CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity (original) (raw)
. Author manuscript; available in PMC: 2013 Aug 29.
Published in final edited form as: Curr Opin Nephrol Hypertens. 2012 Nov;21(6):587–592. doi: 10.1097/MNH.0b013e328358c8d5
Abstract
Purpose of review
Chronic kidney disease (CKD) is associated with oxidative stress and inflammation which contribute to progression of kidney disease and its numerous complications. Until recently, little attention had been paid to the role of the intestine and its microbial flora in the pathogenesis of CKD-associated inflammation. This article is intended to provide an over view of the impact of uremia on the structure and function of the gut and its microbial flora and their potential link to the associated systemic inflammation.
Recent findings
Recent studies conducted in the author’s laboratories have demonstrated marked disintegration of the colonic epithelial barrier structure and significant alteration of the colonic bacterial flora in humans and animals with advanced CKD. The observed disruption of the intestinal epithelial barrier complex can play an important part in the development of systemic inflammation by enabling influx of endotoxin and other noxious luminal contents into the systemic circulation. Similarly via disruption of the normal symbiotic relationship and production, absorption and retention of noxious products, alteration of the microbial flora can contribute to systemic inflammation and uremic toxicity. In fact recent studies have documented the role of colonic bacteria as the primary source of several well known pro-inflammatory/pro-oxidant uremic toxins as well as many as-yet unidentified retained compounds.
Summary
CKD results in disruption of the intestinal barrier structure and marked alteration of its microbial flora –events that play a major role in the pathogenesis of inflammation and uremic toxicity.
Keywords: cardiovascular disease, end-stage renal disease, microbiome, oxidative stress, uremia
INTRODUCTION
Oxidative stress and systemic inflammation play a major role in progression of chronic kidney disease (CKD) and its numerous complications including cardiovascular disease, cachexia, and anemia among others [1–5]. Oxidative stress and inflammation are inseparably linked. This is because by activating the redox-sensitive transcription factor nuclear factor kappa B (NF-κB), which is the master regulator of pro-inflammatory cytokines and chemokines, oxidative stress triggers activation and recruitment of immune cells and thereby promotes inflammation. Inflammation, in turn, initiates or amplifies oxidative stress via production of reactive oxygen, nitrogen, and halogen species by the activated immune cells. Several factors have been shown to contribute to oxidative stress and inflammation in CKD. In this context mitochondrial dysfunction, comorbid conditions (e.g., diabetes and auto-immune diseases), activation of tissue angiotensin system, hypervolemia, hypertension, dyslipidemia, retained uremic toxins and metabolites, and infections (blood access, peritoneal dialysis catheters, hepatitis, etc.) play a major role in the pathogenesis of CKD-associated oxidative stress and inflammation. In addition the recently demonstrated CKD-induced impairment of the nuclear factor-erythroid-2-related factor 2 (Nrf2), pathway, which regulates expression of the antioxidant and cytoprotective enzymes and substrates, plays a major role in this process by disabling the natural anti-oxidant response to oxidative stress and inflammation [6,7]. Finally, iatrogenic factors such as iron overload caused by indiscriminate use of intravenous iron preparations, blood–dialyzer membrane interaction, and influx of impurities from dialysate compartment across the high-flux dialysis membranes can trigger oxidative stress and inflammation in the end-stage renal disease (ESRD) population. However, until recently, little attention had been paid to the role of the intestine and its microbial flora in the pathogenesis of CKD-associated inflammation and oxidative stress. This article is intended to provide an over view of the impact of uremia on the structure/function of the intestinal epithelial barrier complex and the composition of the intestinal microbial flora.
THE INTESTINAL BARRIER STRUCTURE AND FUNCTION
While residing in the most central part of the body, the gastrointestinal tract is in fact an extension of the external environment within the body. Therefore in addition to serving as the engine for processing and import of ingested nutrients and disposal of waste, it serves as a barrier to prevent entry of microbes and their harmful products as well as other noxious compounds into the internal environment. The intestinal barrier structure consists of the apical membrane of the intestinal epithelial cells and the apical junctional complex which fills the gap between the neighboring cells in the way grout seals the opening between the tiles on the floor. Via specific transport channels the apical membrane of the epithelial cells regulates passive and active transcellular transport of solutes, whereas the apical junctional complex serves as the barrier against paracellular permeation of substances from the lumen of the intestinal tract [8]. This complex consists of the tight junction and the subjacent adherens junction components. The tight junction is the most luminal component of the apical junctional complex. It consists of the adhesive transmembrane proteins, that is the occludin and claudin family of proteins, which link the plasma membranes of the adjacent cells to form the barrier to diffusion of fluids and solutes; the actin-binding cytosolic tight junction proteins, that is the zonula occludens (ZO) protein family, which serves as the anchor and regulates organization of the apical junction complex; and finally the peri-junctional ring of actin and myosin which by modulating the structure and function of the tight junction regulates paracellular permeability [9]. The intestinal epithelial tight junction forms an effective barrier against influx of microbes, microbial toxins, bacterial byproducts, antigens, digestive enzymes, degraded food products, and other noxious substances from the gastrointestinal lumen to the internal milieu.
EVIDENCE FOR INTESTINAL BARRIER DYSFUNCTION IN UREMIA
There is mounting evidence supporting the presence of intestinal barrier dysfunction in uremia. First, uremic patients frequently exhibit endotoxemia in the absence of clinical infection [10–12]. The microbial flora of the gastrointestinal tract is the most likely source of endotoxemia in the infection-free uremic patients. If true, the entry of endotoxin from the lumen to the blood stream signifies the intestinal mucosal barrier dysfunction. Second, in their earlier studies Magnusson et al. [13,14] demonstrated increased intestinal permeability to large-molecular-weight polyethylene glycols in the uremic humans and animals as compared to their control counterparts. Third, studies by de Almeida Duarte et al. [15] demonstrated penetration of bacteria across the intestinal wall and their detection in the mesenteric lymph nodes in uremic rats. Fourth, there is histological evidence of chronic inflammation throughout the gastrointestinal tract including esophagitis, gastritis, duodenitis, enteritis, and colitis in the hemodialysis population [16]. Clearly impairment of the intestinal barrier function can contribute to the prevailing inflammation, oxidative stress and uremic toxicity [17].
DISINTEGRATION OF INTESTINAL EPITHELIAL TIGHT JUNCTION IN UREMIA
Given the emerging evidence of increased intestinal permeability in uremia and the critical role of the tight junction in the mucosal barrier function, the author recently tested the hypothesis that uremia may impair the integrity of the intestinal tight junction complex. To this end the abundance and localization of protein constituents of tight junction complex in the colonic tissue were determined by immunohistology and Western blot analysis in rats rendered uremic by subtotal nephrectomy or adenine-induced chronic tubulointerstitial nephritis [18▪▪]. The study revealed marked reductions in protein abundance of claudin-1 (70–90%), occludin (50–70%), and ZO-1 (80–90%) in colonic mucosa in both CKD models compared with the corresponding controls. The reduction in the abundance of the given proteins was confirmed by immunohistological examinations. Unlike the protein abundance, the mRNA abundance of occludin, claudin-1, and ZO-1 was either unchanged or elevated. The latter findings pointed to the post-transcriptional/ post-translational nature of the uremia-induced depletion of the tight junction proteins. Disintegration of the tight junction apparatus in the uremic animals was associated with marked thickening of the colonic wall and heavy infiltration of mononuclear leukocytes in its lamina propria. This study, for the first time, demonstrated the underlying mechanism of the previously documented impaired intestinal barrier function and endotoxemia in uremia.
As noted above, under normal condition, the intestinal epithelial tight junction blocks the entry of microbes and their toxic byproducts, degraded food material, and other noxious agents in the internal milieu. However, the intestinal tight junction barrier is impaired allowing permeation of luminal antigens and pro-inflammatory products into the underlying intestinal tissue in certain disorders such as Crohn’s disease, ulcerative colitis, alcoholic hepatitis, heat stroke, and Clostridium difficile, Escherichia coli, and Vibrio cholera infections [8]. Breakdown of the intestinal tight junction in these conditions leads to activation of the resident macrophages, dendritic cells and T lymphocytes, release of pro-inflammatory cytokines and chemokines, and infiltration of circulating inflammatory cells leading to local and systemic inflammation.
As noted above, there is an indirect but convincing evidence pointing to the association of advanced CKD with increased intestinal permeability. The marked depletions of the key transcellular and cytosolic components of the tight junction, shown in this study, elucidated the molecular mechanism of the previously demonstrated impaired intestinal barrier dysfunction and endotoxemia in uremia and its contribution to the associated systemic inflammation.
FACTORS THAT AGGRAVATE INTESTINAL BARRIER DYSFUNCTION
Bowel wall edema and ischemia have been shown to increase intestinal permeability and result in endotoxemia, systemic inflammation, and even bacterial translocation in patients with uncompensated congestive heart failure or cirrhosis. Therefore when present, severe edema and hypervolemia can further impair intestinal barrier function in CKD patients. Likewise, aggressive ultrafiltration and intradialytic or postdialytic hypotension can intensify the gut barrier dysfunction and endotoxemia by inducing transient bowel ischemia in this population.
THE INTESTINAL MICROBIOME AND ITS BIOLOGICAL FUNCTIONS
The huge microbial community residing in the intestinal tract (microbiome) represents a symbiotic ecosystem that confers trophic functions [19], provides protection against pathogenic organisms [20], contributes to energy metabolism by facilitating absorption of complex carbohydrates [21], and participates in nitrogen [22] and micronutrient homeostasis by synthesizing amino acids (e.g., lysine and threonine) [23] and various vitamins (e.g., vitamin K and group B vitamins) [22,24]. The structure, composition, and function of the microbial flora are heavily influenced by the biochemical milieu in which it resides. The integrity and proper function of the microbiome is critical for good health. In fact, changes in the structure or function of the microbiome contribute to the pathogenesis of various diseases including inflammatory bowel disease [25], chronic inflammation, dyslipidemia, diabetes [26], cardiovascular diseases, neoplasms [27], obesity [28], and atopic disorders [29].
EFFECT OF UREMIA ON THE BIOCHEMICAL MILIEU OF THE INTESTINAL TRACT
As noted above, the biochemical milieu plays a major part in shaping the structure and function of the microbiome. Uremia profoundly alters the biochemical milieu of the gastrointestinal tract by several mechanisms. In this context, reduction in glomerular filtration rate and consequent rise in urea concentration in the intracellular and extracellular fluid compartments result in its heavy influx into the gastrointestinal tract. Within the intestinal lumen, urea is hydrolyzed spontaneously and/or by microbial urease forming large amounts of ammonia [CO(NH2)2 + H2O→CO2 + 2NH3] which is readily converted to ammonium hydroxide [NH3 + H2O→NH4OH]. NH4OH, in turn, raises the luminal fluid’s pH, causes mucosal irritation, and promotes enterocolitis [16,30]. Another factor that contributes to uremia-induced changes in the biochemical environment of the intestinal tract is secretion of significant amounts of uric acid and oxalate by colonic epithelium representing the adaptive response to the reduction or loss of their renal excretion [31–33]. This phenomenon which was elucidated by our group, in part, accounts for the relatively minor rise in serum uric acid despite the totalornear-total loss of renal function in patients with advanced CKD. In addition to changing the gut’s biochemical environment, presence of large amounts of urea and uric acid can serve as substitutes for indigestible carbohydrates which the resident microbes normally feed on. This can, in turn, accommodate the domination of microbes possessing over those lacking the machinery to utilize these substrates.
In addition to the pathophysiological phenomena described above, dietary modifications and medicinal interventions can significantly impact the biochemical milieu of the gastrointestinal tract in patients with advanced CKD. Among them is dietary restriction of fruits and vegetables, which are rich in potassium, to prevent hyperkalemia. These products contain much of the indigestible dietary complex carbohydrates that constitute the primary source of nutrients for the gut microbial flora. Therefore, limitation of their intake can significantly affect the composition and metabolism of the microbiome. Various phosphate-binding agents such as calcium acetate, calcium carbonate, aluminum hydroxide, anion-exchange resins, and iron-based products are commonly consumed with each meal by patients with advanced CKD in an attempt to control hyperphosphatemia. By modifying the luminal milieu of the gut these products can also potentially impact the intestinal microbial community. Another iatrogenic factor that significantly affects the microbiome is antibiotic therapy, which is often used to treat vascular access and other infections in patients with advanced kidney disease.
THE EFFECT OF UREMIA ON THE COMPOSITION OF THE INTESTINAL MICROBIOME
As outlined above, uremia and its treatment with dietary restrictions and medicinal interventions can profoundly alter the biochemical environment of the intestine, which exerts a decisive role in shaping its microbial flora. Accordingly, in a series of studies, we tested the hypothesis that uremia may alter the composition and function of the gut microbial flora [34▪▪]. To this end, microbial DNA was isolated from the stool samples of a group of patients with ESRD maintained on hemodialysis and a group of age, sex, and ethnicity-matched control individuals. A novel phylogenic microarray technique developed by our collaborators was employed to identify the microbial species in the test samples. The study revealed marked differences in the abundance of 190 bacterial operational taxonomic units (OTUs) between the ESRD patients and the normal control groups. The OTUs from Brachybacterium, Cateni-bacterium, Enterobacteriaceae, Halomonadaceae, Moraxellaceae, Nesterenkonia, Polyangiaceae, Pseudomonadaceae, and Thiothrix families were markedly increased in ESRD patients. To isolate the impact of uremia per se from those of interindividual variations, comorbid conditions, and dietary and medicinal interventions, we studied rats 8 weeks after induction of CKD with 5/6 nephrectomy. A group of identical rats maintained under similar condition were subjected to sham operation and used as controls. The studies in rats showed marked differences in the abundance of 175 bacterial OTUs between the uremic and control animals. Taken together, the results of our human and animal studies demonstrated a profound change in the composition of the gut microbiome with advanced CKD. Whereas the biological impact of CKD-induced change in the gut microbiome is presently unknown, it is likely to have adverse consequences. In this context the observed changes in the composition of the gut microbiome in CKD may disturb the normal symbiotic relationship and lead to formation and absorption of pro-oxidant, pro-inflammatory, and otherwise harmful byproducts that contribute to uremic toxicity, inflammation, and cardiovascular, nutritional, and other complications. In addition, the shift in the microbiome can potentially limit the beneficial functions and products conferred by the normal flora which may have as-yet unrecognized consequences.
ROLE OF THE ALTERED MICROBIOME IN THE UREMIC TOXICITY
As noted above, the changes in the composition of the gut microbiome demonstrated in our recent study may result in production and absorption of noxious byproducts that can contribute to the uremic toxicity, inflammation, malnutrition, and other morbidities in the uremic individuals. This supposition is supported by a recent study conducted by Aronov et al. [35▪▪] designed to identify and characterize colon-derived uremic solutes by examining plasma samples from healthy individuals, hemodialysis patients with intact colons, and hemodialysis patients who had undergone total colectomy. Using high-resolution mass spectrometry, they documented the colonic origin of several known uremic toxins and many as-yet unidentified products in the plasma of patients with ESRD, by comparing data obtained from ESRD patients who had undergone colonic resection with those found in ESRD patients with intact colon and normal control individuals. They identified more than 30 individual mass spectroscopic features which were present in the plasma from ESRD patients with colons that were either absent or present in significantly lower concentration in those without colons. Nearly all of these compounds were significantly more prominent in plasma from dialysis patients than normal individuals, suggesting that they represented uremic solutes. In addition by employing HPLC, these investigators verified the colonic origin of p-cresol sulfate and indoxyl sulphate, which are well known uremic toxins. On the basis of these findings they concluded that colonic microbes produce numerous uremic solutes, the great majority of which remain unidentified.
CONCLUSION
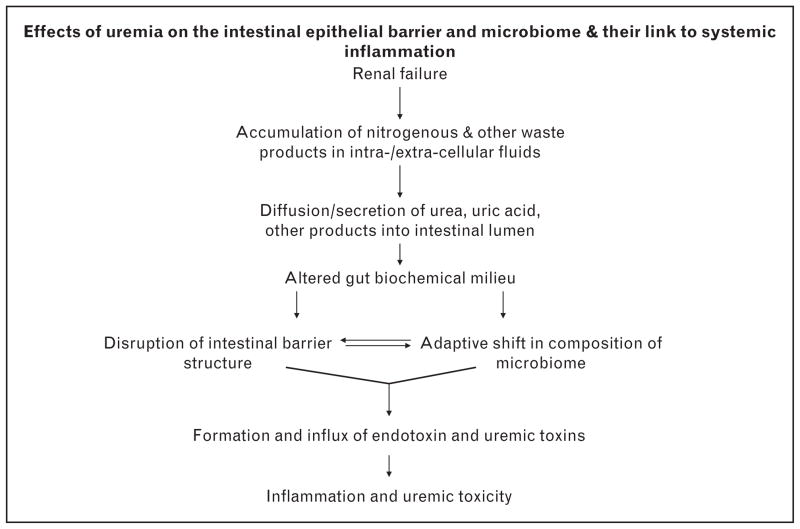
Together, the observed changes in the composition of the gut microbiome and disruption of its barrier structure/function may result in production and absorption of noxious byproducts that can contribute to the uremic toxicity, inflammation, malnutrition, and other morbidities in uremic patients and animals (Fig. 1).
FIGURE 1.
The proposed pathways by which chronic kidney disease may disrupt intestinal epithelial barrier apparatus and alter the intestinal microbiome, thereby causing systemic inflammation and uremic toxicity via formation and influx in the systemic circulation of noxious microbial-derived products.
KEY POINTS.
- Uremia results in disruption of the intestinal epithelial tight junction and its barrier dysfunction, events which can contribute to systemic inflammation by enabling paracellular influx of endotoxin and other noxious luminal products into the systemic circulation.
- By modifying the biochemical milieu of the gut, CKD markedly alters the composition of microbiome, an event which can contribute to the prevailing inflammation and uremic toxicity by disrupting the symbiotic relationship and formation of potentially harmful byproducts.
- In fact, recent studies have identified the colonic microbial flora as the primary source of several well known pro-inflammatory uremic toxins as well as a score of as-yet unindentified retention products in end-stage renal stage (ESRD) patients.
Acknowledgments
The study was in part funded by the NIH grants RR026138 and MD000182.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪ ▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. 2003;12:593–598. doi: 10.1097/00041552-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004;13:93–99. doi: 10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol. 2004;24:469–473. doi: 10.1016/j.semnephrol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Cachofeiro V, Goicochea M, de Vinuesa SG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298:F662–F671. doi: 10.1152/ajprenal.00421.2009. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 activation to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. doi: 10.1038/ki.2012.439. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 9.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 10.Gonçalves S, Pecoits-Filho R, Perreto S, et al. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21:2788–2794. doi: 10.1093/ndt/gfl273. [DOI] [PubMed] [Google Scholar]
- 11.Szeto CC, Kwan BC, Chow KM, et al. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431–436. doi: 10.2215/CJN.03600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raj DS, Carrero JJ, Shah VO, et al. Soluble CD14 levels, interleukin 6, and mortality among prevalent hemodialysis patients. Am J Kidney Dis. 2009;54:1072–1080. doi: 10.1053/j.ajkd.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnusson M, Magnusson KE, Sundqvist T, et al. Increased intestinal permeability to differently sized polyethylene glycols in uremic rats: effects of low- and high protein diets. Nephron. 1990;56:306–311. doi: 10.1159/000186158. [DOI] [PubMed] [Google Scholar]
- 14.Magnusson M, Magnusson KE, Sundqvist T, et al. Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut. 1991;32:754–759. doi: 10.1136/gut.32.7.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, et al. Bacterial translocation in experimental uremia. Urol Res. 2004;32:266–270. doi: 10.1007/s00240-003-0381-7. [DOI] [PubMed] [Google Scholar]
- 16.Vaziri ND, Dure-Smith B, Miller R, et al. Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am J Gastroenterol. 1985;80:608–611. [PubMed] [Google Scholar]
- 17.Ritz E. Intestinal-renal syndrome: mirage or reality? Blood Purif. 2011;31:70–76. doi: 10.1159/000321848. [DOI] [PubMed] [Google Scholar]
- 18.Vaziri ND, Yuan J, Rahimi A, et al. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant. 2012;27:2686–2693. doi: 10.1093/ndt/gfr624. ▪ ▪ This study was the first to demonstrate disruption of the colonic epithelial tight junction in CKD which elucidated the mechanism by which uremia impairs intestinal barrier function and causes endotoxemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 20.Bourlioux P, Koletzko B, Guarner F, Braesco V. The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium ‘The Intelligent Intestine’, held in Paris. Am J Clin Nutr. 2003;78:675–683. doi: 10.1093/ajcn/78.4.675. [DOI] [PubMed] [Google Scholar]
- 21.Savage DC. Gastrointestinal microflora in mammalian nutrition. Annu Rev Nutr. 1986;6:155–178. doi: 10.1146/annurev.nu.06.070186.001103. [DOI] [PubMed] [Google Scholar]
- 22.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 23.Metges CC. Contribution of microbial amino acids to amino acid homeostasis of the host. J Nutr. 2000;130:1857S–11864S. doi: 10.1093/jn/130.7.1857S. [DOI] [PubMed] [Google Scholar]
- 24.Burkholder PR, McVeigh I. Synthesis of vitamins by intestinal bacteria. Proc Natl Acad Sci USA. 1942;28:285–289. doi: 10.1073/pnas.28.7.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brugman S, Klatter FA, Visser JT, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 27.Huycke MM, Gaskins HR. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med (Maywood) 2004;229:586–597. doi: 10.1177/153537020422900702. [DOI] [PubMed] [Google Scholar]
- 28.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isolauri E, Kalliomäki M, Laitinen K, Salminen S. Modulation of the maturing gut barrier and microbiota: a novel target in allergic disease. Curr Pharm Des. 2008;14:1368–1375. doi: 10.2174/138161208784480207. [DOI] [PubMed] [Google Scholar]
- 30.Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci. 1993;38:257–268. doi: 10.1007/BF01307542. [DOI] [PubMed] [Google Scholar]
- 31.Vaziri ND, Freel RW, Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J Am Soc Nephrol. 1995;6:1313–1317. doi: 10.1681/ASN.V641313. [DOI] [PubMed] [Google Scholar]
- 32.Hatch M, Vaziri ND. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin Sci. 1994;86:511–516. doi: 10.1042/cs0860511. [DOI] [PubMed] [Google Scholar]
- 33.Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol. 1994;5:1339–1343. doi: 10.1681/ASN.V561339. [DOI] [PubMed] [Google Scholar]
- 34.Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters the composition of intestinal microbial flora. Kidney Int. (in press). ▪ ▪ Using a sophisticated phylogenic microarray procedure, in this study, the investigators conducted a comprehensive assessment of the effect of CKD on the colonic microbiome in humans and experimental animals and found that uremia profoundly changes the composition of microbial flora. [Google Scholar]
- 35.Aronov PA, Luo FJ, Plummer NS, et al. Colonic contribution to uremic solutes. J Am Soc Nephrol. 2011;22:1769–1776. doi: 10.1681/ASN.2010121220. ▪ ▪ This study was the first to demonstrate the colonic origin of several known uremic toxins and numerous other as-yet unidentified uremic retention products in patients with ESRD. [DOI] [PMC free article] [PubMed] [Google Scholar]