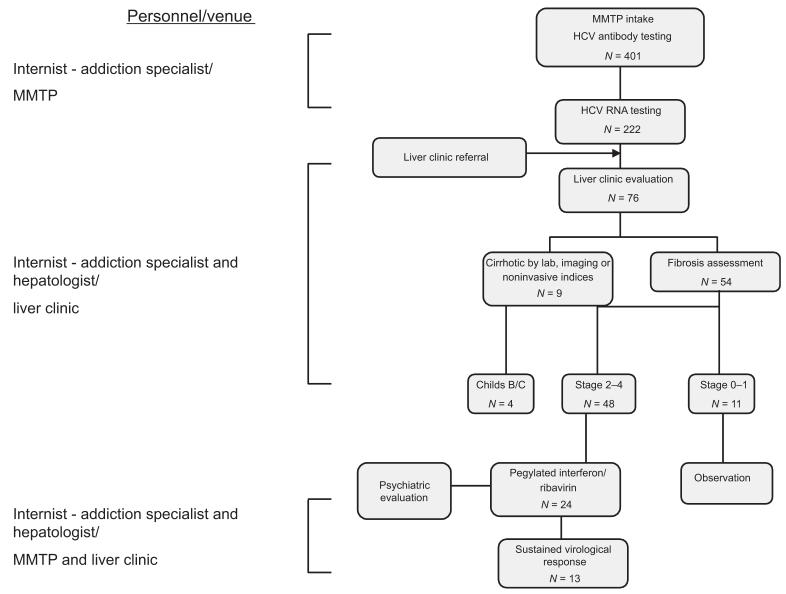
Integrated internist — addiction medicine — hepatology model for hepatitis C management for individuals on methadone maintenance (original) (raw)
. Author manuscript; available in PMC: 2013 Jul 11.
SUMMARY
Despite a high prevalence of hepatitis C virus (HCV) among drug users, HCV evaluation and treatment acceptance are extremely low among these patients when referred from drug treatment facilities for HCV management. We sought to increase HCV treatment effectiveness among patients from a methadone maintenance treatment program (MMTP) by maintaining continuity of care. We developed, instituted and retrospectively assessed the effectiveness of an integrated, co-localized care model in which an internist-addiction medicine specialist from MMTP was embedded in the hepatitis clinic. Methadone maintenance treatment program patients were referred, evaluated by the internist and hepatologist in hepatitis clinic and provided HCV treatment with integration between both sites. Of 401 evaluated patients, anti-HCV antibody was detected in 257, 86% of whom were older than 40 years. Hepatitis C virus RNA levels were measured in 222 patients, 65 of whom were aviremic. Of 157 patients with detectable HCV RNA, 125 were eligible for referral to the hepatitis clinic, 76 (61%) of whom accepted and adhered with the referral. Men engaged in MMTP <36 months were significantly less likely to be seen in hepatitis clinic than men in MMTP more than 36 months (odds ratio = 7.7; 95% confidence interval 2.6-22.9) or women. We evaluated liver histology in 63 patients, and 83% had moderate to advanced liver disease. Twenty-four patients initiated treatment with 19 completing and 13 (54%) achieving sustained response. In conclusion, integrated care between the MMTP and the hepatitis clinic improves adherence with HCV evaluation and treatment compared to standard referral practices.
Keywords: HCV/HIV coinfection, methadone, opiate replacement, substance abuse, viral hepatitis
INTRODUCTION
Five million individuals in the United States are infected with hepatitis C virus (HCV), a virus that can result in cirrhosis, end-stage liver disease and hepatocellular carcinoma. Conventional therapy, consisting of pegylated interferon (PEG-IFN) and ribavirin (RBV), results in viral eradication in roughly one-half of infected individuals [1,2]. Currently, injection drug use is the strongest risk factor for HCV acquisition with HCV seroprevalence >70% among injection drug users (DUs) older than 40 years. However, DUs have been systematically excluded from treatment for HCV owing to stigmatization, physicians’ concerns regarding adherence and patients’ misinformation concerning the importance of a diagnosis of HCV [3,4]. Between 2010 and 2030, the prevalence of cirrhosis is estimated to increase from 25% to 45% among chronic hepatitis C patients [5]. Simultaneously, the number of treated patients is projected to decline [6], unless new strategies are developed to enable DUs to obtain antiviral treatment.
Despite the potential benefits of treatment, surprisingly few HCV-infected DUs are offered anti-HCV therapy, even though expert panels have endorsed HCV treatment in this population [7,8]. Active engagement in therapy for addiction has been shown to increase treatment access for various infectious diseases, such as HIV and tuberculosis, among illicit substance users [9,10]. It has also been demonstrated that the longer a patient is engaged in substance abuse treatment the greater the stability and retention in treatment for medical conditions [11].
Traditional HCV management via referral of DUs to outpatient specialty clinics has resulted in the appearance in the clinic of less than one-third of referred patients [12]. Among DUs, therapeutic effectiveness is an issue of treatment access, acceptance and adherence rather than drug efficacy [13]. Consequently, an approach that integrates the expertise of a variety of disciplines, including specialists in addiction medicine, hepatology, infectious diseases, primary care and psychiatry, has been advocated for the treatment of HCV among DUs [14]. Adherence is likely to be further enhanced if a program offers familiarity, continuity among providers and ready access to health care professionals, as a strong relationship with medical personnel has been shown to be an important determinant of patients receiving preventative care as well as HCV and HIV treatment services [15-17].
To address these concerns, we devised the ‘internist-addiction medicine-hepatology colocalization model’, an integrated, co-located program in which an internist-addiction medicine specialist (ADM) evaluated methadone-maintained patients for HCV infection in the hepatology clinic under the direction of a hepatologist (AHT). We applied our model to patients from our institution’s two methadone maintenance clinics located in close proximity to our viral hepatitis clinic. A primary premise of our model was that methadone-maintained patients would be more likely to accept an HCV evaluation if continuity of care was maintained between the methadone maintenance treatment program (MMTP) and the viral hepatitis clinic by the same physician caring for patients in both venues.
METHODS
Treatment setting and patient selection
A total of 401 patients in our institution’s two MMTP clinics between July 2006 and June 2008 with available HCV serology were eligible for inclusion. Inclusion criteria were broad, and we purposefully did not exclude active drug or alcohol use except in the case of severe incapacitation as determined by either MMTP staff psychiatrists or the internist-addiction medicine specialist as we desired to pursue HCV management in as many MMTP patients as possible. Patients were excluded if HCV serostatus was unavailable or if their active enrolment in the MMTP during the period under study could not be verified. Patients who had poorly compensated psychiatric disease as determined by MMTP staff psychiatrists were also excluded. No patients were excluded for other medical co-morbidities, such as neurological, endocrine or autoimmune conditions.
The two MMTPs are located within a one-block radius of the viral hepatitis clinic and are staffed by internists, psychiatrists, nurses and social workers. The internist-addiction medicine specialist from the MMTP, who provided comprehensive medical services including chronic disease management, was the primary care physician for the majority of patients. All MMTP patients met DSM IV [18] criteria for a diagnosis of opiate dependence and most had an additional diagnosis of dependence on alcohol, benzodiazepines or cocaine. Data were collected retrospectively through chart review. The study was conducted in accordance with a protocol approved by the Institutional Review Board and consistent with the Helsinki Declaration of 1975, as revised in 1983.
Hepatitis C virus antibody testing was performed on all individuals on admission to the MMTP and then annually in seronegative persons. Upon receipt of a positive HCV antibody test result, the patient was informed of their seropositive status, if not previously aware, provided HCV education and offered referral to the hepatitis clinic (Fig. 1). If the patient accepted referral, MMTP staff scheduled the appointment and recorded the date and time in the computerized methadone-dispensing system. During the week preceding the appointment, patients were reminded twice of their upcoming appointment. All patients who missed their initial appointments were questioned as to the reason for failure to appear. For reasons such as forgetfulness or competing priorities at the time of the initial visit, MMTP staff scheduled a second or third appointment as indicated. If a patient missed more than three appointments, they were considered noncompliant. Hepatitis C virus RNA testing was performed in the hepatitis clinic during the first 6 months of our program, and subsequently testing was performed in the MMTP to expedite the referral process. HIV antibody testing was encouraged at admission to the MMTP and repeated every six to twelve months depending upon whether or not the patient continued to engage in high-risk activities.
Fig. 1.
Internist-addiction medicine-hepatology colocalization model for hepatitis C evaluation and treatment among patients in the methadone maintenance treatment program. The number of patients at each step of the HCV management process is indicated. HCV, hepatitis C virus; MMTP, methadone maintenance treatment program.
In the hepatitis clinic, the patient was seen by the internist-addiction medicine specialist under the supervision of a hepatologist. All patients underwent a standard medical examination and comprehensive assessment of HCV status that included HCV RNA measurement, HCV genotyping and liver biopsy, if desired and indicated. Consistent with the 2002 NIH Consensus Conference [19] and American Association for the Study of Liver Disease guidelines [20], patients were strongly encouraged to have histologic assessment of liver disease severity through biopsy as a surrogate marker of adherence. Histology was assessed by staff pathologists using the Scheuer 0–4 point scale [21]. In five patients, we performed FibroSURE™ (LabCorp., Research Triangle Park, NC, USA), a noninvasive test of hepatic fibrosis.
After the biopsy, patients discussed potential HCV treatment in the hepatitis clinic. Besides patient’s willingness, we considered fibrosis stage and potential contraindications to PEG-IFN/RBV prior to initiating therapy. All psychiatrically unstable patients underwent psychiatric assessment prior to PEG-IFN/RBV initiation and were monitored monthly while on therapy by MMTP staff psychiatrists. Staff psychiatrists were available on a daily basis in the MMTP for patient consultation. In addition, a psychosomatic medicine fellow was present in the hepatitis clinic on the designated clinic day for consultation if requested by either the hepatologist or the internist-addiction medicine specialist.
According to standard practice, daily attendance in the MMTP is required 6 days per week. Subsequently, phased reductions in attendance can be initiated if the patient maintains abstinence from the use of illicit substances, complies with the predetermined attendance schedule and actively engages in treatment including attendance at counselling sessions with their assigned social worker. Throughout the stabilization process and treatment course, the patient must comply with random urine toxicology screening at frequent intervals.
Antiviral therapy
Pegylated interferon _α_-2a 180 _μ_g/week was injected subcutaneously. Prior to initiation of treatment, all patients participated in a teaching session with nursing staff from the viral hepatitis clinic during which the patient was instructed in interferon administration. The first dose was administered during the teaching session, and all subsequent doses were self-administered. In no case did the physicians managing the patient deem it necessary that they receive directly observed therapy. Weight-adjusted RBV was taken orally twice daily at a dose of 800–1200 mg. Hematopoietic stimulating factors were utilized as indicated for anaemia and neutropenia. All patients on PEG-IFN/RBV were seen weekly in the MMTP by the internist-addiction medicine specialist and at 6-week intervals in the liver clinic by both the hepatologist and the addiction specialist on the designated clinic day. Patients were monitored for clinical evidence of opiate withdrawal on a weekly basis by the internist-addiction medicine specialist. If a patient missed the follow-up appointment in the hepatitis clinic, they were seen in the MMTP and rescheduled to be seen in the hepatitis clinic at the first availability. Haematologic parameters and aminotransferase levels were measured weekly for the first month and at 6-week intervals thereafter. Patients had the option to have their blood drawn at either the hepatitis clinic or the MMTP. The internist-addiction medicine specialist and the hepatologist maintained a list of all HCV-seropositive patients and reviewed the status of each patient on a weekly basis either by phone or in person.
Statistical analysis
Statistical analysis was performed using SAS (SAS Institute Inc., Cary, NC, USA) and R (R Language, version 2.10.0 http://www.r-project.org). The associations between the variables of interest were determined through chi-square tests, Fisher’s exact tests, logistic regression modelling and Wald tests. Model selection was based on the Akaike information criterion (AIC). Logistic regression was used to model the probability that a patient who was referred to the liver clinic was adherent and to assess the significant factors that influenced adherence with the referral. First, simple logistic regression was used to determine the factors (gender, ethnicity, age and duration in the MMTP) for inclusion in the model. Criterion for inclusion was P ≤ 0.25. Secondly, the AIC criterion was used to select the best multiple logistic model. In addition, we modelled the probability that a patient in whom treatment was indicated actually initiated PEG-IFN/RBV. The significance level for these tests was set at 0.05, two-tailed.
RESULTS
Patient characteristics
Of a total of 401 patients, 51% were Caucasian, 34% were Hispanic, 13% were African American and 66% were male (Table 1). HIV antibody testing results were available for 322 subjects, 48 of whom were positive. Of 48 HIV-positive patients, 2 were HCV seronegative, 13 were HCV seropositive but HCV RNA negative and 33 were co-infected with HCV (both HCV RNA and antibody positive).
Table 1.
Demographic characteristics for methadone maintenance treatment program (MMTP) patients
| Characteristic | N (%)or MedianIQR | HCV Ab+N = 257(%) | HCV Ab−N = 144(%) |
|---|---|---|---|
| Age (N = 401)† | 49 (39–55) | ||
| ≥40 | 300 (75) | 222 (86) | 78 (54) |
| <40 | 101 (25) | 35 (14) | 66 (46) |
| Age at admission | 41 (29–49) | ||
| ≥40 | 218 (54) | 162 (63) | 56 (39) |
| <40 | 183 (46) | 95 (37) | 88 (61) |
| Time in MMTP‡ | |||
| Median (range) | 65 (20–121) | 76 (24–131) | 47 (18–97) |
| Age of HCV antibody testing§ | |||
| Median (range) | 43 (33–50) | 47 (39–52) | 38 (26–46) |
| Gender | |||
| Male | 266 (66) | 165 (64) | 101 (70) |
| Female | 135 (34) | 92 (36) | 43 (30) |
| Ethnicity | |||
| Caucasian | 204 (51) | 123 (48) | 81 (56) |
| Hispanic | 136 (34) | 90 (35) | 46 (32) |
| African | 52 (13) | 40 (16) | 12 (8) |
| American | |||
| Other | 9 (2) | 4 (2) | 5 (3) |
| HAV | |||
| N = 352 | |||
| IgG+ | 155 (44) | 129 (58) | 26 (20) |
| N = 188 | |||
| IgM+ | 2 (1) | 2 (1) | 0 |
| HBV | |||
| HbsAb (N = 350) | |||
| Positive | 144 (41) | 100 (46) | 44 (33) |
| Negative | 206 (59) | 117 (54) | 89 (67) |
| HbsAg (N = 390) | |||
| Positive | 1 (0.2) | 1 (0.4) | 0 |
| Negative | 389 (99.7) | 247 (99.6) | 142 (100) |
| HBV core Ab (N = 204) | |||
| Positive | 114 (56) | 110 (70) | 4 (9) |
| Negative | 90 (44) | 48 (30) | 42 (91) |
| HIV status (N = 322) | |||
| HIV+ | 48 (15) | 46 (23) | 2 (2) |
| CD4 (N = 46) cells/mm3 | 241 (117–467) |
HCV disease characteristics and evaluation
Serology
Hepatitis C virus antibody was obtained at a median age of 43 (33–50) years, on average 2 years after admission to the MMTP. Of 257 HCV-seropositive patients, 86% were older than 40 years, 48% were Caucasian, 35% Hispanic and 16% African American. We found higher HCV seroprevalence with increasing age. Subjects aged 40 years or older were more likely to be HCV antibody positive compared to younger people (odds ratio [OR] = 3.11; 95% CI: 2.00, 4.83, P < 0.0001). However, the association between anti-HCV positivity and age differs significantly among different ethnic groups (P = 0.049). While the likelihood of being anti-HCV positive was higher among Caucasians (OR = 3.5; 95% CI: 1.89, 6.39) and African Americans (OR = 20.7; 95% CI: 2.06, 206.64) older than 40 years, age was not associated with HCV serostatus among Hispanics (OR = 1.51; 95% CI: 0.72, 3.17). Seropositivity did not differ significantly by gender (P = 0.23). Our population was quite stable with a median duration in the MMTP of 65 months among seropositive individuals. We also found that patients who were in the MMTP for more than 36 months were 1.61 times more likely to be HCV seropositive compared to those enrolled in the MMTP for a shorter period (95% CI: 1.06, 2.46, P = 0.026).
HCV RNA
Hepatitis C virus RNA was obtained from 222 (86%) HCV-seropositive patients. Of these, 65 (29%) patients were HCV RNA negative, indicating spontaneous viral eradication. Chronic HCV infection was detected in 157 (71%) patients as indicated by detectable HCV RNA. Of these, 33 patients were HIV/HCV co-infected. In 35 (14%) of seropositive patients, HCV viral quantitation was unattainable: 8 declined testing citing lack of interest, 8 had no insurance, 3 received HCV treatment elsewhere and 16 were inaccessible during the study owing to discharge/transfer from the MMTP (n = 9), death (n = 4) or incarceration (n = 3). Hepatitis C virus genotypes were obtained on 118 (75%) patients with chronic HCV infection. Hepatitis C virus genotype 1 was detected in 91 (77%) patients, followed by genotypes 2 and 3 (11% and 10%, respectively) (Table 2).
Table 2.
Hepatitis C virus disease characteristics
| Characteristic | N (%) |
|---|---|
| HCV Ab (N = 401) | |
| Ab+ | 257 (64) |
| Ab− | 144 (36) |
| HCV RNA (N = 222) | |
| RNA+ | 157 (71) |
| RNA− | 65 (29) |
| Unattainable | 35 |
| HCV genotype (N = 118) | |
| 1 | 91 (77) |
| 2 | 13 (11) |
| 3 | 12 (10) |
| 4 | 2 (2) |
| Liver biopsy (N = 54) | |
| Stage | |
| 0 | 1 (2) |
| 1 | 8 (15) |
| 2 | 18 (33) |
| 3 | 20 (37) |
| 4 | 7 (13) |
| Portal grade | |
| 1 | 7 (13) |
| 2 | 36 (67) |
| 3 | 7 (13) |
| 4 | 4 (7) |
| Lobular grade† | |
| 0 | 1 (2) |
| 1 | 16 (35) |
| 2 | 26 (57) |
| 3 | 2 (4) |
| 4 | 1 (2) |
Liver clinic evaluation
Of the 157 chronically HCV-infected patients, 125 were eligible to be seen in the clinic, 29 of whom were HIV co-infected. Seventy-six individuals adhered with the referral of whom 17 (25%) were HIV/HCV co-infected. The following patients were considered ineligible for referral: 13 received HCV care elsewhere; 12 were not evaluated owing to discharge (n = 5), transfer (n = 4), incarceration (n = 1) or death (n = 2) during the study period; and 7 were uninsured. Twenty-three patients refused hepatitis clinic referral citing lack of interest, and 26 initially accepted referral but failed to appear.
We found that patients who had been enrolled in the MMTP for more than 36 months were more likely to have been seen in liver clinic than those engaged in treatment for <36 months (OR = 3.32, 95% CI: 1.51, 7.28, _P_ = 0.003). However, this relationship depended upon subject gender (_P_ = 0.017). The odds of male patients engaged in MMTP for >36 months accepting and adhering to referral were significantly higher than for men enrolled in MMTP for <36 months (OR = 7.7; 95% CI 2.6–22.9). Among patients who were on methadone treatment for <36 months, men were significantly less likely to be seen in the liver clinic than women (OR = 0.167, 95% CI: 0.04, 0.69). No other demographic or disease-related factors predicted referral adherence. Of the 76 patients with chronic HCV infection evaluated in the viral hepatitis clinic, assessment of fibrosis was achieved in 63 (83%): 54 underwent liver biopsy to assess fibrosis, 4 were diagnosed with cirrhosis based upon laboratory data and/or imaging studies, and 5 preferred noninvasive blood testing to assess the degree of fibrosis. Among 58 patients with histologic assessment based upon biopsy or laboratory/imaging evidence of cirrhosis, 49 (84%) had moderate to advanced liver disease (stage ≥2) indicating the need for urgent treatment. Among patients with liver biopsy, advanced fibrosis (stage >2) differed significantly by ethnicity (P = 0.039); 58% of Caucasians and Hispanics combined had stage >2 vs 20% of African American subjects. Caucasian or Hispanic individuals were 5.5 times more likely to have fibrosis stage >2 when compared to African Americans (95% CI: 1.05, 33.33). Necroinflammatory activity was also significantly associated with fibrosis (P = 0.013 for lobular and P = 0.001 for portal inflammation). Age and gender were not associated with fibrosis.
HCV treatment characteristics
Of the 76 patients who underwent the evaluation process, 24 initiated treatment, including 9 individuals co-infected with HIV (Table 3). Thirty-five patients were ineligible for treatment including 2 (3%) with decompensated psychiatric disease, 6 (11%) with decompensated cirrhosis, 10 (13%) who refused a biopsy, 4 (5%) with no insurance and 2 (3%) who were treated elsewhere. Of these two patients, upon evaluation in the liver clinic, it was determined that one had successfully been treated at an outside institution and the other was treatment ineligible owing to severe thrombocytopenia. Eleven (14%) subjects with mild fibrosis postponed treatment based upon physician recommendation. Twenty-four of 41 treatment eligible patients began treatment. Of the remaining treatment eligible patients, 3 (4%) declined PEG-IFN/RBV citing fear of therapy-related side effects, 4 (5%) had unstable living conditions, 3 (4%) had relocated to different geographic areas complicating pursuit of anti-HCV treatment, 3 declined (4%) owing to co-occurring illnesses and 4 (5%) were lost to follow-up. Patients with stage 3–4 fibrosis were significantly more likely to be treated than those with stage 0–2 (OR = 11.2, 95% CI: 2.89, 43.35, P = 0.0005). Of 24 treatment initiators, 19 completed a full course of therapy with PEG-IFN/RBV. Three patients interrupted treatment owing to reasons unrelated to therapeutic success, including hepatic decompensation (n = 1), severe thrombocytopenia (n = 1) and nonadherence (n = 1). Thirteen patients (54%) achieved sustained virological response (SVR), eight of who were genotype 1 and four were co-infected with HIV. Two HIV/HCV co-infected patients currently remain on treatment.
Table 3.
Treatment outcomes (N = 24)
| Outcomes | N (%) | SVR (%), N = 13 | Relapse/NR (%), N = 6 | Early D/C (%), N = 3 | Ongoing (%), N = 2 |
|---|---|---|---|---|---|
| Genotype | |||||
| 1 | 18 (75) | 8 (61.5) | 5 (83) | 3 (100) | 2 (100) |
| 2 | 3 (12.5) | 2 (15.4) | 1 (17) | ||
| 3 | 3 (12.5) | 3 (23.1) | |||
| HIV Ab | |||||
| Ab+ | 15 (62.5) | 4 (31) | 1 (17) | 2 (67) | 2 (100) |
| Ab− | 9 (37.5) | 9 (61) | 5 (83) | 1 (33) | |
| Stage† | |||||
| <2 | 0 | ||||
| ≥2 | 21 (87.5) | 11 (85) | 5 (83) | 3 (100) | 2 (100) |
| Cirrhotic | 3 (12.5) | 2 (15) | 1 (17) |
DISCUSSION
In this investigation, we demonstrate the effectiveness of an integrated, co-localized care model for HCV utilizing a multidisciplinary approach (Fig. 1). We applied our model to patients from a large MMTP in Manhattan. Overall, HCV seroprevalence was 64% and 61% of those with chronic HCV who were eligible for referral were evaluated in the hepatitis clinic. Of those who initiated treatment, 54% successfully eradicated the virus consistent with the previous studies of HCV treatment in opiate-dependent patients [22-26].
Despite a high prevalence of HCV infection among DUs, less than one-third of eligible individuals receive HCV therapy owing to variety of reasons at the institutional, provider and patient levels [3]. Institutional reasons often include difficulties obtaining or navigating the complexities of the referral system. In addition, many health care providers are concerned about adherence with HCV treatment by DUs, including concerns that they may be disinterested in treatment, that interferon-based therapy may potentiate psychiatric decompensation and that they might be reinfected of continued high-risk practices. Studies of HCV reinfection among successfully treated DUs, however, have shown the converse [27,28]. Using our model, we obtained an HCV evaluation in more than one-half of chronically infected individuals, markedly higher than previous clinic-based cohorts. In addition, we were able to stage the degree of fibrosis in 83% of those evaluated in the liver clinic. Factors that likely contributed to the high degree of acceptance of HCV management and adherence to an evaluation included evaluation by the same physician in both clinics, their geographical proximity and an institution-wide electronic medical record that fosters communication facilitating data access and continuity of care. The fact that these patients had a regular, stable source of medical care that originated in the MMTP and continued to the hepatitis clinic was likely crucial to the success of our program [15,17].
Substance abuse treatment can serve as an entry point into the health care system and is possibly an essential step in preparing DUs for HCV evaluation and treatment. Well-structured MMTPs, with attributes such as access to mental health professionals and general medical staff, likely have advantages for HCV evaluation and treatment over those without such services. Consistent with the findings of prior studies [11,29], we found that men who were engaged in the MMTP for 36 months or more were significantly more likely to appear in the hepatitis clinic than those with shorter duration of opiate substitution therapy.
Our study is limited in its retrospective, non-comparative design and its conduct at a single institution. Our goal, however, was to demonstrate the feasibility and effectiveness of an integrated, co-localized model of care. The health care services offered at our MMTP provided the requisite infrastructure to facilitate HCV evaluation and treatment among MMTP patients. Unfortunately, however, many MMTPs may not be able to offer as wide a spectrum of health care services onsite, which might impact on the ability to offer HCV management except through traditional referral based mechanisms. Our model may be utilized by community-based primary care providers or addiction medicine specialists with immediate access to experts in HCV management who can assist in navigating the complexities of treatment of the infection.
Despite our integrated, colocalized approach, a substantial number of patients did not undergo an HCV evaluation in the hepatitis clinic, with an approximate equal number refusing referral as those initially accepting referral but not appearing for their initial evaluation. Men enrolled in the MMTP for <36 months appeared to be at greatest risk for not accepting or complying with referral to the hepatitis clinic. When questioned, patients indicated that they refused HCV evaluation owing to an apparent lack of interest, reticence or a lack of education or misinformation concerning HCV. Our findings are consistent with previous data that demonstrated significant knowledge gaps among DUs [30]. Implementation of patient-oriented interventions, such as formal, structured HCV educational programs, individual case management to address patient level barriers or staff/peer accompaniment to appointments, might improve adherence with HCV evaluation and treatment and are interventions deserving of further study.
In an effort to inform patients of the severity of their infection and consistent with standard clinical practice in 2006 [20], we strongly encouraged patients to undergo a liver biopsy. On biopsy, we found that the vast majority of patients had at least moderate hepatic fibrosis, indicating the need for urgent treatment. In this model, 41% of patients who had accurate hepatic fibrosis assessment began PEG-IFN/RBV, 79% of whom completed a full course of therapy with an overall SVR rate of 54%. Notably, only one patient had treatment interrupted for issues relating to nonadherence and no patients discontinued treatment because of psychiatric decompensation.
In summary, we demonstrated that localization of addiction medicine specialists and hepatologists in a viral hepatitis clinic is both an effective and efficient model to deliver HCV evaluation and treatment to MMTP patients. This approach could be most appropriate in settings that offer pharmacologically based treatment of addiction which have ready access to expertise in the management of liver disease. As many DUs have advanced stages of hepatic fibrosis, HCV treatment is particularly urgent. Additionally, successful treatment combined with safe injection practices could decrease virus transmission even among individuals who continue to inject. Unless disenfranchised populations with the highest infection prevalence, such as DUs, have access to and accept treatment for HCV, the burden of disease will remain high.
ACKNOWLEDGEMENTS
We acknowledge the contribution of the office and clinical staff of the Hepatology Clinic at Weill Cornell-New York Hospital Medical Center and Annette Ocasio, Hepatology Practice Administrator, for assistance with patient management. Dr. Martinez thanks Drs Steffanie A. Strathdee and Richard Garfein. We also thank the patients who participated in this study.
FUNDING
This study was funded in part by the Greenberg Medical Research Foundation (AHT), by a commitment made at the Clinton Global Initiatives (ABB, AHT), the Adelson Medical Research Foundation (MJK), the Center for HIV/AIDS Minority Pipeline in Substance Abuse (ADM) and National Institutes of Health, NIDA P60DA05130 (MJK), and NIAID K23 AI065319 (KMM). No commercial support was provided for the conduct of this study or writing of the manuscript. Financial support was provided by Vertex Pharmaceuticals for statistical analysis of previously collected data, although the company had no input in the analysis of these data.
Abbreviations
AIC
Akaike Information Criterion
DUs
drug users
HCV
hepatitis C virus
HIV
human immunodeficiency virus
MMTP
methadone maintenance treatment program
OR
odds ratio
PEG-IFN
pegylated interferon
RBV
ribavirin
SVR
sustained virological response
Footnotes
CONFLICT OF INTEREST
Anthony Martinez is on the Speaker’s bureau for Genentech. Rositsa Dimova, Kristen M. Marks, Ann B. Beeder, Marija Zeremski and Mary Jeanne Kreek: No conflict. Andrew H. Talal has received research support from Vertex Pharmaceuticals, is on the Speaker’s bureau for Genentech and has received research support from Merck Inc.
REFERENCES
- 1.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005;20:754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750–1755. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 7.Novick DM, Kreek MJ. Critical issues in the treatment of hepatitis C virus infection in methadone maintenance patients. Addiction. 2008;103:905–918. doi: 10.1111/j.1360-0443.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laine C, Hauck WW, Gourevitch MN, Rothman J, Cohen A, Turner BJ. Regular outpatient medical and drug abuse care and subsequent hospitalization of persons who use illicit drugs. JAMA. 2001;285:2355–2362. doi: 10.1001/jama.285.18.2355. [DOI] [PubMed] [Google Scholar]
- 10.Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: a randomized controlled trial. JAMA. 2001;286:1715–1723. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Rio M, Mino A, Perneger TV. Predictors of patient retention in a newly established methadone maintenance treatment programme. Addiction. 1997;92:1353–1360. [PubMed] [Google Scholar]
- 12.Grebely J, Petoumenos K, Matthews GV, et al. Factors associated with uptake of treatment for recent hepatitis C virus infection in a predominantly injecting drug user cohort: the ATAHC Study. Drug Alcohol Depend. 2010;107:244–249. doi: 10.1016/j.drugalcdep.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broers B, Helbling B, Francois A, et al. Barriers to interferon-alpha therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol. 2005;42:323–328. doi: 10.1016/j.jhep.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Cheruvu S, Beeder AB, Carden M, Edlin BR, Talal AH. Strategies to Control Hepatitis C Infection among Injection Drug Users. In: Negro F, editor. Hot Topics in Viral Hepatitis. FB Communications; Modena, Italy: 2007. pp. 23–30. [Google Scholar]
- 15.Merzel C, Moon-Howard J. Access to health services in an urban community: does source of care make a difference? J Urban Health. 2002;79:186–199. doi: 10.1093/jurban/79.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strathdee SA, Latka M, Campbell J, et al. Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis. 2005;40(Suppl. 5):S304–S312. doi: 10.1086/427445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edn, revised edn. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 19.National Institutes of Health Consensus Development Conference Statement: management of hepatitis C: 2002 – June 10–12, 2002. Hepatology. 2002;36:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 20.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 21.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 22.Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741–747. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- 23.Belfiori B, Ciliegi P, Chiodera A, et al. Peginterferon plus Ribavirin for chronic hepatitis C in opiate addicts on methadone/buprenorphine maintenance therapy. Dig Liver Dis. 2009;41:303–307. doi: 10.1016/j.dld.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37:32–40. doi: 10.1016/j.jsat.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- 26.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009;49:561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- 27.Backmund M, Meyer K, Edlin BR. Infrequent reinfection after successful treatment for hepatitis C virus infection in injection drug users. Clin Infect Dis. 2004;39:1540–1543. doi: 10.1086/425361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalgard O. Follow-up studies of treatment for hepatitis C virus infection among injection drug users. Clin Infect Dis. 2005;40(Suppl. 5):S336–S338. doi: 10.1086/427449. [DOI] [PubMed] [Google Scholar]
- 29.Senn O, Seidenberg A, Rosemann T. Determinants of successful chronic hepatitis C case finding among patients receiving opioid maintenance treatment in a primary care setting. Addiction. 2009;104:2033–2038. doi: 10.1111/j.1360-0443.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 30.Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: knowledge, perceived risk and willingness to receive treatment. Drug Alcohol Depend. 2001;61:211–215. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]