Management of the Patient with Incomplete Response to PPI Therapy (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 1.
Published in final edited form as: Best Pract Res Clin Gastroenterol. 2013 Jun;27(3):401–414. doi: 10.1016/j.bpg.2013.06.005
Abstract
Proton pump inhibitors (PPIs) remove most of the acid from the gastroesophageal refluxate. However, PPIs do not eliminate reflux and the response of specific GERD symptoms to PPI therapy depends on the degree to which acid drives those symptoms. PPIs are progressively less effective for heartburn, regurgitation, chest pain and extra-esophageal symptoms. Hence, with an incomplete PPI response, obtaining an accurate history, detailing which symptoms are ‘refractory’ and exactly what evidence exists linking these symptoms to GERD is paramount. Reflux can continue to cause symptoms despite PPI therapy because of persistent acid reflux or weakly acidic reflux. Given these possibilities, diagnostic testing (pH or pH-impedance monitoring) becomes essential. Antireflux surgery is an alternative in patients if a clear relationship is established between persistent symptoms, particularly regurgitation, and reflux. Treating visceral hypersensitivity may also benefit the subset of GERD patients whose symptoms are driven by this mechanism.
Keywords: Esophagus, Gastroesophageal reflux disease, ambulatory esophageal pH monitoring, ambulatory esophageal pH-impedance monitoring, proton pump inhibitors, visceral hypersensitivity
Introduction
Proton pump inhibitor (PPI) therapy has largely changed the clinical face of gastroesophageal reflux disease (GERD). Prior to the introduction of PPIs in 1989, clinicians struggled to manage reflux patients with the existing pharmacological therapies, dominated at the time by the histamine-2 receptor antagonists. Of course, the ‘refractory patient’ at that time was easily defined with an endoscope: persistent mucosal erosions, ulcers, and recurrent strictures. Almost miraculously, however, these problems succumbed to the potent acid suppression made possible with PPIs. It is now widely accepted that the mucosal manifestations of GERD (other than Barrett’s metaplasia) can be controlled indefinitely with sustained PPI therapy [1]. Not surprisingly, PPI use has subsequently increased tremendously and a number of alternative molecules have been added to the therapeutic armamentarium.
The ensuing PPI euphoria broadened through the turn of the century leading many clinicians to conclude that, not only were these drugs tremendously effective in treating GERD, but that the therapeutic response to PPIs constituted a clinical definition of GERD [2]. If a patient’s symptoms responded to PPIs, they had GERD and conversely, if they did not respond to PPIs, they did not have GERD. Or so the thinking went. This was, of course, flawed thinking, as it would equate to diagnosing rheumatoid arthritis based on improvement with aspirin therapy [3]. While it is true that many cases of rheumatoid arthritis do respond to aspirin, it is also true that many do not and, for that matter, that many other conditions may exhibit a therapeutic response to aspirin. And then there is the placebo response. The analogy with GERD is apparent.
The other evolution that has paralleled the introduction of PPIs, perhaps even made possible by the PPIs, was an improved understanding of the full spectrum of GERD. As the problem of refractory mucosal disease receded, the problem of refractory symptoms blossomed. And the list of symptoms and syndromes potentially attributable to GERD expanded. These developments led to the formation of an international consensus conference tasked with developing a modern definition of GERD. The resultant ‘Montreal definition’ proposed the overarching definition of GERD as ‘a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications’ [4]. The consensus document went on to review health related quality of life data pertinent to the cardinal reflux symptoms, heartburn and regurgitation to define ‘troublesome’. In the case of heartburn, the threshold at which the symptom becomes ‘troublesome’ as evident by a clinically relevant decrement in health related quality of life was ≥ 2 days/week of mild symptoms or ≥ 1 day/week of ≥ moderate symptoms. No thresholds were proposed for any other potential reflux symptoms because no relevant data could be found in the literature. Nonetheless, failure to satisfactorily resolve potential GERD symptoms has become one of the most common reasons for gastroenterological consultations in the US and Western Europe [5]. This treatise will explore the many facets of this clinical scenario and propose a systematic approach to management.
Phenotypes of incomplete PPI response
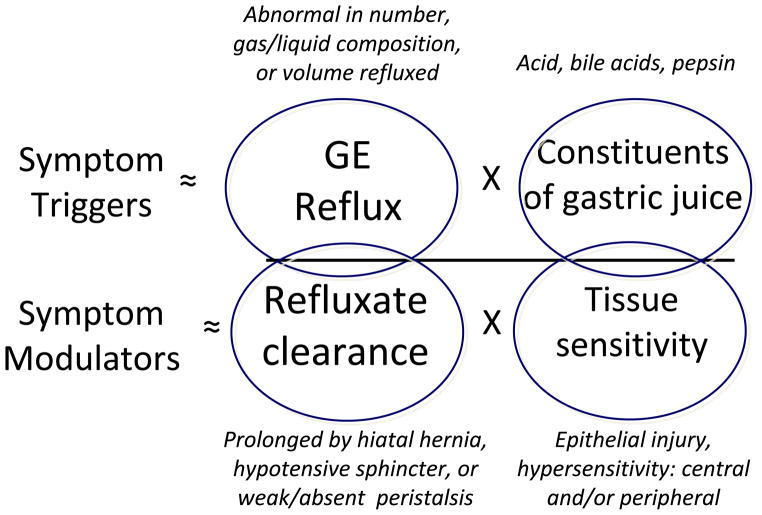
An estimated 10 to 40% of the patients with ‘GERD’ have either an incomplete or no response to a standard dose of PPI [6, 7]. However, while that may be a unifying clinical diagnostic code, this is an extraordinarily heterogeneous group of patients. PPI therapy is, after all, directed at suppressing gastric acid secretion and acid secretion is usually normal in GERD patients. Rather, the primary pathophysiology of GERD usually resides in the domains of excessive or abnormal reflux events, prolonged acid clearance, or altered mucosal sensitivity as conceptualized in Figure 1. Any of these may dominate the pathophysiology of a particular reflux syndrome. Indeed, even prior to treatment most patients with heartburn do not have reflux esophagitis and this disconnect becomes more exaggerated in patients with atypical GERD symptoms. Furthermore, the dominant mechanism distinguishing esophagitis from non-erosive reflux disease is not found in the number of reflux events but rather, in prolonged refluxate (acid) clearance mechanistically attributable to the effects of a hiatal hernia or weak peristalsis [9, 10]. In fact, prolonged acid clearance correlates with both the severity of esophagitis and the presence of Barrett’s metaplasia [11, 12]. The efficacy of acid clearance is particularly impaired in patients with hiatus hernia who exhibit reflux of fluid from the hernia during deglutitive relaxation while in the supine posture [13, 14].
Figure 1.
Conceptual model of the pathophysiological triggers of GERD symptoms. The fundamental abnormalities are of symptomatic reflux events and prolonged clearance. However, the effect of reflux in eliciting symptoms is linked to the toxicity of gastric juice even though this factor is usually normal in GERD patients. Acid clearance and mucosal sensitivity modulate the effect of reflux by prolonging the exposure of the esophageal mucosa to refluxate and diminishing the sensory threshold of what is perceived as painful. Modified from [8].
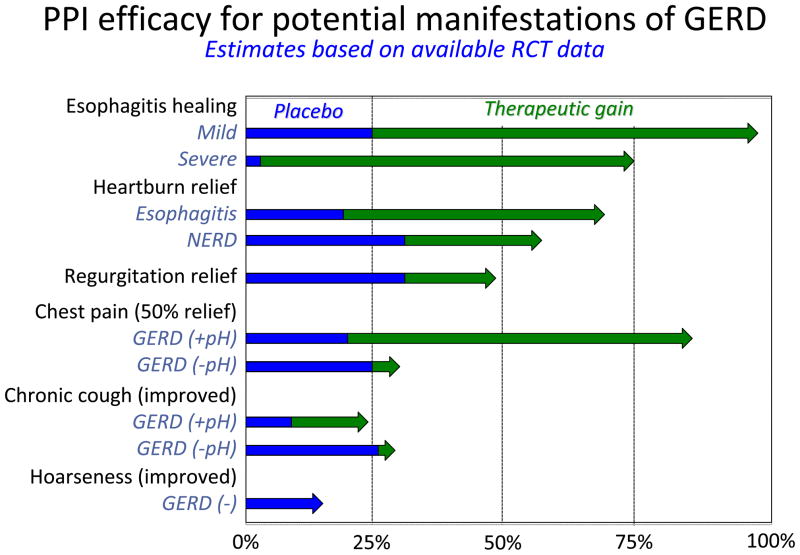
Generally speaking, reflux treatment strategies target individual elements of GERD pathophysiology as represented in Figure 1. Unfortunately, pharmaceutical therapies have minimal effect in the domain of improving acid clearance, but lifestyle modifications such as avoiding post-prandial recumbency and head of bed elevation do target this mechanism [1]. Gaining increasing recognition as an important element of reflux pathophysiology, visceral sensitivity, is also an important modulator of reflux symptom severity and, in the case of reflux-cough, a key pathophysiological feature [15]. However, thus far, treatments targeting this mechanism are rudimentary and non-specific [16]. On the other hand, the lethality of gastric juice to esophageal epithelial cells, a key event in the pathogenesis of esophagitis, proved to be an excellent pharmacological target. However, the dominance of acid as a major pathophysiological determinant diminishes with syndromes other than esophagitis. The therapeutic implications of that observation are summarized in Figure 2 comparing the efficacy of PPIs in treating esophagitis to their efficacy in treating GERD syndromes other than esophagitis [8]. Evident in the figure, PPI efficacy progressively diminishes moving from esophagitis to symptomatic heartburn, regurgitation [18, 19], chest pain [20], cough [21, 22] and laryngitis [17]. Improving these therapeutic outcomes hinges upon finding alternative treatment strategies that are either more efficacious in preventing reflux or in treating disease cofactors that are often equally important with these conditions.
Figure 2.
Summary of PPI efficacy for various GERD syndromes as assessed in randomized controlled trials. In each case, data among trials are averaged to derive estimates of placebo effect and therapeutic gain, defined as the degree to which PPI therapy improved upon the benefit seen with placebo. The blue segments represent the placebo effect and the green arrows the therapeutic gain beyond the placebo effect seen with PPIs. PPI data are grouped in terms of brand and dose, taking some liberties for simplification. However, it is imperative to recognize that the only disease manifestation in which a dose-response curve has been convincingly demonstrated by randomized controlled trial is in healing esophagitis. At the other extreme, in the case of hoarseness, controlled trial data are sparse and the only large trial (which was done in patients without esophagitis or frequent heartburn) failed to show benefit of PPI vs placebo [17]. Modified from [8].
Even within the domain of typical esophageal symptoms (heartburn, regurgitation, chest pain), there are distinct phenotypes of incomplete PPI response to consider. There is the immediate problem that no symptom is 100% specific for GERD. Hence, persistent heartburn, regurgitation or chest pain may alternatively be related to esophagitis of another etiology (eosinophilic, infectious, pill), severe dysmotility such as achalasia, rumination, functional heartburn, or functional chest pain. And then, within the spectrum of GERD there are instances in which reflux continues to cause symptoms despite PPI therapy. This can be result from insufficient acid suppression with persistent acid reflux or symptoms as a result of weakly acidic reflux. Given this wide array of possibilities, and the reality that there is no longer a ‘one size fits all’ therapeutic strategy, diagnostic testing now becomes an essential clinical tool.
Diagnostic Tests
History
In instances of an incomplete symptom response to PPI therapy it is imperative that the patient’s symptoms be revisited. Which were the symptoms allegedly caused by reflux and which of these were incompletely resolved by the PPI? Heartburn, defined as a painful retrosternal burning sensation of relatively short duration, is the most characteristic reflux symptom and the one that responds best to acid inhibition. Regurgitation, defined as backflow of gastric content into the chest or mouth is also a characteristic reflux symptom, but this symptom is known to be less responsive to PPI therapy [18, 19]. Extra-esophageal symptoms such a hoarseness, throat pain, asthma and cough are the least likely to resolve with PPI treatment, in part because they are often not caused by reflux [23]. How severe were the symptoms before the start of the treatment? Patients with severe pretreatment symptoms are less likely to respond satisfactorily to PPI treatment [24].
Various questionnaires have been developed to assess GERD symptom severity and frequency. However, while several of these have been validated and shown to yield reproducible results, their use does not increase the accuracy of the GERD diagnosis [25]. Not surprisingly, higher pretreatment scores for dyspeptic symptoms predict poorer symptom response to PPI therapy [26]. In two large randomized trials using the Reflux Disease Questionnaire (RDQ) other concomitant RDQ items, particularly ‘substernal pain’ and ‘dyspepsia pain’ were associated with a reduced benefit of acid suppression for ‘substernal burning’ [27]. Finally, there are no specific data supporting the clinical use of questionnaires as a diagnostic test in patients with therapy-resistant GERD.
Endoscopy
The American Society of Gastrointestinal Endoscopy recommends endoscopy for patients with “GERD symptoms that are persistent or progressive despite appropriate medical therapy” [28]. In these cases endoscopy can detect alternative diagnoses such as eosinophilic esophagitis, infection and pill injury, even achalasia. Alternatively, endoscopy may detect reflux esophagitis although, admittedly, the likelihood of this is low in a patient compliant with full dose PPI therapy; in a study of 105 patients with incomplete PPI symptomatic response, erosive lesions (Los Angeles A or B) were found in only 6.7% [29]. However, in another recent study, this one restricting the analysis to patients who initially had esophagitis, 31.4% of partial PPI responders were found to have Los Angeles A or B esophagitis [30]. Other potential endoscopic findings that support a diagnosis of GERD are peptic stenosis, Barrett’s metaplasia, or hiatus hernia. However, none of these findings necessarily mean that the patient’s symptoms are caused by reflux.
Histology
Esophageal biopsies in GERD patients may show elongated papillae and hyperplasia of the basal cell layer with dilated intercellular spaces [32, 33]. Hence, although dilated intercellular spaces are reported to be more prevalent in patients with incomplete response to PPI [33], large inter-observer variation, low sensitivity and low specificity strongly limit the value of histology as a diagnostic marker for GERD. Similarly, it is uncertain whether increased numbers of mucosal eosinophils are relevant in the management of therapy-resistant GERD. Nonetheless, it is recommended that esophageal biopsies be taken when eosinophilic esophagitis is a diagnostic consideration, particularly when dysphagia is a presenting symptom.
Esophageal pH monitoring
Esophageal pH monitoring is conventionally carried out for a period of 24 hours, using a portable data logger and a transnasal catheter on which a pH electrode is mounted. The electrode is positioned at 5 cm proximal to the upper border of the manometrically defined LES. More recently, telemetric pH monitoring has become popular (Bravo). With this technique a capsule containing an antimony pH electrode and a radiotransmitter is attached to the esophageal mucosa, positioned with its electrode 6 cm proximal to the endoscopically identified squamocolumnar junction. The advantage of the Bravo technique is that there is no discomfort caused by the presence of the naso-esophageal catheter and it allows longer measurements, e.g. 48–96 hours. However, the wireless pH capsule technology is much more expensive than catheter-based pH-metry. The results obtained with catheter-based and wireless systems correlate well, but may not be identical [34, 35].
Two types of information about gastroesophageal reflux can be obtained with pH monitoring: 1) quantitative data on the magnitude of esophageal acid exposure usually expressed as percentage of time with pH < 4, and 2) pH monitoring makes it possible to assess the temporal association between the patient’s symptom episodes and acid reflux events. A symptom episode is considered to be reflux-related if its onset takes place within two minutes after the onset of an acid reflux episode. The result of such assessment can be expressed in indices such as the Symptom Index or the Symptom Association Probability [36, 37]. The importance of symptom association in a patient with incomplete symptom response to a PPI is that it allows one to distinguish reflux-related symptoms from functional heartburn [38]. The latter condition is defined as the presence of heartburn in the absence of evidence that it is caused by reflux. In the evaluation of a patient with incomplete symptom response to PPIs, esophageal pH monitoring is ideally performed after cessation of acid-suppressive medication for at least 5 days because the primary aim is to determine whether or not the patient really has GERD and reflux events cannot be recognized reliably with pH monitoring when the patient is on a PPI.
Combined pH-impedance monitoring
Whereas pH measurement cannot be used to monitor reflux that is not acidic, the technique of intraluminal impedance monitoring makes it possible to identify all types of reflux (acid and weakly acidic, liquid and gaseous) [39]. Since an array of impedance electrodes covering most of the length of the esophagus is used, the direction in which liquid or gas boluses are propagated can also be established. Impedance monitoring is usually combined with pH monitoring, using a pH electrode incorporated into the impedance catheter, so that the acidity of all reflux events is also measured. Monitoring not only acid (pH < 4) but also weakly acidic (pH 4–7) reflux episodes is relevant because it has been shown that weakly acidic reflux events can cause symptoms, including heartburn [40]. Using impedance monitoring it is possible to detect gastroesophageal reflux whilst the patient is taking a drug that inhibits gastric acid secretion.
There are opposing views on how best to perform impedance monitoring in the patient with incomplete symptom resolution on acid inhibition. One may argue that the purpose of impedance monitoring in such a patient is to establish whether the remaining symptoms on PPI are reflux-related and thus that the measurement should be done while the PPI is continued. However, as with pH monitoring, the chance of finding a positive correlation between the reflux symptoms and reflux events in a pH-impedance study is greatest when the patient is studied ‘off’ acid inhibitors[41]. Therefore, pH-impedance monitoring is best performed off PPI when the diagnosis of GORD has not yet been established beyond doubt. Measurement ‘on’ PPI is reasonable when the diagnosis has been made already and the main question is why the treatment proves to be ineffective.
Manometry
In general, there are no manometric findings that have a sufficiently high specificity and sensitivity for the diagnosis of GERD and there are no data showing that patients with incomplete response to treatment exhibit distinguishing manometric features from patients with a satisfactory response. However, manometry is useful to determine correct positioning for pH electrode placement and to detect the rare case in which achalasia was misdiagnosed as GERD [42]. An erroneous diagnosis of GERD in achalasia is made possible by the report of heartburn in up to 35% of achalasia patients [43]. Finally, manometry may be helpful in diagnosing the rumination syndrome. This is best accomplished with concurrent pH-impedance monitoring [44].
Pharmacological Approaches
Although PPIs are undoubtedly the most widely used drugs to treat GERD, it is still remarkable that patients are not always instructed properly about the timing of PPI intake relative to eating. This is of great relevance as PPIs only selectively interact with and inhibit actively secreting proton pumps. Hence, pre-meal dosing, especially before breakfast has been proven to most effectively reduce acid secretion [45]. However, a recent survey in 100 GERD patients on PPI found only 46% to be dosing optimally (i.e. within one hour before a meal), whereas only 12% dosed in a manner that maximized acid suppression (15–30 min before breakfast) [46]. Hence, optimization of PPI dosing should be the first step in addressing an inadequate symptom response before increasing dose or changing medication.
Double dose PPI
Doubling the PPI dose is one of the most common interventions made to improve symptom control in partial PPI responders. However, objective data supporting this approach are scarce. In fact, several studies have indicated that although increased dosing of PPI (either administered as q.d. or b.i.d.) results in improved suppression of gastric acid secretion, esophageal acid exposure and, most importantly, esophageal symptoms (particularly heartburn) were not more effectively reduced [47]. Alternatively, switching to alternative PPI is often tried to enhance symptom control. In one such trial, patients with persistent symptoms while receiving 30 mg lansoprazole were treated with twice daily 30 mg lansoprazole or single dose 40 mg esomeprazole for 8 weeks. Both treatments were equally effective for controlling heartburn [48]. Finally, the development of new compounds acting through novel mechanisms to reduce acid secretion did not improve symptom control beyond that achieved with PPIs. For example, the potassium-competitive acid blocker AZD0865, capable of nearly complete and immediate acid inhibition, was not superior to esomeprazole with respect to symptom control in patients with either erosive or non-erosive reflux disease [49, 50]. Taken together, these observations suggest that a plateau effect of blockading acid secretion exists with respect to symptom control; more is not better. Rather, alternative strategies beyond increasing the PPI dose should be explored.
PPI plus histamine-2 receptor antagonists (H2RAs)
Nocturnal acid secretion, mainly driven by histamine, is less sensitive to PPIs, as evidenced by a nocturnal drop in gastric pH, even in patients on double dose PPI. This phenomenon is referred to as nocturnal acid breakthrough [51]. Triggered by this observation, nocturnal acid breakthrough has been proposed as a potential mechanism of PPI resistant GERD symptoms. Hence, studies were undertaken in which PPIs were combined with nighttime H2RAs. Proof-of-concept pH monitoring studies indeed showed that acute H2R blockade improved control of nocturnal gastric pH and was associated with improvement of GERD symptoms [52]. However, subsequent studies found that symptom improvement was independent of the degree of nocturnal breakthrough, suggesting this phenomenon to be of limited clinical significance. Moreover, due to the development of tolerance, no difference could be demonstrated in gastric acid control between long term treatment (28 days) with PPI twice daily vs PPI twice daily combined with an H2RA [53], arguing against adding H2RA to PPIs as maintenance therapy in GERD.
PPI plus prokinetics
Accepting that more aggressive acid suppression fails to reduce GERD symptoms, improvement of clearance once the refluxate has entered the esophagus may represent an effective therapeutic approach. Vigneri et al. explored this strategy in GERD patients with endoscopic esophagitis [54]. These investigators reported that in a 12-month trial cisapride plus ranitidine was more effective than ranitidine alone in controlling pain, but that no addititive effect was observed by adding cisapride to omeprazole. A more recent study reported similar findings with mosapride citrate, a prokinetic agent with 5-HT4 agonistic and 5-HT3 antagonistic properties [55]. Two hundred NERD patients were randomized to omeprazole (10 mg o.d.) or omeprazole plus mosapride (5 mg t.i.d.) for 4 weeks, but no difference in symptom control was reported. Taken together, these data do not support the addition of a prokinetic agent to PPI therapy in patients with PPI-persistent GERD symptoms.
Raft-forming agents
A recent observation of great interest is that gastroesophageal reflux, in particular postprandial reflux, originates from a reservoir of gastric acid floating on top of the meal [56, 57]. This ‘acid pocket’ of newly secreted gastric juice does not mix with the meal and can be detected at the esophagogastric junction within 20 minutes after eating making it a novel target to reduce postprandial heartburn [58, 59].
Alginates, i.e. anionic polysaccharides widely distributed in the cell wall of brown seaweed, have been shown to form a floating raft in the presence of acid and calcium ions in vitro. This raft potentially creates a physical barrier against reflux or increases the viscosity of refluxate potentially impeding gastroesophageal reflux. Alginates are currently widely used in combination with an antacid as over-the-counter medication for heartburn and have proven effective in reducing heartburn and esophageal acid exposure time in GERD patients [60]. In line with these observations, Rohof et al. recently demonstrated that alginates co-localize with the acid pocket when administered after the meal and reduce acid reflux episodes and acid exposure more effectively than antacid in GERD patients [61]. These data suggest that alginates have potential to reduce PPI resistant reflux by targeting the acid pocket. Manabe et al. randomly assigned 76 NERD patients to omeprazole 20 mg daily or omeprazole plus sodium alginate [62]. Complete resolution of heartburn for at least 7 consecutive days by the end of 4 weeks treatment was significantly better in the group receiving the combination (57%) compared to omeprazole alone (26%). Although further confirmation is required, this study suggests that alginates may be effective in relieving PPI resistant symptoms. In practice, GERD patients may already apply this strategy, as evidenced by a recent US community-based survey, showing that 42% of patients on PPI use other GERD medication, including over-the-counter medications [63].
Reflux inhibitors
Even though the number of acidic reflux episodes is reduced by PPI treatment, the total number of reflux events is unaffected [64]. Hence, most reflux events detected in GERD patients while taking PPIs are weakly acidic [65]. Given that weakly acidic reflux can cause symptoms, an ideal GERD treatment should reduce both acidic and weakly acidic reflux events. One strategy to achieve this goal is by interfering with the main mechanism underlying gastroesophageal reflux, transient lower esophageal sphincter relaxations (TLESRs). TLESRs are prolonged relaxations of the LES accompanied with inhibition of the crural diaphragm and esophageal shortening, facilitating reflux; they are the physiological mechanism of belching. Interestingly, the gamma-amino butyric acid (GABA) B receptor agonist baclofen not only reduces TLESRs in animal models and humans, but also decreased the number of acidic and weakly acidic reflux events in both healthy volunteers and GERD patients [8]. However, as baclofen treatment can lead to dizziness and sleepiness, more potent and peripherally acting GABAB agonists or prodrugs of baclofen were developed to avoid these central side effects. Unfortunately, although these compounds reduced TLESRs by approximately 30–40%, lack of clinical efficacy and side effects (liver toxicity, central side effects) led to their withdrawal [8, 66, 67] and currently no reflux inhibitors remain in active development programs. If tolerated though, adding baclofen to PPI treatment still represents a valuable therapeutic approach for PPI resistant symptoms [68].
Low dose antidepressants
Antidepressants may modulate esophageal sensitivity at the central nervous system and/or sensory afferents level, potentially benefitting symptomatic patients. Specifically, low dose tricyclic antidepressants have been effective in patients with chest pain that was only partially responsive to PPIs [69]. Similarly, trazodone, a serotonin reuptake inhibitor, was more effective than placebo in patients with esophageal symptoms (chest pain, dysphagia, heartburn, and/or regurgitation) associated with esophageal contraction abnormalities [70]. Citalopram, another selective serotonin reuptake inhibitor, significantly increased the threshold for perception and discomfort after balloon distension in healthy volunteers [71]. Citalopram also prolonged the duration of esophageal acid perfusion required to induce heartburn. Consequently, these medications may be useful adjunctive therapy in the subset of GERD patients with hypersensitivity. However, thus far, there have been no large studies that evaluate antidepressants in GERD patients.
Non-pharmacological Approaches
Acupuncture and hypnotherapy have also been proposed as therapeutic alternatives for GERD. In one series of 30 GERD patients who failed PPI once daily, adding acupuncture (in an uncontrolled study) was significantly better in controlling acid regurgitation and heartburn than was doubling the PPI dose [72]. Response to PPI treatment is also modulated by psychological distress [73]. Consequently, reducing psychological distress may be beneficial in a subset of patients with an inadequate response to PPI. Hypnotherapy has been proposed as such an alternative therapy, especially for patients with atypical GERD symptoms. In a randomized trial including 28 patients with non-cardiac chest pain, patients treated with hypnotherapy experienced a global improvement in pain more frequently than did controls (80% vs 23%). Similarly, in a case series of patients with globus sensation, hypnotherapy appeared to be a beneficial intervention [74]. It remains to determine if this alternative is effective in larger series of patients with GERD-associated functional symptoms.
Surgical fundoplication
High quality evidence on the efficacy of antireflux surgery exists only for esophagitis and/or excessive distal esophageal acid exposure determined by pH monitoring without ongoing PPI therapy [1]. In that scenario, controlled trials have shown antireflux surgery to be at least as effective as PPI therapy in controlling heartburn and acid regurgitation. The best example of this is the recently published LOTUS trial, a large randomized European trial comparing laparoscopic antireflux surgery to esomeprazole treatment for patients with chronic GERD [75]. The diagnosis of GERD was established on the basis of typical symptoms and presence of esophageal mucosal breaks at endoscopy and/or a pathological pH monitoring study. Only patients with clinical response to esomeprazole during a 3-month run-in period were randomized. Over 3 years of follow up, both treatments were similarly effective in achieving complete symptom remission. At 5 years, the remission rates were greater in the esomeprazole group than in the laparoscopic fundoplication group (92% vs 85%, p=0.048) [76]. Differences were also observed when analyzed by specific symptoms. Specifically, regurgitation was significantly worse in the medical group than in the surgical group (13% vs 2% respectively, p<0.001) while there was no significant difference between the groups in heartburn severity. Dysphagia, bloating and flatulence were all significantly more common in the fundoplication group than in the PPI group. Consequently the potential benefits of antireflux surgery should be weighed against the deleterious effect of new symptoms consequent from surgery, particularly dysphagia, flatulence, an inability to belch, and post surgery bowel symptoms.
Another practical limitation of antireflux surgery is that it is acknowledged to be highly operator dependent. Efficacy data from community practice [77] are widely divergent from those of the LOTUS trial with as many as 30% of patients resuming PPI therapy within 5 years of surgery. Revision fundoplication surgery is also common accounting for up to 50% of operations performed at some referral centers [78]. Hence, antireflux surgery should be recommended with restraint. Patients with esophagitis who are intolerant of PPIs will likely benefit from antireflux surgery. Conversely, esophagitis patients who are well maintained on PPIs have nothing to gain from antireflux surgery and incur added risk. Patients with esophageal GERD symptoms poorly controlled by PPIs may benefit from surgery especially in the setting of persistent regurgitation. Even so, the indication must be balanced with the risk of surgery and patients need to be advised of potential dysphagia, inability to belch, flatulence, and bowel symptoms.
Summary
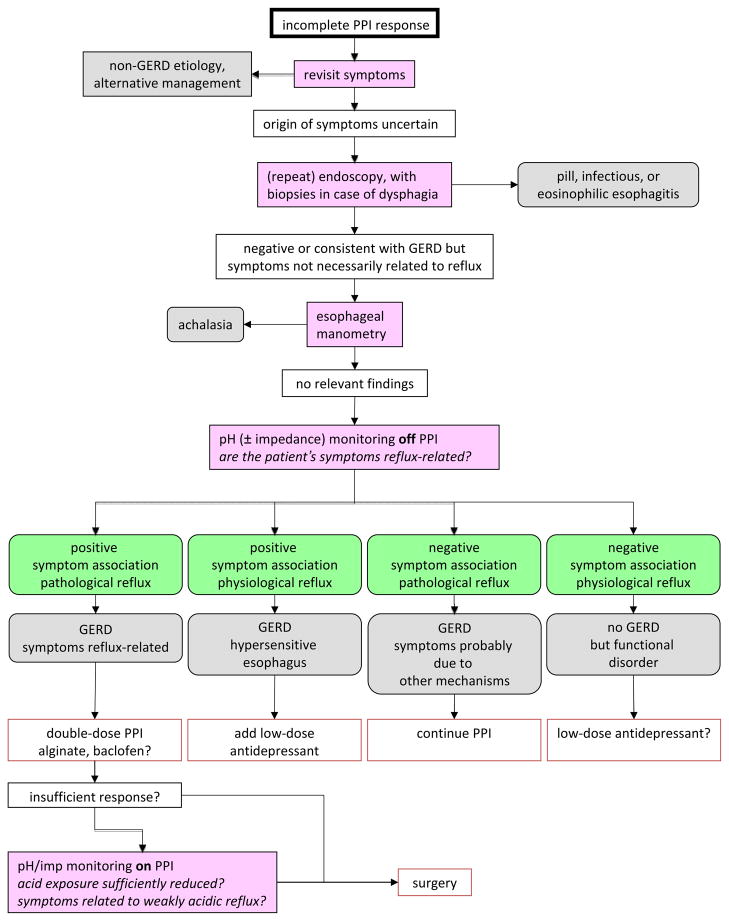
Reflux disease is caused by physiological dysfunction of the EGJ leading to excessive reflux of gastric secretions into the esophagus. Esophagitis can be a direct consequence of this. Hence, reducing gastric acid secretion with PPIs is very effective in esophagitis healing. However, PPIs do not eliminate reflux and the response of specific GERD symptoms to PPI therapy is dependent of the degree to which those symptoms are related to acid. PPIs are most effective for the symptom of heartburn but progressively less so for regurgitation, chest pain and extra-esophageal symptoms. However, even in the case of heartburn, PPI efficacy is substantially less than for healing esophagitis. Hence, with an incomplete response to PPI therapy, a fundamental question is whether or not the persistent symptoms are attributable to reflux. Obtaining an accurate history, detailing which symptoms are ‘refractory’ and exactly what evidence exists linking these symptoms to GERD is of paramount importance. Even within the spectrum of typical esophageal symptoms, persistent heartburn, regurgitation or chest pain may alternatively be related to esophagitis of another etiology (eosinophilic, infectious, pill), severe dysmotility, rumination, functional heartburn, or functional chest pain. Within the spectrum of GERD, reflux can continue to cause symptoms despite PPI therapy because of persistent acid reflux or symptoms as a result of weakly acidic reflux. Given this wide array of possibilities, and the reality that there is no longer a ‘one size fits all’ therapeutic strategy, diagnostic testing with pH or pH-impedance monitoring now becomes an essential clinical tool. Reducing the occurrence of reflux can be an important therapeutic target, especially in patients with persistent regurgitation on PPI therapy. Antireflux surgery is the main alternative in these patients if a clear relationship is established between persistent symptoms, particularly regurgitation, and reflux. Finally treating visceral hypersensitivity may be beneficial in the subset of GERD patients whose symptoms are driven by this mechanism. Figure 3 details an algorithm for managing patients with PPI refractory symptoms.
Figure 3.
Algorithm for management of the patient with incomplete response to PPI therapy. Since the pivotal question in ‘therapy-refractory GERD’ is whether or not the symptoms are attributable to reflux, pH/impedance monitoring plays a central role in the evaluation. The chance of finding a positive correlation between symptoms and reflux events is greatest when the reflux monitoring is carried out ‘off’ acid inhibition therapy. Antireflux surgery is best restricted to patients with pathological reflux and a positive symptom correlation.
Practice Points.
- Patients with a partial response to PPI therapy may have refractory symptoms attributable to persistent reflux or to factors unrelated to reflux disease
- Revisiting the clinical history to characterize symptoms and weigh the evidence that they are even GERD related is of paramount importance
- Reflux testing (pH-metry or pH-impedance monitoring) is essential to phenotype patients with persistent symptoms into those with refractory reflux symptoms vs functional symptoms vs hypersensitivity
Research Agenda.
- Understanding, characterizing, and treating esophageal hypersensitivity
- Characterizing the role of reflux in the domain of extra-esophageal syndromes
- Managing functional heartburn and functional dyspepsia
Acknowledgments
Dr Kahrilas was supported by grant R01 DC00646 from the United States Public Health Service.
Role of the funding source
None.
Footnotes
Potential conflict of interest
P.J. K serves as a paid consultant for AstraZeneca, Ironwood Pharmaceuticals, Reckitt Benckiser, Glaxo Smith Kline, and Torax. A.J.P.M.S. serves as a paid consultant for Reckitt Benckiser and has received financial support for educational meetings from MMS, Given Imaging and Shire Movetis. G.B. is supported by grants from the Research Foundation - Flanders (FWO) (Odysseus program, G.0905.07) and the agency for Innovation by Science and Technology (IWT), Belgium and has received grant support from Reckitt Benckiser.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1*.Kahrilas PJ, Shaheen NJ, Vaezi M in collaboration with the AGAI Medical Position Panel on GERD management. AGAI medical position statement: management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383–1391. doi: 10.1053/j.gastro.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 2.Numans ME, Lau J, de Wit NJ, Bonis PA. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med. 2004;140:518–27. doi: 10.7326/0003-4819-140-7-200404060-00011. [DOI] [PubMed] [Google Scholar]
- 3.Kahrilas PJ. Treatment vs management of gastroesophageal reflux disease (editorial) Am J Gastroenterol. 1997;92:1959–1960. [PubMed] [Google Scholar]
- 4*.Vakil N, Veldhuyzen van Zanten S, Kahrilas P, et al. The Montreal definition and classification of gastro-esophageal reflux disease (GERD) – a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 5.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–11. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 6.Inadomi JM, McIntyre L, Bernard L, et al. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol. 2003;98:1940–4. doi: 10.1111/j.1572-0241.2003.07665.x. [DOI] [PubMed] [Google Scholar]
- 7.Dean BB, Gano AD, Jr, Knight K, et al. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–64. doi: 10.1016/s1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 8*.Kahrilas PJ, Boeckxstaens G. Failure of reflux inhibitors in clinical trials: bad drugs or wrong patients? Gut. 2012;61:1501–1509. doi: 10.1136/gutjnl-2011-301898. [DOI] [PubMed] [Google Scholar]
- 9.Jones MP, Sloan SS, Rabine JC, Huang CF, Kahrilas PJ. Hiatal hernia size is the dominant determinant of esophagitis presence and severity in gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:1711–1717. doi: 10.1111/j.1572-0241.2001.03926.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin S, Ke M, Xu J, Kahrilas PJ. Impaired esophageal emptying in reflux disease. Amer J Gastroenterol. 1994;89:1003–1006. [PubMed] [Google Scholar]
- 11.Gillen P, Keeling P, Byrne PJ, Hennessy TP. Barrett’s oesophagus: pH profile. Br J Surg. 1987;74:774–6. doi: 10.1002/bjs.1800740906. [DOI] [PubMed] [Google Scholar]
- 12.Singh P, Adamopoulos A, Taylor RH, Colin-Jones DG. Oesophageal motor function before and after healing of oesophagitis. Gut. 1992;33:1590–6. doi: 10.1136/gut.33.12.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittal RK, Lange RC, McCallum RW. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology. 1987;92:130–5. doi: 10.1016/0016-5085(87)90849-3. [DOI] [PubMed] [Google Scholar]
- 14.Sloan S, Kahrilas PJ. Impairment of esophageal emptying with hiatal hernia. Gastroenterology. 1991;100:596–605. doi: 10.1016/0016-5085(91)80003-r. [DOI] [PubMed] [Google Scholar]
- 15.Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139(3):754–62. doi: 10.1053/j.gastro.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Clouse RE, Lustman PJ. Use of psychopharmacological agents for functional gastrointestinal disorders. Gut. 2005;54:1332–1341. doi: 10.1136/gut.2004.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006;116(2):254–60. doi: 10.1097/01.mlg.0000192173.00498.ba. [DOI] [PubMed] [Google Scholar]
- 18.Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1419–25. doi: 10.1038/ajg.2011.146. [DOI] [PubMed] [Google Scholar]
- 19.Kahrilas PJ, Jonsson A, Denison H, et al. Regurgitation is less responsive to acid suppression than heartburn in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:612–9. doi: 10.1016/j.cgh.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Kahrilas PJ, Hughes N, Howden CW. Response of unexplained chest pain to proton pump inhibitor treatment in patients with and without objective evidence of gastrooesophageal reflux disease. Gut. 2011;60:1473–1478. doi: 10.1136/gut.2011.241307. [DOI] [PubMed] [Google Scholar]
- 21.Chang AB, Lasserson TJ, Gaffney J, Connor FL, Garske LA. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD004823. doi: 10.1002/14651858.CD004823.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahrilas PJ, Howden CW, Hughes N, Molloy-Bland M. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest. 2013;143(3):605–612. doi: 10.1378/chest.12-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickman R, Boaz M, Aizic S, et al. Comparison of clinical characteristics of patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy versus those who fully responded. J Neurogastroenterol Motil. 2011 Oct;17(4):387–94. doi: 10.5056/jnm.2011.17.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bytzer P, van Zanten SV, Mattsson H, Wernersson B. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis - a post hoc analysis of 5796 patients. Aliment Pharmacol Ther. 2012;36(7):635–43. doi: 10.1111/apt.12007. [DOI] [PubMed] [Google Scholar]
- 25.Aanen MC, Numans ME, Weusten BL, Smout AJ. Diagnostic value of the Reflux Disease Questionnaire in general practice. Digestion. 2006;74:162–8. doi: 10.1159/000100511. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto M, Manabe N, Haruma K. Efficacy of the addition of prokinetics for proton pump inhibitor (PPI) resistant non-erosive reflux disease (NERD) patients: significance of frequency scale for the symptom of GERD (FSSG) on decision of treatment strategy. Intern Med. 2010;49:1469–76. doi: 10.2169/internalmedicine.49.3615. [DOI] [PubMed] [Google Scholar]
- 27.Kahrilas PJ, Jonsson A, Denison H, et al. Concomitant symptoms itemized in the Reflux Disease Questionnaire are associated with attenuated heartburn response to acid suppression. Am J Gastroenterol. 2012;107(9):1354–60. doi: 10.1038/ajg.2012.197. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein DR, Cash BD, Davila R, et al. Role of endoscopy in the management of GERD. Gastrointest Endosc. 2007;66(2):219–24. doi: 10.1016/j.gie.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc. 2010;71(1):28–34. doi: 10.1016/j.gie.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Shaheen NJ, Denison H, Bjöck K, Silberg DG. Esophageal mucosal breaks in gastroesophageal reflux disease partially responsive to proton pump inhibitor therapy. Am J Gastroenterol. 2013;108(4):529–34. doi: 10.1038/ajg.2012.447. [DOI] [PubMed] [Google Scholar]
- 31.Ismail-Beigi F, Horton PF, Pope CE. Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58(2):163–74. [PubMed] [Google Scholar]
- 32.Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111(5):1200–5. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 33.Vela MF, Craft BM, Sharma N, Freeman J, Hazen-Martin D. Refractory heartburn: comparison of intercellular space diameter in documented GERD vs. functional heartburn. Am J Gastroenterol. 2011;106(5):844–50. doi: 10.1038/ajg.2010.476. [DOI] [PubMed] [Google Scholar]
- 34.Pandolfino JE, Schreiner MA, Lee TJ, et al. Comparison of the Bravo wireless and Digitrapper catheter-based pH monitoring systems for measuring esophageal acid exposure. Am J Gastroenterol. 2005;100(7):1466–76. doi: 10.1111/j.1572-0241.2005.41719.x. [DOI] [PubMed] [Google Scholar]
- 35.Hakanson BS, Berggren P, Granqvist S, Ljungqvist O, Thorell A. Comparison of wireless 48-h (Bravo) versus traditional ambulatory 24-h esophageal pH monitoring. Scand J Gastroenterol. 2009;44(3):276–83. doi: 10.1080/00365520802588109. [DOI] [PubMed] [Google Scholar]
- 36.Wiener GJ, Richter JE, Copper JB, Wu WC, Castell DO. The symptom index: a clinically important parameter of ambulatory 24-hour esophageal pH monitoring. Am J Gastroenterol. 1988;83(4):358–61. [PubMed] [Google Scholar]
- 37.Weusten BL, Roelofs JM, Akkermans LM, Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology. 1994;107(6):1741–5. doi: 10.1016/0016-5085(94)90815-x. [DOI] [PubMed] [Google Scholar]
- 38.Galmiche JP, Clouse RE, Balint A, et al. Functional esophageal disorders. Gastroenterology. 2006;130(5):1459–65. doi: 10.1053/j.gastro.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 39.Sifrim D, Holloway R, Silny J, et al. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24-hour pH-impedance recordings. Gastroenterology. 2001;120(7):1588–98. doi: 10.1053/gast.2001.24841. [DOI] [PubMed] [Google Scholar]
- 40*.Bredenoord AJ, Weusten BL, Curvers WL, Timmer R, Smout AJ. Determinants of perception of heartburn and regurgitation. Gut. 2005;55(3):313–8. doi: 10.1136/gut.2005.074690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Hemmink GJ, Bredenoord AJ, Weusten BL, et al. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: ‘on’ or ‘off’ proton pump inhibitor? Am J Gastroenterol. 2008;103(10):2446–53. doi: 10.1111/j.1572-0241.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 42.Kessing BF, Bredenoord AJ, Smout AJ. Erroneous diagnosis of gastroesophageal reflux disease in achalasia. Clin Gastroenterol Hepatol. 2011;9:1020–24. doi: 10.1016/j.cgh.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 43.Ponce J, Ortiz V, Maroto N, et al. High prevalence of heartburn and low acid sensitivity in patients with idiopathic achalasia. Dig Dis Sci. 2011;56(3):773–6. doi: 10.1007/s10620-010-1343-x. [DOI] [PubMed] [Google Scholar]
- 44*.Bredenoord AJ, Tutuian R, Smout AJ, Castell DO. Technology review: Esophageal impedance monitoring. Am J Gastroenterol. 2007;102(1):187–94. doi: 10.1111/j.1572-0241.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 45.Hatlebakk JG, Katz PO, Camacho-Lobato L, Castell DO. Proton pump inhibitors: better acid suppression when taken before a meal than without a meal. Aliment Pharmacol Ther. 2000;14:1267–72. doi: 10.1046/j.1365-2036.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- 46.Gunaratnam NT, Jessup TP, Inadomi J, Lascewski DP. Sub-optimal proton pump inhibitor dosing is prevalent in patients with poorly controlled gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2006;23:1473–7. doi: 10.1111/j.1365-2036.2006.02911.x. [DOI] [PubMed] [Google Scholar]
- 47.Orlando RC, Liu S, Illueca M. Relationship between esomeprazole dose and timing to heartburn resolution in selected patients with gastroesophageal reflux disease. Clin Exp Gastroenterol. 2010;3:117–25. doi: 10.2147/CEG.S12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fass R, Sontag SJ, Traxler B, Sostek M. Treatment of patients with persistent heartburn symptoms: a double-blind, randomized trial. Clin Gastroenterol Hepatol. 2006;4:50–6. doi: 10.1016/s1542-3565(05)00860-8. [DOI] [PubMed] [Google Scholar]
- 49.Dent J, Kahrilas PJ, Hatlebakk J, et al. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. American J Gastroenterol. 2008;103:20–6. doi: 10.1111/j.1572-0241.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 50.Kahrilas PJ, Dent J, Lauritsen K, et al. A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol. 2007;5:1385–91. doi: 10.1016/j.cgh.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Leite LP, Johnston BT, Just RJ, Castell DO. Persistent acid secretion during omeprazole therapy: a study of gastric acid profiles in patients demonstrating failure of omeprazole therapy. Am J Gastroenterol. 1996;91:1527–31. [PubMed] [Google Scholar]
- 52.Mainie I, Tutuian R, Castell DO. Addition of a H2 receptor antagonist to PPI improves acid control and decreases nocturnal acid breakthrough. J Clin Gastroenterol. 2008;42:676–9. doi: 10.1097/MCG.0b013e31814a4e5c. [DOI] [PubMed] [Google Scholar]
- 53.Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 2002;122:625–32. doi: 10.1053/gast.2002.31876. [DOI] [PubMed] [Google Scholar]
- 54.Vigneri S, Termini R, Leandro G, et al. A comparison of five maintenance therapies for reflux esophagitis. N Eng J Med. 1995;333:1106–10. doi: 10.1056/NEJM199510263331703. [DOI] [PubMed] [Google Scholar]
- 55.Miwa H, Inoue K, Ashida K, et al. Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non-erosive reflux disease - a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:323–32. doi: 10.1111/j.1365-2036.2010.04517.x. [DOI] [PubMed] [Google Scholar]
- 56.Fletcher J, Wirz A, Young J, Vallance R, McColl KE. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology. 2001;121:775–83. doi: 10.1053/gast.2001.27997. [DOI] [PubMed] [Google Scholar]
- 57.Beaumont H, Bennink RJ, de Jong J, Boeckxstaens GE. The position of the acid pocket as a major risk factor for acidic reflux in healthy subjects and patients with GORD. Gut. 2010;59:441–51. doi: 10.1136/gut.2009.178061. [DOI] [PubMed] [Google Scholar]
- 58.Rohof WO, Bennink RJ, de Ruigh AA, et al. Effect of azithromycin on acid reflux, hiatus hernia and proximal acid pocket in the postprandial period. Gut. 2012;61:1670–7. doi: 10.1136/gutjnl-2011-300926. [DOI] [PubMed] [Google Scholar]
- 59.Kahrilas PJ, McColl K, Fox M, et al. The acid pocket: a target for treatment in reflux disease? Am J Gastroenterol. 2013 Apr 30; doi: 10.1038/ajg.2013.132. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60.Savarino E, de Bortoli N, Zentilin P, et al. Alginate controls heartburn in patients with erosive and nonerosive reflux disease. World J Gastroenterol. 2012;18:4371–8. doi: 10.3748/wjg.v18.i32.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohof WO, Bennink RJ, Smout AJ, Thomas E, Boeckxstaens GE. An Alginate-Antacid Formulation Localizes to the Acid Pocket to Reduce Acid Reflux in Patients with Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2013 May 10; doi: 10.1016/j.cgh.2013.04.046. pii: S1542-3565(13)00621-6 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 62.Manabe N, Haruma K, Ito M, et al. Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: a randomized clinical trial. Dis Esoph. 2012;25:373–80. doi: 10.1111/j.1442-2050.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- 63.Chey WD, Mody RR, Wu EQ, et al. Treatment patterns and symptom control in patients with GERD: US community-based survey. Current medical research and opinion. 2009;25:1869–78. doi: 10.1185/03007990903035745. [DOI] [PubMed] [Google Scholar]
- 64.Vela MF, Tutuian R, Katz PO, Castell DO. Baclofen decreases acid and non-acid postprandial gastro-oesophageal reflux measured by combined multichannel intraluminal impedance and pH. Aliment Pharmacol Ther. 2003;17:243–51. doi: 10.1046/j.1365-2036.2003.01394.x. [DOI] [PubMed] [Google Scholar]
- 65.Boeckxstaens GE, Smout A. Systematic review: role of acid, weakly acidic and weakly alkaline reflux in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2010;32:334–43. doi: 10.1111/j.1365-2036.2010.04358.x. [DOI] [PubMed] [Google Scholar]
- 66.Boeckxstaens GE, Beaumont H, Hatlebakk JG, et al. A novel reflux inhibitor lesogaberan (AZD3355) as add-on treatment in patients with GORD with persistent reflux symptoms despite proton pump inhibitor therapy: a randomised placebo-controlled trial. Gut. 2011;60:1182–8. doi: 10.1136/gut.2010.235630. [DOI] [PubMed] [Google Scholar]
- 67.Shaheen NJ, Denison H, Bjorck K, Karlsson M, Silberg DG. Efficacy and safety of lesogaberan in gastro-oesophageal reflux disease: a randomised controlled trial. Gut. 2012 doi: 10.1136/gutjnl-2012-302737. [DOI] [PubMed] [Google Scholar]
- 68.Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut. 2003;52:1397–402. doi: 10.1136/gut.52.10.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prakash C, Clouse RE. Long-term outcome from tricyclic antidepressant treatment of functional chest pain. Dig Dis Sci. 1999 Dec;44(12):2373–9. doi: 10.1023/a:1026645914933. [DOI] [PubMed] [Google Scholar]
- 70.Clouse RE, Lustman PJ, Eckert TC, Ferney DM, Griffith LS. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology. 1987;92(4):1027–36. doi: 10.1016/0016-5085(87)90979-6. [DOI] [PubMed] [Google Scholar]
- 71.Broekaert D, Fischler B, Sifrim D, Janssens J, Tack J. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2006;23(3):365–70. doi: 10.1111/j.1365-2036.2006.02772.x. [DOI] [PubMed] [Google Scholar]
- 72.Dickman R, Schiff E, Holland A, et al. Clinical trial: acupuncture vs. doubling the proton pump inhibitor dose in refractory heartburn. Aliment Pharmacol Ther. 2007;26(10):1333–44. doi: 10.1111/j.1365-2036.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- 73.Nojkov B, Rubenstein JH, Adlis SA, et al. The influence of co-morbid IBS and psychological distress on outcomes and quality of life following PPI therapy in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2008;27(6):473–82. doi: 10.1111/j.1365-2036.2008.03596.x. [DOI] [PubMed] [Google Scholar]
- 74.Kiebles JL, Kwiatek MA, Pandolfino JE, Kahrilas PJ, Keefer L. Do patients with globus sensation respond to hypnotically assisted relaxation therapy? A case series report. Dis Esoph. 2010;23(7):545–53. doi: 10.1111/j.1442-2050.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- 75.Lundell L, Attwood S, Ell C, et al. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut. 2008;57(9):1207–13. doi: 10.1136/gut.2008.148833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD. The LOTUS randomized clinical trial. JAMA. 2011;305:1969–77. doi: 10.1001/jama.2011.626. [DOI] [PubMed] [Google Scholar]
- 77.Vakil N, Shaw M, Kirby R. Clinical effectiveness of laparoscopic fundoplication in a U.S. community. Am J Med. 2003;114(1):1–5. doi: 10.1016/s0002-9343(02)01390-6. [DOI] [PubMed] [Google Scholar]
- 78.Hunter JG, Smith CD, Branum GD, et al. Laparoscopic fundoplication failures: patterns of failure and response to fundoplication revision. Ann Surg. 1999;230(4):595–604. doi: 10.1097/00000658-199910000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]