Anillin acts as a bifunctional linker coordinating midbody ring biogenesis during cytokinesis (original) (raw)
. Author manuscript; available in PMC: 2013 Jul 2.
Published in final edited form as: Curr Biol. 2012 Jan 5;22(3):197–203. doi: 10.1016/j.cub.2011.11.062
Summary
Animal cell cytokinesis proceeds via constriction of an actomyosin-based contractile ring (CR) [1, 2]. Upon reaching a diameter of ~1 μm [3], a midbody ring (MR) forms to stabilize the intercellular bridge until abscission [4-6]. How MR formation is coupled to CR closure and how plasma membrane anchoring is maintained at this key transition is unknown. Time-lapse microscopy of Drosophila S2 cells depleted of the scaffold protein, Anillin [7-9], revealed that Anillin is required for complete closure of the CR and formation of the MR. Truncation analysis revealed that Anillin N-termini connected with the actomyosin CR and supported formation of stable MR-like structures, but these could not maintain anchoring of the plasma membrane. Conversely, Anillin C-termini failed to connect with the CR or MR but recruited the septin, Peanut, to ectopic structures at the equatorial cortex. Peanut depletion mimicked truncation of the Anillin C-terminus, resulting in MR-like structures that failed to anchor the membrane. These data demonstrate that Anillin coordinates the transition from CR to MR, and that it does so by linking two distinct cortical cytoskeletal elements. One apparently acts as the core structural template for MR assembly, while the other ensures stable anchoring of the plasma membrane beyond the CR stage.
Results and discussion
The transition from CR to MR requires Anillin
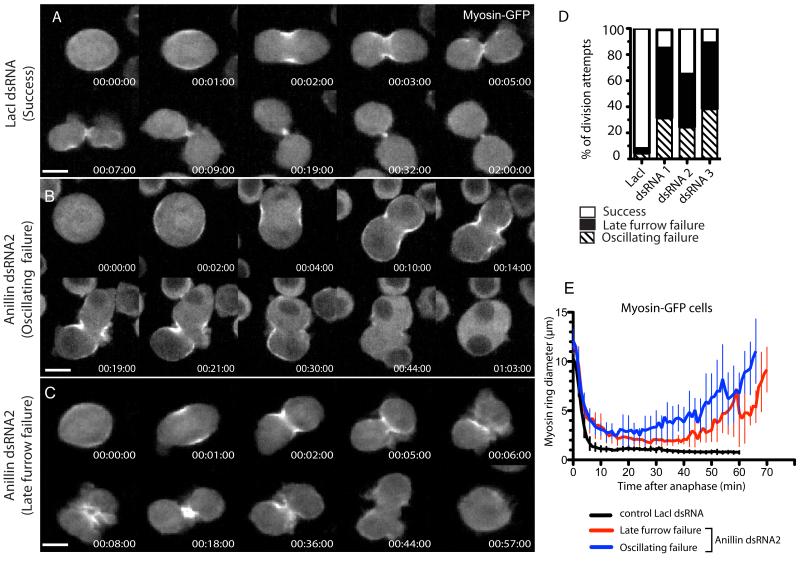
Anillin localizes to the CR and MR [10], and has conserved N-terminal domains shown in Drosophila to bind F-actin [10] and Cindr [11], and in vertebrates to bind myosin II [12] and the formin, mDia2 [13]. Conserved C-terminal AH/PH domains can also bind RacGAP50c/Tum in Drosophila [14, 15], and septins [16, 17] and Rho [18] in mammals. Because loss of Anillin blocks cytokinesis during late furrowing [12, 18-23], we sought to test whether Anillin might play a role in MR formation. We re-examined Anillin depletion phenotypes in Drosophila cells. Each of 3 distinct dsRNAs (Fig. S1A) depleted Anillin by >80% at 72 h (Fig. S1B), and produced penetrant and similar phenotypes, captured by time-lapse spinning disc confocal microscopy of cells stably expressing myosin-GFP (Fig. 1) or GFP-tubulin (Fig. S1D). Images acquired every 4-5 min over several days revealed that cells failed their first division 30-72 h after dsRNA administration. Myosin-GFP recruitment and furrow initiation appeared normal, but cells blebbed excessively and failed cytokinesis via two similar yet distinct phenotypes. In one population, classed as oscillating failures, furrows ingressed to approximately 50% before oscillating laterally (Fig. 1B,D) as described previously in Drosophila [21] and human cells [12, 18, 23], yielding binucleate cells 68±10 min after anaphase. In the second population, furrows ingressed beyond 50% and reached a semi-stable state (albeit with minor oscillations) that persisted for ~20 min before reopening (Fig. 1C and Movie S1), yielding binucleate cells 1h21±16 min after anaphase. These late furrow failures were the more prevalent (Fig. 1D).
Fig. 1. Complete closure of the CR and formation of the MR requires Anillin.
A Time-lapse sequence of control myosin-GFP cell undergoing cytokinesis after incubation with control (LacI) dsRNA. See movie S1, left hand cell. B Anillin-depleted myosin-GFP expressing cell failing cytokinesis via an oscillating furrow phenotype. C Anillin-depleted myosin-GFP expressing cell failing cytokinesis via a late furrow phenotype. See movie S1, right hand cell. D Quantification of phenotypic classes of cells succeeding or undergoing their first failed division following 30-72 h incubation with each dsRNA (n=50 each). E Diameters of myosin-GFP rings from the 3 phenotypic classes plotted over time (mean ± sd, n=10 per condition). See also Fig. S1. Scale bars, 5 μm.
Images acquired at 30-60 sec intervals revealed that control (LacI RNAi) CRs closed within 12±2 min (mean±sd, n=10) of anaphase, producing MR structures of ~1 μm in diameter (Fig. 1E). Initial closure rates of Anillin-depleted CRs were comparable to controls but they progressively slowed such that maximal ingression was reached 25 min post anaphase with a mean diameter of ~3 μm (Fig. 1E). While total myosin-GFP intensity declined sharply in control furrows, the decline slowed markedly in Anillin-depleted furrows, resulting in elevated levels of myosin at the time of maximal ingression (Fig.1C and Fig. S1C).
In addition, Anillin-depleted cells failed to display a thinning of the center of the intercellular bridge (marked by GFP-tubulin, Fig. S1D), an event that normally accompanies MR formation.
Thus, in addition to its role in preventing cleavage furrow oscillations, Anillin is required to maintain the normal rate and extent of CR closure and to allow formation of the MR, even when furrows do not oscillate.
C-terminally truncated Anillin supports formation of MR-like structures that fail to stably anchor the plasma membrane
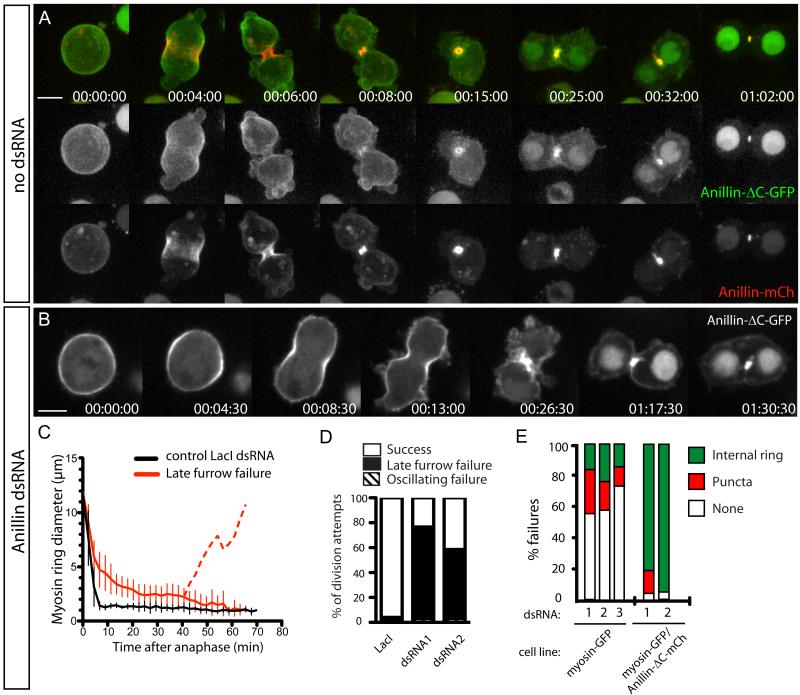
Towards functionally dissecting Anillin, we generated stable cell lines inducibly expressing Anillin amino acids 1-758 fused to GFP or mCherry (Anillin-ΔC-FP, Fig. 2).
Fig. 2. Anillin-ΔC supports formation of MR-like structures that fail to anchor the plasma membrane.
A Time-lapse sequence of a typical cell expressing Anillin-mCh (lower panels, red in merged) and Anillin-ΔC-GFP (middle panels, green in merged) undergoing furrowing and MR formation. See also movie S2, upper cell. B Timelapse sequence of a typical cell depleted of endogenous Anillin and expressing Anillin-ΔC-GFP. See also movie S2, lower cell. C Myosin-GFP ring diameters plotted over time from cells co-expressing Anillin-ΔC-mCh following control or endogenous Anillin RNAi (mean ± sd, n=10 per condition). Dotted line represents the equatorial diameter from mean time of furrow regression. D Quantification of phenotypic classes observed in Anillin-ΔC-GFP-expressing cells succeeding or undergoing their first failed division following 30-72 h control (LacI) or Anillin RNAi (n=50 per condition). E quantification from time-lapse records of each terminal binucleate phenotype (shown in Fig. S2A) for the indicated cell lines depleted of endogenous Anillin using the indicated dsRNAs (n≥30 failed division attempts per condition). See also Fig. S2. Scale bars, 5 μm.
In cells co-expressing endogenous Anillin and/or full-length Anillin-FP (Fig. 2A and Movie S2, upper cell), Anillin-ΔC-FP localized to nuclei during interphase (not shown) and to the cell cortex at metaphase (Fig. 2A). However, it was poorly recruited to the furrow cortex during ingression (Fig. 2A), indicating that although the Anillin N-terminus can localize to the cortex, the deleted AH/PH region must play a major role in recruiting Anillin during furrowing. Although barely detectable at the furrow apex at CR closure (Fig. 2A, 00:06:00), Anillin-ΔC-FP was still recruited to the MR during the following 10-20 min (Fig. 2A) and remained there until after abscission (not shown).
In Anillin-depleted cells, Anillin-ΔC-FP localized to the cell cortex at metaphase, became enriched at the equatorial cortex during early anaphase, and remained cortical throughout furrowing (Fig. 2B). Furrowing was slowed to a similar extent to cells depleted of all sources of Anillin, blebbing of the plasma membrane persisted and cytokinesis failed almost exclusively with the late furrow phenotype (Fig. 2B-D and Movie S2, lower cell). At the close of the prolonged furrowing, Anillin-ΔC-FP localized robustly to a structure resembling a MR, although its diameter was generally greater than a bona fide MR (Fig. 2B-C). With a mean time of 40 min after anaphase (n=10), the plasma membrane lost attachment to the rings and regressed, resulting in binucleate cells harboring internal MR-like structures (Fig. 2B and Movie S2, lower cell), whose diameters decreased over ~20 min to that approaching control MRs (Fig. 2C). These MR-like structures persisted for many hours and were observed in 75% of failed division attempts, captured by time-lapse microscopy (n>50).
Myosin-GFP provided an independent marker of these ring structures (Fig. S2B,C). From timelapse recordings, 80-95% of cells co-expressing Anillin-ΔC-mCherry and myosin-GFP failed cytokinesis with internal MR-like structures (n>50, Fig. 2E and Fig. S2A), when depleted of endogenous Anillin. Conversely, only 18-25% (depending on dsRNA used) of cells expressing only myosin-GFP, depleted of Anillin, displayed such internal rings after failure: 12-30% displayed puncta (Fig. 2E and Fig. S2A); while 50-75% exhibited no persistent structures (Fig. 2E and Fig. S2A), even though myosin had been present at elevated levels during furrowing (Fig. 1D and Fig. S1C). Thus Anillin-ΔC-FP promoted the recruitment or stabilization of myosin-GFP to the structures. Myosin is a bona fide MR marker, consistent with these structures being analogous to MRs. Additional support includes reduced accessibility of both structures to Anillin antibodies (Fig. S2D,E), reduced levels of phalloidin-staining of both structures (Fig. S2F,G), and shared resistance of both structures to 1μg/ml of the inhibitor of actin polymerization, Latrunculin A (not shown).
N-terminally truncated Anillin localizes to cortical material that is disconnected from ring structures
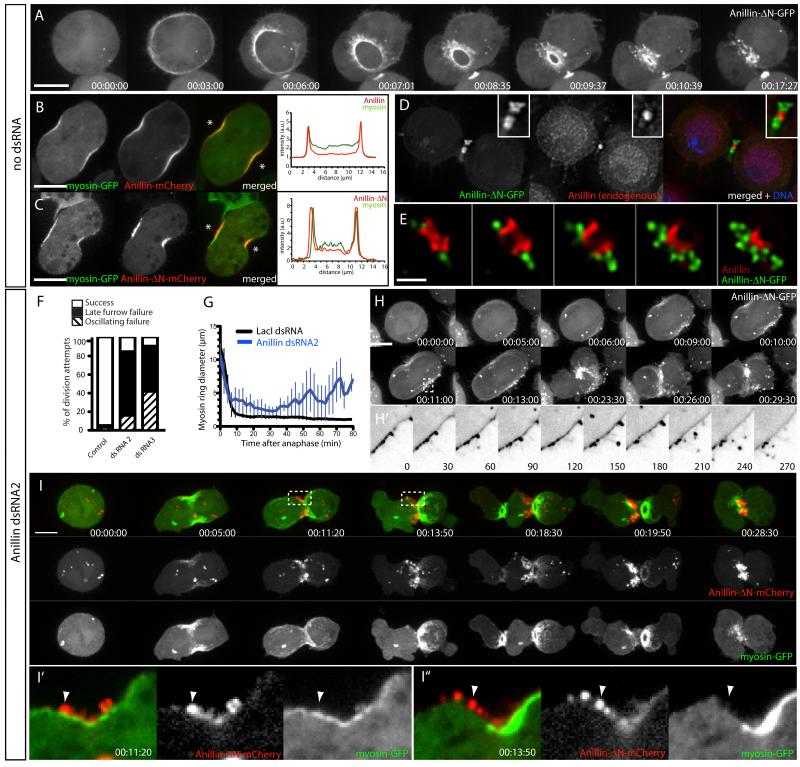
The reciprocal truncation, comprising only the AH/PH region, (Anillin-ΔN-FP) was cytoplasmic at metaphase (unlike full-length Anillin) and robustly recruited to the equatorial cortex during anaphase (Fig. 3A and Movie S3, upper cell), consistent with the above experiments and an analogous human truncation [18]. However, during furrowing, when Anillin-FP and myosin-FP normally colocalize (Fig. 3B), Anillin-ΔN-FP localized to cortical protrusions that extended outwards beyond co-expressed myosin-FP (Fig 3C) and endogenous Anillin (Fig. S3B), appearing as puncta that trailed the apex of the closing CR (Fig. 3A and Movie S3, middle cell). Anillin-ΔN-FP partially colocalized with a plasma membrane probe during furrowing (mCherry-PLCδ-PH, Fig. S3A) but was excluded from the nascent MR marked by endogenous Anillin (Fig. 3D-E), rather displaying a peri-MR localization that was progressively lost by 60-90 min post-furrowing (not shown). Anillin-ΔN-GFP puncta colocalized poorly with phalloidin-labeled F-actin (Fig. S3C-E).
Fig. 3. Anillin-N forms cortical material that is disconnected from ring structures.
A Time-lapse sequence of a cell expressing Anillin-ΔN-GFP and mCh-tubulin (not shown). See also movie S3, upper cell. B-C Cell co-expressing myosin-GFP and Anillin-mCh (B) or Anillin-ΔN-mCh (C) during early furrowing. Intensity profiles along a line drawn between the asterisks are shown. D-E Deconvolved single Z planes (or montage of Z planes, E) of MRs fixed and stained for endogenous Anillin (red in merged) in cells co-expressing Anillin-ΔN-GFP (green). F Quantification of phenotypic classes observed in Anillin-ΔN-GFP-expressing cells succeeding or undergoing their first failed division following 30-72 h control (LacI) or Anillin RNAi (n=50 per condition). G Myosin-GFP ring diameters plotted over time in cells co-expressing Anillin-ΔN-mCh following control or endogenous Anillin RNAi (late furrow failures only, mean±sd, n=10 per condition). H Time-lapse sequence of a typical cell depleted of endogenous Anillin and expressing Anillin-ΔN-GFP and mCh-tubulin (not shown). Maximum intensity projection of 6 Z planes. See also movie S3, middle cell. H’ Inverted LUT of single optical section of boxed region in H, enlarged and with higher temporal resolution as indicated (sec). I Time-lapse sequence of a typical cell depleted of endogenous Anillin and expressing Anillin-ΔN-mCh (red) and myosin-GFP (green) attempting cytokinesis (maximum intensity projection). See also movie S3, lower cell. I’-I” Magnified views of single z planes from boxed regions in I. See also Fig. S3. Scale bars, 5 μm.
Cells overexpressing Anillin-ΔN-FP furrowed with normal kinetics and completed cytokinesis (Fig. 3F-G). However, upon depletion of endogenous Anillin (using dsRNA2 or dsRNA3), 90% of division attempts failed (n=50) in a manner similar to Anillin-depleted control cells (Fig. 3F-G). Anillin-ΔN-FP was still recruited to the equatorial cortex and formed puncta that were associated with protrusions. Some of these protrusions clearly extended outwards for several μm (Fig. 3H-H’ and Movie S3, middle cell), reminiscent of the microvillus-like projections that emanate from CRs of sea urchin embryos [24]. Spatial segregation of Anillin-ΔN-FP and myosin-GFP was striking after endogenous Anillin depletion. While both FPs were simultaneously recruited to the equatorial cortex (Fig. 3I and Movie S3, lower cell), Anillin-ΔN-mCherry abruptly appeared in puncta external to the myosin-GFP. Myosin-GFP then oscillated back and forth across the equator, while Anillin-ΔN-mCherry remained equatorial (see Fig. S3F for additional example). Anillin-ΔN-FP presumably remained anchored via additional membrane and/or cytoskeletal components such as microtubules [14, 15, 21] or septins (see below). Anillin-ΔN-FP localized to the extreme cell periphery, even in cells treated with 1 μg/ml LatA to disrupt the cortical actin cytoskeleton (Fig. S3G). Given that the puncta were external to actomyosin, we interpret them as representing plasma membrane-associated material that was inappropriately disconnected from the underlying actomyosin cytoskeleton.
Collectively, these data show that the Anillin N-terminus localizes to the CR and is sufficient to allow its transformation into an MR structure, while the C-terminal domains of Anillin are required for robust recruitment during furrowing, timely closure of the CR and, crucially, for stable anchoring of the plasma membrane by the MR.
We also co-expressed Anillin-ΔN-FP and Anillin-ΔC-FP in the same cells. Each truncation localized to the same distinct cortical structures as when expressed alone, and co-expression did not rescue loss of Anillin, indicating that the N- and C-termini need to be physically linked to faithfully coordinate the transition from CR to MR (data not shown).
The septin, Peanut, acts with the Anillin C-terminus to anchor the plasma membrane to cytokinetic rings
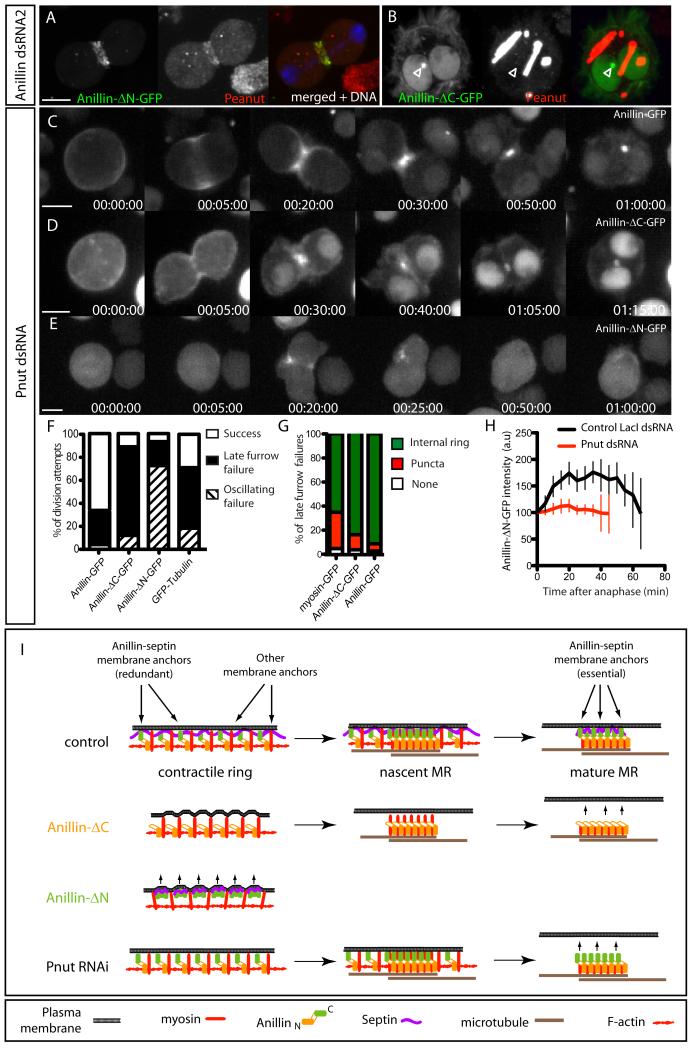
The Anillin C-terminus binds septins [17] and Anillin recruits septins to the CR [21, 25, 26] and to actin filaments in vitro [16]. We tested whether septins were constituents of the cortical material to which Anillin-ΔN-FP localized. Immunofluoresence confirmed Pnut colocalization with Anillin-ΔN-FP during cytokinesis (Fig. 4A), and also in cortical linear structures in interphase cells (Fig. S4), similar to those reported for a human Anillin C-terminal fragment [17], and that we interpret as being plasma-membrane associated. Conversely, Pnut was undetectable in the Anillin-ΔC-FP-dependent MR-like structures that formed when endogenous Anillin was depleted (Fig. 4B, the localization of Pnut to tubular cytoplasmic structures is normal for S2 cells [21]).
Fig. 4. The septin, Peanut, acts with the Anillin C-terminus to anchor the plasma membrane to the MR.
A Pnut immunofluorescence of a cell expressing Anillin-ΔN-GFP during furrowing. B Pnut immunofluorescence in a cell expressing Anillin-ΔC-GFP following Anillin RNAi. Open arrowhead marks an Anillin-ΔC-FP-positive MR-like structure that does not label with Pnut antibody. Note that the localization of Pnut to linear cytoplasmic structures is normal for S2 cells [21]. C-E Time-lapse sequences of cells expressing Anillin-GFP (C), Anillin-ΔC-GFP (D) and Anillin-ΔN-GFP (E) following a 7-day incubation with Pnut dsRNA. See also movie S4. F Quantification from time-lapse records of each phenotypic class for the indicated cell lines depleted of pnut (n=30 division attempts per condition). G Percentages of failures that resulted in GFP localizing to persistent internal midbody ring-like structures in the indicated cell lines. H Quantitation over time of total Anillin-ΔN-GFP intensities at the cytokinetic apparatus following control or Pnut RNAi. I Cartoon summary of results and model for Anillin and septin action during the transition from CR to MR. See also Fig. S4. Scale bars, 5 μm.
Live imaging of Anillin-GFP expressing cells following 7-8 day incubation with Pnut dsRNAs (Fig. 4 C) revealed a 34% cytokinesis failure rate (n>80, Fig. 4F). In 87% of these failed attempts (n>20), Anillin-GFP localized to persistent MR-like structures that failed to anchor the plasma membrane (Fig. 4C,G and Movie S4), indicating that Pnut depletion phenocopies truncation of the Anillin C-terminus. Parallel depletions of Pnut in control cells marked only by GFP-tubulin resulted in a markedly higher cytokinesis failure rate (71%, n>50, Fig. 4F), suggesting that Anillin-GFP overexpression can partially rescue loss of Pnut.
In Anillin-ΔC-FP-expressing cells, Pnut depletion induced an 89% failure rate (n>40), predominantly with a late furrow phenotype (Fig. 4D,F). In 91% of failed cells, Anillin-ΔC-FP localized to internal MR-like structures following furrow regression (Fig. 4G).
In Anillin-ΔN-FP-expressing cells, Pnut depletion induced a 93% failure rate (n>50), predominantly with an oscillating furrow phenotype (Fig. 4F). Pnut depletion also diminished recruitment of Anillin-ΔN-FP to the cleavage furrow, where it no longer formed puncta (Fig. 4E,H), and abolished the ectopic structures in interphase cells (Fig. S4E).
Thus the C-terminus of Anillin and Pnut are each dispensable for forming stable MR-like structures, but they cooperate to form ectopic structures and each is required for the MR to anchor the plasma membrane. Determining the precise mechanism of membrane anchoring requires further study. However, given that Anillin can localize to the MR independently of Pnut (Fig. 4), yet is insufficient to tether the membrane in the absence of Pnut, it seems likely that Anillin organizes a septin-dependent membrane anchor [27].
In summary, we show that Anillin is required for complete CR closure and concomitant MR formation. The Anillin N-terminus (Anillin-ΔC-FP), recruited to CRs, was sufficient to form stable structures that bore hallmarks of bona fide MRs but that failed to anchor the plasma membrane. The Anillin C-terminus (Anillin-ΔN-FP) was independently targeted to the equatorial cortex where it formed septin-dependent structures that were excluded from the actomyosin CR and the nascent MR.
These observations suggest that the MR derives directly from the CR, rather than forming as a separate entity, and lead to a model (Fig. 4I) in which Anillin N-termini guide the transformation of the CR into a stable core MR structure, while the Anillin C-termini simultaneously organize septins to form a robust membrane anchor. The data support early suggestions that Anillin might link the plasma membrane to the CR [10, 28], but suggest that Anillin organizes a redundant membrane anchor at the CR stage that becomes essential at the MR stage. Further studies of these Anillin-dependent structures will likely yield deeper insight into the architecture of the cortical cytokinetic machinery.
Supplementary Material
01
02
03
04
05
Highlights.
- Anillin depletion prevents contractile ring completion and midbody ring biogenesis.
- Anillin N-termini support midbody ring biogenesis but not membrane anchoring.
- Anillin C-termini localize to the cortex but are disconnected from ring structures.
- Septins are required to anchor the plasma membrane to midbody rings.
Acknowledgements
We are indebted to Patrick O’Farrell in whose lab this work was initiated (NIH grant R01-GM037193). We thank C. Field, J. Brill, R. Vale, A. Kiger and the Developmental Studies Hybridoma Bank for reagents. We thank C. Field, U. Eggert, A. Maddox, S. Carreno, A. Piekny, E. Kritikou and C. Sheppard for helpful discussions and comments on the manuscript. GRXH was a Special Fellow of the Leukemia & Lymphoma Society and holds a Fonds de la Recherche en Santé du Québec (FRSQ) junior 1 salary award. This work was supported by the CIHR (MOP-97788), the Canadian Fund for Innovation, the FRSQ, and the Fondation Centre de Cancérologie Charles Bruneau.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–566. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 2.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol. 2010;22:50–56. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullins JM, Biesele JJ. Terminal phase of cytokinesis in D-98s cells. J Cell Biol. 1977;73:672–684. doi: 10.1083/jcb.73.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Schweitzer JK, D’Souza-Schorey C. Finishing the job: cytoskeletal and membrane events bring cytokinesis to an end. Exp Cell Res. 2004;295:1–8. doi: 10.1016/j.yexcr.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Steigemann P, Gerlich DW. Cytokinetic abscission: cellular dynamics at the midbody. Trends Cell Biol. 2009;19:606–616. doi: 10.1016/j.tcb.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 7.D’Avino PP. How to scaffold the contractile ring for a safe cytokinesis - lessons from Anillin-related proteins. J Cell Sci. 2009;122:1071–1079. doi: 10.1242/jcs.034785. [DOI] [PubMed] [Google Scholar]
- 8.Hickson GR, O’Farrell PH. Anillin: a pivotal organizer of the cytokinetic machinery. Biochem Soc Trans. 2008;36:439–441. doi: 10.1042/BST0360439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piekny AJ, Maddox AS. The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol. 2010;21:881–891. doi: 10.1016/j.semcdb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haglund K, Nezis IP, Lemus D, Grabbe C, Wesche J, Liestol K, Dikic I, Palmer R, Stenmark H. Cindr interacts with anillin to control cytokinesis in Drosophila melanogaster. Curr Biol. 2010;20:944–950. doi: 10.1016/j.cub.2010.03.068. [DOI] [PubMed] [Google Scholar]
- 12.Straight AF, Field CM, Mitchison TJ. Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol Biol Cell. 2005;16:193–201. doi: 10.1091/mbc.E04-08-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe S, Ando Y, Yasuda S, Hosoya H, Watanabe N, Ishizaki T, Narumiya S. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol Biol Cell. 2008;19:2328–2338. doi: 10.1091/mbc.E07-10-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Avino PP, Takeda T, Capalbo L, Zhang W, Lilley KS, Laue ED, Glover DM. Interaction between Anillin and RacGAP50C connects the actomyosin contractile ring with spindle microtubules at the cell division site. J Cell Sci. 2008;121:1151–1158. doi: 10.1242/jcs.026716. [DOI] [PubMed] [Google Scholar]
- 15.Gregory SL, Ebrahimi S, Milverton J, Jones WM, Bejsovec A, Saint R. Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr Biol. 2008;18:25–29. doi: 10.1016/j.cub.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of Mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- 17.Oegema K, Savoian MS, Mitchison TJ, Field CM. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol. 2000;150:539–552. doi: 10.1083/jcb.150.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol. 2008;18:30–36. doi: 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 19.Echard A, Hickson GR, Foley E, O’Farrell PH. Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol. 2004;14:1685–1693. doi: 10.1016/j.cub.2004.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldbach P, Wong R, Beise N, Sarpal R, Trimble WS, Brill JA. Stabilization of the actomyosin ring enables spermatocyte cytokinesis in Drosophila. Mol Biol Cell. 2010;21:1482–1493. doi: 10.1091/mbc.E09-08-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickson GR, O’Farrell PH. Rho-dependent control of anillin behavior during cytokinesis. J Cell Biol. 2008;180:285–294. doi: 10.1083/jcb.200709005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somma MP, Fasulo B, Cenci G, Cundari E, Gatti M. Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Mol Biol Cell. 2002;13:2448–2460. doi: 10.1091/mbc.01-12-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao WM, Fang G. Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J Biol Chem. 2005;280:33516–33524. doi: 10.1074/jbc.M504657200. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder TE. The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J Cell Biol. 1972;53:419–434. doi: 10.1083/jcb.53.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field CM, Coughlin M, Doberstein S, Marty T, Sullivan W. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development. 2005;132:2849–2860. doi: 10.1242/dev.01843. [DOI] [PubMed] [Google Scholar]
- 26.Maddox AS, Habermann B, Desai A, Oegema K. Distinct roles for two C. elegans anillins in the gonad and early embryo. Development. 2005;132:2837–2848. doi: 10.1242/dev.01828. [DOI] [PubMed] [Google Scholar]
- 27.Gilden J, Krummel MF. Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton (Hoboken) 2010;67:477–486. doi: 10.1002/cm.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giansanti MG, Bonaccorsi S, Gatti M. The role of anillin in meiotic cytokinesis of Drosophila males. J Cell Sci. 1999;112(Pt 14):2323–2334. doi: 10.1242/jcs.112.14.2323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
04
05