Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells (original) (raw)
Abstract
We investigated the effects of transient receptor potential M8 (TRPM8) channel on the proliferation and motility of androgen-independent prostate cancer PC-3 cells. After being permanently transfected with an empty vector and cDNA encoding the TRPM8 protein, cells were analysed for cell cycle distribution and motility using flow cytometry and scratch assay. Immunocytochemistry and Ca2+ imaging analysis revealed the overexpression of functional TRPM8 channel on both endoplasmic reticulum and plasma membrane of PC-3-TRPM8 cells. Cell cycle distribution and scratch assay analysis revealed that TRPM8 induced cell cycle arrest at the G0/G1 stage (P < 0.05) and facilitated the cell apoptosis induced by starvation (P < 0.05). Furthermore, TRPM8 inhibited the migration of PC-3-TRPM8 cells (P < 0.01) through the inactivation of focal-adhesion kinase. It appears that TRPM8 was not essential for the survival of PC-3 cells; however, the overexpression of TRPM8 had negative effects on the proliferation and migration of PC-3 cells. Thus, TRPM8 and its agonists may serve as important targets for the treatment of prostate cancer.
Keywords: migration, proliferation, prostate cancer, transient receptor potential (TRP) channels
Introduction
Prostate cancer (PC) is one of the leading threats to men's health 1. In its early stages, PC cells depend on androgens for growth and survival, and androgen ablation therapy at that time may be effective in causing tumours to regress; however, in the late androgen-independent stage, there is currently no successful therapy.
The important role of Ca2+ in global cancer-related cell signalling pathways is well accepted. Fluctuations in Ca2+ homeostasis may lead to an increase in cell proliferation 2, 3, and even induce differentiation 4 and apoptosis 5, 6, 7. According to a growing number of studies, cationic channels from the transient receptor potential (TRP) family are key players in calcium homoeostasis and cell physiopathology. The Trpm8 gene, initially known as trp-p8, encodes for a so-called 'cold' receptor protein belonging to the melastatin (TRPM) subfamily of TRP channels, which is activated by cool temperatures and by menthol. Aside from sensory neurons, in which the role of TRPM8 involves mediating cold-evoked excitation 8, this channel is most abundantly expressed in the prostate tissue. In fact, TRPM8 was first cloned from the human prostate as a prostate-specific gene 9, even before its role in mediating the cold sensation response was established. Moreover, while remaining at moderate levels in a normal prostate, TRPM8 expression strongly increases in PC cells, and the expression levels have been shown to be highly correlated with specific PC tumour stages 9.
It has been shown that anti-androgen therapy greatly reduced the expression of TRPM8, suggesting that TRPM8 is regulated by androgens 10. Bidaux et al. 11 also showed that in PC cell lines, and in primary cultures of normal, hyperplasic and cancerous prostate epithelial cells, TRPM8 was a target gene of the androgen receptor (AR). TRPM8 expression-silencing experiments using small interference RNA (siRNA) suggested that Ca2+ influx through the TRPM8 channel plays an essential role in cellular Ca2+ homoeostasis in prostate epithelial cells and is involved in cell survival 12.
However, upon administration of anti-androgen therapy, the prostate epithelial cells downregulate the expression of AR and, consequently, that of TRPM8 mRNA. Prostate cancer and metastasis then progress into an androgen-independent stage, resulting in cancer relapse with a more aggressive phenotype rather than apoptosis or a simple regress.
This study was designed to investigate the possible effects of TRPM8 on the proliferation and motility of androgen-independent cancer PC-3 cells that are characterized by a decreased or complete lack of expression of AR and TRPM8.
Materials and methods
Cell culture
Human prostate carcinoma PC-3 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). PC-3 cells were routinely cultured in RPMI 1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 100 IU mL−1 penicillin G sodium, 100 μg mL−1 streptomycin sulphate and 10% foetal bovine serum (FBS) (Gibco BRL, Grand Island, NY, USA) in a humidified atmosphere consisting of 95% air and 5% CO2 at 37°C.
Permanent transfection of PC-3 cells with TRPM8 cDNA
PC-3 cells were plated on a six-well plate and transfected at about 90% confluence with the rat TRPM8 encoding vector pcDNA3 (a kind gift from Dr David Julius of the University of California, San Francisco, CA, USA) using 10 μL Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) as recommended by the manufacturer's protocol. Stably transfected clones were selected using Geneticin G-418 (Sigma, St. Louis, MO, USA) at a concentration of 500 μg mL−1. Nine colonies were identified using reverse transcription-polymerase chain reaction (RT-PCR) and western blot analyses, and were then subcloned and maintained under the selected pressure for an additional week.
Immunofluorescence and microscopy
The PC-3-vector (permanently transfected with plasmid encoding an empty vector) and PC-3-TRPM8 cells (permanently transfected with plasmid encoding the TRPM8 complementary DNA) were plated on 12-mm coverslips and incubated overnight before being fixed with 100% methanol at −20°C for 10 min. After three washes in phosphate buffered solution (PBS), the cells were incubated in 5% bovine serum albumin (BSA) at room temperature for 30 min and then washed in PBS thrice. Afterwards, the cells were incubated with a primary rabbit polyclonal anti-TRPM8 antibody (code: ACC-049, Alomone Labs, Jerusalem, Israel) and diluted (1:100) in 1% BSA at room temperature for 1 h. After thorough washes in PBS, the FITC-anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was added to the mixture and incubated for 1 h, and the cells were subsequently washed four times with PBS, followed by incubation with 4,6-diaminodino-2-phenylinodel (Sigma). Coverslips were analysed using a confocal microscope.
Ca2+ imaging
The cytosolic Ca2+ concentration ([Ca2+]c) was measured using the ratiometric dye Fura-3 (Molecular Probes, Leiden, The Netherlands). The temperature was maintained at 37°C using a temperature controller (Cell Microcontrol System, Norfolk, VA, USA). For confocal Ca2+ imaging, the cells were loaded in the presence of 2.5 μmol L−1 Fluo-3AM dye in the culture medium at 37°C for 2 h. During measurements, the cells were incubated in Hanks' balanced salt solution (HBSS) containing 150 mmol L−1 NaCl, 5.4 mmol L−1 KCl, 2 mmol L−1 CaCl2, 1 mmol L−1 MgCl2, and 10 mmol L−1 Hepes (pH adjusted to 7.4 with 1 mol L−1 NaOH). In order to produce Ca2+-free conditions, CaCl2 was removed from this solution, and 0.5 mmol L−1 ethylene glycol tetraacetic acid (EGTA) was added in its place. The emission intensity was measured for 90 s at 3-s intervals using excitation wavelengths of 340 and 380 nm at an emission wavelength of 520 nm. Menthol (Sigma) was added after 15 s. Analyses of the emission/excitation intensity ratios at 340 nm/380 nm were performed using the ImageMaster suite of software.
Cell cycle and cell apoptosis induced by starvation were examined by flow cytometry
Approximately 5 × 105 cells per well were incubated until 85% confluence was achieved and were then digested with 0.25% trypsin (Gibco BRL, Grand Island, NY, USA). Cells were subsequently harvested and fixed gently (drop by drop) by the addition of 70% ethanol (in PBS) at 4°C overnight and then re-suspended in PBS containing 40 μg mL−1 propidium iodide (PI), 0.1 mg mL−1 RNase and 0.1% Triton X-100 in a dark room. After incubation at 37°C for 30 min, the cells were analysed through flow cytometry (Becton-Dickinson, San Jose, CA, USA) equipped with an argon ion laser at a wavelength of 488 nm. The cell cycle stage was then determined and analysed.
For cell apoptosis analysis, cells were seeded in a 75-mm flask at 40%–50% confluence. After 24-h incubation in complete medium, the old medium was aspirated and a culture medium containing 1% FBS was added, after which the cells were incubated for an additional 48 h. After treatment, the cells were incubated in a binding buffer containing FITC-conjugated Annexin V and PI (Abcam, Cambridge, MA, USA) at room temperature for 5 min in the dark, according to the manufacturer's protocol. The percentages of apoptotic cells were then determined using flow cytometry.
Scratch motility assay
Cells were cultured as confluent monolayers in complete medium for 24 h, and then wounded by removing cells across the well with a standard 200 μL pipette tip 13. The wounded monolayers were washed twice to remove the non-adherent cells. The wound closure was monitored using an inverted phase contrast microscope (Leica, Wetzlar, Germany) at the time the wound was created and 24 h later. This 24-h time interval was chosen as it is shorter than the PC-3 doubling time. Four different fields from each sample were considered for quantitative estimation of the distance between the borderlines, and in each image, four different equidistant points were measured to obtain a better estimate of the true width of the wounded area. The migration rate is expressed as a percentage of the control (PC-3), and was calculated as the proportion of the mean distance between both borderlines caused by scratching to the distance that remained cell-free after re-growing. Two independent series of experiments were performed in quadruplicates.
Western blot assay
Cultured cells were pooled and lysed in lysis buffer (50 mmol L−1 Tris-HCl pH 7.4, 5 mmol L−1 ethylenediaminetetraacetic acid, 1 mmol L−1 EGTA, 10 mmol L−1 2-mercaptoethanol) containing protease inhibitors (5 μg mL−1 leupeptin, 5 μg mL−1 aprotinin, 10 μg mL−1 soybean trypsin inhibitor and 1 mmol L−1 phenylmethylsulphnyl fluoride) and were subsequently sonicated. After centrifugation at 12 000 × g for 15 min to remove all organelles, the supernatant was recovered, and the total protein content was measured using a bicinchoninic acid (BCA) kit. The protein expression of TRPM8, cyclin-dependent kinase (Cdk) 4, Cdk6, focal-adhesion kinase (FAK)-pY397 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was assayed using western blot analysis using anti-TRPM8-specific (code: ACC-049, Alomone labs, Jerusalem, Israel), anti-Cdk4-specific (Neomarkers, Union City, CA, USA), anti-Cdk6-specific (Neomarkers), anti-FAK-pY397-specific (Biosource, Camarillo, CA, USA) and anti-GAPDH-specific (Neomarkers) antibodies, as described earlier 7.
Statistical analysis
The SPSS version 11.5 for Windows (SPSS, Chicago, IL, USA) was used for the statistical analysis. All of these data have been presented as the mean ± SEM. Statistical analysis was performed using unpaired _t_-test, with P< 0.05 taken as statistically significant.
Results
Detection of TRPM8 protein expression in PC-3, PC-3-vector and PC-3-TRPM8 cells
Using the specific TRPM8 antibody, we investigated the TRPM8 protein expression in PC-3, PC-3-vector and PC-3-TRPM8 cells through analysis of western blot assays (Figure 1A). As expected, we detected the expression of the TRPM8 protein in PC-3-TRPM8 cells and in human embryonic KIDNEY-TRPM8 cells, the latter of which served as a positive control. Although it has been reported earlier that TRPM8 is expressed and is functionally active in PC-3 cells 12, we failed to detect marked TRPM8 protein expression. Immunocytochemistry studies using the same antibody showed that TRPM8 immunofluorescence was clearly observed in almost all of the PC-3-TRPM8 cells examined, and that the majority of TRPM8 protein was found to be associated with a reticular structure in the intracellular restricted areas and outside of the nucleus (Figure 1B). Similarly, there was no visible immunofluorescence in the PC-3-vector cells examined.
Figure 1.
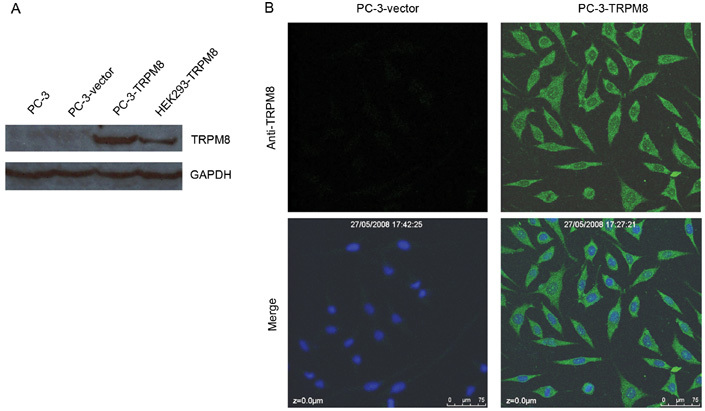
Expression and intracellular distribution of the transient receptor potential M8 (TRPM8) protein in PC-3-vector and PC-3-TRPM8 cells. (A): The detection of TRPM8 in PC-3, PC-3-vector and PC-3-TRPM8 cells using Western blotting analysis with an anti-TRPM8 antibody as described in the Materials and methods section. HEK-TRPM8 cells were used as the positive control and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. (B): Immunocytochemistry using an anti-TRPM8 antibody as described above. The fluorescence of TRPM8 was determined using confocal microscopy. The results shown are representative of those obtained in each of two independent experiments. Results similar to those shown were obtained for 13 of 15 cells examined in one experiment and for 14 of 15 cells examined in a second experiment.
Increase in [Ca2+]c in response to menthol in PC-3-vector and PC-3-TRPM8 cells
In a solution containing Ca2+, both PC-3-vector and PC-3-TRPM8 cells exhibited an increase in the Fluo-3AM fluorescence ratio (representing [Ca2+]c) in response to administration of the TRPM8 agonist menthol (100 μmol L−1). However, the increase in [Ca2+]c in PC-3-TRPM8 cells was much higher than that observed for the PC-3-vector cells (ΔRatio (340/380)mean of 2.546 ± 0.363 vs. 0.659 ± 0.293, P < 0.001); notably, these increases appeared as a single peak followed by a low-level sustained plateau (Figure 2).
Figure 2.
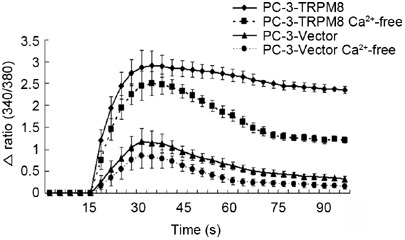
The increase in [Ca2+]c in PC-3-vector and PC-3-TRPM8 cells in response to menthol at room temperature (25°C). PC-3-vector and PC-3-TRPM8 cells were treated with 100 μmol L−1 menthol in HBBS in the absence and presence of calcium (HBBS-Ca2+ and -Ca2+-free, respectively). Each point represents the mean maximal [Ca2+]c produced by menthol administration during a 75-s application period. Menthol was added after 15 s.
To test whether the menthol-induced increase in [Ca2+]c was the result of the influx of Ca2+ from the extracellular region, cells were incubated in HBSS in the absence of Ca2+ and the effects of menthol were compared with those of cells in a Ca2+-containing solution. Perhaps of considerably more interest, the increase in Fura-3AM fluorescence in response to the administration of menthol, when compared with those cells in Ca2+-containing solution, decreased by 32.4% and 39.8% in PC-3-TRPM8 and PC-3-vector cells, respectively (Figure 2).
These results indicate that in both PC-3-vector and PC-3-TRPM8 cells, the substantial increase in [Ca2+]c in response to menthol is mainly caused by the release of Ca2+ from intracellular compartments and is only partly due to the influx of extracellular Ca2+. This means that the TRPM8 protein may be primarily expressed and functionally active on the endoplasmic reticulum (ER) membrane, and only partly expressed on the plasma membrane (PM), although we did not detect its visible expression on the PM when performing the immunofluorescence assay.
Cell cycle and apoptosis were examined using flow cytometry
After cells had been fixed and stained with PI, the DNA content and cell cycle distribution were measured using flow cytometry. The results indicated that the percentage of cells in the G0/G1 stage increased for the PC-3-TRPM8 cells when compared with the PC-3 and PC-3-vector cells (71.89% ± 3.43% vs. 54.67% ± 4.59% and 71.89% ± 3.43% vs. 51.48% ± 3.54%, respectively, P < 0.05, Figures 3A and C). The effect of the TRPM8 protein on the pro-apoptosis of the PC-3 cells was also investigated. After incubation in RPMI 1640 medium supplemented with 1% FBS in a humidified atmosphere consisting of 95% air and 5% CO2 at 37°C for 48 h, TRPM8 was found to have a significant pro-apoptotic effect on PC-3 cells, as indicated by the Annexin V-positive ratio in PC-3-TRPM8 cells that was higher than the ratios calculated for PC-3 and PC-3-vector cells (12.56% ± 1.78% vs. 4.67% ± 1.49% and 12.56% ± 1.78% vs. 5.28 ± 1.35%, respectively; P < 0.01, Figures 3B and D).
Figure 3.
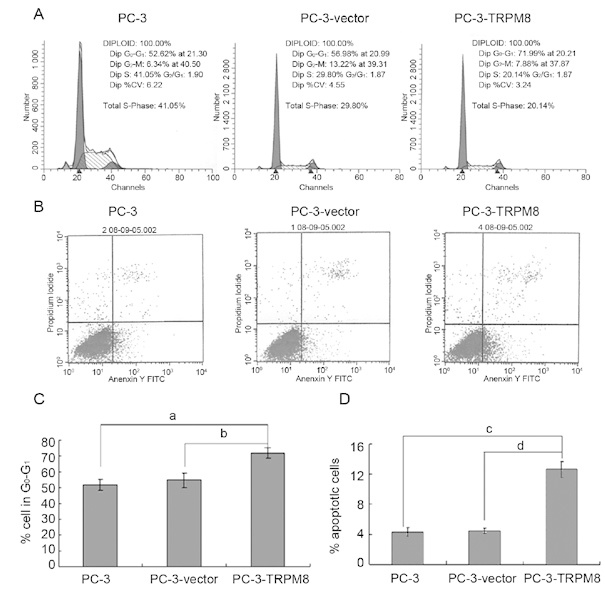
The cell cycle distribution and 1% foetal bovine serum (FBS)-induced apoptosis of PC-3, PC-3-vector and PC-3-transient receptor potential M8 (TRPM8) cells. (A) and (C): Cells stained with PI were analysed to determine the progression through the cell cycle using flow cytometry (a, b_P_ < 0.05, n_= 3, in separate cell cultures). (B) and (D): After treatment with RPMI 1640 supplemented with 1% FBS for 48 h, the cells were incubated with FITC-conjugated Annexin V and PI at room temperature for 5 min in the dark. The percentages of cell apoptosis were then determined using flow cytometry. The data are the mean of three independent experiments performed in duplicate (c, d_P < 0.01).
Cell migration assay
Using the scratch-wound assay, a continuous and rapid movement was observed for all cells, but a resultant movement of the PC-3 cell migration front was clearly evident at 24 h, in which a highly confluent (90%–100%) monolayer region gradually migrated to the cell-free 'scratch' region (Figure 4A). Upon comparison with PC-3 and PC-3-vector cells, the migration of PC-3-TRPM8 cells was significantly reduced after 24 h of incubation (P < 0.01, Figures 4A and B).
Figure 4.
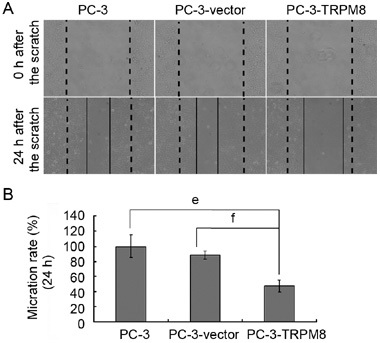
Transient receptor potential M8 (TRPM8) reduces PC-3 cell motility. (A): Photomicrographs showing the representative views of motility assays, obtained at 0 and 24 h. The broad dashed lines represent the original borderlines at the time of scratching, whereas the thin and solid lines represent the borderlines 24 h after scratching (original magnification: × 200). In the control (PC-3 cells) and in the PC-3-vector cells, the distance between the borderlines becomes significantly shorter 24 h after generation of the wound, whereas it is still elevated in PC-3-TRPM8 samples. (B): Wound areas were measured and normalized relative to the control values (PC-3 at 24 h) that were assumed at 100%. The data from six independent experiments were collected and the mean values were plotted with SEM. The graph shows that the cell migration rates of the PC-3-TRPM8 cells are significantly reduced in comparison with the PC-3 and PC-3-vector cells (e, f_P_ < 0.01).
Cell proliferation and migration behaviours, which are both targets of anti-cancer agent development, are important characteristics of cancer cells and serve as indicators of malignance. These observations revealed that the TRPM8 protein resulted in a statistically significant inhibition of PC-3-TRPM8 cell migration as compared with the cell migration observed for either the PC-3 or the PC-3-vector cells (Figure 4B).
Effect of TRPM8 on the expression of proliferation and motility-related proteins
To increase our understanding of the molecular mechanism of TRPM8-induced cell cycle arrest and migration changes in PC-3 cells, we examined the expression of proteins associated with cell cycle and migration after PC-3 cells were transfected with an empty vector and a TRPM8-encoding plasmid. In the group of cell cycle-related proteins, the CDK family is one of the most important and is known for its ability to regulate the cell cycle. However, it is generally thought that FAK plays a key role in cell migration 14, a function required for the invasion and metastasis of cancer cells.
In our study, TRPM8 downregulated the expression of Cdk4 and Cdk6, and thus induced cell cycle arrest at the G0/G1 stage (Figure 5). At the same time, TRPM8 was shown to decrease the activation of FAK (Figure 5).
Figure 5.
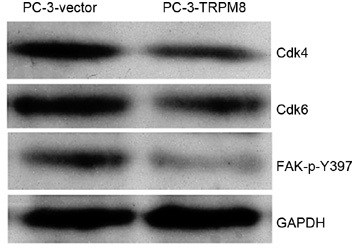
Changes in the protein expression of Cdk4, Cdk6 and FAK-p-Y397. Immunoblot analysis of the protein expression of Cdk4, Cdk6, FAK-p-Y397 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The protein expression of GAPDH in the same samples was used as a loading control.
Discussion
TRPM8 is a so-called 'cold' receptor protein belonging to the melastatin (TRPM) subfamily of TRP channels and is activated by both cool temperatures and menthol. Apart from sensory neurons, in which the role of TRPM8 in mediating the cold-evoked excitation has been fairly well established 8, 15, this channel is most abundantly expressed in the prostate. Moreover, although remaining at moderate levels in a normal prostate, TRPM8 expression significantly increases in PC cells. Despite a growing number of studies, the precise role of TRPM8 in the prostate remains unclear, although it has been suggested to be involved in the secretion functions of the prostate, and in the regulation of proliferation and/or apoptosis 11, 12.
Bidaux et al. 16 showed earlier that only highly differentiated human prostate primary luminal epithelial cells expressed functional PM TRPM8 channels [PMTRPM8 (PM TRPM8)], whereas ER TRPM8 channels [ERTRPM8 (ER TRPM8)] remained functional as an ER Ca2+ release channel independent of its differentiation status. In other words, these results indicate that localization may depend on the epithelial cell phenotype (i.e., fully differentiated secretory apical cells versus non-differentiated basal cells) and on the androgen status (i.e., androgen-dependent vs. hormone refractory cells highly resistant to apoptosis).
Although we were unable to detect marked expression of TRPM8 in PC-3-vector cells through immunocytochemistry and western blotting studies, the present investigation confirms that PC-3 cells may express an extremely low level of functional TRPM8, as the specific agonist menthol was able to induce an increase in [Ca2+]c, both in the presence and absence of Ca2+. Although the reasons for the absence of TRPM8 detection in the current study are unclear, this may be related to the antibody itself, in terms of its sensitivity, for example, or to the specific cell line used herein, as we did not detect the expression of TRPM8 in PC-3 cells at the mRNA level through routine RT-PCR. Furthermore, the increase in [Ca2+]c induced by menthol in the Ca2+-containing solution was significantly higher than that observed in the Ca2+-free solution. The extremely low level of AR expression in androgen refractory prostate cancer PC-3 cells probably contributed to the much lower level of TRPM8 gene expression and the lack of response to androgen regulation 12.
Our results, coupled with those reported by Zhang and Barritt 12, indicate that TRPM8 may be expressed not just from the PM, but also from the ER membrane. Dual localization and the channel-like function of the TRPM8 protein in the two membranes significantly broaden the spectrum of physiological and pathological processes in which it may be involved.
Antagonist experiments using capsazepine and TRPM8 expression-silencing experiments using siRNA 11, 12 suggested that the Ca2+ influx mediated by TRPM8 plays an essential role in cellular Ca2+ homoeostasis in LNCaP cells and is involved in cell survival. These results indicate that TRPM8 is an important determiner of Ca2+ homoeostasis in prostate epithelial cells, and may be a potential drug target for the management of PC. However, in this study, we found that the increased expression of TRPM8 in androgen-independent PC-3 cells induced cell cycle arrest at the G0/G1 stage, as opposed to functioning as an essential component for cell survival as reported earlier 11, 12, or for promoting the proliferation of PC-3 cells. Similarly, a report by Altuwaijri et al. 17 has shown that dihydrotestosterone at a concentration of 0.001–10 nmol L−1 induces cell cycle arrest or inhibits proliferation of PC-3-AR cells, which overexpress heterologous AR. In addition, it has been shown that heterologous AR overexpression in PC-3 cells strongly upregulates the expression of functional TRPM8 at both the levels of transcription and translation 16.
Cell cycle checkpoints are important control mechanisms that ensure the proper execution of cell cycle events. In addition, cell cycle progression is precisely regulated by a series of CDKs whose activities are strongly dependent on their association with cyclin subunits 18. We found herein that the overexpression of TRPM8 arrested the cell cycle at the G1 to S phase transition through the downregulation of Cdk4 and Cdk6. We also found that the overexpression of TRPM8 did not induce cell apoptosis (data not shown), but rather facilitated cell apoptosis induced by starvation in PC-3 cells. These results indicate that TRPM8, although expressed at an extremely low level on both the PM and the ER, may play an important role in maintaining the cytosolic Ca2+ homoeostasis in PC-3 cells. However, once the expression level is significantly increased, TRPM8 may function to perturb the Ca2+ homoeostasis in PC-3 cells, and thus induce an anti-proliferation and pro-apoptotic effect in PC-3 cells.
Death from cancer is generally associated with metastasis, a biological phenomenon linked to the tumour cell's ability to migrate, seed and colonize distant sites. FAK is an important regulator of cell migration 14, and is absolutely required from a functional perspective for the invasion and metastasis of cancer cells. This migration requires individual cells, or more likely small groups of cells, to initially move three-dimensionally through the extracellular matrix (ECM) surrounding the region of the primary tumour. This often, but not always, requires the combined action of matrix metalloproteinases that function to degrade ECM barriers. However, recent evidence suggests that there are also proteolysis-independent invasion pathways, and thus it is possible that FAK may be differentially required for distinct modes of tumour cell migration and invasion. The present investigation indicated that overexpression of TRPM8, through inactivation of FAK, reduced the motility of PC-3 cells.
Recent work by Zhang and Barritt 12 suggested that TRPM8 was required for the survival of the androgen-dependent LNCaP cell line, which strongly expresses TRPM8 on both the PM and the ER under the control of androgen. However, we found that as an androgen-independent cell line, PC-3 cells may express an extremely low level of functional TRPM8 on both the PM and the ER. Overexpression of TRPM8 in PC-3 cells induced cell cycle arrest and facilitated starvation-induced cell apoptosis, whereby TRPM8 itself did not directly induce apoptosis. Furthermore, the overexpression of TRPM8 in PC-3 cells also decreased the motility of the cell through the inactivation of FAK.
Although the natural TRPM8 activators in the prostate have not yet been identified, it is assumed that this cationic Ca2+-permeable ion channel plays a functional role in Ca2+ homoeostasis in prostate cells, especially in androgen-dependent cells such as normal prostate cells and LNCaP. We also assume that in PC-3 cells, which express an extremely low level of TRPM8, this cationic Ca2+-permeable ion channel is also of importance and its overexpression may perturb the Ca2+ homoeostasis. Thus, TRPM8 has an anti-proliferation effect on PC-3 cells, and furthermore, TRPM8 has an inhibitive effect on malignant progression through the inactivation of FAK, a protein that mediates both tumour formation and malignant progression.
In summary, this study shows that PC-3 cells express an extremely low level of TRPM8 on both the PM and the ER. Our results have also indicated that overexpression of TRPM8 has negative consequences on the proliferation and malignant progression of PC-3 cells. Therefore, for patients in the late androgen-independent stage, although there is currently no successful therapy, the activation of the existing channels or the overexpression of the channel may serve as a potential alternative treatment that is worthy of further investigation.
Acknowledgments
This work was supported by the Natural Science Foundation of Guangdong Province, China (No. 7001197) and National Natural Science Foundation of China (No. 30872572). We thank Dr David Julius, Department of Cellular and Molecular Pharmacology, University of California, San Francisco, USA, for the gift of the rat TRPM8 cDNA construct, which was critical for the completion of this study.
Footnotes
Edited by Dr Robert H. Getzenberg
References
- Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin. 1996;46:5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- Legrand G, Humez S, Slomianny C, Dewailly E, Vanden Abeele F, et al. Ca2+ pools and cell growth. Evidence for sarcoendoplasmic Ca2+-ATPases 2B involvement in human prostate cancer cell growth control. J Biol Chem. 2001;276:47608–14. doi: 10.1074/jbc.M107011200. [DOI] [PubMed] [Google Scholar]
- Thebault S, Flourakis M, Vanoverberghe K, Vandermoere F, Roudbaraki M, et al. Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 2006;66:2038–47. doi: 10.1158/0008-5472.CAN-05-0376. [DOI] [PubMed] [Google Scholar]
- Vanoverberghe K, Vanden Abeele F, Mariot P, Lepage G, Roudbaraki M, et al. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ. 2004;11:321–30. doi: 10.1038/sj.cdd.4401375. [DOI] [PubMed] [Google Scholar]
- Skryma R, Mariot P, Bourhis XL, Coppenolle FV, Shuba Y, et al. Store depletion and store-operated Ca2+ current in human prostate cancer LNCaP cells: involvement in apoptosis. J Physiol. 2000;527 Pt 1:71–83. doi: 10.1111/j.1469-7793.2000.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele F, Roudbaraki M, Shuba Y, Skryma R, Prevarskaya N. Store-operated Ca2+ current in prostate cancer epithelial cells. Role of endogenous Ca2+ transporter type 1. J Biol Chem. 2003;278:15381–9. doi: 10.1074/jbc.M212106200. [DOI] [PubMed] [Google Scholar]
- Vanden Abeele F, Skryma R, Shuba Y, Van Coppenolle F, Slomianny C, et al. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–79. doi: 10.1016/s1535-6108(02)00034-x. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001;61:3760–9. [PubMed] [Google Scholar]
- Henshall SM, Afar DE, Hiller J, Horvath LG, Quinn DI, et al. Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res. 2003;63:4196–203. [PubMed] [Google Scholar]
- Bidaux G, Roudbaraki M, Merle C, Crepin A, Delcourt P, et al. Evidence for specific TRPM8 expression in human prostate secretory epithelial cells: functional androgen receptor requirement. Endocr Relat Cancer. 2005;12:367–82. doi: 10.1677/erc.1.00969. [DOI] [PubMed] [Google Scholar]
- Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–73. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–92. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004. 1692. pp. 77–102. [DOI] [PubMed]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–15. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Bidaux G, Flourakis M, Thebault S, Zholos A, Beck B, et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest. 2007;117:1647–57. doi: 10.1172/JCI30168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuwaijri S, Wu CC, Niu YJ, Mizokami A, Chang HC, et al. Expression of human AR cDNA driven by its own promoter results in mild promotion, but not suppression, of growth in human prostate cancer PC-3 cells. Asian J Androl. 2007;9:181–8. doi: 10.1111/j.1745-7262.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995;308 Pt 3:697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]