Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy (original) (raw)
Abstract
Most tumor-associated antigens represent self-proteins and as a result are poorly immunogenic due to immune tolerance. Here we show that tolerance to carcinoembryonic antigen (CEA), which is overexpressed by the majority of lethal malignancies, can be reversed by immunization with a CEA-derived peptide. This peptide was altered to make it a more potent T cell antigen and loaded onto dendritic cells (DCs) for delivery as a cellular vaccine. Although DCs are rare in the blood, we found that treatment of advanced cancer patients with Flt3 ligand, a hematopoietic growth factor, expanded DCs 20-fold in vivo. Immunization with these antigen-loaded DCs induced CD8 cytotoxic T lymphocytes that recognized tumor cells expressing endogenous CEA. Staining with peptide-MHC tetramers demonstrated the expansion of CD8 T cells that recognize both the native and altered epitopes and possess an effector cytotoxic T lymphocyte phenotype (CD45RA+CD27−CCR7−). After vaccination, two of 12 patients experienced dramatic tumor regression, one patient had a mixed response, and two had stable disease. Clinical response correlated with the expansion of CD8 tetramer+ T cells, confirming the role of CD8 T cells in this treatment strategy.
In recent years, development of cancer vaccines has met with limited success. The majority of the progress has been seen in the more immunogenic tumors such as lymphoma, melanoma, and renal cell carcinoma (1–3). For other malignancies, such as lung and colorectal cancer, clinical success has been elusive. Unfortunately, these two malignancies represent the two most common cancers and the two leading causes of cancer deaths in the United States. Carcinoembryonic antigen (CEA) is a 180-kDa membrane intercellular adhesion glycoprotein that is overexpressed by a significant proportion of human tumors including >90% of colorectal, gastric, and pancreatic cancers, 70% of nonsmall cell lung cancer, and 50% of breast cancer (4, 5). Although many approaches have been used to vaccinate against CEA, none of the reported trials has led to objective responses (6–8). A clinical trial using viral vectors expressing CEA to immunize patients led to the identification of cytotoxic T lymphocytes (CTLs) specific for an HLA*A0201-restricted epitope from CEA (CEA605–613) (7). Nevertheless, vaccine trials with this specific epitope have failed to induce clinical responses (8).
As a “self-antigen,” CEA is poorly immunogenic, which likely explains the failure of clinical trials in which self-proteins are used as immunogens. One approach to this problem is to immunize with altered peptide ligands (APLs), peptides derived from native T cell epitopes that possess amino acid substitutions that either increase peptide affinity for the MHC peptide-binding groove or modify interactions through the T cell receptor (9, 10). Although the former approach has been studied, particularly in experimental melanoma vaccines (2, 11), the latter approach has not been explored clinically as a vaccination. Recently, an agonist epitope of CEA605–613 has been identified with aspartate substituting for asparagine at position 610 (610D). Although this substitution does not increase class I MHC binding of the peptide, the resulting APL possesses increased potency in inducing CTLs against CEA in vitro, presumably through altered interactions at the level of the T cell receptor, leading to enhanced ZAP-70 phosphorylation (12, 13). Importantly, CTLs raised to this agonist epitope maintain their ability to lyse tumor cells expressing native CEA. In the study described herein, this APL was tested for its ability to overcome immune tolerance to CEA.
Development of effective cancer vaccines relies not only on the identification of target antigens, but approaches to deliver these antigens to generate immunity as well. Dendritic cells (DCs) represent unique antigen-presenting cells that are capable of sensitizing T cells to both new and recall antigens (14). In recent years, several groups have begun to explore the use of DCs loaded in vitro with tumor-associated antigens as therapeutic vaccines (15). To date, DC vaccination has produced promising results primarily in patients with immunogenic tumors, but no success has been seen in poorly immunogenic tumors such as colorectal and lung cancer.
This report describes a phase I clinical trial combining APL vaccination with an approach to the acquisition of large numbers of DCs: the systemic administration of Flt3 ligand (Flt3L) to expand DCs in vivo followed by their isolation, antigen loading, and infusion as a vaccine. Flt3L initially was described as a growth factor for multipotent hematopoietic stem cells as well as for committed myeloid and lymphoid progenitors (16). More recently, preferential expansion of DCs was noted in Flt3L-treated mice and humans (17, 18). The clinical trial described in this report examines the ability of Flt3L to increase the number of DCs in cancer patients as well as the ability of these expanded DCs to be harvested and used to immunize patients against CEA. We demonstrate that immunization with Flt3L-expanded DCs loaded with an APL derived from CEA can lead to CEA-specific immunity and clinical responses.
Materials and Methods
Patients.
Twelve patients were enrolled in a phase I study of immunotherapy for colon or nonsmall cell lung cancer. All patients were required to have metastatic or recurrent cancer, have an abnormal or rising serum CEA, and be age ≤70 years. Before participating in the trial, study subjects provided signed informed consent that fulfilled Institutional Review Board guidelines at Stanford University. Patients were required to express the HLA A*0201 allele, have adequate hepatic and renal function, and have a life expectancy of more than 6 months. Patients also were tested for delayed type hypersensitivity to recall antigens tetanus toxoid, mumps, and candida (Connaught Laboratories) at study entry. A positive skin test was defined as ≥5 mm of induration 48 h after injection. Patients were followed until evidence of progression.
Antigens and Cell Lines.
CEA605–613 (YLSGANLNL), tyrosinase-derived Tyr368–376 (YMDGTMSQV), and cytomegalovirus (CMV) pp65 (NLVPMVATV) peptides were synthesized at the PAN facility (Stanford, CA). GMP grade 610D (YLSGADLNL) peptide was produced by Multiple Peptide Systems (San Diego). GMP grade keyhole limpet hemocyanin (KLH) was purchased from Intracel (Rockville, MD). The A221 cell line is an HLA-A, -B, and -C null mutant human B lymphoblastoid cell line devoid of costimulatory molecules and transfected with the HLA*A0201 gene (gift of D. Gerahty, Fred Hutchison Cancer Research Center, Seattle). The T2 cell line is a B and T lymphoblastoid hybrid cell line that expresses HLA*A0201 (gift of P. Cresswell, Yale University, New Haven, CT). The cell lines SW403 and SW1417 were derived from colorectal adenocarcinoma (American Type Culture Collection). Although both cell lines express CEA, only SW403 expresses HLA*A0201. The cell line A375 (gift of Y. Kawakami, National Cancer Institute, Bethesda, MD) expresses HLA*A0201 but lacks CEA expression. All cell lines were propagated in RPMI supplemented with 10% FBS, glutamine, antibiotics, and 2-mercaptoethanol.
Flt3L Administration.
Flt3L was supplied by Immunex. Patients received 20 mcg/kg of Flt3L up to a maximum dose of 1.5 mg via daily s.c. injections for the 10 days preceding their leukapheresis.
Fluorescence-Activated Cell Sorter (FACS) Analysis.
Four-color FACS analyses were performed by using a Becton Dickinson FACSCaliber. Fluorochrome-conjugated anti- CD3, CD11c, CD14, CD19, CD40, CD62L, CD80, CD86, anti-HLA-DR (Becton Dickinson); anti-CD44 and -CD83 (Immunotech); and anti-CD11c and goat anti-mouse IgM antibody (Caltag) were used for staining. CMRF44 antibody was kindly supplied by D. Hart, Brisbane, Australia. Anti-CCR7 antibody was kindly supplied by L. Wu (Millennium Pharmaceuticals, Cambridge, MA). A total of 1 × 106 cells were suspended in staining buffer (DPBS with 1% FCS and 0.1% sodium azide) with human IgG at 1 mg/ml (Sigma) for 20 min at 4°C, and then stained with the described antibodies. Data were analyzed and presented by using flowjo software (Tree Star, San Carlos, CA).
DC Preparation.
The patients underwent peripheral blood leukapheresis, consisting of processing two total body blood volumes (8–14 liters of blood), with a COBE cell separator. DCs were enriched by sequential density centrifugation modified from our previously described protocol (17). Briefly, peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation with Ficoll-Hypaque (Amersham Pharmacia) and cultured at a cell density of 10 × 106/ml in media (RPMI 1640, 10% human serum) containing KLH (2 mcg/ml) (Intracel). After 24 h of culture in a humidified incubator at 37°C with 10% CO2, DCs were depleted of lymphocytes by centrifugation through a 15% (wt/vol) metrizamide gradient (Sigma). The enriched DCs then were cultured overnight in media containing 50 mcg/ml KLH and 20 mcg/ml 610D peptide. Enriched DCs then were washed free of antigen and resuspended in normal saline with 5% autologous serum. The DCs were defined with flow cytometry by their expression of HLA DR and lack of lineage markers CD3, CD14, CD19, and CD56.
DC Immunization.
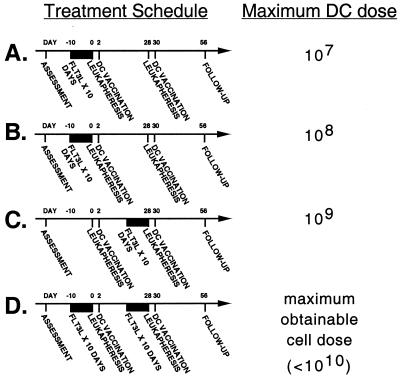
Patients underwent a series of two DC vaccinations that were separated by 1 month. These DC vaccinations were performed with unmobilized or Flt3L mobilized DCs as shown in Fig. 1. Patients received their autologous DCs i.v. in the General Clinical Research Center at Stanford Medical Center. Designed as a phase I DC dose escalation trial, the first study cohort of three patients received a maximum dose of 107 DC/injection. The second cohort received 108 DC/injection. The third cohort received 109 DC/injection. The fourth cohort received all DCs obtained with each vaccine preparation, which was <1010. The DC dose obtainable without Flt3L was <107 DC/injection.
Figure 1.
Treatment timeline. Study subjects received two monthly DC vaccinations enriched from autologous PBMCs obtained 2 days before by a leukapheresis procedure. Patients underwent 10 days of s.c. Flt3L injections before one or both of their initial leukapheresis procedures depending on the cohort assigned. (A) The first cohort (n = 3) received a 10-day course of Flt3L before their first leukapheresis and a maximal DC dose of 107 DC/vaccination. (B) The second cohort (n = 3) received a 10-day course of Flt3L before their first leukapheresis and a maximal DC dose of 108 DC/vaccination. (C) The third cohort (n = 3) received a single 10-day course of Flt3L before their second leukapheresis and a maximal DC dose of 109 DC/vaccination. (D) The fourth cohort (n = 3) received 10-day courses of Flt3L before both of their leukaphereses and the maximal DC dose obtainable (<1010 DC/vaccination).
T Cell Proliferation Assay.
PBMCs were cultured at 100,000 cells per well in triplicate in 96-well U-bottom plates (Costar) in RPMI containing 10% pooled human serum. KLH at varying concentrations or tetanus toxoid at a concentration of 2.5 units/ml (Connaught) was added at the initiation of culture. Proliferation was assessed on the basis of 18-h [3H]thymidine incorporation after 6 days of culture.
CTL Assays.
Cryopreserved PBMCs from each patient obtained before or after immunization were cultured in parallel with irradiated (8,000 R) A221 cells pulsed with 610D peptide at a 10:1 ratio in 48-well plates (Costar) at 106 cells/0.5 ml of media. IL-2 (R&D Systems) was added to the media 2 days later to a final concentration of 10 units/ml. This in vitro restimulation was repeated on day 7, and the subsequent effectors were analyzed for cytolytic activity in a [51Cr] release assay on day 14. Before their use as targets, cell lines SW403, SW1417, A375, and T2 were incubated in 250 μCi of [51Cr] for 2 h. T2 cells also were incubated without or with target peptides during this labeling step. Cell lines then were washed three times with RPMI and plated in triplicate with at least 5,000 targets/well in 96-well U-bottom plates (Costar). Effector cells then were coincubated with the 51Cr-labeled target cells at the described effector/target ratios. After a 4-h culture, supernatants were harvested and counted in a Microbeta counter (Wallac, Turku, Finland). Percent specific lysis was calculated by the formula: 100% × (experimental release − spontaneous release)/(maximum release − spontaneous release). Maximum release was determined by lysis of target cells in PBS containing 0.5% Triton X-100 (Sigma).
Tetramer Staining.
Production of MHC/peptide tetramers has been described (19). Briefly, a 15-aa BirA substrate peptide for BirA-dependent biotinylation has been engineered onto the COOH terminus of HLA-A*0201. The A2- BirA substrate peptide fusion protein and human β2-microglobulin were expressed in Escherichia coli and folded in vitro with the specific peptide ligand. The properly folded MHC-peptide complexes were extensively purified and biotinylated on a single lysine within the BirA substrate peptide by using the BirA enzyme (Avidity, Denver). Tetramers were produced by mixing the biotinylated MHC-peptide complexes with phycoerythrin-streptavidin (Prozyme, San Leandro, CA) at a molar ratio of 4:1. Tetramers presenting CEA605–613, 610D, and CMV pp65 were produced. Each tetramer reagent was titered individually and used at the optimum concentration, generally 10 μg/ml. CEA605–613 and 610D tetramers were validated by staining against CTL lines specific for HLA-A*0201 in association with CEA605–613 and 610D. CMV pp65 tetramer was validated by staining PBMCs from CMV immune patients. Cryopreserved PBMCs obtained before or after vaccination were thawed and analyzed in parallel. A total of 1 × 106 cells were stained with the corresponding HLA-A*0201 tetramer for 30 min at room temperature. Antibodies then were added at the recommended concentrations and incubated for 30 min at 4°C. Samples then were washed twice and analyzed with four-color flow cytometry. To establish the background for tetramer staining, 20 volunteer blood donors were assessed with the same methodology and had 0.30% ± 0.18% and 0.27% ± 0.14% to CEA605–613 and 610D tetramers, respectively. The monomer blocking experiments were performed by incubating cells with 610D monomer at concentrations of 10–50 mcg/ml for 30 min at room temperature before the addition of the tetramer and antibodies as described.
Statistical Analysis.
Statistical analyses were performed with the indicated tests by using statview (SAS Institute, Cary, NC). Significance was set at P < 0.05.
Results
Demographics and Trial Design.
Candidates for this clinical trial had metastatic cancer assessable by computed tomography scans and/or rising serum levels of CEA. Twelve patients with recurrent colorectal or nonsmall cell lung cancer have completed the treatment (summarized in Table 1). All patients were no longer responding to conventional treatment with chemotherapy. Three patients had been treated with two or more different chemotherapy regimens whereas four also had received radiation therapy. Five patients had persistent lymphopenia upon study entry, and six patients did not mount delayed type hypersensitivity skin reactions to candida, mumps, or tetanus.
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Age (median, range) | 53 (33–71) |
| Karnofsky Performance Status (median, range) | 90 (80–100) |
| Sex | 6 M, 6 F |
| Baseline serum CEA (mean, range) | 91.3 (4–380) |
| Primary tumor | 9 colon, 1 rectum, 2 lung |
| Courses of immunosuppressive treatment | |
| Chemotherapy | 15 |
| Radiation therapy | 4 |
| Baseline lymphopenia | 5/12 |
| Baseline delayed type hypersensitivity anergy* | 6/12 |
Patients participating in the trial underwent two DC immunizations, 28 days apart. To study the ability of Flt3L to increase the number of DCs for vaccination, patients were enrolled into sequential cohorts with differing schedules of Flt3L administration. To also explore the safety and effects of increasing DC dose, the successive cohorts were given increasing doses of DCs (Fig. 1).
Flt3L Administration for DC Mobilization.
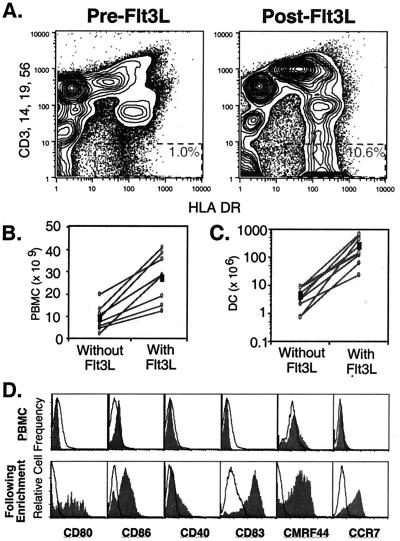
Patients' PBMCs were assessed for DCs by flow cytometry before and after 10 days of Flt3L administration. DCs were characterized by their expression of HLA DR and the absence of lineage markers CD3, CD14, CD19, and CD56. Study subjects had increases in their circulating DCs of between 5- and 10-fold as a percentage of PBMCs (Fig. 2A). In addition to expanding the percentage of circulating DCs, Flt3L treatment also increased the total number of PBMCs obtained in a standardized leukapheresis from patients in the first three cohorts (Fig. 2B). Two blood volume leukaphereses without Flt3L yielded 9.2 × 109 PBMCs on average (range 2.3 to 19.5 × 109), whereas leukaphereses after Flt3L treatment yielded 27.0 × 109 PBMCs on average (range 12.2 to 40.5 × 109), an increase of almost 3-fold that was statistically significant (paired t test, P = 0.0002). This leukapheresis product subsequently was subjected to sequential density gradient centrifugation to enrich for DCs. The number of DCs obtainable from each leukapheresis after enrichment rose from an average of 4.6 × 106 (range 0.7 to 9.4 × 106) to 271.2 × 106 (range 23.3 to 654.8 × 106) (Fig. 2C). This represented a 60-fold increase of enriched DCs on average that was also statistically significant (paired t test, P = 0.0089). DCs in Flt3L-mobilized PBMCs lacked expression of costimulatory molecules CD80, CD86, and CD40 as well as activation markers CD83 and CMRF-44. After ex vivo enrichment and 2 days of in vitro culture, Flt3L-mobilized DCs developed a mature phenotype with up-regulation of all of these markers (Fig. 2D). Moreover, DCs up-regulated expression of CCR7, which presumably would be required for DC homing to secondary lymphoid tissues through putative interactions with MIP-3β.
Figure 2.
Expansion of circulating blood DCs with Flt3L administration. Patients' PBMCs were evaluated either before (PreFlt3L) or after 10 days of Flt3L administration (PostFlt3L) to assess DC expansion in vivo with Flt3L. (A) DC were characterized with four-color flow cytometry for their expression of HLA DR without expression of lineage markers, CD3, CD14, CD19, and CD56 (dashed rectangles). (B) Total PBMCs obtained from a standardized two blood volume leukapheresis with and without Flt3L treatment were compared and were significantly increased with Flt3L (P = 0.0002). Cell numbers are shown for each patient (○) and for the mean of the nine patients (■). (C) The number of DCs enriched for vaccine preparation with and without Flt3L treatment also was compared and was significantly increased (P = 0.0089). Cell numbers are shown for each patient (○) and for the mean of the nine patients (■). DCs were evaluated for activation markers by gating on their expression of HLA DR and lack of lineage markers. (D) Flt3L expanded DCs were assessed either directly in the patients' PBMCs (Upper) or in the DC product after 2 days of enrichment and ex vivo culture in antigen (Lower) for expression level of CD80, CD86, CD40, CD83, CMRF44, and CCR7. Unfilled histograms represent isotype-matched controls.
Vaccination- and Treatment-Related Side Effects.
Patients were monitored during and after the infusions in the General Clinical Research Center at Stanford Hospital and assessed for toxicity by the National Cancer Institute common toxicity scale. Patients tolerated the treatment with only minor side effects. During DC infusions, seven of the 12 patients developed transfusion-like reactions manifesting as self-limited rigors and fever that were either National Cancer Institute grade I or II in severity. Five patients also developed grade I or II diarrhea that typically began 2–6 days after vaccination, lasted 2–3 days, and resolved spontaneously.
Induction of Immunity to the Vaccine Antigens.
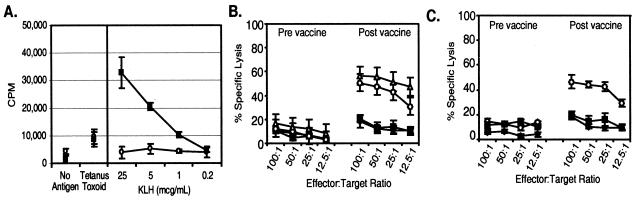
To assess whether Flt3L-mobilized DCs are capable of priming antigen-specific immunity in vivo, PBMCs obtained from the patients before and 4 weeks after the initial vaccination were assessed for immune responses to KLH and the CEA epitope. Induction of CD4 T cell immunity specific for KLH was assessed by proliferation of PBMCs to antigen (Fig. 3A). Significant proliferative responses to KLH, while absent before immunization, were evident after a single DC immunization in all patients. No difference in the response to tetanus toxoid, a recall control antigen, was seen, consistent with antigen-specific immunity.
Figure 3.
In vivo induction of specific immunity with Flt3L-expanded DCs. Patients were immunized with autologous Flt3L-expanded DCs that had been cultured ex vivo with 610D peptide and KLH as target antigens. PBMCs were obtained preimmunization and 4 weeks after the initial immunization and assessed for immunity to the target antigens. (A) PBMCs were obtained prevaccination (○) and postvaccination (■). CD4 T cell responses were assessed by proliferative responses to varying concentrations of KLH by [3H] thymidine incorporation over 18 h after 6 days of in vitro culture. Results represent the mean ± SEM for triplicate wells from one set of experiments and are representative of six. (B) CTL activity was assessed from PBMC prevaccination or postvaccination against 51Cr-labeled T2 cells loaded with 610D (▵), CEA605–613 (○), an irrelevant HLA-A*0201 binding peptide Tyr368–376 (■), and unpulsed T2 cells (⧫) in a standard 4-h [51Cr] release assay. Results represent the mean + SEM for triplicate wells from one set of experiments, representative of patients with measurable CTL activity. (C) Cytolytic activity was assessed against human tumor-derived cell lines SW403 (HLA*A0201+, CEA+) (○), SW1417 (HLA*A0201−, CEA+) (■), or A375 (HLA*A0201+, CEA−) (⧫). Results represent the mean ± SEM for triplicate wells from one set of experiments, representative of patients with measurable CTL activity.
To assess for the induction of CEA-specific CD8 T cell immunity, PBMCs obtained before and 3 weeks after the first DC vaccination also were assessed for CTL activity in standardized 4-h [51Cr] release assays. When peptide-loaded T2 cells that express HLA-A*0201 were used as targets, no CTLs could be detected above background lysis of T2 before vaccination (Fig. 3B). However, after vaccination, significant lytic activity (specific lysis ≥ 20%) was seen in seven of the 12 patients. CTLs recognized not only 610D-pulsed T2 cells, but also CEA605–613-pulsed targets as well. The lytic activity was similar in magnitude between the two peptide-loaded targets. Lysis of T2 cells loaded with an irrelevant MHC class I binding peptide derived from tyrosinase was similar to background. Moreover, when tumor-derived cell lines were used as labeled targets, CTL activity could be detected against a cell line expressing both HLA A*0201 and endogenous CEA, SW403, only after vaccination (Fig. 3C). No killing over background was seen against tumor cell lines that either lacked expression of CEA (A375) or the relevant MHC haplotype HLA-A*0201 (SW1417). Patient sera were assessed for the induction of CEA-specific antibodies, but none could be detected before or after vaccination (data not shown).
Detection and Characterization of Antigen-Specific CD8 T Cells with MHC/Peptide Tetramers.
We stained patients' blood before and after DC administration with fluorochrome-labeled HLA-A*0201 tetramers bearing 610D or CEA605–613 peptide. Tetramer synthesis and validation was performed as described (23). Five of 12 patients had >1% 610D-tetramer+ CD8+ T cells after vaccination (Table 2). Moreover, patients with 610D-tetramer+ T cells had a similar percentage of CEA605–613-tetramer+ T cells (Fig. 4A). Tetramer staining was specific for the peptides as staining with HLA-A*0201 tetramers bearing irrelevant HLA-A*0201 binding peptides such as CMV pp65 tetramer failed to stain cells in CMV seronegative patients (Fig. 4B). To determine whether the 610D-tetramer+ T cells were an identical or distinct population from CEA605–613-tetramer+ T cells, postvaccination PBMCs were incubated with 610D-MHC monomer before staining with CEA605–613 tetramer. As shown in Fig. 4C, 610D-MHC monomer blocked staining with the CEA605–613 tetramer in a concentration-dependent fashion. 610D-tetramer+ and CEA605–613-tetramer+ T cells were further characterized phenotypically and found to bear the same expression pattern of a variety of T cell markers, again indicating that these populations of T cells are the same (data not shown). Although the tetramer+ cells expressed high levels of CD45RA and no CD45RO, they expressed CD57 and CD44, but also lacked CD62L and CD27 (Fig. 4D). The CD45RA+CD45RO− phenotype has historically been associated with naïve T cells, but more recent studies have demonstrated that this paradigm does not hold for human CD8 T cells (20–22). The CD45RA+CD44+CD27− pattern of expression is consistent with prior reports as the phenotype of effector CTLs (23). These tetramer+ T cells also lack expression of the chemokine receptor CCR7, which is reported to be absent on effector CTLs (24). However, these tetramer+ T cells express CD28, a marker usually absent in terminally differentiated CTLs, suggesting that these CTLs may maintain their capacity to proliferate (25).
Table 2.
Immune and clinical responses
| Patient | Clinical response | CTL prevaccine, % | CTL postvaccine, % | Tetramer+ prevaccine, % | Tetramer+ postvaccine, % | Fold expansion |
|---|---|---|---|---|---|---|
| 1 | PD | 0 | IC | 0.08 | 0.25 | — |
| 2 | PD | −3 | 16 | 0.03 | 0.08 | — |
| 3 | SD-4 mo | 5 | 33 | 0.15 | 1.11 | 7.4 |
| 4 | PD | 13 | 11 | 0.18 | 0.04 | — |
| 5 | CR-10 mo | 5 | 26 | 0.40 | 1.03 | 2.6 |
| 6 | PD | 0 | 0 | 0.10 | 0.31 | — |
| 7 | PD | 0 | 6 | 0.26 | 0.49 | 1.9 |
| 8 | SD-6 mo | 8 | 41 | 0.43 | 1.05 | 2.4 |
| 9 | PD | 6 | 32 | 0.16 | 0.07 | — |
| 10 | PD | 12 | 43 | 0.24 | 0.50 | 2.1 |
| 11 | CR-10 mo+ | 3 | 38 | 0.28 | 1.03 | 3.7 |
| 12 | MR | 5 | 27 | 0.12 | 1.68 | 14.0 |
Figure 4.
Identification and characterization of antigen-specific CD8 T cells with MHC/peptide tetramers. PBMCs were stained with CD8 (positive gate), CD4, CD14, CD19, and CD56 (negative gate) as well as with a phycoerythrin-labeled MHC/tetramer, and analyzed by four-color flow cytometry. (A) 610D tetramer (Upper) and CEA605–613 tetramer (Lower) were used to assess PBMCs obtained prevaccine and postvaccine. (B) Tetramers with irrelevant peptides including CMV pp65 were used in the appropriate seronegative hosts for tetramer specificity. (C) To assess whether 610D tetramers and CEA605–613 tetramers stained the same CD8 T cells, CEA605–613 tetramer staining was performed without or with preincubation with 610D/MHC monomer for 1 h at concentrations of 10 mcg/ml and 50 mcg/ml. (D) Tetramer+ CD8+ cells were characterized phenotypically for functional markers of CD8 T cells to delineate between naïve, effector, and memory CD8 T cells. Unfilled histograms represent isotype-matched controls. Results are representative of three patients assessed.
Clinical Responses to Treatment.
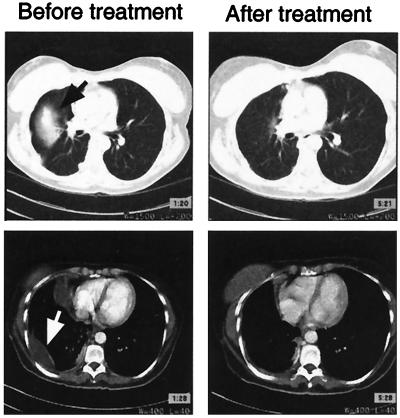
While participating in the clinical trial, none of the patients received any other form of cancer treatment and would be expected to have progressive disease. Patients were followed monthly with serial visits and testing including monitoring of serum CEA levels. Radiographic imaging was obtained every 3 months. Objective responses were observed in two of the 12 patients. One patient with progressive metastatic colorectal cancer had a complete resolution of her pulmonary metastasis and malignant pleural effusion 4 months after vaccination (Fig. 5). She ultimately went on to develop intraabdominal metastasis 10 months later, although she did not redevelop metastasis in her lung. Another patient, who had metastatic colorectal cancer evaluable with a rising serum CEA level, developed a drop in his CEA levels to below the limits of detection by our clinical assay and has remained free of malignancy beyond 10 months after vaccination. Additionally, one patient with colon cancer developed a mixed response after vaccination with regression of some but not all liver metastases. Finally, two of the 12 patients had disease stabilization as assessed by serum CEA levels and radiographic imaging. This clinical status lasted 3 months for one patient and 6 months for the other.
Figure 5.
Clinical response to treatment. Computed tomography scans were performed on the study subjects within 1 month before their first vaccination and 4 months after their final vaccinations and then every 3 months thereafter. Patients did not receive any other concurrent treatments for their cancer. One patient with growing lung metastases (black arrow) and malignant pleural effusion (white arrow) before vaccination developed resolution of these metastases after vaccination.
Correlation Between CTL, Tetramer+ CD8 T Cell, and Clinical Responses.
Although no patients had significant CTL activity before vaccination, seven (58%) of the 12 patients developed CTL activity (defined as >20% specific lysis) after vaccination (paired t test, P = 0.0013) (Table 2). Similarly, whereas no patients had significant levels of tetramer+ CD8 cells before vaccination, five (41%) patients had >1% tetramer+ CD8 cells, and six had ≥0.5% tetramer+ CD8 cells after vaccination (paired t test, P = 0.011). CTL lytic activity and tetramer positivity were correlated (paired sign test, P = 0.038), although a linear relationship between these results could not be demonstrated (simple regression, P = 0.099). For the purpose of determining the relationship between measured immune response and clinical response, the five patients with tumor regression, mixed response, or stable disease were defined as clinical responders. Although CTL activity after vaccination did not directly correlate with clinical responses (ANOVA, P = 0.216), the percentage of tetramer+ CD8 cells did (ANOVA, P = 0.002). The magnitude of tetramer+ CD8 T cell expansion (up to 14-fold in patient 12) also correlated with clinical response (ANOVA, P = 0.02). Conversely, patients who failed to develop measurable CTL or tetramer+ CD8 cells after vaccination had no respite from their progressive cancer. Of the five patients who responded clinically, one patient (patient 3) was lymphopenic and two (patients 3 and 5) were anergic to skin testing before vaccination, suggesting that a high level of immunocompetence is not required to respond to DC vaccination.
Discussion
As a result of negative selection in the thymus, the T cells that comprise the peripheral T cell pool are depleted of most high-affinity autoreactive T cells (26). Any residual autoreactive T cells that survive thymic selection may then be rendered tolerant through various mechanisms active in the periphery (27). Evidence that nonresponsive, antigen-specific T cells can occur in human cancer has been reported (28).
In the work described here, we attempted to break tolerance to CEA, a self-antigen, by immunizing cancer patients with an APL derived from that antigen. The APL used in this study, 610D, alters interactions with the T cell receptor and may represent the “agonist” for CEA-specific T cells surviving thymic selection. Alternatively, 610D may serve to activate T cells with other specificities that are ignorant to CEA605–613, but once activated, these T cells are capable of ultimately recognizing the native determinant.
In our trial, DCs were used as a means to deliver and present antigen to naïve T cells. Patients received increasing doses of DC ranging from ≤107 to 109. Within this range, we did not detect a statistically significant relationship between DC dose and immune or clinical response, suggesting that antigen-presenting cell dose may not be limiting. Nevertheless, the number of patients enrolled was small in this study; additional trials are needed to further evaluate this.
Our results show that a vaccine comprised of these expanded, antigen-loaded DCs can prime antigen-specific immunity. CEA-specific CTLs could be detected by conventional chromium release assays. We also demonstrate the utility of MHC/peptide tetramer staining to identify antigen-specific CD8 T cells after vaccination. This approach allowed us to show that, in fact, T cell receptor that recognize 610D also recognize CEA605–613. Moreover, the tetramer+ CD8 T cells expanded with the vaccination possess a novel CTL phenotype that may possess continued proliferative capacity given their surface expression of CD28 and CD57 (29, 30). Immunologic assays have long been used as surrogate endpoints in vaccine trials. However, no vaccine trial has clearly demonstrated the utility of immune monitoring in predicting clinical responses (2). Our trial demonstrates a statistically significant correlation between the percentage of tetramer+ T cells and clinical response.
Spontaneous regression is not seen in patients with colorectal cancer, supporting a therapeutic effect from the treatment. These results demonstrate that APLs can be used to immunize against self-antigens, resulting in both immunologic and clinical responses in nonimmunogenic malignancies. Given these promising results, additional clinical investigation is warranted to confirm and further characterize the efficacy of our approach.
Acknowledgments
We are grateful to the patients who participated in the study. We also thank Avani Patel, Pat Grant, and the nurses of the Stanford Blood Center and General Clinical Research Center for their help in patient care. We thank Dr. Dania Caron for her support of the clinical trial, Peter Lee for initial discussions on tetramer staining, and Jim Allison for reviewing the manuscript. L.F. is supported by a physician-scientist award from the National Cancer Institute (K23 CA82584–01). This investigation also was supported in part by National Institutes of Health Grant P01-HL56443, research funding from Immunex Corporation, and Human Health Service Grant M01-RR00070 (General Clinical Research Centers, National Center for Research Resources, National Institutes of Health).
Abbreviations
CEA
carcinoembryonic antigen
CTL
cytotoxic T lymphocyte
APL
altered peptide ligand
DC
dendritic cell
Flt3L
Flt3 ligand
CMV
cytomegalovirus
KHL
keyhole limpet hemocyanin
PBMC
peripheral blood mononuclear cell
References
- 1.Kwak L W, Campbell M J, Czerwinski D K, Hart S, Miller R A, Levy R. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S A, Yang J C, Schwartzentruber D J, Hwu P, Marincola F M, Topalian S L, Restifo N P, Dudley M E, Schwarz S L, Spiess P J, et al. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller C A, Becker V, et al. Nat Med. 2000;6:332–336. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 4.Hammarstrom S. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell E P. Semin Oncol. 1998;25:12–20. [PubMed] [Google Scholar]
- 6.Foon K A, John W J, Chakraborty M, Sherratt A, Garrison J, Flett M, Bhattacharya-Chatterjee M. Clin Cancer Res. 1997;3:1267–1276. [PubMed] [Google Scholar]
- 7.Tsang K Y, Zaremba S, Nieroda C A, Zhu M Z, Hamilton J M, Schlom J. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 8.Morse M A, Deng Y, Coleman D, Hull S, Kitrell-Fisher E, Nair S, Schlom J, Ryback M E, Lyerly H K. Clin Cancer Res. 1999;5:1331–1338. [PubMed] [Google Scholar]
- 9.Zugel U, Wang R, Shih G, Sette A, Alexander J, Grey H M. J Immunol. 1998;161:1705–1709. [PubMed] [Google Scholar]
- 10.Kessler B M, Bassanini P, Cerottini J C, Luescher I F. J Exp Med. 1997;185:629–640. doi: 10.1084/jem.185.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkhurst M R, Salgaller M L, Southwood S, Robbins P F, Sette A, Rosenberg S A, Kawakami Y. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 12.Zaremba S, Barzaga E, Zhu M, Soares N, Tsang K Y, Schlom J. Cancer Res. 1997;57:4570–4577. [PubMed] [Google Scholar]
- 13.Salazar E, Zaremba S, Arlen P M, Tsang K Y, Schlom J. Int J Cancer. 2000;85:829–838. doi: 10.1002/(sici)1097-0215(20000315)85:6<829::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y J, Pulendran B, Palucka K. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 15.Fong L, Engleman E G. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 16.Lyman S D, Jacobsen S E. Blood. 1998;91:1101–1134. [PubMed] [Google Scholar]
- 17.Maraskovsky E, Brasel K, Teepe M, Roux E R, Lyman S D, Shortman K, McKenna H J. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski C R, Hoek J, Caron D, Lebsack M E, McKenna H J. Blood. 2000;96:878–884. [PubMed] [Google Scholar]
- 19.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 20.Hamann D, Kostense S, Wolthers K C, Otto S A, Baars P A, Miedema F, van Lier R A. Int Immunol. 1999;11:1027–1033. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 21.Hamann D, Baars P A, Rep M H, Hooibrink B, Kerkhof-Garde S R, Klein M R, van Lier R A. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoflich C, Docke W D, Busch A, Kern F, Volk H D. Int Immunol. 1998;10:1837–1845. doi: 10.1093/intimm/10.12.1837. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman C, Brduscha-Riem K, Blaser C, Zinkernagel R M, Pircher H. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature (London) 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 25.Casamayor-Palleja M, Khan M, MacLennan I C. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jameson S C, Hogquist K A, Bevan M J. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 27.Van Parijs L, Abbas A K. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 28.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, et al. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 29.Wang E C, Moss P A, Frodsham P, Lehner P J, Bell J I, Borysiewicz L K. J Immunol. 1995;155:5046–5056. [PubMed] [Google Scholar]
- 30.Azuma M, Phillips J H, Lanier L L. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]