vildagliptin (NDVil) 62 ± 4 au, ND group treated with sitagliptin (NDSi) 78 ± 10 au, Figure 3], the red/green fluorescent intensity ratio, which indicated cardiac mitochondrial membrane potential change (NDV 0.35 ± 0.01, NDVil 0.35 ± 0.02, NDSi 0.35 ± 0.01, Figure 4), and the absorbance, which indicated mitochondrial swelling (NDV 0.95 ± 0.03 au, NDVil 0.94 ± 0.02 au, NDSi 0.95 ± 0.01 au, Figure 5), were not different among the three treatment groups of the ND rats. In HFD rats, an increase in cardiac mitochondrial ROS production (HFDV 164 ± 11 au, P < 0.05 versus ND group, Figure 3), cardiac mitochondrial depolarization (HFDV 0.27 ± 0.01, P < 0.05 versus ND group, Figure 4), and cardiac mitochondrial swelling (HFDV 0.83 ± 0.02 au, P < 0.05 versus ND group, Figure 5) were observed. Both vildagliptin (HFDVil 73 ± 7 au) and sitagliptin (HFDSi 86 ± 18 au) returned cardiac mitochondrial ROS production to the normal level (Figure 3). Moreover, vildagliptin and sitagliptin prevented cardiac mitochondrial depolarization (Figure 4) and cardiac mitochondrial swelling (Figure 5). In HFD rats, representative electron microscope picture illustrated unfolded cristae in cardiac mitochondrion, comparison with that in ND rats (Figure 6), indicating cardiac mitochondrial swelling. Both vildagliptin and sitagliptin prevented cardiac mitochondrial swelling in HFD rats (Figure 6).
Figure 3.
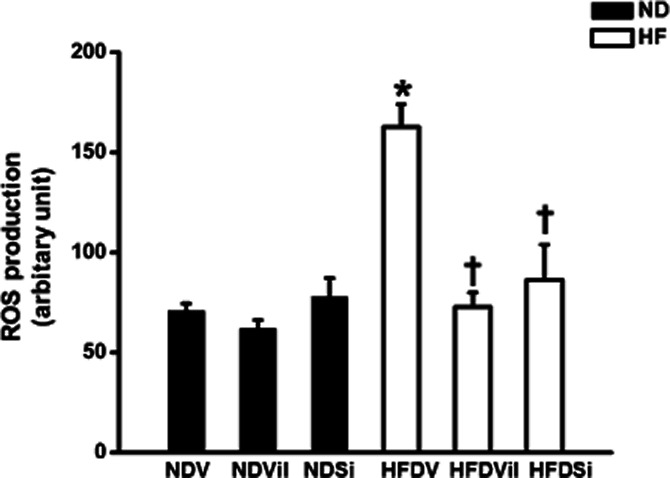
Cardiac mitochondrial ROS production in ND and HFD rats treated with vehicle, vildagliptin, and sitagliptin. In HFD rats, vildagliptin and sitagliptin reduced cardiac mitochondrial ROS production, in comparison with the vehicle. *P < 0.05 versus NDV, †P < 0.05 versus HFDV.
Figure 4.
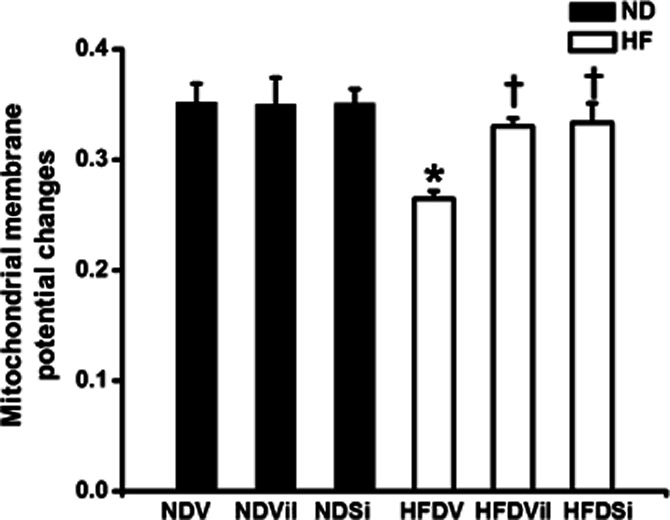
Cardiac mitochondrial membrane potential changes in ND and HFD rats treated with vehicle, vildagliptin and sitagliptin. In HFD rats, vildagliptin and sitagliptin prevented cardiac mitochondrial membrane depolarization, in comparison with the vehicle. *P < 0.05 versus NDV, †P < 0.05 versus HFDV.
Figure 5.
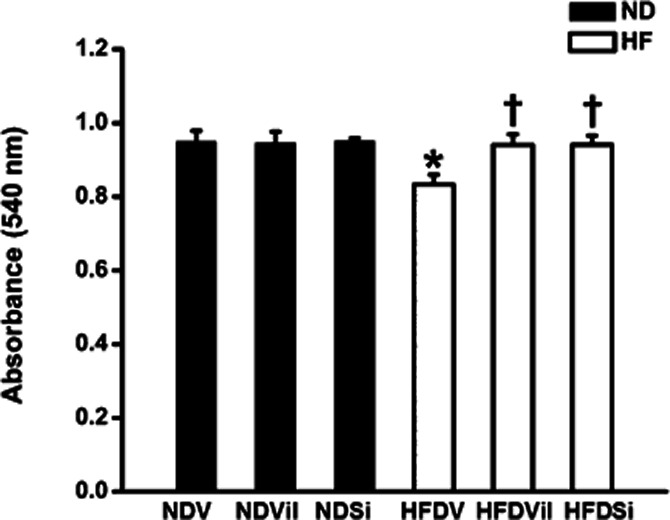
Cardiac mitochondrial swelling in ND and HFD rats treated with vehicle, vildagliptin and sitagliptin. In HFD rats, vildagliptin and sitagliptin reduced cardiac mitochondrial swelling, in comparison with the vehicle. *P < 0.05 versus NDV, †P < 0.05 versus HFDV.
Figure 6.
Electron microscope pictures of cardiac mitochondria in NDV (A) and HFD rats treated with vehicle (B), vildagliptin (C) and sitagliptin (D). In HFD rats, vildagliptin and sitagliptin prevented cardiac mitochondrial morphology changes, in comparison with the vehicle.
Discussion
Findings from this study show that vildagliptin and sitagliptin improve metabolic parameters and decrease oxidative stress in HFD-induced insulin-resistant rats. Vildagliptin and sitagliptin completely preserved HRV, and attenuated cardiac dysfunction in HFD-induced insulin-resistant rats. However, only vildagliptin returned HR to the normal level. Moreover, vildagliptin and sitagliptin prevented cardiac mitochondrial dysfunction and preserved cardiac mitochondrial morphology in rats with insulin resistance induced by HFD consumption.
In the present study, our model is a high-fat diet-induced insulin resistance, which is characterized by hyperinsulinaemia with euglycaemia. Our result showed that plasma glucose level was not different between ND and high-fat diet group at baseline and at week 12, whereas plasma insulin level was significantly increased in rats at week 12 of high-fat diet consumption. This result confirmed that these rats developed an insulin resistance at week 12 after high-fat diet consumption. These findings were also consistent with previous reports using high-fat diet-induced insulin-resistant rats (Pratchayasakul et al., 2011; Apaijai et al., 2012; Pipatpiboon et al., 2012).
Long-term HFD consumption is known to induce insulin resistance (Pongchaidecha et al., 2009; Pratchayasakul et al., 2011; Pipatpiboon et al., 2012). Previous studies have shown that DPP-4 inhibitors, vildagliptin and sitagliptin specifically, improved metabolic parameters, and reduced plasma insulin levels in animal and clinical studies (Ahren et al., 2004; Mari et al., 2005; Dobrian et al., 2011; Briand et al., 2012). In the current study, plasma insulin levels were decreased in HFD rats treated with both vildagliptin and sitagliptin. Moreover, we found that both vildagliptin and sitagliptin reduced total plasma cholesterol levels in HFD rats. This finding is consistent with previous studies reporting that vildagliptin and sitagliptin decreased plasma cholesterol levels in normal rats (Yin et al., 2011), HFD mice (Flock et al., 2007) and type 2 diabetes patients (Kleppinger and Helms, 2007; Tremblay et al., 2011). Furthermore, our study is the first showing that vildagliptin and sitagliptin could increase plasma HDL levels in long-term HFD-induced insulin-resistant rats, whereas both drugs did not alter plasma glucose and LDL/VLDL levels. Previous clinical studies as well as in normal and type 2 diabetes rats also showed that vildagliptin and sitagliptin reduced levels of oxidative stress (Read et al., 2010; Matsui et al., 2011; Chinda et al., 2012; Goncalves et al., 2012). In this study, we found that plasma and cardiac MDA levels were decreased in HFD rats treated with vildagliptin and sitagliptin. These findings indicate that both vildagliptin and sitagliptin could attenuate insulin-resistant condition and reduce oxidative stress in obese insulin-resistant rats induced by long-term HFD consumption.
HRV is an indicator used to determine cardiac sympathovagal balance, a commonly used metric associated with autonomic regulatory function (Chattipakorn et al., 2007; Incharoen et al., 2007; Kumfu et al., 2012). Our study demonstrated that the LF/HF ratio was increased in HFD rats during week 8 and markedly increased during week 12 of HFD consumption, indicating cardiac sympathovagal imbalance. This study showed that both vildagliptin and sitagliptin restore the HRV by returning the LF/HF ratio to normal levels. Because increased sympathetic activity such as stress and insulin resistance could cause depressed HRV (i.e. increased LF/HF ratio; McCann et al., 1995; Sun et al., 2011; Apaijai et al., 2012), HRV improvement by vildagliptin and sitagliptin could be due to the modulation of autonomic regulatory function derived from improved insulin sensitivity and reduced oxidative stress levels. The improved HRV found in this study could also be due to the anti-inflammatory effect. Future studies are needed to investigate the effects of the DPP-4 inhibitors on the association between anti-inflammatory role and HRV.
Previous studies and ours have shown that cardiac dysfunction can be induced by various diets in obese insulin-resistant rats (McCann et al., 1995; Sun et al., 2011; Apaijai et al., 2012). Consistent with previous reports, this study demonstrated that long-term HFD consumption leads to cardiac dysfunction. Treatment with vildagliptin and sitagliptin significantly improved cardiac function in HFD-induced insulin-resistant rats. This cardioprotective effects could be due to their protection of cardiac mitochondrial dysfunction.
Cardiac mitochondria are responsible for supplying appropriate energy for maintaining normal cardiac function. Previous studies have reported that insulin resistance is related to mitochondrial dysfunction in various insulin target tissues including myocardium (Kraegen et al., 2008; Coletta and Mandarino, 2011). We found that cardiac mitochondrial dysfunction occurred in HFD rats, as characterized by increased cardiac mitochondrial ROS production, mitochondrial membrane depolarization, and cardiac mitochondrial swelling (Thummasorn et al., 2011; Chinda et al., 2012). Chinda et al. reported that vildagliptin could attenuate cardiac mitochondrial ROS production and mitochondrial membrane depolarization in isolated cardiac mitochondria experiencing oxidative stress (Chinda et al., 2012). Moreover, Thummasorn et al. reported that severe oxidative stress could damage cardiac mitochondrial ultrastructure (Thummasorn et al., 2011). In the present study, we found that both vildagliptin and sitagliptin prevented cardiac mitochondrial dysfunction in obese insulin-resistant rats caused by long-term HFD consumption.
In this study, we demonstrated that our obese insulin-resistant rats demonstrated cardiac autonomic dysfunction as shown by depressed HRV, cardiac mechanical dysfunction as shown by abnormal cardiac pressure–volume data, and cardiac mitochondrial dysfunction as shown by increased mitochondrial ROS production, mitochondrial membrane depolarization and mitochondrial swelling. In the present study, we found that both vildagliptin and sitagliptin provided cardioprotection via their benefits on prevention of cardiac mitochondrial dysfunction including decreased ROS production, restored mitochondrial membrane potential and prevented mitochondrial swelling. Because it is known that increased ROS production is mainly contributed to mitochondrial depolarization and mitochondrial swelling, the major effect that both drugs attenuated mitochondrial membrane depolarization could be due to their ability to decrease the mitochondrial ROS production in these obese insulin-resistant rats. The anti-oxidative effect of both drugs could also be seen in their ability to decrease plasma MDA as well. These findings suggested that the anti-oxidative effects of these DPP-4 inhibitors could play a major role in their cardioprotective effects in these obese insulin-resistant rats.
In conclusion, insulin resistance, increased oxidative stress, cardiac sympathovagal imbalance, cardiac dysfunction and cardiac mitochondrial dysfunction were observed in long-term HFD-fed rats. Both vildagliptin and sitagliptin ameliorated these undesirable effects by decreasing oxidative stress and cardiac mitochondrial dysfunction, and improving insulin-resistant condition, HRV and cardiac function in long-term HFD consumption induced insulin-resistant rats.
Acknowledgments
The authors would like to thank Ms. Nusara Chomanee at the Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University for her technical assistance on electron microscopy. This work was supported by grants from the Thailand Research Fund (TRF) RTA5580006 (NC) and BRG5480003 (SC). Vildagliptin was provided by Novartis (Thailand).
Glossary
DBP
diastolic BP
DPP-4
dipeptidyl peptidase-4
EDP
end-diastolic pressure
ESP
end-systolic pressure
FFT
fast Fourier transform
GLP-1
glucagon-like peptide-1
HDL
high-density lipoprotein
HF
high-frequency
HFD
high-fat diet
HFDSi
high-fat diet group treated with sitagliptin
HFDV
high-fat diet group treated with vehicle
HFDVil
high-fat diet group treated with vildagliptin
HOMA
homeostasis model assessment
HR
heart rate
HRV
heart rate variability
JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra ethylbenzimidazolcarbocyanine iodide
LDL
low-density lipoprotein
LF
low-frequency
MDA
malondialdehyde
ND
normal diet
NDSi
normal-diet group treated with sitagliptin
NDV
normal-diet group treated with vehicle
NDVil
normal-diet group treated with vildagliptin
ROS
reactive oxygen species
SBP
systolic BP
SV
stroke volume
SW
stroke work
TBA
thiobarbituric acid
TBARS
thiobarbituric acid reactive substances
VLDL
very low-density lipoprotein
VLF
very low-frequency
Conflict of interest
None.
References
- Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–2084. doi: 10.1210/jc.2003-031907. [DOI] [PubMed] [Google Scholar]
- Apaijai N, Pintana H, Chattipakorn SC, Chattipakorn N. Cardioprotective effects of metformin and vildagliptin in adult rats with insulin resistance induced by a high-fat diet. Endocrinology. 2012;153:3878–3885. doi: 10.1210/en.2012-1262. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- Briand F, Thieblemont Q, Burcelin R, Sulpice T. Sitagliptin promotes macrophage-to-faeces reverse cholesterol transport through reduced intestinal cholesterol absorption in obese insulin resistant CETP-apoB100 transgenic mice. Diabetes Obes Metab. 2012;14:662–665. doi: 10.1111/j.1463-1326.2012.01568.x. [DOI] [PubMed] [Google Scholar]
- Burkey BF, Li X, Bolognese L, Balkan B, Mone M, Russell M, et al. Acute and chronic effects of the incretin enhancer vildagliptin in insulin-resistant rats. J Pharmacol Exp Ther. 2005;315:688–695. doi: 10.1124/jpet.105.087064. [DOI] [PubMed] [Google Scholar]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- Chattipakorn N, Incharoen T, Kanlop N, Chattipakorn S. Heart rate variability in myocardial infarction and heart failure. Int J Cardiol. 2007;120:289–296. doi: 10.1016/j.ijcard.2006.11.221. [DOI] [PubMed] [Google Scholar]
- Chen B, Moore A, Escobedo LV, Koletsky MS, Hou D, Koletsky RJ, et al. Sitagliptin lowers glucagon and improves glucose tolerance in prediabetic obese SHROB rats. Exp Biol Med (Maywood) 2011;236:309–314. doi: 10.1258/ebm.2010.010161. [DOI] [PubMed] [Google Scholar]
- Chinda K, Palee S, Surinkaew S, Phornphutkul M, Chattipakorn S, Chattipakorn N. Cardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia-reperfusion injury. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.01.011. (in press). doi: 10.1016/j.ijcard.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Coletta DK, Mandarino LJ. Mitochondrial dysfunction and insulin resistance from the outside in: extracellular matrix, the cytoskeleton, and mitochondria. Am J Physiol Endocrinol Metab. 2011;301:E749–E755. doi: 10.1152/ajpendo.00363.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrian AD, Ma Q, Lindsay JW, Leone KA, Ma K, Coben J, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300:E410–E421. doi: 10.1152/ajpendo.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Li Q, Sreejayan N, Nunn JM, Ren J. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes. 2007;56:2201–2212. doi: 10.2337/db06-1596. [DOI] [PubMed] [Google Scholar]
- Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56:3006–3013. doi: 10.2337/db07-0697. [DOI] [PubMed] [Google Scholar]
- Goncalves A, Leal E, Paiva A, Teixeira Lemos E, Teixeira F, Ribeiro CF, et al. Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood–retinal barrier in a type 2 diabetes animal model. Diabetes Obes Metab. 2012;14:454–463. doi: 10.1111/j.1463-1326.2011.01548.x. [DOI] [PubMed] [Google Scholar]
- Incharoen T, Thephinlap C, Srichairatanakool S, Chattipakorn S, Winichagoon P, Fucharoen S, et al. Heart rate variability in beta-thalassemic mice. Int J Cardiol. 2007;121:203–204. doi: 10.1016/j.ijcard.2006.08.076. [DOI] [PubMed] [Google Scholar]
- Kleppinger EL, Helms K. The role of vildagliptin in the management of type 2 diabetes mellitus. Ann Pharmacother. 2007;41:824–832. doi: 10.1345/aph.1H460. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Cooney GJ, Turner N. Muscle insulin resistance: a case of fat overconsumption, not mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2008;105:7627–7628. doi: 10.1073/pnas.0803901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfu S, Chattipakorn S, Chinda K, Fucharoen S, Chattipakorn N. T-type calcium channel blockade improves survival and cardiovascular function in thalassemic mice. Eur J Haematol. 2012;88:535–548. doi: 10.1111/j.1600-0609.2012.01779.x. [DOI] [PubMed] [Google Scholar]
- Lenski M, Kazakov A, Marx N, Bohm M, Laufs U. Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol. 2011;51:906–918. doi: 10.1016/j.yjmcc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- McCann JD, Margolis TP, Wong MG, Kuppermann BD, Luckie AP, Schwartz DM, et al. A sensitive and specific polymerase chain reaction-based assay for the diagnosis of cytomegalovirus retinitis. Am J Ophthalmol. 1995;120:219–226. doi: 10.1016/s0002-9394(14)72610-8. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, et al. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4888–4894. doi: 10.1210/jc.2004-2460. [DOI] [PubMed] [Google Scholar]
- Matsui T, Nishino Y, Takeuchi M, Yamagishi S. Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol Res. 2011;63:383–388. doi: 10.1016/j.phrs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA, et al. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia. 2005;48:1229–1237. doi: 10.1007/s00125-005-1755-x. [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. 2012;153:329–338. doi: 10.1210/en.2011-1502. [DOI] [PubMed] [Google Scholar]
- Pongchaidecha A, Lailerd N, Boonprasert W, Chattipakorn N. Effects of curcuminoid supplement on cardiac autonomic status in high-fat-induced obese rats. Nutrition. 2009;25:870–878. doi: 10.1016/j.nut.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88:619–627. doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- Singh SK, Suresh MV, Prayther DC, Moorman JP, Rusinol AE, Agrawal A. Phosphoethanolamine-complexed C-reactive protein: a pharmacological-like macromolecule that binds to native low-density lipoprotein in human serum. Clin Chim Acta. 2008;394:94–98. doi: 10.1016/j.cca.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Pan H, Tan H, Yu Y. High free fatty acids level related with cardiac dysfunction in obese rats. Diabetes Res Clin Pract. 2011;95:251–259. doi: 10.1016/j.diabres.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Thummasorn S, Kumfu S, Chattipakorn S, Chattipakorn N. Granulocyte-colony stimulating factor attenuates mitochondrial dysfunction induced by oxidative stress in cardiac mitochondria. Mitochondrion. 2011;11:457–466. doi: 10.1016/j.mito.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:366–373. doi: 10.1111/j.1463-1326.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- Yin M, Sillje HH, Meissner M, van Gilst WH, de Boer RA. Early and late effects of the DPP-4 inhibitor vildagliptin in a rat model of post-myocardial infarction heart failure. Cardiovasc Diabetol. 2011;10:85. doi: 10.1186/1475-2840-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]