DEVELOPMENT OF CD27+ MARGINAL ZONE B CELLS REQUIRES GALT (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 1.
Published in final edited form as: Eur J Immunol. 2013 Apr 19;43(6):1484–1488. doi: 10.1002/eji.201243205
Summary
In species other than mouse, little is known about the origin and development of marginal zone (MZ) B cells. Using cross-reactive antibodies, we identified and characterized splenic MZ B cells in rabbits as CD27+CD23−. In rabbits in which organized GALT was surgically removed at birth, we found only CD23+ follicular (FO) B cells and almost no CD27+ MZ B cells in the spleen, indicating that GALT is required for the development of splenic MZ B cells. These findings lead us to suggest that commensal microbiota contribute to development of MZ B cells.
Keywords: B cell development, Marginal zone B cells, Commensal microbiota, GALT
Introduction
With the exception of mice, little is known about the origin and development of MZ B cells in mammals, including humans. The gradual decline in the number of circulating MZ B cells in splenoctomized patients suggests that spleen may play a role in the development and/or maintenance of MZ B cells in humans [1, 2]. Because MZ B cells with somatically diversified Ig genes are found in young children [2–4] and in patients unable to form T-dependent germinal centers [2, 3, 5, 6], Weill et al [7] proposed that human MZ B cells develop in GALT in a T-independent manner, analogous to the strategy used by B cells in sheep and rabbits [7, 8]. Unlike spleen, however, the requirement and/or role of GALT for peripheral B cell development cannot be directly addressed in humans. Because we previously used rabbits in which organized GALT (comprised of appendix, sacculus rotundus, and Peyer’s patches) was surgically removed at birth (GALTless) [9], we thought to use these rabbits to investigate the role of GALT in the development of B cell subsets. These GALTless rabbits appeared healthy, with no apparent signs of infection, and exhibited a growth rate that was similar to control littermates. The frequency of peripheral IgM+ B cells in these GALTless rabbits was however, significantly reduced, but the identity of the B cells that were reduced or missing could not be determined. In this study, we analyzed frozen tissues preserved from the spleen of these GALTless rabbits and found that both the FO and MZ B cell compartments were perturbed.
Results and discussion
Identification and characterization of MZ B cells
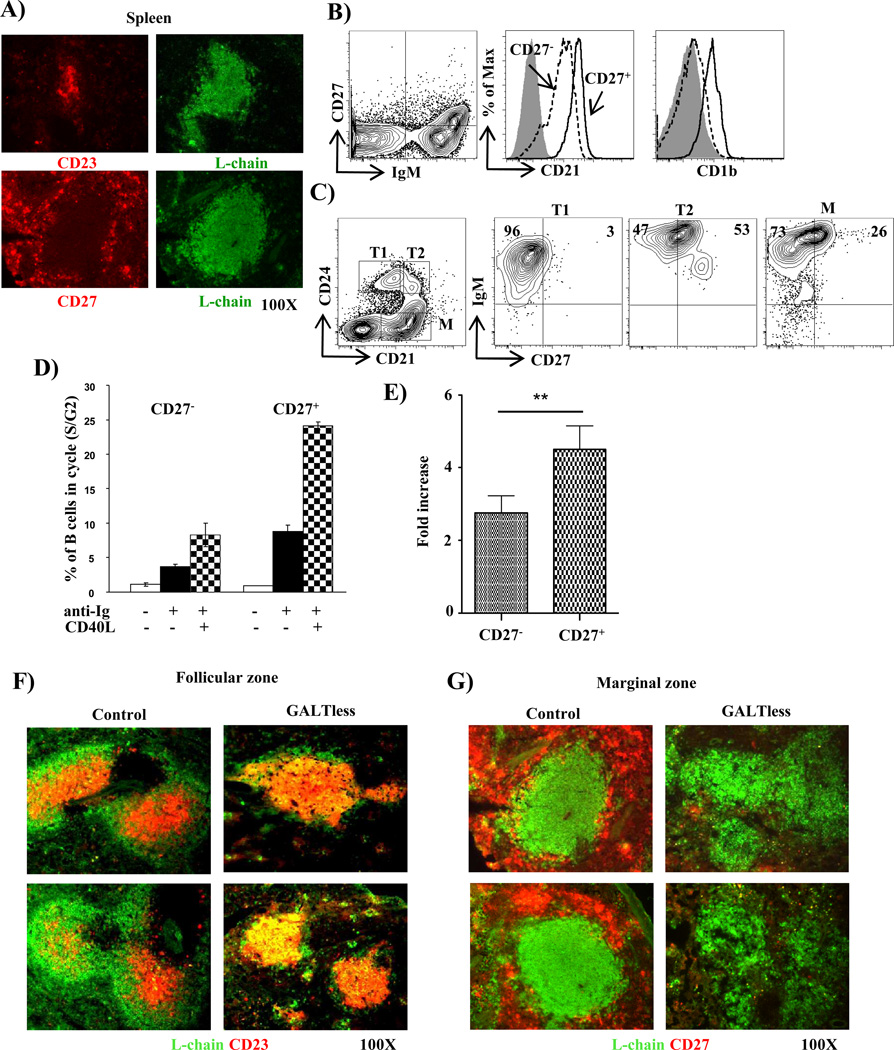
MZ B cells are identified by expression (or lack thereof) of surface Ig, CD23 and CD27[7, 10]. Because IgD is not found in rabbits [11], we tested if the expression of IgM, CD23 and CD27 can be used to distinguish rabbit MZ B cells from FO B cells, that we previously [12, 13] described as CD23+ (Fig 1A). Using a crossreactive anti-human CD27 mAb and anti-rabbit L chain Ab, we found B cells in the margin of B cell follicles (Fig 1A) and these were IgMhi (Fig 1B, left). We used anti-L chain Ab instead of anti-IgM for immunohistology, because the polyclonal anti-L chain Ab provides a stronger signal than does the mAb anti-μ chain Ab. The CD27+ B cells expressed higher levels of complement receptor, CD21 than CD27− B cells (Fig 1B, middle), and most CD27+ B cells expressed CD1b (Fig 1B, right), similar to the expression of CD1 isoforms on human (CD1c) and murine (CD1d) MZ B cells [7, 14]. We conclude that MZ B cells in the spleen can be identified as a CD27+CD23−IgMhi phenotype. Essentially all CD27+ cells in the spleen were B cells, and most of them were IgM+ (Fig 1B); a few were class-switched B cells (Table 1). We also found CD27+ B cells in peripheral blood and other lymphoid tissues, including GALT (Table 2). We previously found that some transitional B cells in rabbit spleen localize to the MZ [13]. Human transitional B cells are CD27− [15], and we found that most rabbit T1 B cells were also CD27− (Fig 1C); surprisingly, however, approximately 50% of the T2 B cells were CD27+ (Fig 1C). We suggest that the CD27+ T2 B cells may be precursors to CD27+ mature MZ B cells. T2 B cells in mice are similarly thought to contain precursors for MZ B cells as well as for FO cells [10]. Functionally, 24 hrs after anti-Ig and CD40L stimulation, we found more CD27+ B cells in cell cycle than CD27− B cells (Fig 1D), indicating that CD27+ B cells enter cell cycle more readily than CD27− B cells. Upon stimulation with CD40L and IL4 for 8 days, we found significantly more total Ig in the culture supernatant of sorted CD27+ B cells than CD27− B cells (Fig 1E), suggesting that CD27+ B cells secrete more Ig than CD27− B cells. We conclude that rabbit CD27+ and CD27− B cells represent distinct subsets that differ by virtue of their anatomical location, phenotype, and functional properties.
Figure 1. Identification and characterization of splenic marginal zone B cells.
A) Immunofluorescent staining of spleen from normal rabbits for CD23 and L-chain (LC) (top), and CD27 and LC (bottom). Original magnification = 100× for all images. Flow cytometric analysis of splenocytes from normal rabbits for B) IgM, and CD27 (upper); CD21 and CD1b histograms (lower) of cells in the IgM+CD27+ gate (solid line) and IgM+CD27− gate (dotted line)[Shaded histograms = staining with isotype control], and C) CD21, CD24 (left); expression of IgM and CD27 on transitional (T1 and T2) and mature (M) B cells (right) from normal rabbits; D) Bar graph showing % CD27+ and CD27− B cells from normal rabbits in S/G2 cell cycle after 24 hr culture alone (medium), with anti-Ig or with anti-Ig plus CD40L. Error bars indicate the SEM from two independent experiments and a total of 2 rabbits; E) Bar graph showing the fold-increase in total Ig found in the culture supernatants of CD40L and IL4 stimulated CD27+ and CD27− B cells, relative to B cells cultured in medium alone. Error bars indicate SEM calculated from fold changes obtained from four independent experiments and a total of 4 rabbits. **P = 0.006, two-tailed student’s t test. The data in A) to C) are representative of 2–3 independent experiments; F & G) Staining of frozen spleen sections from control (normal) and GALTless rabbit for LC and CD23 (F), LC and CD27 (G). Panels F and G are from different areas of the tissue. Original magnification for F and G = 100×. The data in F & G are representative of staining obtained from each of 3 GALTless rabbits.
Table I.
Frequency of class-switched CD27+ B cells in Spleena
| CD27+ | CD27− | |
|---|---|---|
| IgA+ | 5 ± 0.6 | 1.6 ± 0.2 |
| IgG+ | 7.4 ± 1.3 | 4 ± 1.8 |
Table II.
Frequency of CD27+ B cells in adult rabbit tissues
| Tissuea | IgM+CD27+b | IgM+CD27−b |
|---|---|---|
| Spleen (10) | 20 ± 2.3 | 31.5 ± 3 |
| Appendix (3) | 13 ± 4.1 | 70 ± 0.7 |
| Sacculus rotundus (3) | 7.5 ± 5 | 65 ± 10.6 |
| Peyer’s patches (3) | 4.5 ± 2.3 | 53.2 ± 10 |
| Mesenteric lymph nodes (3) | 8 ± 1.8 | 51 ± 3 |
| Peripheral Blood (5) | 5.8 ± 0.7 | 31 ± 5.2 |
Immunohistological analysis of spleen from GALTless rabbits
To determine if there was a perturbation in the splenic B cell compartment after neonatal removal of GALT, we stained frozen spleen tissues with anti-CD23 and anti-CD27 mAbs to identify FO and MZ B cells, respectively. Unlike control rabbits that had well-defined CD23+ and CD23− areas (Fig 1F, left), nearly all B cells in the follicles of GALTless rabbits were CD23+ (Fig 1F, right). Consistent with this observation, we found almost no CD27+ MZ B cells in the GALTless rabbits (Fig 1G), indicating that GALT is required for development of MZ B cells.
The intestinal microbiota is required for development of GALT [16] and in the absence of intestinal microbiota, follicles of proliferating B cells are not found in GALT, and the number of peripheral B cells is makedly reduced [9]. In GALTless rabbits, only organized GALT, appendix, sacculus rotundus and Peyer’s patches are removed; isolated lymphoid follicles (ILF) [17] and cryptopatches would remain in the GALTless rabbits and be exposed to intestinal microbiota. The apparent absence of MZ B cells in GALTless rabbits indicates that ILF and cryptopatch B cells either do not mature into MZ B cells, or that they give rise to only small numbers of MZ B cells.
Notch 2 is important for both murine and human MZ B cell development [18–21], and its ligand delta-like-1 (DL1) is expressed by intestinal epithelial cells [22]. We suggest that transitional B cells enter the follicle-associated epithelium and domes of the appendix [13], interact with DL1+ epithelial cells, and become committed to a MZ fate; these cells would then migrate to the spleen and possibly other tissues. The CD27+ T2 B cells in spleen may represent putative MZ precursors derived from T1 B cells in GALT. In addition to notch2 signals, commensal microbiota in GALT may influence the development and/or maintenance of B cell subsets. Recently, Puga et al[23] identified a subpopulation of commensal-dependent neutrophils, called, “B cell-helper neutrophils” (NBH) that are required for the development of MZ B cells. They found fewer NBH cells in germfree (GF) and _Trif_−/−_Myd_88−/− mice than conventional mice and proposed that NBH cells are recruited to the spleen through TLR signals derived from commensal bacteria. While investigating the role of resident microbial communities on B cell populations in vivo, Wei et al [24] observed that mice with a restricted microflora (RF) have significantly reduced numbers of MZ B cells compared to specific pathogen-free (SPF) or conventional mice. They demonstrated that an expanded population of cytotoxic CD8+ T cells in the RF mice, rapidly and selectively killed MZ B cells. In other studies, Mazmanian et al [25] elegantly demonstrated that polysaccharide PSA from Bacteroides fragilis influences the balance of CD4+ Th1 and Th2 subsets in vivo, and Ivanov et al [26] showed that segmented filamentous bacterium induces the appearance of CD4+ T helper cells in the lamina propria. Similarly, we suggest that products from the microbial community normally present in the appendix and SR may influence the development and/or maintenance of B cell subsets in vivo. In support of this idea, germfree-appendix rabbits and remote colony rabbits with altered microbiota have reduced numbers of peripheral B cells [27]. Whether these rabbits have a perturbation in the MZ B cell compartment remains to be determined.
Although we defined CD27+ B cells as MZ B cells based on anatomical localization, these cells may also include a large proportion of memory B cells and/or the recently described CD27+ human B1-like cells [28]. The expression of CD27 on a large fraction of class-switched B cells, and the rapid activation of CD27+ B cells with either Ig cross-linking or CD40L engagement is suggestive of an activated/memory phenotype. However, unlike in other species, the presence of somatic hypermutation in the Ig V-region genes cannot be used to identify memory B cells in rabbits, because all B cells are somatically diversified after a few weeks of age [29]. The expression of surface IgD and CD27 is used to classify human B cells into CD27−IgDhigh (Naïve), CD27+IgDlow (IgM memory) and CD27+IgD− (Switched memory) B cells [7, 30]. Rabbits, however do not appear to have IgD [11] and no markers are yet known to specifically identify rabbit memory B cells. Recently, members of the FcR-like (FCRL) family of proteins have emerged as another marker for memory B cells [31–33]. The FCRL1–6 molecules are highly conserved in humans, dogs, and oppossums, but not mice [34]. Whether these molecules are expressed in rabbits, and can be used to identify memory B cells remains to be determined. Further characterization of CD27+ B cells in peripheral tissues (Table 2) may reveal if CD27 in combination with other markers can be used to identify memory B cells in rabbit.
Concluding Remarks
We identified and characterized MZ B cells in rabbits and showed that their absence in GALTless rabbits reveals a hitherto unknown link between GALT and splenic MZ B cells. Further, these studies suggest that rabbits can potentially be used as a model to study human MZ B cell development.
Materials and methods
Rabbits and reagents
Rabbits (4 months to 2 years of age) were maintained at Loyola University Chicago. All studies were reviewed and approved by the Institutional Animal Care and Use Committee of Loyola University Chicago. GALTless rabbits were as described previously [9]. In those studies, the appendix and the ileocecal junction were surgically excised from 1-day-old rabbits. After 3–5 wks, the macroscopically visible Peyer’s patches from these rabbits were surgically removed using purse-string sutures. After surgery, these rabbits were maintained under conventional conditions in the colony. At the time of sacrifice, no residual GALT in these rabbits was macroscopically visible.
Reagents used were as follows: anti-IgM (367; BD Biosciences), goat anti-L chain (KLK stock), anti-CD1b (LAT-3; kindly provided by Dr. Steward Sell, Albany Medical College, NY); and cross-reactive, anti-CD21 (BL13), anti-CD23 (9P25; Immunotech), anti-CD24 (M1/169; eBiosciences) and anti-CD27 (LT27; AbD Serotec). Additional reagents were Dylight 649, 549-conjugated and/or biotinylated goat Fab anti-mouse IgG, and streptavidin PE/APC (Jackson ImmunoResearch). Although the specificity of crossreactive anti-CD27, anti-CD23 and anti-CD24 mAbs used in this study has not been determined, these reagents all bind subsets of IgM+ B cells and thus identify B cell subsets in rabbits [13] and data described herein].
Flow cytometry and Immunohistochemistry
All flow cytometry data were acquired with FACSCanto or FACSAria (BD Biosciences), gated on live lymphocyte-sized cells on the basis of forward and side scatter, and analyzed using FlowJo software (Tree star). For immunohistochemistry, cryosections (7–8µm) were stained with primary Ab and indirect reagents: Cy2- or Cy3-streptavidin and Cy2- or Dylight 549-goat (Fab) anti-mouse IgG (Jackson ImmunoResearch). Slides were viewed and images processed as described earlier [13]. Frozen spleen tissues were obtained from GALTless rabbits described previously [9].
Cell cycle analysis
FAC-Sorted splenic CD21+CD27+ and CD21+CD27− B cells were cultured with anti-Ig (10µg/ml) or with irradiated murine CD40L-transfected chinese hamster ovary (CHO) cells in a 100:1 ratio, respectively. After 24 h, cells were fixed with cold 70% ethanol, treated with RNase (50µg/ml), stained with propidium iodide (50µg/ml) and analyzed by flow cytometry.
ELISA
To measure total secreted Ig, sorted splenic CD21+CD27+ and CD21+CD27− B cells (104–105) were cultured in 200–500µl complete RPMI with murine CD40L-transfected CHO cells (100:1), and human IL-4 (100ng/ml) (R&D Systems Inc). The culture supernatants were harvested after 8 days and the total amount of Ig secreted was determined by ELISA using anti rabbit-L chain-coated microtiter plates. The ELISA was developed using biotinylated anti-L chain Ab and streptavidin HRP (Jackson ImmunoResearch) plus ABTS (Sigma Aldrich) as substrate.
Acknowledgements
We acknowledge the help of Patricia Simms in the FACS Core Facility at Loyola University Chicago. This work was supported by the National Institute of Health Grants AI50260 and AI068390 to KLK.
Abbreviations used in this article
FO
follicular
MZ
marginal zone
GALT
gut-associated lymphoid tissues
T1
transitional type 1
T2
transitional type 2
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, Quinti I, Carsetti R. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, Weill JC. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weller S, Faili A, Garcia C, Braun MC, Le Deist FF, de Saint Basile GG, Hermine O, Fischer A, Reynaud CA, Weill JC. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller S, Mamani-Matsuda M, Picard C, Cordier C, Lecoeuche D, Gauthier F, Weill JC, Reynaud CA. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008;205:1331–1342. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agematsu K, Nagumo H, Shinozaki K, Hokibara S, Yasui K, Terada K, Kawamura N, Toba T, Nonoyama S, Ochs HD, Komiyama A. Absence of IgD-CD27(+) memory B cell population in X-linked hyper-IgM syndrome. J Clin Invest. 1998;102:853–860. doi: 10.1172/JCI3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brezinschek HP, Dorner T, Monson NL, Brezinschek RI, Lipsky PE. The influence of CD40-CD154 interactions on the expressed human V(H) repertoire: analysis of V(H) genes expressed by individual B cells of a patient with X-linked hyper-IgM syndrome. Int Immunol. 2000;12:767–775. doi: 10.1093/intimm/12.6.767. [DOI] [PubMed] [Google Scholar]
- 7.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 8.Severson KM, Mallozzi M, Driks A, Knight KL. B cell development in GALT: role of bacterial superantigen-like molecules. J Immunol. 2010;184:6782–6789. doi: 10.4049/jimmunol.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vajdy M, Sethupathi P, Knight KL. Dependence of antibody somatic diversification on gut-associated lymphoid tissue in rabbits. J Immunol. 1998;160:2725–2729. [PubMed] [Google Scholar]
- 10.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 11.Lanning DK, Zhai SK, Knight KL. Analysis of the 3' Cmu region of the rabbit Ig heavy chain locus. Gene. 2003;309:135–144. doi: 10.1016/s0378-1119(03)00500-6. [DOI] [PubMed] [Google Scholar]
- 12.Yeramilli VA, Knight KL. Requirement for BAFF and APRIL during B cell development in GALT. J Immunol. 2010;184:5527–5536. doi: 10.4049/jimmunol.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeramilli VA, Knight KL. Somatically diversified and proliferating transitional B cells: implications for peripheral B cell homeostasis. J Immunol. 2011;186:6437–6444. doi: 10.4049/jimmunol.1003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 15.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182:5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172:1118–1124. doi: 10.4049/jimmunol.172.2.1118. [DOI] [PubMed] [Google Scholar]
- 17.Keren DF, Holt PS, Collins HH, Gemski P, Formal SB. The role of Peyer's patches in the local immune response of rabbit ileum to live bacteria. J Immunol. 1978;120:1892–1896. [PubMed] [Google Scholar]
- 18.Witt CM, Won WJ, Hurez V, Klug CA. Notch2 haploinsufficiency results in diminished B1 B cells and a severe reduction in marginal zone B cells. J Immunol. 2003;171:2783–2788. doi: 10.4049/jimmunol.171.6.2783. [DOI] [PubMed] [Google Scholar]
- 19.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 20.Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, Nakagami-Yamaguchi E, Hamada Y, Aizawa S, Hirai H. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 21.Scheeren FA, Nagasawa M, Weijer K, Cupedo T, Kirberg J, Legrand N, Spits H. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med. 2008;205:2033–2042. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama J, Okamoto R, Iwasaki M, Zheng X, Yui S, Tsuchiya K, Nakamura T, Watanabe M. Delta-like 1 expression promotes goblet cell differentiation in Notch-inactivated human colonic epithelial cells. Biochem Biophys Res Commun. 2010;393:662–667. doi: 10.1016/j.bbrc.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 23.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei B, Su TT, Dalwadi H, Stephan RP, Fujiwara D, Huang TT, Brewer S, Chen L, Arditi M, Borneman J, Rawlings DJ, Braun J. Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells. Eur J Immunol. 2008;38:3411–3425. doi: 10.1002/eji.200838432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanning D, Sethupathi P, Rhee KJ, Zhai SK, Knight KL. Intestinal microflora and diversification of the rabbit antibody repertoire. J Immunol. 2000;165:2012–2019. doi: 10.4049/jimmunol.165.4.2012. [DOI] [PubMed] [Google Scholar]
- 28.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight KL, Barrington RA. Somatic diversification of IgH genes in rabbit. Immunol Rev. 1998;162:37–47. doi: 10.1111/j.1600-065x.1998.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 30.Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or"memory" B cells? J Immunol. 2007;179:13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- 31.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson TA, Haga CL, Ehrhardt GR, Davis RS, Cooper MD. FcR-like 2 Inhibition of B cell receptor-mediated activation of B cells. J Immunol. 2010;185:7405–7412. doi: 10.4049/jimmunol.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, Cooper MD. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrhardt GR, Cooper MD. Immunoregulatory roles for fc receptor-like molecules. Curr Top Microbiol Immunol. 2011;350:89–104. doi: 10.1007/82_2010_88. [DOI] [PubMed] [Google Scholar]