Theaflavin-3,3′-Digallate and Lactic Acid Combinations Reduce Herpes Simplex Virus Infectivity (original) (raw)
Abstract
The present study examined the efficacy of using multiple mechanisms as part of a topical microbicide to inactivate herpes simplex virus (HSV) by combining theaflavin-3,3′-digallate (TF-3) and lactic acid (LA) over the pH range of 4.0 to 5.7 to mimic conditions in the female reproductive tract. Six clinical isolates of HSV-2 and two clinical isolates of HSV-1 were almost completely inactivated when TF-3 (100 μM) was present with LA over the pH range of 4.5 to 5.7, whereas four additional HSV-1 clinical isolates required TF-3 concentrations of 250 to 500 μM for comparable virus titer reduction. LA (1%) alone at pH 4.0 reduced the titers of laboratory and clinical isolates of HSV-1 and HSV-2 by ≥5 log10, but most LA-dependent antiviral activity was lost at a pH of ≥4.5. When HSV-1 and HSV-2 were incubated at pH 4.0 without LA virus titers were not reduced. At pH 4.0, HSV-1 and HSV-2 titers were reduced 5 log10 in 20 min by LA alone. TF-3 reduced HSV-2 titers by 5 log10 in 20 to 30 min at pH 4.5, whereas HSV-1 required 60 min for comparable inactivation. Mixtures of TF-3 and LA stored at 37°C for 1 month at pH 4.0 to 5.7 maintained antiviral activity. Semen, but not cervical vaginal fluid, decreased LA-dependent antiviral activity at pH 4.0, but adding TF-3 to the mixture reduced HSV titers by 4 to 5 log10. These results indicate that a combination microbicide containing TF-3 and LA could reduce HSV transmission.
INTRODUCTION
Herpes simplex virus type 2 (HSV-2) is the primary causative agent of genital herpes worldwide (1), but HSV-1, which is usually orally located, has been shown recently to be responsible for an increasing proportion of first episode HSV infections (2). HSV is the major cause of genital ulcers in the developed world (3).
Recent trials of an HSV vaccine found that the vaccine did not prevent HSV-2 disease or infection but was somewhat effective against HSV-1 (4). In the absence of an effective HSV vaccine, microbicides represent an alternative strategy to reduce HSV transmission which is important not only for preventing HSV transmission but also for reducing human immunodeficiency virus (HIV) transmission. There is a link between HSV and HIV so that people with an HSV infection are at greater risk for HIV acquisition (5). It has been suggested that increased HIV infection in the presence of HSV is the result of ulcerations caused by HSV that can provide an entryway for HIV through the mucosa. However, daily acyclovir therapy did not reduce HIV-1 transmission despite a 73% reduction in HSV-2 caused genital ulcers (6). Other studies have shown that acyclovir therapy to reduce HSV-2 shedding did not reduce the risk of HIV infection, possibly as the result of continued innate immune activation (7) and the resultant continued presence of HIV-1 receptor-positive cells in the genitalia (8). These studies suggest that preventing initial HSV-2 infection may be the sole way to break the link between HSV-2 infection and increased risk of HIV infection.
Recent studies have indicated that HSV-2 shedding is accompanied by a constant release of virus (9) at widely spaced regions in the genital tract (10) and that, even though HSV-2 shedding decreases after the initial clinical episode, high virus numbers (>3 log10/ml) of HSV-2 are shed for years after initial infection (11). Even in patients receiving high-dose antiviral therapy to suppress HSV, reactivation was frequent and HSV-2 shedding was 2.5 to 3.0 log10/ml of buffer used to extract HSV DNA from genital swabs (12).
We have previously shown that the digallate dimer of (−)epigallacatechin gallate (EGCG), theaflavin-3,3′digallate (TF-3), which is the primary catechin in black tea (Fig. 1), has the potential to function as a microbicidal agent against HSV at acidic and neutral pH (13). However, it has been shown that reducing the transmission of other enveloped viruses, such as HIV (14) and hepatitis C virus (15), is more effectively accomplished using multiple drug therapy. Combining three or even four drugs can synergize the action of the drugs interacting with different molecular targets and reduce the risk of drug resistance development (14).
Fig 1.
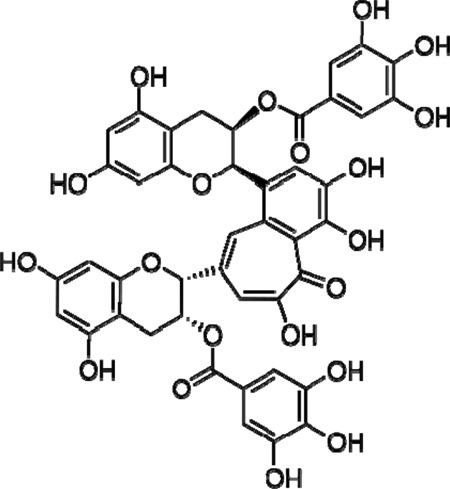
Structure of theaflavin-3,3′-digallate (TF-3).
Vaginal fluid has been shown to inactivate a number of bacterial pathogens through the combined mediated activities of lactic acid (LA), low pH, and antimicrobial peptides (16). HIV transmission is reduced by acidic pH combined with LA (17). The importance of LA at physiological concentrations (55 to 111 mM) and acidic pH has been shown for protection against bacteria associated with bacterial vaginosis (18).
In the present study we showed that combining TF-3 with LA at pH 4.0 reduced HSV titers by ≥5 log10 and that between pH 4.5 and 5.7, where LA loses antiviral activity, TF-3 substantially reduced HSV titers. HSV inactivation was rapid and occurred at a concentration for both TF-3 and LA that can easily be delivered to the female reproductive tract.
MATERIALS AND METHODS
Cells and viruses.
HSV-1 (strain F1) was obtained from R. Rubenstein (Downstate Medical Center, NY), and HSV-2 (strain 333) was obtained from Mary K. Howett (Penn State University). HSV-1 and HSV-2 were grown in Vero and CV-1 cells, respectively. Cells were obtained from the American Type Culture Collection (Manassas, VA) and were grown in RPMI 1640 medium with 10% fetal calf serum (FCS) (19). The maintenance medium for Vero and CV-1 cells was as described above but with 1% FCS. Clinical isolates of HSV-1 and HSV-2 were obtained from Lisa Rohan and Jeanne Jordan at the Magee-Womens Hospital (Pittsburgh, PA) by using an institutional review board-approved protocol. In response to a physician order, independent of this protocol, swabs were collected from patients and put in M-4 medium, and samples not needed for HSV diagnosis were frozen at −20°C and shipped to our laboratory coded but without identifiers, as outlined in our institutional review board protocol.
Virus purification.
Vero and CV-1 cells were grown in RPMI 1640 medium supplemented with 1% FCS (13). Monolayers of Vero cells were infected with HSV-1 at a multiplicity of infection of 1:100, as were CV-1 cells with HSV-2. After 1 h adsorption, the cells were washed and then incubated for 48 h at 37°C in 5% CO2. The cells were then frozen at −80°C and thawed at 4°C, and the suspensions were clarified by centrifugation at 3,000 rpm for 10 min.
HSV-1 and HSV-2 titration.
HSV-1 and HSV-2 were incubated under the indicated experimental conditions for 1 h, with the exception of the time course study, in RPMI 1640 medium with 1% FCS at the appropriate pH. Virus was titrated by inoculation of 10-fold dilutions into Vero cell cultures in 96-well microtiter tissue culture plates (Falcon). A virus dilution (0.1 ml) in maintenance medium was inoculated into each well with three wells per dilution (20). Each experiment was performed in triplicate, and each datum point presented is the mean ± the standard deviation of three separate experiments. The plates were incubated for 5 days at 37°C and examined daily for a cytopathic effect. Virus titers were calculated by the method of Reed and Muench (21). To ensure that combination of TF-3 and LA were not toxic to the Vero cells used for titration, incubation mixtures at pH 4.0, 4.5, 5.0, and 5.7 were diluted with medium as described above; the cells were incubated for 24 h and then tested using a Cell Titer 96 aqueous nonradioactive cell proliferation assay (Promega, Madison, WI). No cell toxicity was found.
Virus inactivation with TF-3.
TF-3 (Wako Chemicals USA, Richmond, VA) was dissolved in dimethyl sulfoxide (DMSO) and diluted in phosphate-buffered saline (PBS) or 1% FCS medium just before use. The final DMSO concentration in both control and TF-3 samples was 0.5% and did not affect cell growth or survival. HSV isolates were incubated with TF-3 at the indicated concentration for 1 h at 37°C in PBS at the indicated pH. Addition of TF-3 to the medium did not change the pH.
pH treatment of virions.
PBS with LA was made by adding 1% LA to PBS and then adjusting pH to 4.0 to 5.7 with NaOH. To make PBS with HCl, HCl was added to PBS to reach the desired pH. The virus was treated with the appropriate buffer for 1 h and then titrated using Vero cells. Prior to conducting these experiments, we determined that the pH of the PBS remained at 4.0 or 4.5 in the presence of 1% LA for at least 24 h (see Table S1 in the supplemental material).
Storage studies.
The TF-3 stock solution in DMSO was either diluted with PBS containing 1% LA at pH 4.0 to 5.7 or with PBS adjusted to pH 4.0 with HCl and then stored at 4°C, 25°C and 37°C for 1 week or 1 month in the dark. Control samples of HSV-1 and TF-3 were made immediately before assay, incubated at 37°C with 5% CO2 for 1 h, and then titrated using Vero cells.
Treatment with semen and CVL.
Semen (Lee Biosolutions, St. Louis, MO) or cervical vaginal fluid (CVL; provided by Bernard J. Moncla and Charlene S. Dezzutti, Magee Womens Research Institute, Pittsburgh, PA, and collected using institutional review board-approved protocol PRO11020218) was diluted with PBS alone or PBS with 1% LA. The virus was incubated with semen and/or CVL for 1 h at 37°C and then titrated using Vero cells. CVL did not change the pH of the incubation mixture, and semen raised the pH 0.3 to 0.5 U (see Table S1 in the supplemental material).
Statistics.
Results are expressed as the means ± the standard deviations of three separate experiments. Treatment differences were measured using unpaired Student t tests. Differences with a P value of <0.05 were considered significant.
RESULTS
Effect of pH and LA on the inactivation of HSV by TF-3.
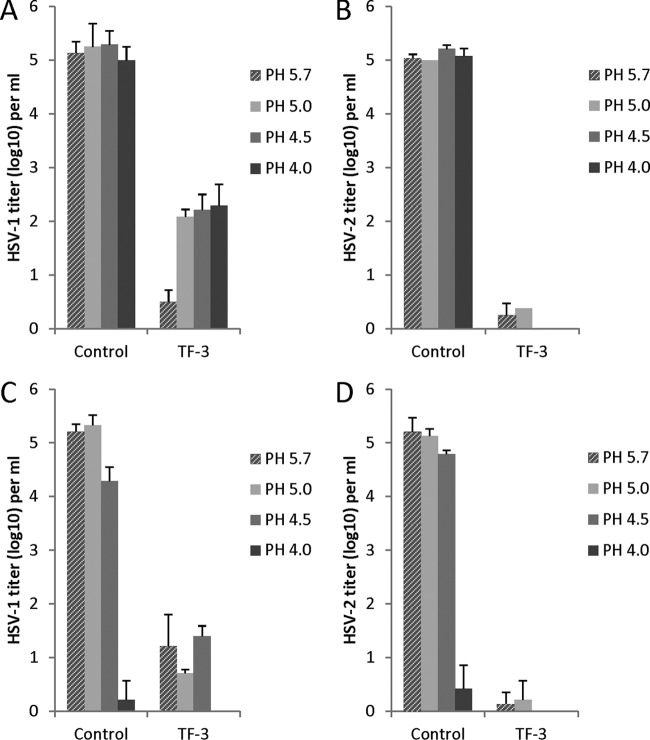
HSV-1 and HSV-2 were inactivated by TF-3 in PBS over the pH range of 4.0 to 5.7 with a reduction in virus titer of 3 to 5 log10 (Fig. 2A and B). HSV-2 and HSV-1 were equally inactivated by TF-3 at pH 5.7 but HSV-2 was 1.5 to 2.5 log10 more sensitive to TF-3 inactivation between pH 4.0 and pH 5.0. When 1% LA (111 mM) was added to the incubation mixture (Fig. 2C and D) with TF-3, the virus titers were reduced by 4 to 5 log10 from pH 4.5 to 5.7 and, interestingly, 1% LA by itself (control samples) reduced the titers of HSV-1 and HSV-2 by ∼5 log10 at pH 4.0 (Fig. 2C and D) without added TF-3. As mentioned in Materials and Methods, 1% LA and TF-3 were not toxic to the Vero cells used for HSV titration and cell numbers were not reduced. These results indicate that a combination of TF-3 and 1% LA very effectively inactivates HSV from pH 4.0 to 5.7.
Fig 2.
Efficacy of TF-3 and LA for inactivation of HSV-1 and HSV-2. TF-3 was used at a concentration of 100 μM, and LA was used at 1%. Virus from a clarified cell supernatant was incubated for 1 h at 37°C under the indicated condition prior to titration with Vero cells. (A) HSV-1 and TF-3; (B) HSV-2 and TF-3; (C) HSV-1, 1% LA, and TF-3; (D) HSV-2, 1% LA, and TF-3. Control samples in panels A and B are without LA, while in panels C and D controls have 1% LA. Titers are the means ± the standard deviations of results of three separate experiments. The laboratory strains of HSV-1 and HSV-2 were used in these experiments.
When the concentration of LA was decreased to 0.3% at pH 4.0 (Fig. 3) HSV-1 and HSV-2 titers were still reduced by 4 to 5 log10. At a concentration of 0.1% LA HSV-1 titers are not reduced, while the titer of HSV-2 is reduced by ∼2 log10, suggesting that HSV-2 may be somewhat more sensitive to inactivation by LA than HSV-1.
Fig 3.
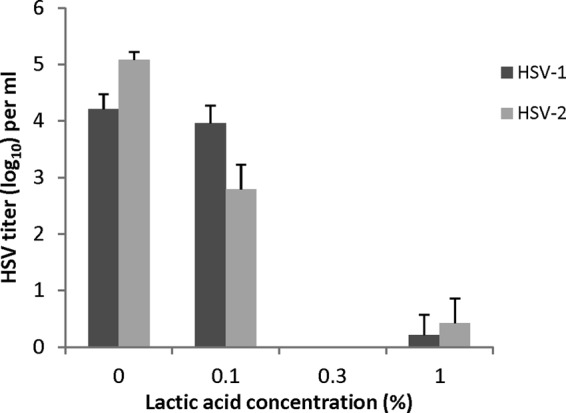
Effect of LA concentration on the inactivation of laboratory strains of HSV-1 and HSV-2 at pH 4.0. Isolates were prepared and titers were determined as described for Fig. 2.
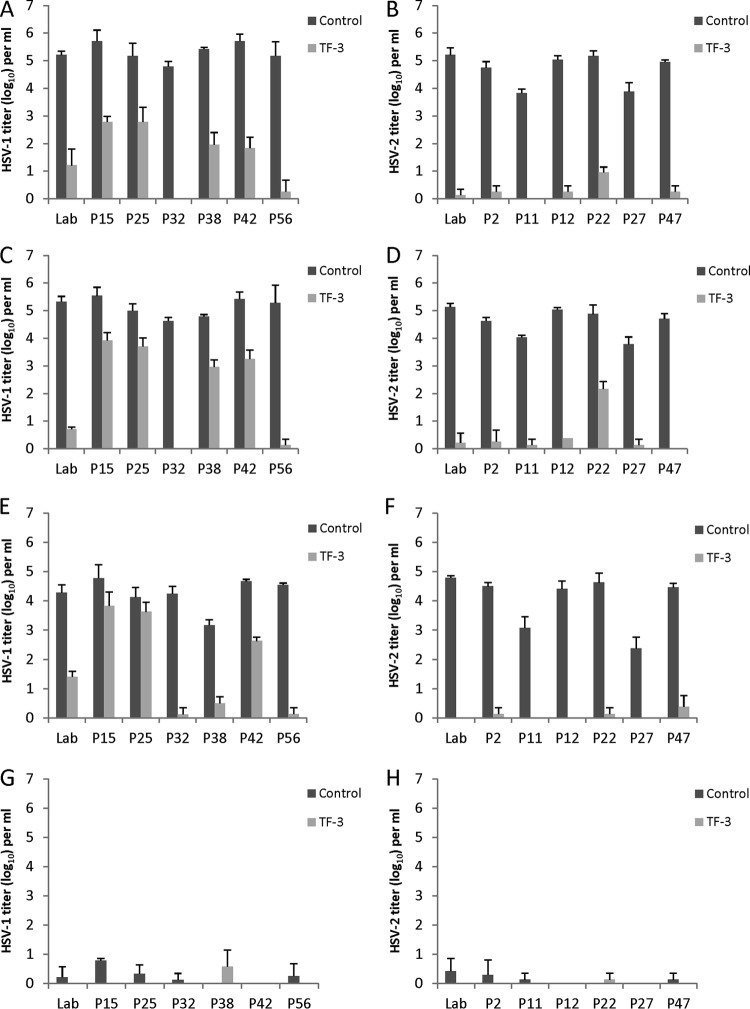
Inactivation of clinical isolates of HSV-1 and HSV-2 by TF-3 and LA between pH 4.0 and 5.7.
Experiments were performed to determine whether there was variability in the sensitivity of clinical isolates of HSV-1 and HSV-2 to inactivation by TF-3 in the presence of LA between pH 4.0 and pH 5.7 (Fig. 4). Clinical isolates and the laboratory strain of HSV-2 were sensitive to inactivation by TF-3 at pH 5.7 and 5.0 with most titer reductions of ca. 4 to 5 log10 (Fig. 4B and D). At pH 4.5, four of the six clinical isolates as well as the laboratory strain were inactivated by >4 log10, while the titers of the other two HSV-2 clinical isolates (P11, P27) were reduced by 2 to 3 log10 (Fig. 4F). However, P11 and P27 control titers were lower than the other isolates at pH 4.5, allowing for less total decrease in virus titers. Both the laboratory strain and the clinical isolates showed significant TF-3-dependent titer decreases (P < 0.001) between pH 4.5 and 5.7. At pH 4.0, all of the HSV-2 clinical isolates were inactivated in the presence of 1% LA, and therefore no effect of TF-3 could be measured (Fig. 4H).
Fig 4.
Efficacy of combinations of TF-3 and LA for the inactivation of clinical isolates of HSV-1 and HSV-2 in the pH range found vaginally. All control samples contained 1% LA, and treated samples had TF-3 (100 μM) and 1% LA. (A and B) pH 5.7; (C and D) pH 5.0; (E and F) pH 4.5; (G and H) pH 4.0. Panels A, C, E, and G show results for HSV-1 isolates, and panels B, D, F, and H show results for HSV-2 isolates. “Lab” indicates the laboratory strains of HSV-1 and HSV-2 utilized in other figures. Isolates were prepared and titers were determined as described for Fig. 2.
HSV-1 clinical isolates showed variability in sensitivity to TF-3 inactivation (Fig. 4A, C, and E) between pH 5.7 and 4.5. At pH 5.7, the titers of the six clinical isolates were all ∼5 log10 and showed titer reductions in the presence of TF-3 from 3 to 5 log10. The titer decreases of the six clinical isolates and the laboratory strain at pH 5.7 were significant (P < 0.001). HSV-1 isolates P32 and P56 appeared to be most sensitive to TF-3 inactivation at pH 5.7. At pH 5.0, the titers of P32, P56, and the laboratory isolate were reduced by 4 to 5 log10 (P < 0.001), whereas the P15, P25, P38, and P42 titers declined by 1-2 log10 (P < 0.01). When the pH was reduced to 4.5, three clinical isolates (P56, P38, and P32) and the laboratory isolate showed titer reductions of 3 to 5 log10 (P < 0.001), whereas isolates P15 and P25 showed infectivity decline of <1 log10. At pH 4.0, all of the HSV-1 isolates were reduced by 4 to 5 log10 by 1% LA (Fig. 4G). The results at pH 4.0 (Fig. 4G and H) show that all clinical isolates of HSV-1 and HSV-2 are almost completely inactivated by 1% LA.
The three HSV-1 clinical isolates (P15 [25, 42]) which showed less inactivation by 100 μM TF-3 at pH 4.5 and 5.0 (Fig. 4C and E) than the other HSV-1 clinical isolates were treated with TF-3 concentrations of 250 and 500 μM (Fig. 5A and B). TF-3 at 250 μM decreased the titers of P15 and P25 by an additional 1 to 1.5 log10 at both pHs but did not further decrease the titer of P42. When the TF-3 concentration was raised to 500 μM the infectivities of P15 and 25 were almost completely eliminated. P42 showed greater resistance to TF-3 inactivation than any of the other HSV clinical isolates, but its infectivity was reduced by ≥3 log10 at pH 4.5 and 5.0 using 500 μM TF-3 and was reduced by ≥2.5 log10 by 100 and 250 μM TF-3. When the HSV-2 clinical isolate P22, which showed less sensitivity to 100 μM TF-3 at pH 5.0 (Fig. 4D) than the other isolates was treated with 250 μM TF-3 its titer was reduced by 5 log10 (results not shown). These results indicated that HSV clinical isolates that are more resistant to TF-3 can be further inactivated by increasing the TF-3 concentration.
Fig 5.
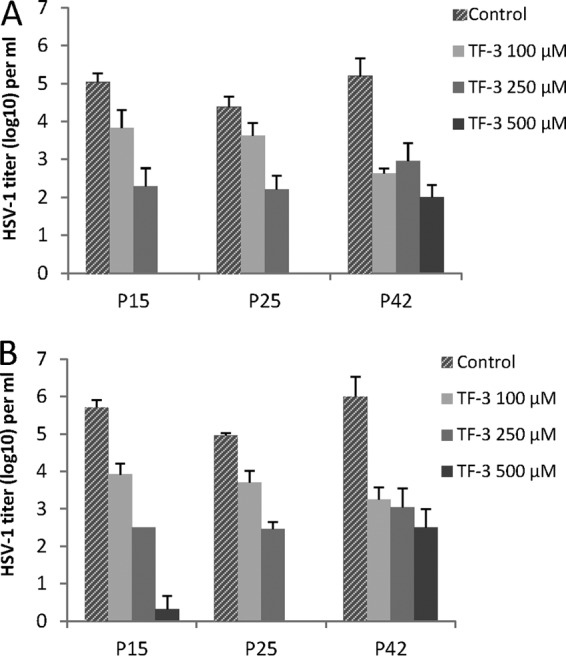
Effect of increased TF-3 concentrations on TF-3 resistant HSV-1 clinical isolates. (A) pH 4.5; (B) pH 5.0. Isolates were prepared and titers were determined as described in Fig. 2.
Time course of HSV-1 and HSV-2 inactivation at pH 4.0 and pH 4.5.
The rate of HSV-1 and HSV-2 inactivation was examined over a 1-h time period in the presence of 1% LA or 1% LA+TF-3 (100 μM) (Fig. 6). With 1% LA alone at pH 4.0 the titers of both HSV-1 and HSV-2 are reduced by 5 log10 in 60 min, while at pH 4.5 titer reductions of 1.0 to 1.5 log10 were seen in the same time interval. When TF-3 is present with LA at pH 4.5, the inactivation curve of HSV-2 is similar to those seen for both HSV-1 and HSV-2 at pH 4.0 with 1% LA. Within 15 min, 3 to 4 log10 of HSV-2 is inactivated and within 20 to 30 min HSV-2 titers are decreased by ≥5 log10. When HSV-1 was incubated with TF-3 and LA at pH 4.5, while the virus was almost completely inactivated in 60 min the rate of infectivity decline is slower than for HSV-1 and HSV-2 at pH 4.0 with LA alone and HSV-2 at pH 4.5 with TF-3 and LA. Between 20 and 30 min of incubation HSV-1 infectivity at pH 4.5 is reduced by 2.5 to 3.0 log10 less compared to both HSVs at pH 4.0 (1% LA) and HSV-2 at pH 4.5 (TF-3, 1% LA). The results in Fig. 6 in conjunction with those in Fig. 4 and 5 suggest that some HSV-1 clinical isolates are more resistant to TF-3 inactivation than HSV-2.
Fig 6.
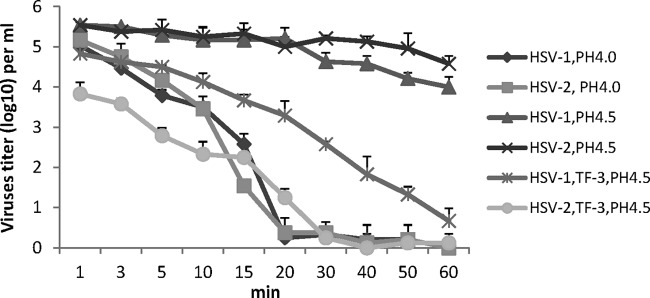
Time required for inactivation of HSV-1 and HSV-2 by LA and TF-3. All samples at pH 4.0 and 4.5 have 1% LA and, where indicated, TF-3 (100 μM) as well. Laboratory isolates were prepared and titers were determined as described in Fig. 2.
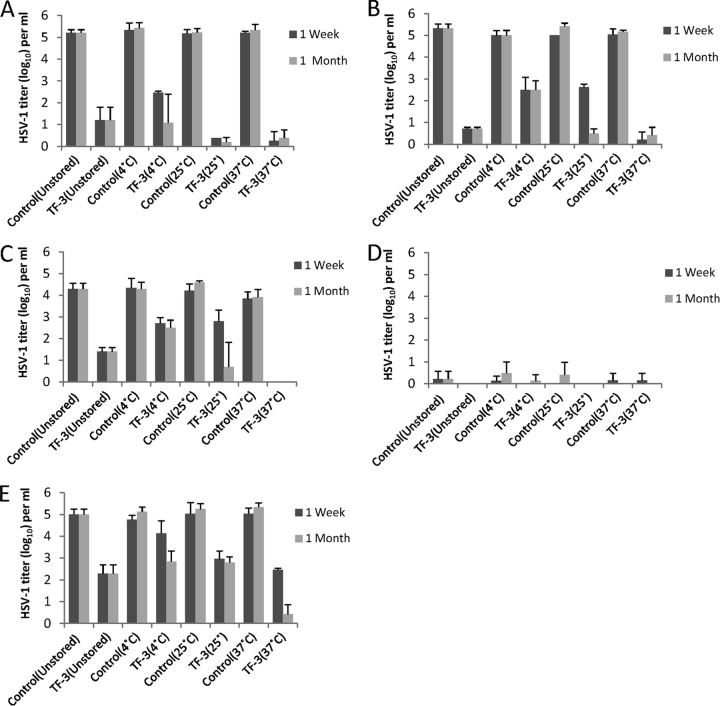
Effect of storage on the antiviral activity of TF-3.
TF-3 was stored with LA under conditions of various temperature and pH, which a microbicide product could encounter during storage and use, after which anti-HSV activity was measured at 1 week and 1 month (Fig. 7A to D). TF-3 stored with LA and kept at 37°C consistently inactivated 4 to 5 log10 of HSV-1 between pH 4.5 and 5.7 at 1 week (P < 0.001) and 1 month (P < 0.01). Antiviral mixtures stored at 25°C for 1 week inactivated 2 to 3 log10 of virus at pH 5.7 and 5.0 (P < 0.001) but at 1 month showed comparable activity to samples stored at 37°C (P < 0.001). When antiviral mixtures were stored at 4°C HSV-1 titers were reduced by 2 to 3 log10 at both 1 week and 1 month between pH 4.5 and 5.7. Controls which contained 1% LA but no TF-3 showed that LA maintained its antiviral activity at pH 4.5 and 4.0 (Fig. 7C and D). At pH 4.0 LA reduced virus titers by ≥5 log10 at all storage temperatures and at pH 4.5 LA reduced HSV titers by 1 to 2 log10. LA by itself had no antiviral activity at pH 5.0 and 5.7. TF-3 was also stored at 4°C without LA for 1 week and 1 month (Fig. 7E). After 1 week, stored TF-3 decreased HSV titers by ∼2.5 log10 at 25°C (P < 0.01) and 37°C (P < 0.001), which was comparable to the unstored sample, whereas the sample stored at 4°C had decreased antiviral activity. After 1 month of storage, samples at 4 and 25°C showed comparable antiviral activity to unstored samples, reducing infectivity by 2 to 3 log10, while the sample stored at 37°C inactivated virus by 4 to 5 log10 (P < 0.001) (Fig. 4E). These results indicated that storage of TF-3 and LA or TF-3 alone for 1 month did not result in the loss of antiviral activity of either compound.
Fig 7.
Effect of storage at various temperatures and pHs on the antiviral activity of TF-3 and LA. Samples were stored at either 4, 25, or 37°C at pH 5.7 (A), pH 5.0 (B), pH 4.5 (C), or pH 4.0 (D) with 1% LA present in both controls and TF-3 (100 μM)-treated samples. Unstored samples were made fresh directly before assay at the indicated pH. (E) Samples were also stored without LA at pH 4.0. Isolates were prepared and titers were determined as described in Fig. 2.
Effect of semen and cervical vaginal fluid on TF-3 antiviral activity.
Since semen and CVL will be present in vivo, the antiviral efficacy of TF-3 and LA were examined in their presence (Table 1). When HSV-1 and HSV-2 were incubated at pH 4.0 with LA but without TF-3 the titer of both viruses was reduced by >3 log10 in the presence of only semen or CVL. Semen appeared to slightly inhibit the anti-HSV activity of LA against HSV-2 at pH 4.0, while CVL had no effect on viral inactivation. We have found that 12.5% semen raises the pH slightly to 4.3 to 4.4, and this may partially explain why semen somewhat reduces the anti-HSV activity of LA (data not shown). When semen and CVL were present together at pH 4.0 with 1% LA, HSV-1 inactivation was 2 log10 less effective than when semen or CVL was present alone. However, when TF-3 was added to the 1% LA at pH 4.0 both HSV-1 and HSV-2 titers were reduced by 4 to 5 log10 in the presence of semen and CVL.
Table 1.
Effect of semen and CVL on the inactivation of HSV by TF-3 and LAa
| HSV sampleb | Mean HSV titer ± SDc at a TF-3 concn of: | |||
|---|---|---|---|---|
| 0 μM | 100 μM | 250 μM | 500 μM | |
| HSV-1 | ||||
| Semen + LA | 0.66 ± 0.77 | 0 | 0 | |
| CVL + LA | 0.35 ± 0.26 | 0 | 0 | |
| Semen + CVL + LA | 2.63 ± 0.69 | 0.83 ± 1.44 | 0.58 ± 1.01 | 0.83 ± 0.88 |
| HSV-2 | ||||
| Semen + LA | 1.59 ± 0.48 | 0.54 ± 0.94 | 0 | 0 |
| CVL + LA | 0.69 ± 1.38 | 0.46 ± 0.80 | 0 | 0 |
| Semen + CVL + LA | 2.03 ± 1.51 | 0 | 0.13 ± 0.22 | 0.58 ± 1.01 |
DISCUSSION
The results of the present study establish that all clinical isolates of HSV-1 and HSV-2 examined were effectively inactivated at pH 4.0 by 1% LA but that antiviral LA activity was diminished at a pH of ≥4.5. The difference seen in the effectiveness of LA at pH 4.0 and 4.5 may be due to the change in distribution at the two pHs between lactic acid and lactate. The pKa of l-lactic acid used in these experiments is 3.85 so that while the concentration of 1% LA (including both lactate and lactic acid) is 111 mM at pH 4.0 and pH 4.5, the concentrations of the acid forms are 44.8 mM at pH 4.0 and 19.6 mM at pH 4.5. Previous studies with bacteria have shown that it is the acid form of the molecule which has antimicrobial activity (16). However, when TF-3 was utilized in combination with LA, HSV titers could be reduced by as much as 5 log10 from pH 4.0 to pH 5.7. We have previously shown that TF-3 inactivates HSV from pH 5.7 to pH 8.0 (13) so that a combination of TF-3, LA, and acidic pH would be effective over the entire pH continuum found vaginally (22). The ability of semen to inhibit inactivation of HSV and HIV by candidate microbicides, as well as the endogenous antiviral activity in CVL, has been found in a number of studies (23, 24, 25). These previous results suggested that by combining multiple mechanisms for viral inactivation HSV and HIV could be effectively inactivated in the presence of semen. We also show here that the presence of semen alone or with CVL can reduce the efficacy of LA against HSV at pH 4.0 but that the addition of TF-3 at pH 4.0 with LA reduces virus titers as effectively as when semen is absent. These studies also established that storage of TF-3 with 1% LA at 37°C for 1 week to 1 month consistently maintained TF-3-dependent anti-HSV activity from pH 4.5 to 5.7 with no apparent loss of LA-dependent antiviral activity at pH 4.0.
Our results showing that it is not acidic pH by itself, i.e., pH 4.0, that inactivates HSV, but the acid form of lactic acid is in agreement with other studies that found that at pH 4.0 or below the acid form of total lactate reduces the infectivity of HIV in the presence of human cervicovaginal mucus (17) and inactivates or reduces the growth rate of Escherichia coli (16) and other Gram-negative bacteria (26). It has also been found that LA in its acidic form increases the release of antiviral mediators from vaginal epithelial cells (27). This cytokine response is specific for LA and occurs solely at an acidic pH. It is the presence of LA in the vagina that is important for maintaining protective function, e.g., preventing bacterial vaginosis, and it does not appear to matter which bacterium produces LA (18, 28). The importance of acidic pH by itself, however, should not be discounted since it has been shown that human papillomavirus diffusion through mucin is 2.5 times slower at pH 3.0 than pH 7.0 (29).
TF-3 has been shown in our previous studies to inactivate a number of enveloped viruses, including HIV (30), measles virus, Semliki Forest virus, respiratory syncytial virus, and vesicular stomatitis virus (13). A number of recent studies have shown that the TF-3 precursor EGCG inhibits the enveloped virus hepatitis C from cell entry and confirm our previous studies showing EGCG inactivation of HSV (31, 32). The mechanism of EGCG and TF-3 viral and bacterial inactivation appears to involve interaction with components of cell membranes (33, 34). TF-3 has the ability to bind to lipid bilayers without being absorbed into the interior of the bilayer, as does EGCG, and both can therefore be delivered to membrane proteins (34), including viral membrane proteins required for fusion such as HSV gB and gD (35). The present study focused on TF-3 since we have previously shown that whereas TF-3 and EGCG inactivate HSV at neutral pH, only TF-3 inactivates HSV at acidic pH (13, 19).
Since TF-3 binds to lipid bilayers and can also inactivate bacteria (36), it is likely that it also can bind cell membranes and raises the question of toxicity. There is, however, evidence arguing against TF-3 toxicity in addition to the fact that TF-3 is present in black tea, which is widely consumed and is on the U.S. Food and Drug Administration's list of compounds generally recognized as safe (GRAS) and approved for human consumption. We have previously shown using confocal and electron microscopy that TF-3 does not damage Vero cells (13). TF-3 in combination with other theaflavins from black tea has been shown to have low mucosal toxicity and lack systemic absorption when applied vaginally in a gel to rabbits as well as showing no cytotoxicity to human vaginal and cervical epithelial cells (37). When TF-3 and other theaflavins were consumed orally by healthy volunteers, only minute amounts could be detected in plasma and urine (38, 39). This is similar to results found with EGCG, where studies with healthy volunteers showed oral consumption was safe and well tolerated (40), and no dermal toxicity was found after daily topical application with mice (41).
Although all HSV-2 isolates examined in the present study were equally sensitive to TF-3 inactivation from pH 4.0 to 5.7, some HSV-1 clinical isolates required higher TF-3 concentrations for comparable inactivation. The numbers of isolates used here are not sufficient to draw a broad conclusion that HSV-2 isolates will on average be more sensitive to TF-3 inactivation than HSV-1. However, this would not be entirely unique since differences between the interaction of HSV-1 and HSV-2 with other compounds such as the HSV receptor heparin sulfate, and neomycin have also been found indicating that HSV-2 is more sensitive to inhibition by polyanions than HSV-1, while polycations more effectively inhibit HSV-1 than HSV-2 (42, 43, 44). This suggests that there may be differences in the structure of HSV envelope glycoproteins required for cell binding and fusion between the clinical HSV isolates so that TF-3 has a lower binding affinity for the envelope glycoprotein(s) of some HSV isolates.
Shedding of HSV-2 in the genital tract has been found to be characterized by frequent, intermittent episodes and not solely by larger less frequent episodes (9). Although antiviral therapy is clinically effective, it does not change the frequency of virion shedding occurrences, and it was found that 20% of patients receiving standard dose antiviral therapy had HSV titers of ≥104/ml (12). That study also showed that even with high-dose valaciclovir or acyclovir, virion shedding remained at comparable levels with standard dose therapy, and the authors suggested that genital HSV shedding cannot be further reduced with current treatment regimens. It is therefore necessary to inactivate shed HSV virions to further reduce HSV transmission.
A microbicide utilizing TF-3, LA, and buffered to an acidic pH (45) would inactivate shed HSV virions and supplement innate mucosal protection, including LA already present in the vagina, as well as potentially help inactivate acyclovir resistant HSV virions (46). Decreasing the size of the HSV inoculum is important for reducing viral transmission since it is likely that reducing HSV titers close to an infectious threshold will reduce the likelihood of infection. Vaccine challenge studies in a guinea pig model showed protection from infection using 5 × 104 PFU/ml, but no protection with a 20-fold-higher challenge dose (47). The importance of the shed HSV titer for viral transmission has also been found for mother-to-infant transmission during birth, where differences in shed HSV titers can result in 10-fold differences in the incidence of viral transmission (48). Viral load has also been shown to be the primary predictor of HIV transmission (49). Therefore, the more mechanisms that can be utilized to reduce shed HSV titers provided both by the microbicide and the mucosal immune system, the greater the probability of reducing viral transmission. Work supporting this principle has been provided recently by a study using combinations of vaccines and microbicides to reduce HIV transmission in a macaque model (50). The vaccine reduced the HIV load but not the acquisition of the virus, whereas the microbicide reduced HIV acquisition somewhat but had little effect on viral loads. TF-3 reduction of HSV-1 and HSV-2 inoculum sizes, even if the titers of some HSV-1 isolates may not be reduced as much as HSV-2 isolates, should decrease HSV transmission at all pHs in the female genital tract. Potentially, this will also reduce HIV transmission as well since the probability of HIV infection is three times higher in people with previous HSV-2 infection (5).
In summary, to reduce HSV transmission in the genital tract multiple antiviral mechanisms should function simultaneously to inactivate both HSV-1 and HSV-2 virions because of the increasing prevalence of HSV-1 infections in the anogenital tracts (2). TF-3 inactivates HSV at a neutral and acidic pH, is stable in this pH range (51), and when combined with LA at low pH inactivates HSV in the presence of semen and CVL. A microbicide that maintains low vaginal pH and provides additional LA to maintain a vaginal LA concentration of ≥1% (111 mM) is supplementing antiviral mechanisms already present in the vagina. If the antiviral activity of TF-3 and LA are added to the natural low pH and innate mucosal immunity present in the female reproductive tract, it will be possible to reduce the transmission of HSV.
Supplementary Material
Supplemental material
ACKNOWLEDGMENTS
CVL samples were kindly provided by the Magee-Womens Research Institute, University of Pittsburgh as part of the FAB research program supported by Public Health Service grant AI-082639 from the National Institute of Allergy and Infectious Diseases. This study was also supported by the New York State Office of Mental Retardation and Development Disabilities.
We thank Richard J. Kascsak for helpful suggestions during the preparation of the manuscript and Anna Parese and Mary Ellen Cafaro for helping to prepare the manuscript.
Footnotes
Published ahead of print 28 May 2013
REFERENCES
- 1.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973 [DOI] [PubMed] [Google Scholar]
- 2.Ryder N, Jin F, McNulty AM, Grulich AE, Donovan B. 2009. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992–2006. Sex. Transm. Infect. 85:416–419 [DOI] [PubMed] [Google Scholar]
- 3.Mindel A. 1998. Genital herpes: how much of a public-health problem? Lancet 351:16–18 [DOI] [PubMed] [Google Scholar]
- 4.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83 [DOI] [PubMed] [Google Scholar]
- 6.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, Mujugira A, Baeten JM, Mullins JI, Hughes JP, Bukusi EA, Cohen CR, Katabira E, Ronald A, Kiarie J, Farquhar C, Stewart GJ, Makhema J, Essex M, Were E, Fife KH, de Bruyn G, Gray GE, McIntyre JA, Manongi R, Kapiga S, Coetzee D, Allen S, Inambao M, Kayitenkore K, Karita E, Kanweka W, Delany S, Rees H, Vwalika B, Stevens W, Campbell MS, Thomas KK, Coombs RW, Morrow R, Whittington WLH, McElrath MJ, Barnes L, Ridzon R, Corey L, for the partners in the Prevention HSV/HIV Transmission Study Team 2010. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N. Engl. J. Med. 362:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naranbhai V, Karim SSA, Altfeld M, Samsunder N, Durgiah R, Sibeko S, Karim QA, Carr WH, and the CAPRISA004 TRAPS team 2012. Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J. Infect. Dis. 206:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat. Med. 15:886–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Magaret A, Wald A, Corey L. 2009. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci. Transl. Med. 1:7ral6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tata S, Johnston C, Huang ML, Selke S, Magaret A, Corey L, Wald A. 2010. Overlapping reactivations of herpes simplex virus type 2 in the genital and perianal mucosa. J. Infect. Dis. 201:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phipps W, Saracino M, Magaret A, Selke S, Remington M, Huang ML, Warren T, Casper C, Corey L, Wald A. 2011. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J. Infect. Dis. 203:180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang ML, Schiffer JT, Koelle DM, Corey L, Wald A. 2012. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomized, open-label, crossover trials. Lancet 379:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacs CE, Xu W, Merz G, Hillier S, Rohan L, Wen GY. 2011. Digallate dimers of (−)-epigallocatechin gallate inactivate herpes simplex virus. Antimicrob. Agents Chemother. 55:5646–5653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Clercq E. 2012. Where rilpivirine meets with tenofovir, the start of a new anti-HIV drug combination era. Biochem. Pharmacol. 84:241–248 [DOI] [PubMed] [Google Scholar]
- 15.Swiss Association for the Study of the Liver 2012. Review Article. Treatment of chronic hepatitis C genotype 1 with triple therapy comprising telaprevir or boceprevir. Swiss Med. Wkly. 142:1w13516. [DOI] [PubMed] [Google Scholar]
- 16.Valore EV, Park CH, Igreti SL, Ganz T. 2002. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 187:561–568 [DOI] [PubMed] [Google Scholar]
- 17.Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. 2009. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol. 83:11196–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witkin SS, Ledger WJ. 2012. Complexities of the uniquely human vagina. Sci. Transl. Med. 4:132fs11. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs CE, Wen GY, Xu W, Jia JH, Rohan L, Corbo C, Di Maggio V, Jenkins EC, Jr, Hillier S. 2008. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 52:962–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thormar H, Isaacs CE, Brown HR, Barshatzky MR, Pessolano T. 1987. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 31:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed LJ, Muench M. 1938. A simple method of estimating 50 per cent end points. Am. J. Hyg. (Lond.) 27:493–497 [Google Scholar]
- 22.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A. 108:4680–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neurath AR, Strick N, Li YY. 2006. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect. Dis. 6:1471–2334/6/1501-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel S, Hazrati E, Cheshenko N, Galen B, Yang H, Guzman E, Wang R, Herold BC, Keller MJ. 2007. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J. Infect. Dis. 196:1394–1402 [DOI] [PubMed] [Google Scholar]
- 25.Herold BC, Mesquita PM, Madan RP, Keller MJ. 2011. Female genital tract secretions and semen impact the development of microbicides for the prevention of HIV and other sexually transmitted infections. Am. J. Reprod. Immunol. 65:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alakomi H-L, Skyttä E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66:2001–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mossop H, Linhares IM, Bongiovanni AM, Ledger WJ, Witkin SS. 2011. Influence of lactic acid on endogenous and viral RNA-induced immune mediator production by vaginal epithelial cells. Obstet. Gynecol. 118:840–846 [DOI] [PubMed] [Google Scholar]
- 28.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, Koenig SSK, Fu L, Ma Z, Zhou X, Abdo Z, Forney LJ, Ravel J. 2012. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieleg O, Lieleg C, Bloom J, Buck CB, Ribbeck K. 2012. Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules 13:1724–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Lu H, Zhao Q, He Y, Niu J, Debnath AK, Wu S, Jiang S. 2005. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Biophys. Acta 1723:270–281 [DOI] [PubMed] [Google Scholar]
- 31.Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, Manns MP, Ott M, Wedemeyer H, Meuleman P, Pietschmann T, Steinmann E. 2011. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 504:1947–1955 [DOI] [PubMed] [Google Scholar]
- 32.Calland N, Albecka A, Belouzard S, Wychowski C, Duverlie G, Descamps V, Hober D, Dubuisson J, Rouillé Y, Séron K. 2012. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 55:720–729 [DOI] [PubMed] [Google Scholar]
- 33.Kajiya K, Kumazawa S, Naito A, Nakayama T. 2008. Solid-state NMR analysis of the orientation and dynamics of epigallocatechin gallate, a green tea polyphenol, incorporated into lipid bilayers. Magn. Reson. Chem. 26:174–177 [DOI] [PubMed] [Google Scholar]
- 34.Sirk TW, Friedman M, Brown EF. 2011. Molecular binding of black tea theaflavins to biological membranes: relationship to bioactivities. J. Agric. Food Chem. 59:3780–3787 [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpesvirus fusion and entry: a story with many characters. Viruses 4:800–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman M, Henika PR, Levin CE, Mandrell RE, Kozukue N. 2006. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus cereus. J. Food Prot. 69:354–361 [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Li L, Jin H, Tan S, Qiu J, Yang L, Ding Y, Jiang ZH, Jiang S, Liu S. 2012. Vaginal gel formulation based on theaflavin derivatives as a microbicide to prevent HIV sexual transmission. Aids Res. Hum. Retrovir. 11:1498–1508 [DOI] [PubMed] [Google Scholar]
- 38.Mulder TPJ, van Platerink CJ, Schuyl PJW, van Amelsvoort JMM. 2001. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J. Chromatogr. 760:271–279 [DOI] [PubMed] [Google Scholar]
- 39.Yang CS, Wang X, Lu G, Picinich SC. 2009. Cancer prevention by tea: animal studies molecular mechanisms and human relevance. Cancer 9:429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, Pineau B, Weber P. 2003. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 31:88–101 [DOI] [PubMed] [Google Scholar]
- 41.Stratton SP, Bangert JL, Alberts DS, Dorr RT. 2000. Dermal toxicity of topical (−)epigallocatechin-3-gallate in BALB/c and SKH1 mice. Cancer Lett. 158:47–52 [DOI] [PubMed] [Google Scholar]
- 42.Herold BC, Gerber SI, Belval BJ, Siston AM, Shulman N. 1996. Differences in the susceptibility of herpes simplex virus types 1 and 2 to modified heparin compounds suggest serotype differences in viral entry. J. Virol. 70:3461–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trybala E, Liljeqvist JÅ, Svennerholm B, Bergström T. 2000. Herpes simplex virus types 1 and 2 differ in their interaction with heparan sulfate. J. Virol. 74:9106–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langeland N, Holmsen H, Lillehaug JR, Haarr L. 1987. Evidence that neomycin inhibits binding of herpes simplex virus type 1 to the cellular receptor. J. Virol. 61:3388–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patton DL, Sweeney YC, Cummings PK, Meyn L, Rabe LK, Hillier SL. 2004. Safety and efficacy evaluations for vaginal and rectal use of buffer gel in the macaque model. Sex. Transm. Dis. 31:290–296 [DOI] [PubMed] [Google Scholar]
- 46.Strand M, Islam K, Edlund K, öberg CT, Allard A, Bergström T, Mei Elofsson Y-FM, Wadell G. 2012. 2-[4,5-difluoro-2-(2-fluorobenzoylamino)-benzoylamino] benzoic acid, an antiviral compound with activity against acyclovir-resistant isolates of herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 56:5735–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prichard MN, Kaiwar R, Jackman WT, Quenelle DC, Collins DJ, Kern ER, Kemble GM, Spaete RR. 2005. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine 24:5424–5431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitley RJ. 1994. Herpes simplex virus infections of women and their offspring: implications for a developed society. Proc. Natl. Acad. 91:2441–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921–929 [DOI] [PubMed] [Google Scholar]
- 50.Barouch DH, Klasse PJ, Dufour J, Veazey RS, Moore JP. 2012. Macaque studies of vaccine and microbicide combinations for preventing HIV-1 sexual transmission. Proc. Natl. Acad. Sci. U. S. A. 109:8694–8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J, Li L, Tan S, Jin H, Qiu J, Mao Q, Li R, Xia C, Jiang Jiang Z-HS, Liu S. 2012. A natural theaflavins preparation inhibits HIV-1 infection by targeting the entry step: potential applications for preventing HIV-1 infection. Fioterapia 83:348–355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material