Sequential Regulation of DOCK2 Dynamics by Two Phospholipids During Neutrophil Chemotaxis (original) (raw)
. Author manuscript; available in PMC: 2013 Sep 4.
Published in final edited form as: Science. 2009 Mar 26;324(5925):384–387. doi: 10.1126/science.1170179
Abstract
During chemotaxis, activation of the small guanosine triphosphatase Rac is spatially regulated to organize the extension of membrane protrusions in the direction of migration. In neutrophils, Rac activation is primarily mediated by DOCK2, an atypical guanine nucleotide exchange factor. Upon stimulation, we found that DOCK2 rapidly translocated to the plasma membrane in a phosphatidylinositol 3,4,5-trisphosphate–dependent manner. However, subsequent accumulation of DOCK2 at the leading edge required phospholipase D–mediated synthesis of phosphatidic acid, which stabilized DOCK2 there by means of interaction with a polybasic amino acid cluster, resulting in increased local actin polymerization. When this interaction was blocked, neutrophils failed to form leading edges properly and exhibited defects in chemotaxis. Thus, intracellular DOCK2 dynamics are sequentially regulated by distinct phospholipids to localize Rac activation during neutrophil chemotaxis.
Chemotaxis regulates a wide range of biological functions, including developmental morphogenesis, wound healing, and immune responses (1). During chemotaxis, filamentous actin (F-actin) polymerizes asymmetrically at the leading edge of the cell, providing the force necessary to extend membrane protrusions in the direction of migration (1, 2). This morphologic polarity is regulated by Rac, a member of the small guanosine triphosphatases (GTPases) that cycle between inactive guanosine diphosphate (GDP)–bound and active guanosine triphosphate (GTP)–bound states (3). Rac is preferentially activated at the leading edge (1, 4, 5), which is achieved in part by regulating the subcellular localization of guanine nucleotide exchange factors (GEFs) (1, 3). The GEFs contain a variety of localization motifs such as pleckstrin homology (PH) domains and the DOCK homology region (DHR)–1, both of which bind to phosphatidylinositol 3,4,5-trisphosphate (PIP3) (3, 6), a lipid product of phosphoinositide 3-kinases (PI3Ks). Upon stimulation, PIP3 transiently accumulates at the plasma membrane edge facing the highest level of chemoattractant (1, 2). However, a functional leading edge is established even in neutrophils lacking PI3Kγ, the major generator of PIP3 in this cell type (7, 8). Thus, other factors may alternately suffice to localize Rac GEFs at the leading edge during neutrophil chemotaxis.
DOCK2 is a member of the CDM family of proteins (Caenorhabditis elegans, CED-5; mammals, DOCK180; and Drosophila melanogaster, Myoblast city) and is predominantly expressed in hematopoietic cells (9). Although DOCK2 does not contain the PH and Dbl homology domains typically found in GEFs, DOCK2 can bind to PIP3 through its DHR-1 domain (10) and mediates the GTP-GDP exchange reaction for Rac by means of its DHR-2 domain (11, 12). DOCK2 is a major Rac GEF that controls motility and polarity during neutrophil chemotaxis (10). In response to chemoattractants, neutrophils polarize and accumulate DOCK2 at the plasma membrane edge (fig. S1). To explore the mechanism controlling intracellular DOCK2 dynamics, we first analyzed the role of PIP3 by crossing PI3Kγ−/− mice with mice that had been made by a “knock-in” strategy to express endogenous DOCK2 as a fusion protein with green fluorescent protein (GFP) (10, 13). When neutrophils from DOCK2-GFP mice were stimulated in suspension with chemotactic factors such as _N_-formyl-Met-Leu-Phe (fMLP) and C5a, DOCK2 rapidly translocated to the plasma membrane at 15 s in the presence, but not in the absence, of PI3Kγ (fig. S2). Thus, the initial membrane translocation of DOCK2 is mediated by PIP3. However, despite the absence of PI3Kγ expression, DOCK2 and F-actin nonetheless still accumulated preferentially at the pseudopod at later time points (fig. S2).
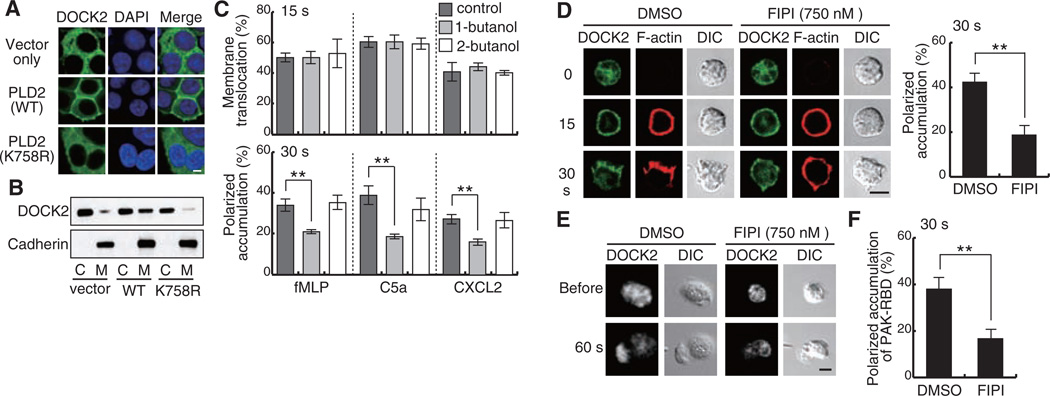
Phosphatidic acid (PA) is a negatively charged phospholipid that can function as a lipid anchor by binding directly to positively charged sites on effector proteins (14). In response to many types of external stimuli, signaling pools of PA are formed through hydrolysis of phosphatidylcholine (PC) by phospholipase D (PLD) or phosphorylation of diacylglycerol (DAG) by diacylglycerol kinase (DGK) (14, 15). Although PLD has been implicated in migratory responses of Dictyostelium discoideum, epithelial cells, and neutrophils (16–18), the mechanistic basis is largely unknown. To investigate whether subcellular localization of DOCK2 is influenced by PLD, GFP-tagged DOCK2 was expressed in human embryonic kidney (HEK) 293T cells with or without coexpression of PLD2, a PLD isoform that localizes primarily to the plasma membrane (19). DOCK2 was localized mainly in the cytosol when expressed alone; however, it readily accumulated at the plasma membrane when PLD2 was coexpressed (Fig. 1, A and B). In contrast, the catalytically inactive PLD2 mutant K758R in which Lys758 is replaced by Arg (20) failed to alter DOCK2 localization (Fig. 1, A and B). Thus, coexpressing PLD2 induces plasma membrane accumulation of DOCK2 through a mechanism dependent on its catalytic activity.
Fig. 1.
PA controls DOCK2 localization during neutrophil chemotaxis. (A and B) Effect of coexpressed PLD2 on localization of GFP-tagged DOCK2 in HEK293T cells. 4′,6′-Diamidino-2-phenylindole (DAPI) was used to stain nuclei. Membrane (M) and cytosol (C) fractions were analyzed by immunoblot. (C and D) Effect of 0.2% 1-butanol (C) or 750 nM FIPI (D) on DOCK2 localization was analyzed at 15 s or 30 s after stimulation with 10 µM fMLP (C), 25 nM C5a (C and D), or 5 nM CXCL2 (C). Dimethyl sulfoxide (DMSO), vehicle. DIC, differential interference contrast. (E) Effect of FIPI on DOCK2 localization in neutrophils stimulated with a micropipette containing 10 µM fMLP. (F) Effect of FIPI on localization of GFP-tagged PAK-RBD was analyzed 30 s after stimulation with 25 nM C5a. Data in (C), (D), and (F) are means ± SD of triplicate experiments, in each of which at least 50 cells were analyzed. **P < 0.01. Scale bar in (A), (D), and (E), 5 µm.
Primary alcohols such as 1-butanol compete with water in the hydrolysis of PC by PLD (15). To examine whether PLD-generated PA is involved in control of intracellular DOCK2 dynamics, we treated neutrophils of DOCK2-GFP mice with 1-butanol and, as a control, with 2-butanol. In response to fMLP, C5a, and CXCL2, 25 to 35% of neutrophils treated with 2-butanol exhibited polarized morphology with focused distribution of DOCK2. However, treatment with 1-butanol significantly inhibited accumulation of DOCK2 and F-actin at the pseudopods (Fig. 1C and fig. S3). Similar results were obtained when neutrophils were treated with 5-fluoro-2-indolyl des-clorohalopemide (FIPI), a PLD-specific inhibitor (21) (Fig. 1D), but not with the DGK inhibitor R59 022 (fig. S4). Although DOCK2 accumulated at the leading edge in control cells in response to a point source of fMLP, the majority of neutrophils treated with 1-butanol or FIPI displayed aberrant morphology with extremely thin lamellae and poorly focused DOCK2 distribution (Fig. 1E and fig. S5). Moreover, treatment with 1-butanol or FIPI impaired localization to the pseudopods of a fluorescent probe that detects activated Rac, the GFP-tagged Rac-binding domain (RBD) of p21-activated kinase (PAK) (22) (Fig. 1F and fig. S6). On the other hand, neither 1-butanol nor FIPI inhibited the initial membrane translocation of DOCK2 (Fig. 1, C and D). Thus, PA acts selectively in the late phase to control polarized DOCK2 localization during neutrophil chemotaxis.
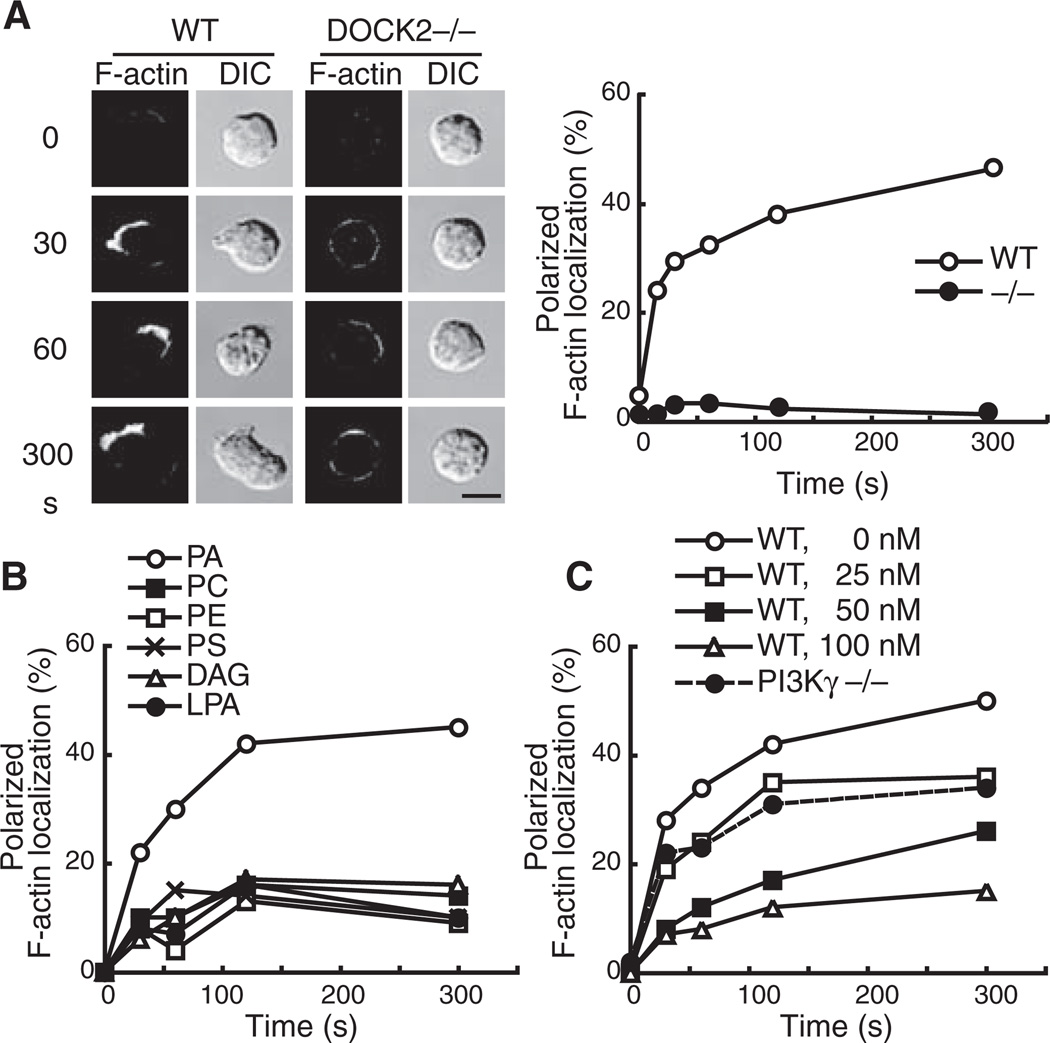
Exogenous PA added to culture medium is rapidly incorporated into the plasma membrane and can elicit cellular responses (23) (fig. S7). Wild-type (WT) neutrophils treated with PA exhibited polarized morphology with focused distribution of F-actin (Fig. 2A). This morphological change appeared to be mediated by PA itself, because other phospholipids including lysophosphatidic acid (LPA) and DAG, the two major metabolites of PA, failed to induce actin polymerization (Fig. 2B and fig. S8). The effect of PA was insensitive to pertussis toxin, an inhibitor of Gi and Go proteins (fig. S9), and PA addition did not increase phosphorylation of Akt, a downstream effector of PI3Ks (fig. S10). However, PA-induced DOCK2 accumulation and actin polymerization were attenuated by treating neutrophils with the PI3K inhibitor wortmannin (Fig. 2C and fig. S11), and the ability of PA to stimulate F-actin assembly and morphologic change was almost totally lost for DOCK2−/− neutrophils (Fig. 2A). Thus, PA induces actin polymerization in unstimulated neutrophils and makes “micro-polarity” visible in a manner dependent on both basal PI3K activity and DOCK2.
Fig. 2.
Exogenous PA induces actin polymerization through a mechanism dependent on DOCK2. (A) WT or DOCK2−/− neutrophils were stimulated with PA (10 µg/ml). Scale bar, 5 µm. (B) WT neutrophils were stimulated with varied phospholipids (10 µg/ml). (C) WT or PI3Kγ−/− neutrophils were stimulated with PA (10 µg/ml) at the indicated concentrations of wortmannin. At least 100 cells were analyzed in each experiment.
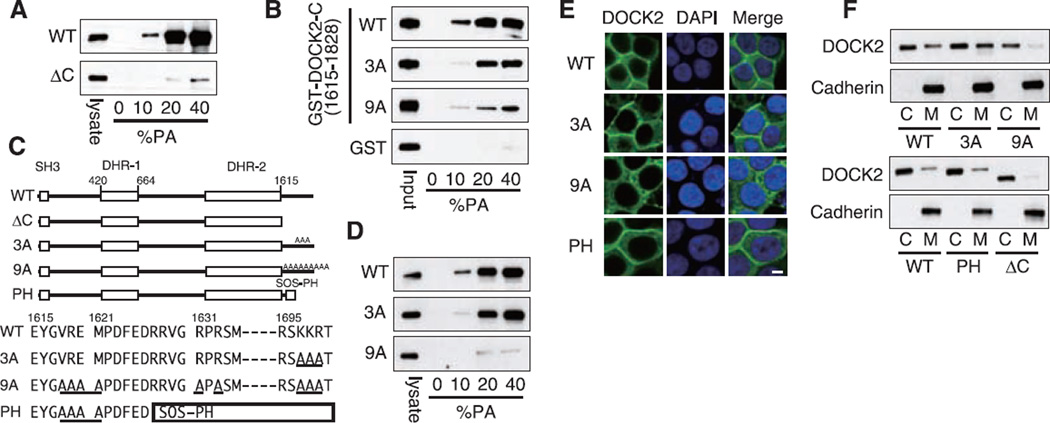
This finding led us to examine whether DOCK2 physically interacts with PA. Although no binding was found when HEK293T cell lysates containing DOCK2 were incubated with lipid vesicles composed solely of PC and phosphatidylethanolamine (PE), DOCK2 bound to PA-containing vesicles in a concentration-dependent manner (Fig. 3A). The PA binding was almost totally abolished by deleting the C-terminal 214 amino acid residues (1615 to 1828) (DOCK2-ΔC) (Fig. 3A), and the C-terminal fragment bound in vitro to lipid vesicles containing PA (Fig. 3B) but not to other acidic phospholipids including PIP3, phosphatidylserine (PS), and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] (fig. S12). Thus, the C-terminal region of DOCK2 interacts directly and selectively with PA.
Fig. 3.
DOCK2 binds to PA through the C-terminal polybasic amino acid cluster. (A) Extracts from HEK293T cells expressing GFP-tagged DOCK2-WT or DOCK2-ΔC were pulled down with PA-containing lipid vesicles. (B) Glutathione _S_-transferase (GST) fusion proteins encoding the C-terminal fragment of DOCK2 or its mutants were pulled down with PA-containing lipid vesicles. (C) Schematic representation of DOCK2 mutants used in this study (20). The mutated amino acid residues are underlined. (D) The ability to bind to PA was assayed for DOCK2-WT and its mutants as in (A). (E and F) Localization of DOCK2-WT or its mutants was visualized in HEK293T cells overexpressing PLD2. Scale bar, 5 µm.
The only motif known for PA-binding proteins is the presence of one or more basic amino acid residues (14). Both mouse and human DOCK2 encode the amino acid sequence Ser-Lys-Lys-Arg (residues 1696 to 1699), which mediates PA binding for the yeast protein Opi1p (24). When the three basic residues were all mutated to Ala (designated 3A), PA binding was diminished, but only to a modest extent (Fig. 3B). However, by mutating six additional resides to Ala (designated 9A), we found that the binding capacity of the DOCK2 C-terminal fragment to PA decreased to 20% of the WT level (Fig. 3, B and C). Similar results were obtained when full-length DOCK2 protein bearing the 9A mutations (DOCK2-9A) was assayed for PA binding (Fig. 3D). Although the expression of DOCK2-9A in HEK293T cells induced Rac activation fully (fig. S13), the DOCK2-9A failed to localize to the plasma membrane even when PLD2 was coexpressed (Fig. 3, E and F). In contrast, when the C-terminal region of DOCK2 was replaced with a known PA-binding module, the PH domain of Son of sevenless (SOS) (25) (Fig. 3C and fig. S14), this chimeric molecule (DOCK2-PH) accumulated effectively at the plasma membrane in the presence of PLD2 (Fig. 3, E and F). Thus, PA controls subcellular localization of DOCK2 by directly binding to the C-terminal polybasic amino acid cluster.
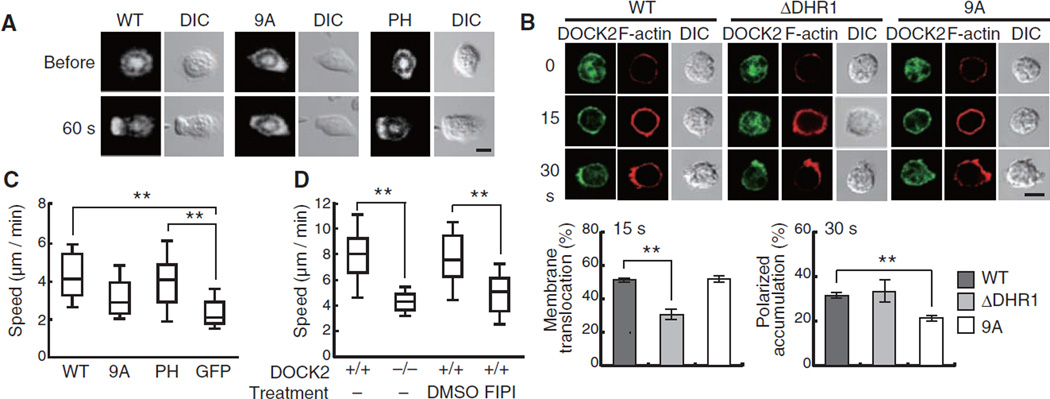
We next examined whether the DOCK2-PA interaction is functionally important by expressing GFP-tagged DOCK2-WT, DOCK2-9A, or DOCK2-PH in DOCK2−/− neutrophils. Although more than 85% of DOCK2−/− neutrophils expressing DOCK2-WT or DOCK2-PH extended lamellipodia containing accumulated DOCK2 toward the fMLP source, the expression of DOCK2-9A only partially restored the leading edge formation (Fig. 4A). Because GFP-tagged SOS-PH localized to the leading edge even in DOCK2−/− neutrophils (fig. S15), this defect appeared to result from the inability of PA to tether DOCK2-9A. Indeed, DOCK2-9A translocated normally to the plasma membrane, but accumulated less effectively at the pseudopods of WT neutrophils than did DOCK2-WT and a DOCK2 mutant lacking DHR-1 domain (DOCK2-ΔDHR1; Fig. 4B). When DOCK2-WTor DOCK2-PH was expressed in DOCK2−/− neutrophils, the migration speed increased by 75% or 65%, respectively, compared with that of the control expressing GFP alone (Fig. 4C). However, the expression of DOCK2-9A failed to significantly increase the motility of DOCK2−/− neutrophils (Fig. 4C). Consistent with this finding, treatment of WT neutrophils with the PLD inhibitor FIPI reduced the migration speed to a level almost comparable to that of the DOCK2−/− neutrophils (Fig. 4D).
Fig. 4.
The DOCK2-PA interaction is required to stabilize the leading edge during neutrophil chemotaxis. (A) DOCK2−/− neutrophils expressing GFP-tagged DOCK2 constructs were stimulated with a micropipette containing 10 µM fMLP. (B) WT neutrophils expressing GFP-tagged DOCK2 constructs were stimulated with C5a (25 nM). Data are means ± SD of triplicate experiments, in each of which at least 50 cells were analyzed. **P < 0.01. (C) After expression of GFP-tagged DOCK2 constructs or GFP alone, DOCK2−/− neutrophils were allowed to migrate in an EZ-Taxiscan chamber along an fMLP gradient (0 to 50 µM). The migration speed was analyzed for at least 24 GFP-positive cells that moved more than 20 µm over 15 min. **P < 0.01. (D) WT or DOCK2−/− neutrophils were allowed to migrate as in (C) in the presence or absence of FIPI (750 nM). The migration speed was analyzed for at least 40 cells. **P < 0.01. Scale bar in (A) and (B), 5 µm.
We showed here, during neutrophil chemotaxis, that intracellular DOCK2 dynamics were sequentially regulated by two distinct phospholipids: Although the plasma membrane translocation of DOCK2 was initially mediated by PIP3, PA acted at a late phase to focus DOCK2 localization and to stabilize the leading edge. This two-step regulation fits well with the kinetics of these phospholipids in response to chemoattractants (fig. S16). Lamellipodia formation in epithelial cells is mediated by DOCK180, another GEF, and is inhibited by 1-butanol (17, 26). The C-terminal region of DOCK180 also bound to PA (fig. S17), which suggested a common mechanism for its recruitment. Thus, PA may be involved in spatial regulation for Rac activation in varied biological settings by controlling localization of GEFs.
Supplementary Material
Suppl. Mat.
Acknowledgments
This work was supported by grants for Targeted Proteins Research Project, Genome Network Project, Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Toray Science Foundation; CREST program of Japan Science and Technology Agency; the Global Center of Excellence program of the Japan Society for the Promotion of Science; and NIH award R01GM71520.
Footnotes
References and Notes
- 1.Ridley AJ, et al. Science. 2003;302:1704. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Trends Cell Biol. 2000;10:466. doi: 10.1016/s0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt A, Hall A. Genes Dev. 2002;16:1587. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 4.Kraynov VS, et al. Science. 2000;290:333. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 5.Gardiner EM, et al. Curr. Biol. 2002;12:2029. doi: 10.1016/s0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- 6.Côté J-F, Motoyama AB, Bush JA, Vuori K. Nat. Cell Biol. 2005;7:797. doi: 10.1038/ncb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishio M, et al. Nat. Cell Biol. 2007;9:36. doi: 10.1038/ncb1515. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson GJ, et al. Nat. Cell Biol. 2007;9:86. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 9.Fukui Y, et al. Nature. 2001;412:826. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 10.Kunisaki Y, et al. J. Cell Biol. 2006;174:647. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brugnera E, et al. Nat. Cell Biol. 2002;4:574. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 12.Côté J-F, Vuori K. J. Cell Sci. 2002;115:4901. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 13.Materials and methods are available as supporting material on Science Online.
- 14.Stace CL, Ktistakis NT. Biochim. Biophys. Acta. 2006;1761:913. doi: 10.1016/j.bbalip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 15.McDermott M, Wakelam MJO, Morris AJ. Biochem. Cell Biol. 2004;82:225. doi: 10.1139/o03-079. [DOI] [PubMed] [Google Scholar]
- 16.Zouwail S, et al. Biochem. J. 2005;389:207. doi: 10.1042/BJ20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santy LC, Casanova JE. J. Cell Biol. 2001;154:599. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman N, et al. Blood. 2006;108:3564. doi: 10.1182/blood-2006-02-005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du G, Huang P, Liang BT, Frohman MA. Mol. Biol. Cell. 2004;15:1024. doi: 10.1091/mbc.E03-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Single-letter abbreviations for the amino acid residues are as follows: A, Ala; C, Cys; D, Asp; E, Glu; F, Phe; G, Gly; H, His; I, Ile; K, Lys; L, Leu; M, Met; N, Asn; P, Pro; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; W, Trp; and Y, Tyr.
- 21.Su W, et al. Mol. Pharmacol. 2009;75:437. doi: 10.1124/mol.108.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan S, et al. J. Cell Biol. 2003;160:375. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Science. 2001;294:1942. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 24.Loewen CJR, et al. Science. 2004;304:1644. doi: 10.1126/science.1096083. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Nat. Cell Biol. 2007;9:706. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 26.Santy LC, Ravichandran KS, Casanova JE. Curr. Biol. 2005;15:1749. doi: 10.1016/j.cub.2005.08.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Mat.