Roles of different pools of the mitotic checkpoint complex and the mechanisms of their disassembly (original) (raw)
Abstract
The mitotic (or spindle assembly) checkpoint system prevents premature separation of sister chromatids in mitosis. When the checkpoint is turned on, the mitotic checkpoint complex (MCC) inhibits the ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C). MCC is composed of the checkpoint proteins BubR1, Bub3, and Mad2 associated with the APC/C activator Cdc20. The mechanisms of the assembly of MCC when the checkpoint is turned on, and of its disassembly when the checkpoint is inactivated, are not sufficiently understood. Previous reports indicated that APC/C-mediated polyubiquitylation of Cdc20 in MCC is required for the dissociation of APC/C-associated MCC, but not of free MCC. The pool of free MCC is disassembled by an ATP-dependent process stimulated by the Mad2-binding protein p31comet. It remained unknown whether free MCC is the precursor or the dissociation product of APC/C-bound MCC. By characterizing the mechanisms of the disassembly of APC/C-bound MCC in a purified system, we find that it cannot be the source of free MCC, because it is bound at high affinity and is released only in ubiquitylated or partially disassembled forms. By the use of a cell-free system from Xenopus eggs that reproduces the mitotic checkpoint, we show that MCC can be assembled in the absence of APC/C in a checkpoint-dependent manner. We propose that when the checkpoint is turned on, free MCC is the precursor of APC/C-bound MCC. When the mitotic checkpoint is extinguished, both APC/C-bound and free MCC pools have to be disassembled to release APC/C from inhibition.
The mitotic (or spindle assembly) checkpoint is a surveillance mechanism that prevents premature separation of sister chromatids in mitosis and thus ensures the fidelity of chromosome segregation. It senses the existence of kinetochores that are not yet attached correctly to the mitotic spindle and inhibits the action of the anaphase-promoting complex/cyclosome (APC/C), a ubiquitin ligase that targets for degradation the anaphase inhibitors securin and cyclin B (reviewed in refs. 1–3). APC/C is inhibited by the mitotic checkpoint complex (MCC), which consists of the checkpoint proteins Bub1 related protein 1 (BubR1), Budding uninhibited by benzimidazole 3 protein (Bub3), and Mitotic arrest deficient protein 2 (Mad2) associated with the APC/C activator cell division cycle protein 20 (Cdc20) (4). The molecular mechanisms of the assembly of MCC when the checkpoint is turned on, and of its disassembly when it is turned off, are not sufficiently understood. It has been reported that APC/C-catalyzed polyubiquitylation of Cdc20 in MCC is required for the disassembly of MCC and for the inactivation of the mitotic checkpoint (5, 6). This finding has been challenged, based on the observation that preventing Cdc20 ubiquitylation by mutating all its lysine residues to arginines did not prevent MCC disassembly (7). However, more recently it has been shown that the APC/C subunit Mnd2/APC15 promotes the ubiquitylation of MCC-associated Cdc20 and is required for efficient inactivation of the mitotic checkpoint (8, 9). These apparently conflicting observations may be reconciled by assuming the existence of parallel pathways for MCC disassembly, one that requires Cdc20 ubiquitylation and another that does not (10). Notably, ubiquitylation and degradation of MCC-associated Cdc20 have been observed not only in checkpoint inactivation, but also during active checkpoint (7, 11–14). It has been suggested that the role of this process may be to provide a dynamic situation for rapid MCC disassembly when checkpoint-induced assembly of MCC is turned off (14).
We have been studying the mechanisms of the inactivation of the mitotic checkpoint and of MCC disassembly in extracts from checkpoint-arrested HeLa cells. When checkpoint extracts were incubated in the presence of ATP, MCC was disassembled and APC/C regained activity (15, 16). We noted that polyubiquitylation was required only for the disassembly of APC/C-bound MCC (APC/C–MCC). Another pool of free MCC was disassembled rapidly, without requirement for polyubiquitylation. The disassembly of both pools of MCC required ATP cleavage at the β−γ position (17). We also found that p31comet, a Mad2-binding protein that was known to be involved in the inactivation of the mitotic checkpoint (18, 19), promoted the disassembly of free MCC in a process that required ATP (20). A part of the ATP requirement was accounted for by the finding that p31comet stimulated the phosphorylation of Cdc20 by cyclin-dependent kinase (Cdk) (21). We found that this process caused the dissociation of Cdc20 from BubR1 in MCC and suggested that the binding of p31comet to MCC may trigger a conformational change in Cdc20 that facilitates its phosphorylation by Cdk and thus promotes its dissociation from BubR1 (20, 21). The complex and energy-dependent processes involved in the disassembly of both APC/C-bound and free MCC may be required to overcome high-affinity protein-protein interactions in these complexes.
Although the APC/C–MCC complex presumably reflects the checkpoint-inhibited state of APC/C, the role of the free pool of MCC remained obscure. It may serve as precursor for association with APC/C, a product of dissociation from APC/C, or both. Here, we examined the relationship between the two pools of MCC and the mechanisms of their disassembly in checkpoint inactivation.
Results
Relative Amounts of APC/C-Bound and Free Pools of MCC in Extracts from Checkpoint-Arrested Cells.
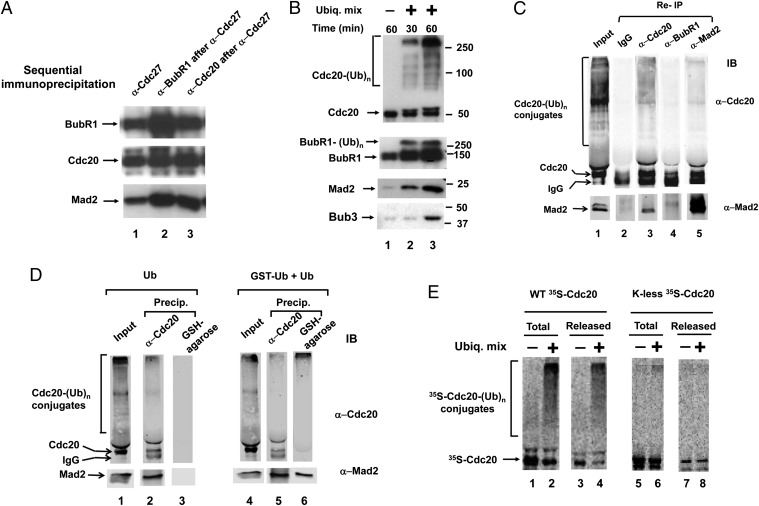
Previous observations indicated the existence of APC/C-bound and unbound pools of MCC that are disassembled in different ways when the checkpoint is inactivated (17). We first tried to estimate the relative sizes of these pools by sequential immunoprecipitations. Extracts from nocodazole-arrested HeLa cells were first immunoprecipitated with antibody directed against the APC/C subunit Cdc27, for estimation of APC/C-bound MCC. The resulting supernatants were then subjected to precipitation with anti-BubR1 or anti-Cdc20 antibodies, for the estimation of free MCC. The different immunoprecipitates were analyzed by quantitative immunoblotting of different MCC components. The amounts of BubR1 and Mad2 were ∼3- to 4-fold higher in the pool of free MCC than in APC/C-bound MCC (Fig. 1_A_, lanes 2 and 3 vs. lane 1). Levels of Cdc20 were only slightly higher in free MCC than in the APC/C-bound species; this may be due to the portion of APC/C observed in checkpoint extracts that binds Cdc20, but not MCC (22). These data indicate that the majority of MCC in checkpoint-arrested cells is in the free pool.
Fig. 1.
Characteristics of the disassembly of APC/C-bound MCC. (A) Comparison of sizes of APC/C-bound and free pools of MCC. Extracts from nocodazole-arrested HeLa cells were subjected to immunoprecipitation with anti-Cdc27 (lane 1). The resulting supernatants were precipitated with anti-BubR1 (lane 2) or anti-Cdc20 (lane 3). Equivalent samples of all immunoprecipitates were subjected to immunoblotting for the indicated MCC components. The estimation of quantities was corrected for the efficiency of immunoprecipitations, as determined by quantification of the amounts remaining in supernatants after immunoprecipitation. These were ∼85% with anti-Cdc27 and anti-BubR1, and ∼70% with anti-Cdc20. (B) Ubiquitylation and the release from APC/C–MCC of different MCC components. Anti-Cdc27 immunoprecipitates from checkpoint extracts were incubated and the release of MCC components was estimated as described in Methods. Where indicated, a ubiquitylation mixture (“Ubiq. Mix”; Methods) was added. Following incubation for the time periods indicated, samples of the supernatant were separated by SDS/PAGE and were immunoblotted for the indicated proteins. (C) Ubiquitylation of APC/C–MCC releases Mad2 associated with Cdc20. Purified APC/C–MCC on anti-Cdc27 beads was first incubated with ubiquitylation mixture for 60 min as described in A, and the supernatants containing released material were collected (“Input”). Samples of 50 μL of the supernatants were subjected to reimmunoprecipitation with 5 μL of Affi-prep protein A beads that contained 2.5 μg of the indicated antibodies. Following mixing at 4 °C for 60 min, immunoprecipitated material was analyzed by SDS/PAGE and immunoblotting. (D) Mad2 released from APC/C–MCC is bound to ubiquitylated Cdc20. The experiment was similar to C, except that release incubation was carried out either with 100 μM ubiquitin (lanes 1–3) or with a mixture of GST-ubiquitin and ubiquitin (50 μM, each, lanes 4–6). Samples of released material (“Input”) were precipitated with either anti-Cdc20 or GSH-agarose beads, as indicated. (E) Ubiquitylation and release of 35S-labeled wild-type or K-less Cdc20 exchanged into APC/C–MCC. 35S-Cdc20s were prepared and exchanged into APC/C–MCC as described in Methods, and the preparations were incubated (30 °C, 45 min) with or without ubiquitylation mixture as in B. Equivalent samples of the total reaction mixture (including beads) and of the supernatants containing released material were subjected to SDS/PAGE and radioactivity detection.
Characterization of Ubiquitylation and Release of MCC Components from APC/C in a Purified System.
To gain an insight into the roles the two pools of MCC, we next examined the mode of the dissociation of APC/C-bound MCC. For this purpose, APC/C–MCC immunopurified from checkpoint extracts was incubated with or without ubiquitylation mixture and the release and ubiquitylation of MCC components was determined in supernatants (Methods). As shown in Fig. 1_B_, the supplementation of ubiquitylation mixture greatly increased the release of MCC components, indicating that the purified system reproduced the process observed in extracts (5, 17). Most of released Cdc20 was polyubiquitylated. A smaller amount of released Cdc20 was in a free form, but this was not dependent on ubiquitylation and may be derived from a species of Cdc20 bound to APC/C but unrelated to MCC. A portion of BubR1 was also polyubiquitylated, but most of BubR1 released in the presence of the ubiquitylation system was in a free form. A small fraction of released Mad2 was monoubiquitylated, but no significant polyubiquitylation of either Mad2 or Bub3 could be detected under these conditions (for the entire length of these immunoblots, see Fig. S1). Thus, the polyubiquitylation of Cdc20 was best correlated with the release of MCC components from APC/C.
We next examined whether polyubiquitylation causes the release from APC/C of complete MCC, in which all components are still associated with each other, or whether this process involves the disassembly of MCC into individual components or subcomplexes. Immunopurified APC/C–MCC was first incubated with ubiquitylation mixture, supernatants containing released material were collected and then subjected to reimmunoprecipitation with antibodies against different MCC components. These precipitates were immunoblotted for Cdc20 and Mad2. As shown in Fig. 1_C_, lane 2, in control precipitation with IgG beads, only IgG heavy chains were detected, which migrated slightly ahead of Cdc20. Reimmunoprecipitation with anti-Cdc20 pulled down free and ubiquitylated Cdc20, as well as Mad2 (Fig. 1_C_, lane 3). This result suggested that following release from APC/C, at least a part of Mad2 is still bound to Cdc20. This result was corroborated by the finding that reimmunoprecipitation with anti-Mad2 pulled down Cdc20, as well as a part of ubiquitylated Cdc20 (Fig. 1_C_, lane 5). By contrast, reimmunoprecipitation with anti-BubR1 did not precipitate significant amounts of Cdc20 or Mad2 (Fig. 1_C_, lane 4). The latter observation suggests that BubR1 is dissociated from Cdc20 and Mad2 in polyubiquitylation-induced release of MCC components from APC/C.
The association maintained between Mad2 and Cdc20 resembles a similar association that we have detected following the disassembly of purified free MCC with p31comet and ATP (20). Because the released material also contained free Cdc20, it was possible that most of Mad2 was bound to free, rather than to ubiquitylated Cdc20. We therefore further examined this problem by the use of GST-ubiquitin and pull-down with glutathione beads. In the experiment shown in Fig. 1_D_, immunopurified APC/C–MCC was treated with ubiquitylation mixtures containing either native ubiquitin (lanes 1–3), or a mixture of GST-ubiquitin and ubiquitin (lanes 4–6). Subsequently, released material was pulled down with either anti-Cdc20 or glutathione-agarose beads and precipitated material was immunoblotted for Cdc20 and Mad2. Control treatments showed that following incubation with native ubiquitin, Cdc20 derivatives were pulled down with anti-Cdc20, but not with glutathione beads (Fig. 1_D_, lanes 2 and 3). Following release from APC/C with a mixture of GST-ubiquitin and ubiquitin, glutathione beads bound high-molecular weight derivatives of Cdc20 (presumably containing GST-ubiquitin), but not free Cdc20 (Fig. 1_D_, lane 6). In this case, the efficiency of binding of Cdc20-GST-ubiquitin conjugates was actually better with glutathione beads than by immunoprecipitation with anti-Cdc20 (Fig. 1_D_, compare lanes 5 and 6). These results indicate that following the release of MCC components from APC/C by ubiquitylation with GST-ubiquitin, at least part of released Mad2 molecules are bound to ubiquitylated material, presumably ubiquitylated Cdc20. By contrast, BubR1 is released free of Cdc20 or Mad2 (Fig. 1_C_), indicating that the ubiquitylation-dependent release of Mad2 from APC/C is accompanied by the partial disassembly of MCC.
The question arose whether the ubiquitylation of Cdc20 is directly required for its release from APC/C–MCC. Although we observed that among MCC subunits, only Cdc20 is released predominantly ubiquitylated (Fig. 1_B_), it was possible that the ubiquitylation of some other target protein, such as an APC/C subunit involved in MCC binding, causes the release of MCC from APC/C. We have examined this problem by the use of a mutant Cdc20 in which all lysine residues have been converted to arginines (7). In vitro translated, 35S-labeled wild-type or K-less Cdc20 were exchanged into APC/C–MCC on anti-Cdc27 beads and were incubated in the presence or absence of ubiquitylation mixture. 35S-labeled derivatives were examined in the total reaction mixture and in material released from APC/C. As shown in Fig. 1_E_, Left, wild-type 35S-Cdc20 was polyubiquitylated and released, similarly to that observed with endogenous Cdc20. This result suggests that 35S-Cdc20 exchanged into APC/C–MCC faithfully reproduces ubiquitylation-induced release from APC/C. K-less 35S-Cdc20 was exchanged well into APC/C–MCC, but its polyubiquitylation was greatly reduced (Fig. 1_E_, Right). We observed the formation of a small amount of high molecular sized conjugates of K-less 35S-Cdc20 (Fig. 1_E_, lane 6), which is likely due to ubiquitylation on a nonlysine residue, such as the N-terminal amino group (23). If ubiquitylation of some other protein (such as a subunit of APC/C) were responsible for the release of Cdc20, it would be expected that the supplementation of ubiquitylation system would cause the release of free K-less 35S-Cdc20. This is clearly not the case (Fig. 1_E_, lane 8), indicating that the ubiquitylation of Cdc20 itself is required for its release from APC/C–MCC.
Role of p31comet in the Release of MCC Components from APC/C.
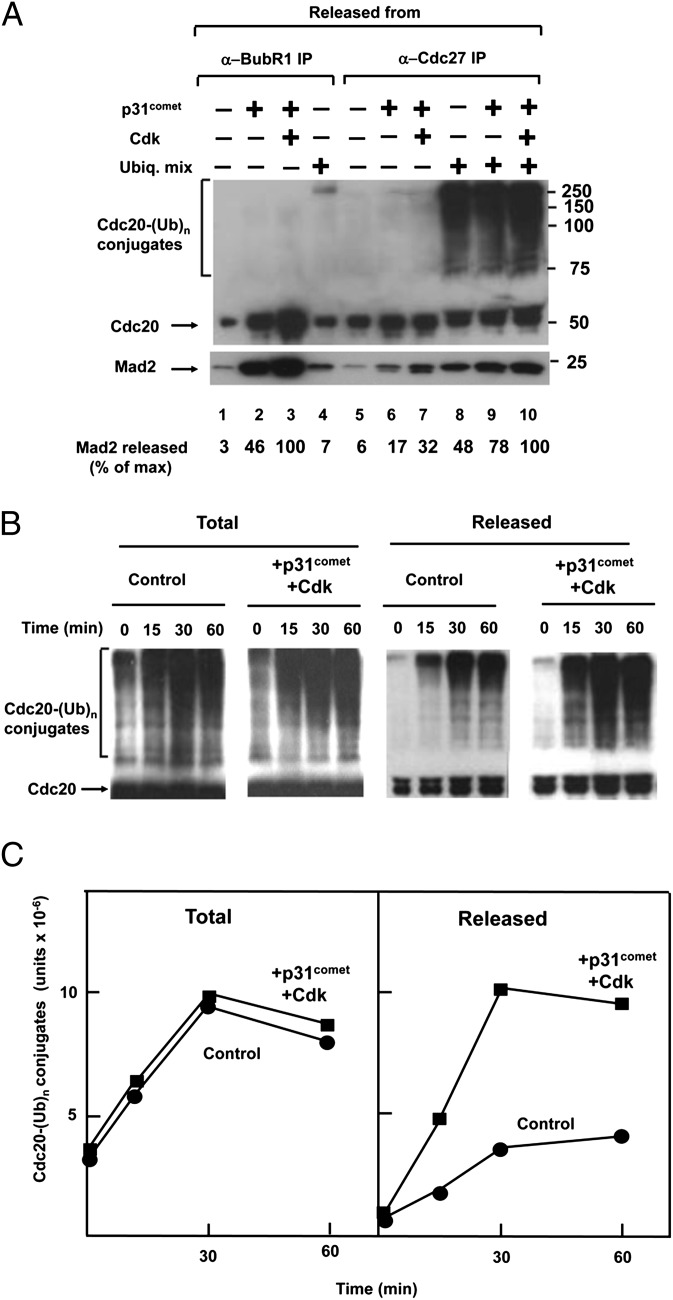
We next examined the influence of p31comet on the release of MCC from APC/C–MCC in the purified system. In the experiment shown in Fig. 2_A_, lanes 5–10, immunopurified APC/C–MCC on anti-Cdc27 beads was incubated with p31comet and Cdk, in the presence or absence of ubiquitylation mixture, and the release of MCC components to supernatants was determined. The results were compared with the previously known effects of p31comet and Cdk on the disassembly of free MCC, immunopurified with anti-BubR1 (Fig. 2_A_, lanes 1–4). As described (20, 21), p31comet promoted the release of Cdc20 and Mad2 from BubR1 in free MCC, and this was further stimulated by Cdk (Fig. 2_A_, lanes 1–3). The supplementation of ubiquitylation mixture to anti-BubR1 immunoprecipitate caused small stimulation of the release of Mad2, accompanied by slight ubiquitylation of Cdc20 (Fig. 2_A_, lane 4); this was presumably due to the presence of a small amount of residual APC/C in this preparation. By contrast, the release of MCC components from APC/C–MCC was mostly ubiquitylation-dependent. Without ubiquitylation mixture, p31comet and Cdk stimulated the release of Mad2 and Cdc20 from anti-Cdc27 immunoprecipitate to a limited extent (Fig. 2_A_, lanes 5–7). In the presence of ubiquitylation mixture, p31comet and Cdk markedly stimulated the release of Mad2 and of ubiquitylated Cdc20, synergistically with that obtained with ubiquitylation mixture alone (Fig. 2_A_, lanes 8–10).
Fig. 2.
Influence of p31comet and Cdk on ubiquitylation-dependent release of MCC components from APC/C–MCC. (A) Effects of p31comet and Cdk in the presence or absence of ubiquitylation mixture. Immunopurified preparations of free MCC on anti-BubR1 beads (lanes 1–4) or of APC/C–MCC on anti-Cdc27 beads (lanes 5–10) were incubated with the indicated additions and the release of MCC components was determined as described in Methods. Cdk, 1,000 units of Cdk1-Δ88cyclin B (27). Numbers on the right side indicate the position of molecular mass marker proteins (kDa). (B) p31comet and Cdk stimulate the release of ubiquitylated Cdc20 from APC/C–MCC. Reaction conditions were as in A. Following incubation for the time periods indicated, samples were taken for from the total reaction mixture and from released material in supernatants. Equivalent samples were subjected to SDS/PAGE and immunoblotting. (C) Quantification of results. Results are expressed in units of the ImageQuant RT ECL apparatus (GE Healthcare).
The question arose whether p31comet facilitates the release of MCC components by stimulating the ubiquitylation of Cdc20, as reported previously (5), or by stimulating the release from APC/C of ubiquitylated Cdc20. For this purpose, we have compared the time course of ubiquitylation of Cdc20 in the total reaction mixture with the release of ubiquitylated Cdc20. As shown in Fig. 2 B and C, there was no significant stimulation by p31comet and Cdk of the formation of ubiquitylated Cdc20 in the total reaction mixture, which included APC/C-bound and released species of ubiquitylated Cdc20. By contrast, p31comet and Cdk markedly stimulated the release of polyubiquitylated Cdc20 from APC/C. The stimulation by p31comet and Cdk of the release of ubiquitylated Cdc20 was especially prominent at early times of incubation. These results indicated that p31comet and Cdk stimulate the release of Cdc20 after it is ubiquitylated.
Checkpoint-Dependent Formation of MCC in the Absence of APC/C and High-Affinity Binding of Free MCC to APC/C.
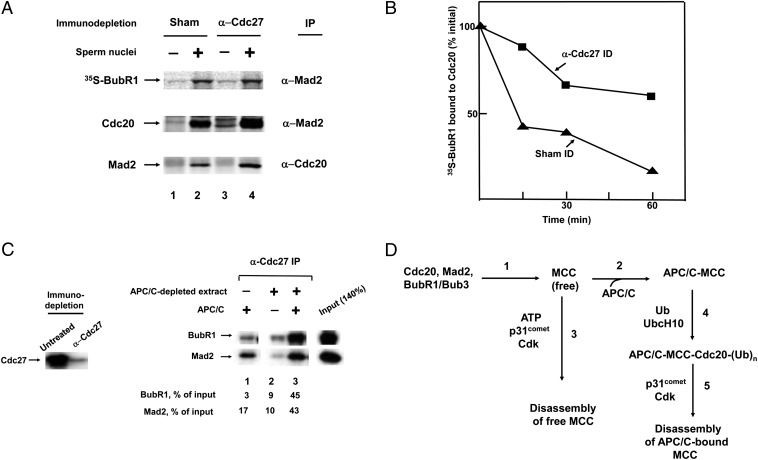
The results described above bear not only on the mechanisms of the disassembly of the different pools of MCC, but also on the role of the free MCC pool. Because MCC bound to APC/C is not released at a significant rate in the absence of ubiquitylation (Fig. 1_B_) and ubiquitylation-dependent release of MCC components from APC/C is accompanied by partial disassembly of MCC (Fig. 1 C and D), it seemed unlikely that the pool of free MCC originates by dissociation from APC/C. We therefore examined the possibility that this pool may serve as the precursor for the formation of APC/C–MCC. Several reports have shown that MCC can be assembled on APC/C from individual components in vitro (22, 24), but it is not known whether assembly of MCC on APC/C is obligatory, or that the mitotic checkpoint stimulates the formation of free MCC in the absence of binding to APC/C. We examined this problem in a cell-free system from Xenopus eggs, in which mitotic checkpoint can be reproduced by supplemented sperm nuclei and nocodazole (25). In the experiment shown in Fig. 3_A_, Xenopus egg extracts were subjected to immunodepletion with anti-Cdc27 (that removed ∼90% of APC/C), or to sham immunodepletion, and then were incubated with in vitro-translated 35S-labeled BubR1 in the absence or presence of sperm nuclei. 35S-BubR1 was used because of the lack of a suitable antibody for the detection of Xenopus BubR1. The formation of MCC was monitored by immunoprecipitation with antibodies directed against Xenopus Mad2 or Cdc20. With sham-treated egg extract, sperm nuclei induced the formation of MCC, as indicated by immunoprecipitation of 35S-BubR1 and of endogenous Cdc20 with anti-Mad2 and by immunoprecipitation of Mad2 with anti-Cdc20 (Fig. 3_A_, lanes 1 and 2). Immunodepletion of APC/C by anti-Cdc27 did not decrease the formation of MCC, but actually increased it about twofold. In this case, too, the assembly of 35S-BubR1, Cdc20 and Mad2 into MCC was dependent upon supplemented sperm nuclei (Fig. 3_A_, lanes 3 and 4). These results suggested that the presence of APC/C is not necessary for the checkpoint-signal induced assembly of MCC.
Fig. 3.
Checkpoint-dependent assembly of free MCC and its binding to APC/C. (A) MCC is assembled in APC/C-depleted extracts of Xenopus eggs. Extracts of Xenopus eggs were prepared as described in Methods, and were subjected to immunodepletion with anti-Cdc27 or to sham treatment. Samples (20 μL) of extracts were supplemented with 1 μL of in vitro translated 35S-BubR1 and were incubated (23 °C, 10 min), with or without sperm nuclei (5,000 per μL), as indicated. Subsequently, nocodazole (1 ng/μL) was added and samples were incubated for further 20 min. Samples were subjected to immunoprecipitation with anti-XCdc20 or anti-XMad2 antibodies. Immunoprecipitates were subjected to SDS/PAGE and were probed for radioactivity or by immunoblotting. (B) Immunodepletion of APC/C slows down the disassembly of MCC in Xenopus extracts. Extracts from Xenopus eggs were subjected to immunodepletion with anti-Cdc27 or to sham treatment and then were incubated with 35S-BubR1, sperm nuclei and nocodazole, as in A. Subsequently, samples were centrifuged (12,600 × g 1 min, twice); this treatment lowered the concentration of sperm nuclei four- to fivefold. Samples were incubated at 23 °C for the time periods indicated and were subjected to immunoprecipitation with anti-XCdc20. (C) High-affinity binding of free MCC to APC/C. Extracts form checkpoint-arrested HeLa cells were depleted of APC/C by three treatments with anti-Cdc27. This treatment removed more than 90% of APC/C (Left). The amount of MCC in this preparation was estimated by immunoprecipitation with anti-Cdc20 and immunoblotting for BubR1 and Mad2 (“Input”). Samples of 10 μL of APC/C-depleted extract were mixed with 15 μL of APC/C purified from mitotic HeLa cells by affinity chromatography on p13suc1 as described (27). The samples were allowed to stay on ice for 1 h, and then were precipitated with anti-Cdc27, washed with buffer containing 300 mM NaCl and 10% Nonidet P-40 and were subjected to immunoblotting. Numbers below lanes 1–3 indicate the percentage of input precipitated with anti-Cdc27. (D) Proposed sequence of events in the formation and disassembly of free and APC/C-bound MCC. See Discussion for details.
We further examined the cause for the increased accumulation of MCC in APC/C-depleted Xenopus extracts. It seemed reasonable that this could be due to decreased rate of MCC disassembly, caused by the lack of the APC/C-catalyzed, ubiquitylation-dependent disassembly pathway. To examine this problem, 35S-BubR1 was first assembled into MCC in either APC/C-depleted or sham-treated Xenopus egg extracts incubated with sperm nuclei. Subsequently, the checkpoint was terminated by removal of sperm by centrifugation, incubation was continued and the dissociation of 35S-BubR1 from Cdc20 was monitored by immunoprecipitation. As shown in Fig. 3_B_, the rate of the dissociation of 35S-BubR1 from Cdc20 was markedly reduced by the depletion of APC/C. This finding suggested the existence of an APC/C-catalyzed MCC disassembly pathway in Xenopus eggs, similar to that observed in HeLa cell extracts.
The above experiments suggested that the mitotic checkpoint signal can stimulate the assembly of free MCC, but did not show that free MCC can actually be a precursor for high-affinity binding to APC/C. To formally address this issue, we mixed APC/C-depleted checkpoint extracts from HeLa cells, which contain MCC, with APC/C purified from the same source, and monitored the binding of MCC to APC/C by immunoprecipitation with anti-Cdc27 and immunoblotting for BubR1 and Mad2. As shown in Fig. 3_C_, Right, lane 1, APC/C purified from checkpoint extracts contained some residual MCC components. Small amounts of BubR1 and Mad2 were also precipitated from APC/C-depleted extract (Fig. 3_C_, lane 2). However, after mixing these two preparations, the amounts of BubR1 and Mad2 precipitated by anti-Cdc27 greatly exceeded the sum of the values obtained by the two separate preparations (Fig. 3_C_, lane 3). Because the immunoprecipitates were subjected to washes under stringent conditions, these data suggested that free MCC binds to APC/C at high affinity.
Discussion
The data presented in this paper provide insights into the relationship between of APC/C-bound and free pools of MCC and also into the mechanisms of their disassembly in the inactivation of the mitotic checkpoint. In checkpoint-arrested HeLa cells, the size of the pool of free MCC greatly exceeds that of APC/C–MCC (Fig. 1_A_). In view of the high affinity of the binding of MCC to APC/C and the complex and energy-dependent mechanisms of disassembly, it does not seem likely that free MCC is derived by simple dissociation from APC/C on which MCC may be assembled. It rather seems that the pool of free MCC serves as precursor for binding to APC/C. The scheme in Fig. 3_D_ shows our proposal on the processes involved in the formation and disassembly of these two pools of MCC. We assume that when the checkpoint is turned on, free MCC is first formed (Fig. 3_D_, step 1), which then rapidly binds to APC/C and inhibits its activity (Fig. 3_D_, step 2). This suggestion is supported by the finding that the presence of APC/C is not required for the checkpoint-dependent assembly of MCC (Fig. 3_A_). The large pool of free MCC may be derived from the excess formed after all APC/C is bound to MCC. In view of the rapid turnover of APC/C-associated Cdc20 when the checkpoint is turned on (7, 11–14), the large excess of free MCC may ensure effective and total inhibition of APC/C during active mitotic checkpoint.
After the checkpoint is satisfied, both APC/C-bound and free pools of MCC have to be disassembled, otherwise free MCC would continue to bind to and to inhibit APC/C. Free MCC is disassembled rapidly in a process that is stimulated by the Mad2-binding protein p31comet and by Cdk-catalyzed phosphorylation of Cdc20 (ref. 21 and Fig. 3_D_, step 3). In addition, free MCC is also disassembled in a process that requires ATP hydrolysis, but not Cdk action (21). The mode of the action of this ATP-dependent disassembly of MCC remains to be elucidated.
We have confirmed the involvement of APC/C-catalyzed ubiquitylation in the disassembly of APC/C-bound MCC and found that Cdc20 is the only component of MCC that is released mostly in polyubiquitylated forms; among other MCC components, only a small fraction of BubR1 is polyubiquitylated (Fig. 1_B_). The polyubiquitylation of Cdc20 is directly required for its release (Fig. 1_E_). The role of the ubiquitylation of Cdc20 does not seem to be its dissociation from Mad2, as suggested previously (5), because we found that at least a part of released Mad2 is still bound to polyubiquitylated Cdc20 (Fig. 1 C and D). It appears more likely that the polyubiquitylation of Cdc20 in MCC interferes with its interaction with APC/C. It may also dissociate Cdc20 from BubR1, because free BubR1 is released under these conditions (Fig. 1_C_). We also found that p31comet and Cdk strongly stimulate the release of MCC components from APC/C–MCC under conditions of ubiquitylation (Fig. 2_A_). p31comet and Cdk do not stimulate the ubiquitylation of Cdc20, but markedly accelerate the rate of the release of ubiquitylated Cdc20 (Fig. 2 B and C). We propose a two-step mechanism in which the polyubiquitylation of Cdc20 in MCC first partially decreases its affinity to APC/C (Fig. 3_D_, step 4); this is followed by the action of p31comet and Cdk, which further disassemble and release MCC components (Fig. 3_D_, step 5). Assuming that the release of polyubiquitylated Cdc20 is required for its degradation, this may explain the finding that p31comet is required for Cdc20 degradation in mitotic checkpoint (14).
It should be noted that additional processes, not shown in the above scheme, apparently also participate in the disassembly of the different pools on MCC. We observed that ubiquitylation system, by itself, caused considerable release of MCC components from immunopurified APC/C–MCC, although maximal rates required p31comet and Cdk (Figs. 1 and 2). This may be due to partial reduction of the affinity of ubiquitylated MCC to APC/C, or to the presence of endogenous p31comet in the preparation of APC/C–MCC, because p31comet binds strongly to MCC (14). We have also found some release of MCC components from APC/C–MCC by p31comet and Cdk in the absence of ubiquitylation (Fig. 2_A_). The existence of such alternate, ubiquitylation-independent pathways for the disassembly of APC/C-bound MCC may explain the report that the expression of K-less Cdc20 does not prevent checkpoint inactivation or MCC disassembly (7). Further investigation is required to assess the contribution of alternative pathways of MCC disassembly in the inactivation of the mitotic checkpoint.
Methods
Extracts from nocodazole-arrested HeLa cells were prepared as described (15). Extracts from Xenopus eggs and sperm nuclei were prepared as described (26). His6-p31comet was expressed in bacteria and was purified as described (20). His6-tagged UbcH10 and p27Kip1 were expressed in bacteria and were purified on Ni-NTA-agarose (Qiagen). Cdk1-Δ88-cyclin B was prepared and purified as described (27). The following polyclonal antibodies were raised in rabbits and were purified by affinity chromatography on the respective antigens: anti-Cdc27, the 17-aa C-terminal peptide of human and Xenopus Cdc27; anti-XCdc20, 27-aa C-terminal peptide of Xenopus Cdc20; antiXMad2, full-length Xenopus Mad2. Polyclonal antibodies against human Cdc20 and BubR1 were as described (15, 16). In all cases, rabbit polyclonal antibodies were used for immunoprecipitation and immunodepletion, which were carried out as described (15–17), except for immunodepletion of Xenopus extracts, in which case we used Protein A magnetic beads (New England Biolabs). For immunoblotting of human proteins, the following mouse monoclonal antibodies were used: Cdc27, BubR1, and Bub3 (BD Transduction Laboratories); Cdc20 (Santa Cruz Biotechnology sc-13162); Mad2 (MBL Laboratories K0167). For detection of Xenopus proteins we used anti-Cdc27 and anti-Mad2 from BD Transduction Laboratories and anti-Cdc20 from Abcam (18217). Immunoblots were quantified with ImageQuant RT ECL (GE Healthcare) or with Odyssey (Li-Cor) instruments.
For immunopurification of APC/C–MCC, samples of 100 μL of extracts from checkpoint-arrested HeLa cells were precipitated with 10 μg of anti-Cdc27 antibody bound to 20 μL of Affi-Prep Protein A beads (Bio-Rad). Following rotation at 4 °C for 2 h, beads were washed twice with 1-mL portions of Buffer A [50 mM Tris⋅HCl (pH 7.2), 1 mg/mL BSA, 20% (vol/vol) glycerol and 0.5 mM DTT] that contained 300 mM NaCl and twice with Buffer A without salt. Beads were resuspended in 4 volumes of Buffer A and stored at −70 °C in small samples. To determine the release of MCC components from immunopurified APC/C–MCC, samples of 3 μL of beads were suspended in 30 μL of a reaction mixture that contained 40 mM Tris⋅HCl (pH 7.6), 1 mg/mL BSA, 5 mM MgCl2, 1 mM DTT, 10% (vol/vol) glycerol, 1 mM ATP, 10 mM phosphocreatine, and 100 mg/mL creatine phosphokinase. Where indicated, a ubiquitylation mixture consisting of 0.2 μM E1, 1 μM UbcH10, and 100 μM ubiquitin was added. Other additions were as described in the figure legends. Samples were incubated at 23 °C with shaking at 1,400 rpm for the time periods specified and centrifuged briefly, and supernatants were passed through 0.45 μM Ultra-free centrifugal filters (Millipore) to ensure complete removal of beads from supernatants. Samples of the supernatants were analyzed by immunoblotting for MCC components.
The plasmid for the expression of mutant (“K-less”) Cdc20 in which all lysines were converted to arginines (7) was generously provided by Jakob Nilsson and Jonathon Pines (University of Cambridge, UK); it was subcloned into pcDNA3. 35S-labeled wild-type and K-less Cdc20s were expressed by in vitro transcription-translation, using TnT T7 Quick kit (Promega). These preparations were subjected to immunodepletion with anti-Cdc27, as described (21). To exchange 35S-Cdc20s into APC/C–MCC, samples of 15 μL of the preparations were mixed with 100 μL of immunopurified APC/C–MCC on anti-Cdc27 beads (20% suspension, beads/Buffer A). Following rotation at 4 °C for 90 min, beads were thoroughly washed as described above for the preparation of affinity-purified APC/C–MCC.
Supplementary Material
Supporting Information
Acknowledgments
We thank Dr. Rey-Huei Chen for valuable advice on the use of the Xenopus cell-free system, Dr. Michael Fry for helpful comments on the manuscript, and Drs. Jakob Nilsson and Jonathon Pines for providing the plasmid for the expression of K-less Cdc20. This work was supported by grants from the Israel Science Foundation and the Diane and Guilford Glazer Distinguished Chair of the Israel Cancer Research Fund.
Footnotes
The authors declare no conflict of interest.
References
- 1.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8(5):379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, Yu H. Mutual regulation between the spindle checkpoint and APC/C. Semin Cell Dev Biol. 2011;22(6):551–558. doi: 10.1016/j.semcdb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray AW. A brief history of error. Nat Cell Biol. 2011;13(10):1178–1182. doi: 10.1038/ncb2348. [DOI] [PubMed] [Google Scholar]
- 4.Sudakin V, Chan GKT, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001;154(5):925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446(7138):921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 6.Stegmeier F, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446(7138):876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol. 2008;10(12):1411–1420. doi: 10.1038/ncb1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster SA, Morgan DO. The APC/C subunit Mnd2/Apc15 promotes Cdc20 autoubiquitination and spindle assembly checkpoint inactivation. Mol Cell. 2012;47(6):921–932. doi: 10.1016/j.molcel.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzunova K, et al. APC15 mediates CDC20 autoubiquitylation by APC/C(MCC) and disassembly of the mitotic checkpoint complex. Nat Struct Mol Biol. 2012;19(11):1116–1123. doi: 10.1038/nsmb.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia L, et al. Defining pathways of spindle checkpoint silencing: Functional redundancy between Cdc20 ubiquitination and p31(comet) Mol Biol Cell. 2011;22(22):4227–4235. doi: 10.1091/mbc.E11-05-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J, Chen RH. Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev. 2004;18(12):1439–1451. doi: 10.1101/gad.1184204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King EM, van der Sar SJ, Hardwick KG. Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS ONE. 2007;2(4):e342. doi: 10.1371/journal.pone.0000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge S, Skaar JR, Pagano M. APC/C- and Mad2-mediated degradation of Cdc20 during spindle checkpoint activation. Cell Cycle. 2009;8(1):167–171. doi: 10.4161/cc.8.1.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varetti G, Guida C, Santaguida S, Chiroli E, Musacchio A. Homeostatic control of mitotic arrest. Mol Cell. 2011;44(5):710–720. doi: 10.1016/j.molcel.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Braunstein I, Miniowitz S, Moshe Y, Hershko A. Inhibitory factors associated with anaphase-promoting complex/cylosome in mitotic checkpoint. Proc Natl Acad Sci USA. 2007;104(12):4870–4875. doi: 10.1073/pnas.0700523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eytan E, et al. Two different mitotic checkpoint inhibitors of the anaphase-promoting complex/cyclosome antagonize the action of the activator Cdc20. Proc Natl Acad Sci USA. 2008;105(27):9181–9185. doi: 10.1073/pnas.0804069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miniowitz-Shemtov S, Teichner A, Sitry-Shevah D, Hershko A. ATP is required for the release of the anaphase-promoting complex/cyclosome from inhibition by the mitotic checkpoint. Proc Natl Acad Sci USA. 2010;107(12):5351–5356. doi: 10.1073/pnas.1001875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habu T, Kim SH, Weinstein J, Matsumoto T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 2002;21(23):6419–6428. doi: 10.1093/emboj/cdf659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia G, et al. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 2004;23(15):3133–3143. doi: 10.1038/sj.emboj.7600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teichner A, et al. p31comet Promotes disassembly of the mitotic checkpoint complex in an ATP-dependent process. Proc Natl Acad Sci USA. 2011;108(8):3187–3192. doi: 10.1073/pnas.1100023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miniowitz-Shemtov S, et al. Role of phosphorylation of Cdc20 in p31(comet)-stimulated disassembly of the mitotic checkpoint complex. Proc Natl Acad Sci USA. 2012;109(21):8056–8060. doi: 10.1073/pnas.1204081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lara-Gonzalez P, Scott MIF, Diez M, Sen O, Taylor SS. BubR1 blocks substrate recruitment to the APC/C in a KEN-box-dependent manner. J Cell Sci. 2011;124(Pt 24):4332–4345. doi: 10.1242/jcs.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciechanover A, Ben-Saadon R. N-terminal ubiquitination: More protein substrates join in. Trends Cell Biol. 2004;14(3):103–106. doi: 10.1016/j.tcb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Kulukian A, Han JS, Cleveland DW. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell. 2009;16(1):105–117. doi: 10.1016/j.devcel.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994;79(3):475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 26.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 27.Sudakin V, Shteinberg M, Ganoth D, Hershko J, Hershko A. Binding of activated cyclosome to p13(suc1). Use for affinity purification. J Biol Chem. 1997;272(29):18051–18059. doi: 10.1074/jbc.272.29.18051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information