Oxidative Stress and Protein Carbonylation in Adipose Tissue - Implications for Insulin Resistance and Diabetes Mellitus (original) (raw)
. Author manuscript; available in PMC: 2014 Oct 30.
Published in final edited form as: J Proteomics. 2013 Apr 11;92:10.1016/j.jprot.2013.04.002. doi: 10.1016/j.jprot.2013.04.002
Abstract
While historically considered simply as a depot for excess energy, white adipose tissue is a dynamically active endocrine organ capable of responding to a variety of efferent stimuli resulting in the synthesis and secretion of peptides, proteins and metabolites that serve as signal transducers to the peripheral and central circulation. Such regulation controls a variety of physiological processes including energy expenditure, food intake, reproductive capacity and responsiveness to insulin. Indeed, accumulation of inflammatory cells in white adipose tissue is considered to be causative in the development of insulin resistance and eventually type 2 diabetes mellitus. A large body of evidence suggests that oxidative stress in adipose tissue not only correlates with insulin resistance but is also causative in its development. Moreover, using the available plasma oxidative stress biomarkers, many clinical studies have shown the presence of systemic oxidative stress in obese insulin resistant subjects, and its decrease after the successful treatment of obesity. In this review we emphasize the role of protein carbonylation in dysfunctional obese white adipose tissue and its metabolic implications. We focus on glutathione S-transferase A4 as the key enzyme for trans-4-hydroxy-2-nonenal and trans-4-oxo-2-nonenal removal from the cell, thus preventing protein carbonylation.
Keywords: Oxidative Stress, Reactive Oxygen Species, Protein Carbonylation, Glutathione S-transferase, Adipose Tissue, Insulin Resistance
Overweight and obesity leading to insulin resistance and type 2 diabetes mellitus
The prevalence of overweight (defined with body mass index which is greater than or equal to 25 kg/m2) and obesity (defined with body mass index which is greater than or equal to 30 kg/m2) has dramatically increased in the past few decades in both developed and developing countries. In 2008, more than 1.4 billion adults age of 20 and older were overweight. Of these, over 200 million men and nearly 300 million women were obese and more than 40 million children under the age of five were overweight [1]. Because of the low cost and ready availability of calorie-dense, but nutritionally poor processed foods, it is not unusual to meet both obesity and under-nutrition existing side-by-side in many low- and middle-income countries. Moreover, obesity is often followed by a lack of essential nutrients in low-income subgroups in developed countries [2, 3]. Although recognized by public and media as a primarily esthetic problem, overweight and obesity have serious health implications because there is strong evidence that raised body mass index is a major risk factor for non-communicable diseases such as: diabetes, cardiovascular diseases, musculoskeletal disorders (especially osteoarthritis), and some cancers [1].
Obesity may lead to insulin resistance [4] that is defined as a state of reduced responsiveness to normal circulating levels of insulin and is a key factor in the pathogenesis of Type 2 Diabetes Mellitus (T2DM) [5, 6]. In the insulin resistant but not diabetic state, islet β-cells have capacity to secrete sufficient insulin for maintenance of euglycemia resulting in hyperinsulinemia. As such, insulin resistance is often clinically overlooked when exclusively assessing fasting blood glucose or HbA1c levels as determinants. However, as the syndrome progresses β-cell function diminishes, resulting in a relative or absolute insulin deficiency. This in turn leads to the well known characteristics of T2DM including increased hepatic glucose output, diminished insulin secretion and peripheral insulin resistance [7].
Diabetes mellitus itself is one of the most challenging health problems globally in the 21st century. It is estimated that in 2011 about 366 million adults worldwide had diabetes, which is approximately 8.3% of the global adult population. It is also projected that by 2030 the number of people with diabetes will rise to 552 million [8]. Most of the patients diagnosed as diabetics will have T2DM. Data from the International Diabetes Federation indicates that type 2 diabetes accounts for at least 90% of all cases [9]. Whether the type 2 diabetes phenotype will occur depends on many factors, including genetic predisposition, previously mentioned obesity, sedentary lifestyle and/or β-cell cytotoxic factors [10, 11, 12]. Thus, the T2DM is considered as a heterogeneous cluster of conditions, rather than a uniform entity, with obesity induced insulin resistance being only one of the contributing factors. Complications from diabetes, such as coronary artery and peripheral vascular disease, stroke, diabetic neuropathy, renal failure, and blindness are a serious health problem for modern health system and society, resulting in increasing disability and reduced life expectancy.
Oxidative stress in obesity and insulin resistance
Recent evidence implicates a central role for oxidative stress and inflammation in obesity, insulin resistance, diabetes and its complications [13]. Oxidative stress is defined as a significant imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses. ROS are metabolites of molecular oxygen (O2) generated as by-products of normal aerobic metabolism, and consist of: superoxide radical (˙O2−), nitric oxide radical (˙NO), hydroxyl radical (˙OH−) and non-radicals such as hydrogen peroxide (H2O2) and peroxynitrite (ONOO−) [14]. Although usually regarded as toxic, ROS serve as mediators of signal transduction and are essential for normal physiology [15]. Moreover, they also have a protective function in the body, as in the case with the stimulation of ROS production by macrophages as an innate immune response to bacterial infection [16].
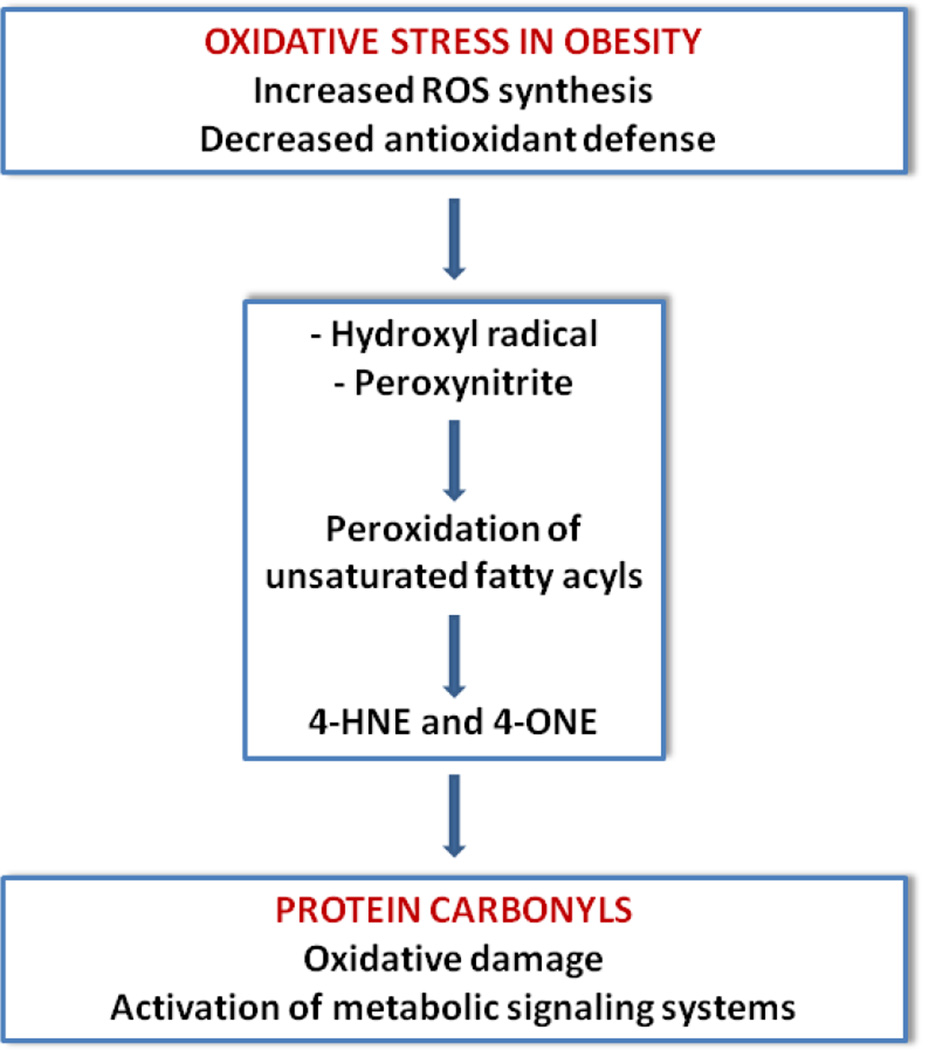
However, chronic exposure to excessive ROS levels can lead to direct oxidative modification of proteins, lipids, carbohydrates, and DNA, resulting in tissue damage. It is well known that hydroxyl radical and peroxynitrite can induce lipid peroxidation, attacking conjugated double bonds of membrane unsaturated fatty acids, primarily linoleic and arachidonic. Lipid peroxidation is autocatalytic and produces a chain reaction that simultaneously generates lipid hydroperoxides and aldehydes of various chain lengths. It is generally accepted that lipid peroxidation consists of three phases: initiation, propagation and termination. In the phase of initiation the reaction of hydroxyl radical or peroxynitrite with an unsaturated fatty acid results in generation of new, less reactive carbon-centered lipid radical (L˙). In the second phase the lipid radical reacts with molecular oxygen, which results in formation of lipid peroxyl radical (LOO˙). The lipid peroxyl radical has ability to abstract hydrogen from another unsaturated fatty acid, and both lipid hydroperoxide (LOOH) and new carbon-centered lipid radical are formed. Lipid hydroperoxides react with trace metals and form lipid alkoxyl radicals (LO˙). Both lipid peroxyl radicals and lipid alkoxyl radicals undergo cyclization and/or degradation, generating reactive aldehydes [91, 92] such as trans-4-hydroxy-2-nonenal (4-HNE), trans-4-oxo-2-nonenal (4-ONE), acrolein, malondialdehyde, glyoxal, crotonaldehyde, and 2-hexenal. The lipid peroxidation terminates when lipid peroxyl radical reacts with other radical or some non-radical antioxidant, such as vitamin E.
Two of the most reactive and certainly most well studied of the aldehyde products of lipid peroxidation are 4-HNE [17] and 4-ONE that are recognized as mediators and markers of the cellular dysfunction and degeneration in many disorders including obesity, insulin resistance and diabetes type 2. There is experimental evidence that in some systems 4-ONE is more reactive and more toxic than 4-HNE [93]. Although formed in membranes, as relatively high soluble compounds, 4-HNE and 4-ONE can diffuse into the cytoplasm and nucleus and react with a variety of proteins and DNA. In the case of proteins, 4-HNE and 4-ONE can be covalently bound to the sulfur atom of cysteine, imidizole nitrogen of histidine, or, to a lesser extent, the amine nitrogen of lysine residues by Michael addition reaction. This process is generically termed as secondary protein carbonylation and most typically leads to loss of protein function. However, there are examples when such modification results in activation of certain metabolic signaling systems [18] (Figure 1). For instance, there is experimental evidence that 4-HNE acts as an endogenous ligand for peroxisome proliferator-activated receptor delta (PPAR-δ) in pancreatic β-cells. More specifically, it has been demonstrated that treatment of cultured β-cells with glucose in high concentrations induces release of arachidonic and linoleic acid from membrane phospholipids, and increases concentration of 4-HNE in the cell culture media. Further experiments have demonstrated that 4-HNE increases insulin secretion through activation of PPAR-δ in pancreatic β-cells. Antioxidant N-acetylcysteine markedly reduces 4-HNE generation, blocks PPAR-δ activation, and abolishes the effect of high concentration of glucose on insulin secretion [102].
Figure 1. Mechanism of formation of adipocyte protein carbonyls.
Schematic representation protein carbonylation derived from increased oxidative stress leading to hydroxyl radical formation and production or reactive aldehydes such as 4-HNE and 4-ONE.
Apart from the secondary protein carbonylation, the so-called “primary protein carbonylation” [19] (or “direct protein carbonylation”), which refers to hydrogen peroxide and metal mediated oxidation of amino acid side chains, predominantly of lysine, arginine, proline and threonine, forming semialdehyde amino acids. Recent studies however suggest that protein carbonylation which results from addition of lipid derived aldehydes is more prevalent that direct carbonylation [18].
For maintenance of redox balance, cells have developed antioxidant defense that is comprised of enzymatic (superoxide dismutase, catalase, thioredoxin, glutathione peroxidase) and non-enzymatic (glutathione, ascorbate, α-tocopherol, resveratrol) compounds [14]. Lipid hydroperoxides can be reduced and thus reactive aldehydes formation prevented by peroxiredoxins and glutathione peroxidases. 4-HNE itself can be converted into carboxylic acid by (fatty) aldehyde dehydrogenase [94] and also into corresponding alcohol by alcohol dehydrogenase, aldehyde reductase, or aldose reductase. However, the major route for 4-HNE and 4-ONE removal is via glutathionylation by glutathione S-transferase (GST), i.e. via Michael addition of glutathione to the double bond of 4-HNE/4-ONE. There are few different isoforms of GST, and it is demonstrated that GSTA4 has the greatest specificity for 4-HNE/4-ONE [18, 20]. Once formed, glutathione/4-HNE and glutathione/4-ONE adducts are readily removed from the cell.
Notably, the mitochondrial electron transport chain is one of the major sites of ROS production within the cell. The transfer of electrons through the mitochondrial transport chain at sites other than Complex IV produces superoxide anions as primary ROS species. The potential deleterious effect of the superoxide anions is prevented by the mitochondrial manganese superoxide dismutase which converts them to the hydrogen peroxide which is further metabolized by the antioxidant enzymes catalase, glutathione peroxidase and peroxiredoxin. However, in the conditions of oxidative stress, and in the presence of ferrous ions, the hydrogen peroxide can accumulate and be oxidized to the hydroxyl radical, which can initiate the lipid peroxidation.
Clinical studies have shown presence of an increased systemic oxidative stress in obesity [21], which is further enhanced in association with abdominal adiposity [22], and which can be reduced by use of polyphenolic antioxidants [23, 24], hypocaloric diet and regular moderate aerobic exercise [25]. Moreover, there is a tendency for an increase of oxidative stress among obese subjects, in accordance with the severity of insulin resistance [26]. Plasma protein carbonyls, as one of the biomarkers of systemic oxidative stress are also found increased in obesity [27, 28], with a positive correlation to the insulin resistance [29], and significant decrease after different kinds of treatments of obese patients [30, 31].
Along with the oxidative stress, a chronic low-intensity inflammation is clinically evident in obese [32] and insulin resistant subjects [33], manifesting itself by increased values of the most commonly used plasma inflammatory biomarkers, such as C-reactive protein, tumor necrosis factor-α, interleukin-6 and fibrinogen. There is evidence that in patients with morbid obesity a significant weight lost is followed by improvement of the inflammatory state [34]. Even a moderate weight lost through dietary changes and physical exercise leads to improvement of blood inflammatory biomarkers in obese children [35].
However, there are still “metabolically healthy obese” or “insulin sensitive obese” subjects who are usually protected against T2DM and its complications, and who are “inappropriately healthy for their degree of obesity” [36]. Such individuals do not exhibit increased oxidative stress in visceral adipose tissue and maintain increased levels of antioxidant enzymes. It is estimated that in the USA about 30% of obese adults are actually metabolically healthy [37]. All these recent, and many other complementary data from numerous clinical studies, emphasize the need for extensive basic research of mechanisms involving oxidative stress and inflammation in the pathogenesis of obesity induced insulin resistance. Of particular interest is the research focused on the differences between obese insulin sensitive and obese insulin resistant subjects that may lead to discovery of new therapeutic approaches.
White adipose tissue in obesity and insulin resistance
For many years the white adipose tissue was considered as metabolically inactive, dedicated exclusively for fat storage. However, this classical concept has been completely abandoned over the past two decades.
The white adipose tissue, as the most abundant and most extensively studied form of adipose tissue in the human body [38], consists primarily of white adipocytes, but also macrophages, fibroblasts, endothelial cells, pre-adipocytes, and other types of cells. There are also stem cells from which the pre-adipocytes originate having potential to generate new adipocytes throughout the entire human life [39]. The white adipocytes have a unique ability to store the dietary energy in a highly concentrated form - triglycerides, that are found in the cell as a single lipid droplet. In response to lipolytic stimulation, either endocrine or from the sympathetic nervous system, adipocytes release stored energy mainly in the form of free fatty acids that can meet the energy requirements of the body during a state of increased physical activity, or supply it with energy in a fasting state.
Apart from the fat storage, the adipose tissue has many other important functions as an active endocrine organ under both physiological and pathophysiological conditions, playing one of the central roles in energy homeostasis and insulin sensitivity. Namely, adipose tissue produces a wide range of biologically active molecules, collectively known as adipokines, which are involved in the glucose metabolism (adiponectin, resistin), inflammation (tumor necrosis factor-α, interleukin-6), coagulation (plasminogen activator inhibitor-1), blood pressure regulation (angiotensinogen, angiotensin II), and feeding behavior (leptin), thus communicating with other central and peripheral organs and tissues, such as muscle, liver, nervous and vascular system, and affecting their metabolism and function [40]. Notably, the list of adipokines comprises of numerous signaling protein molecules, and is ever increasing [41, 42]. The functions of some of the adipokines are well studied. Thus, tumor necrosis factor-α and interleukin-6, which are not exclusively secreted by adipocytes, have pro-inflammatory effect, and are closely related to insulin resistance. On the other hand, adiponectin expression and secretion is unique to differentiated adipocytes. In contrast to other adipokines, adiponectin exerts an anti-inflammatory effect, and its plasma concentrations are decreased in obesity. By its complex influence on liver and muscle, adiponectin markedly improves insulin sensitivity. It also inhibits the adhesion of monocytes to endothelial cells, thus acting in an antiatherogenic manner. Tumor necrosis factor-α and interleukin-6 are potent inhibitors of adiponectin expression and secretion.
However, there is a process of “remodeling of the white adipose tissue” in obesity, which is characterized by adipocyte hypertrophy, macrophage and other immune cells infiltration, increased angiogenesis, and extracellular matrix overproduction [43]. This “remodeling” makes the white adipose tissue dysfunctional, with unbalanced production of pro- and anti-inflammatory cytokines, resulting in chronic low-intensity inflammatory state. Thus, along with the increased secretion of free fatty acids, the enlarged adipocytes of obese adipose tissue secrete high quantities of tumor necrosis factor-α and interleukin-6 and diminished levels of adiponectin, all of which lead to insulin resistance [40].
Infiltrated macrophages, and especially the adipocyte-macrophage interactions, have an important role in remodeling of the obese white adipose tissue. Namely, as a result of increased levels of chemokines in the obese white adipose tissue, an accumulation of macrophages occurs, which further participate in inflammatory pathways. In fact, there is a paracrine loop involving free fatty acids from adipocytes and tumor necrosis factor-α from macrophages, which establishes a vicious cycle that further accelerates the inflammation [44]. Recent evidence points out the existence of two types of macrophages in the white adipose tissue, referred as M1 – pro-inflammatory and M2 – anti-inflammatory, with occurrence of a phenotypic change from M2 to M1 in the obese adipose tissue [45].
There is also an ectopic lipid accumulation in obesity, particularly in the skeletal muscle [46] and liver [47], but in endothelial cells, heart and pancreas as well. This kind of lipid accumulation is a consequence of increased lipolysis and decreased capacity for free fatty acids accumulation in dysfunctional adipocytes from the obese adipose tissue [48], where the extracellular matrix overproduction (or the white adipose tissue fibrosis) also play a role. The ectopic lipid accumulation impairs the normal function of the affected cells, tissues and organs, contributing in the metabolic consequences of the obesity. It appears that lipid metabolites such as long-chain acyl-CoA, diacylglycerols and ceramides have more deleterious effect on affected cells and organs than the accumulated lipids itself. Ectopic lipids can be accumulated both intracellularly and intercellularly, but it is intracellular lipid accumulation that is associated with decreased insulin sensitivity.
And finally, there is a difference between the visceral and subcutaneous white adipose tissue. Apart from their different localization, significant structural and functional differences exist between them [49], which are manifested by differences in gene expression. Namely, in overweight subjects the visceral adipose tissue is enriched by inflammation and oxidative stress related pathways, while subcutaneous adipose tissue is enriched by those related to insulin homeostasis [50]. Moreover, in obese subjects, the number of infiltrated macrophages into the visceral adipose tissue is significantly higher than into the subcutaneous one [51].
However, visceral adipose tissue from healthy obese (insulin sensitive obese) subjects exhibits less pro-inflammatory profile compared to the insulin resistant obese subjects, which manifests itself by decreased macrophage infiltration and decreased expression of interleukin-6 and interleukin-1β [52].
Oxidative stress and protein carbonylation
Experimental data demonstrate a very close interplay between the white adipose tissue inflammation and its level of oxidative stress, although the causal relationship is not completely clear. Thus, in comparison to their weight-matched female counterparts, obese male mice exhibit an increased number of infiltrated immune cells in the white adipose tissue, as well as an increased expression of adipose tissue oxidative stress biomarkers [53]. Moreover, there is evidence that an increased physical activity decreases expression of inflammation-related adipokines with concomitant reduction of oxidative stress in rat white adipose tissue [54].
Adipose tissue oxidative stress in obesity has been studied extensively, especially in the past ten years [55]. One of the most comprehensive of the early experimental studies dealing with adipose tissue pathophysiology clearly demonstrates the presence of an increased oxidative stress in the adipose tissue of obese mice [56], although there are some opposite findings [57, 58]. It was further demonstrated that the pathways for ROS production are up-regulated in both the liver and adipose tissue of mice fed with high fat diet before the onset of insulin resistance, and preceded the elevation of tumor necrosis factor-α and free fatty acids in plasma and liver, suggesting that ROS might be an initial factor in obesity induced insulin resistance [59]. A significant decrease in expression of the antioxidant enzyme glutathione peroxidase isoform 3, and increased levels of total ROS and thiobarbituric acid-reactive substances were found in the white adipocytes of obese mice [60], and dysregulated glutathione metabolism leads to impaired insulin action in adipocytes [61]. There are also data that different kinds of standard and/or alternative treatments can reduce the oxidative stress in dysfunctional white adipose tissue [62, 63, 64] (Figure 2).
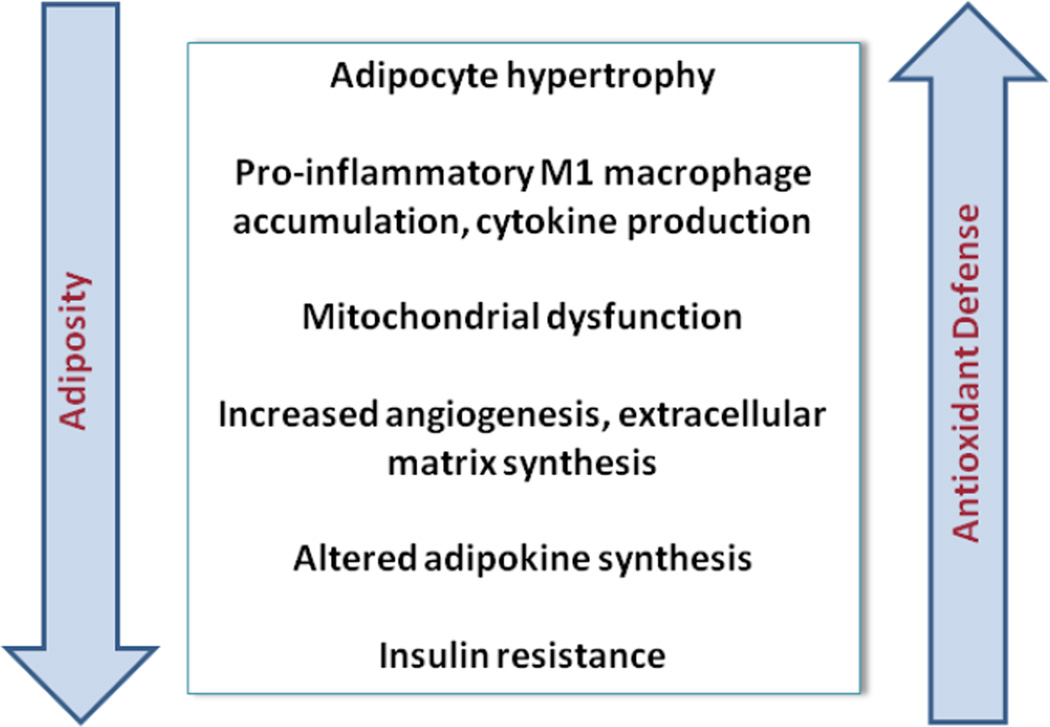
Figure 2. Characteristics of the white adipose tissue in obesity.
Progression of adipose tissue from lean to obese states is accompanied by increased macrophage infiltration and acquisition of the classically activated phenotype and cytokine synthesis. Associated with inflammation is increased oxidative stress in the adipocyte fraction, mitochondrial dysfunction and increased ER stress leading to insulin resistance.
Protein carbonyls in the adipose tissue have only been recently evaluated as critical factors regulating cellular function [65]. In a recent experimental study [66] protein carbonyls were measured in the white adipose tissue of obese Zucker rats, showing their significant decrease as one of the beneficial effects of moderate physical exercise. Previously it has been demonstrated that a diet induced obesity-linked insulin resistance in mice results in approximately 2–3 fold increase in total protein carbonyls in the white adipose tissue, with concomitant 3–4 fold decrease in the abundance of the white adipose tissue GSTA4 protein [67]. This study was in fact the first one focused on protein carbonylation in obese white adipose tissue. The discovery that GSTA4 levels are highly regulated, as well as preceding complementary data about the expression of glutathione S-transferase in the white adipose tissue [68], suggest that carbonylation plays a significant role in the development of metabolic disease.
In a clinical setting protein carbonyls serve as a reliable biomarker of visceral adipose tissue oxidative stress, negatively correlating with plasma adiponectin levels [69, 70]. Moreover, the level of adipose tissue protein carbonyls was found positively correlated with adiposity and serum free fatty acids in human obesity [71].
Oxidative stress and insulin resistance in cultured 3T3-L1 adipocytes
The earliest studies of cultured 3T3-L1 adipocytes have demonstrated the influence of oxidative stress on adipocyte insulin resistance. Treatment of cultured 3T3-L1 adipocytes with micromolar concentrations of H2O2 reduced their metabolic responses to insulin stimulation and altered the expression of glucose transporters [72]. The effect of H2O2 was attenuated by lipoic acid pretreatment, but not by troglitazone, its vitamin E moiety or with vitamin C [73]. Unlike troglitazone, glicazide--a second generation sulfonylurea, restored insulin-stimulated glucose transporter 4 (GLUT4) translocation [74] and improved insulin action.
The effect of oxidative stress on adipocyte insulin signaling was confirmed by treatment of cultured 3T3-L1 adipocytes with non-toxic concentrations of 4-HNE, which impaired adipocyte insulin transduction pathway by carbonylation of insulin receptor substrate-1 and insulin receptor substrate-2 proteins. It was also demonstrated that the effects of 4-HNE are partially reversed with adenovirus induced increase of expression of adipocyte fatty aldehyde dehydrogenase (FALDH), one of the enzymes involved in 4-HNE detoxification [94]. The same research group had previously demonstrated that in high fat diet induced insulin resistance in mice the stimulatory action of insulin on adipose tissue FALDH expression is lost [95]. Thus, authors suggest existence of vicious cycle between 4-HNE accumulation and insulin resistance. Additional experiments with cultured 3T3-L1 adipocytes showed that treatment with anti-diabetic drug rosiglitazone increased FALDH expression [94], which is one of numerous mechanisms of its action. Unfortunately, due to serious adverse effects, rosiglitazone is already withdrawn from the market in many countries [96]. Still, experimental findings suggest that FALDH could be considered as one of the possible candidate targets for development of novel therapeutic approaches to combat insulin resistance, and this hypothesis should be further carefully investigated.
In addition, there is experimental evidence that chronic insulin treatment of cultured 3T3-L1 adipocytes significantly increases the intracellular generation of superoxide anion, hydrogen peroxide and hydroxyl radical, which inhibit insulin signaling and glucose uptake. These effects are further reversed by N-acetylcysteine, superoxide dismutase or catalase [75]. It has also been demonstrated that insulin-like growth factor 1 treatment of cultured 3T3-L1 adipocytes induces insulin resistance of the cultured cells via ROS overproduction, which can be attenuated by pre-incubation of the cells with the antioxidant N-acetylcysteine [76].
Similarly, tumor necrosis factor-α induced oxidative stress and insulin resistance in cultured 3T3-L1 adipocytes was attenuated when pre-accumulated with β-carotene [77]. Similarly, fermented rice bran rich in phenolic antioxidants is able to ameliorate oxidative stress induced insulin resistance in cultured 3T3-L1 adipocytes [78] while the green tea catechins attenuate tumor necrosis factor-α and dexamethasone induced ROS generation and insulin resistance [79].
Given the endocrine function of the white adipocyte in regulating insulin sensitivity and energy metabolism, it is important to study the secretion of adipokines under the state of oxidative stress and insulin resistance in the white adipocyte itself, as well as the cellular signal transduction pathways participating in the oxidative stress induced adipokine dysregulation. To that end it was demonstrated that the production of adiponectin is suppressed, whereas of plasminogen activator inhibitor and interleukin 6 is enhanced in cultured 3T3-L1 adipocytes exposed to exogenous H2O2 [80]. In a recent study cultured adipocytes were exposed to exogenous 4-HNE resulting in decreased adiponectin secretion as well as significantly decreased intracellular adiponectin protein abundance. At the same time, adiponectin gene expression was found significantly elevated, with a concomitant increase in peroxisome proliferator-activated receptor gamma (PPAR-γ) gene expression. This study further revealed that the 4-HNE exposure accelerated the adiponectin protein degradation by increasing ubiquitinated adiponectin protein levels [81].
There is also recent experimental evidence that natural dietary antioxidants p-coumaric acid, quercetin and resveratrol inhibit tumor necrosis factor-α induced increase of interleukin-6 in cultured 3T3-L1 adipocytes. These antioxidants also demonstrated inhibition of tumor necrosis factor-α induced changes in the levels of plasminogen activator inhibitor-1, monocyte chemoattractant protein-1 and intracellular ROS, with concomitant increase of adiponectin and antioxidant enzymes [82].
As stated before, GSTA4 protein levels are significantly decreased in murine white adipose tissue concomitant with obesity-induced insulin resistance. In addition, GSTA4 was shown down-regulated in cultured 3T3-L1 adipocytes treated with tumor necrosis factor-α in a time- and concentration-dependent manner [83], which further relates inflammation to insulin resistance. This finding is in accordance with previous experimental data about tumor necrosis factor-α induced ROS production and insulin resistance in cultured 3T3-L1 adipocytes [77, 79]. Silencing of GSTA4, the key enzyme for 4-HNE and 4-ONE removal from the cell, has been used as a suitable model for study of metabolic consequences of an increased protein carbonylation in cultured 3T3-L1 adipocytes [83]. Extending these studies, the expression of other 4-HNE metabolizing enzymes, including FALDH, was not significantly different in obese white adipose tissue compared to lean and it was further demonstrated that GSTA4 is down-regulated only in the visceral and subcutaneous white adipose tissue, but not in the brown adipose tissue, liver and muscle. Given the presence of many different types of cells in the white adipose tissue, it was further demonstrated that the tissue GSTA4 down-regulation actually derives from the adipocytes. Further experiments demonstrated that GSTA4 in the white adipose tissue is downregulated not only in obese C57BL/6J mice, but in many other animal models of obesity and insulin resistance as well. Moreover, in a clinical study, GSTA4 expression was found decreased in visceral and subcutaneous white adipose tissue from obese patients, with statistically significant negative correlation to the level of insulin resistance.
The metabolic study of GSTA4 silenced 3T3-L1 adipocytes has demonstrated significant alterations in their glucose and lipid metabolism. Namely, GSTA4 silenced adipocytes exhibited a significant increase in basal glucose transport, as well as net decrease in insulin-stimulated hexose uptake, which is in accordance with previous studies of glucose metabolism in cultured adipocytes exposed to an exogenous oxidant [72]. Moreover, the GSTA4 silenced adipocytes were found to have increased intracellular levels of small organic acids: lactate, pyruvate, citrate and succinate, suggesting an impairment of the tricarboxylic acid cycle, possibly at few points, leading to diminished flux through it. This finding is in accordance with results from a previous study that demonstrated that 4-HNE inactivates some of the enzymes of the tricarboxylic acid cycle [84]. Regarding the lipid metabolism, the GSTA4 silenced 3T3-L1 adipocytes exhibited approximately 50% increase in the level of lipolysis, in both basal and insulin-stimulated conditions. Indeed, increased lipolysis is a common finding in the state of oxidative stress and insulin resistance of white adipocytes. The basal (but not insulin-stimulated) fatty acid uptake in the GSTA4 silenced 3T3-L1 adipocytes was found slightly increased, whereas total lipid storage and de novo lipogenesis were unchanged. Moreover, the mitochondrial β-oxidation was significantly decreased in the GSTA4 silenced cells.
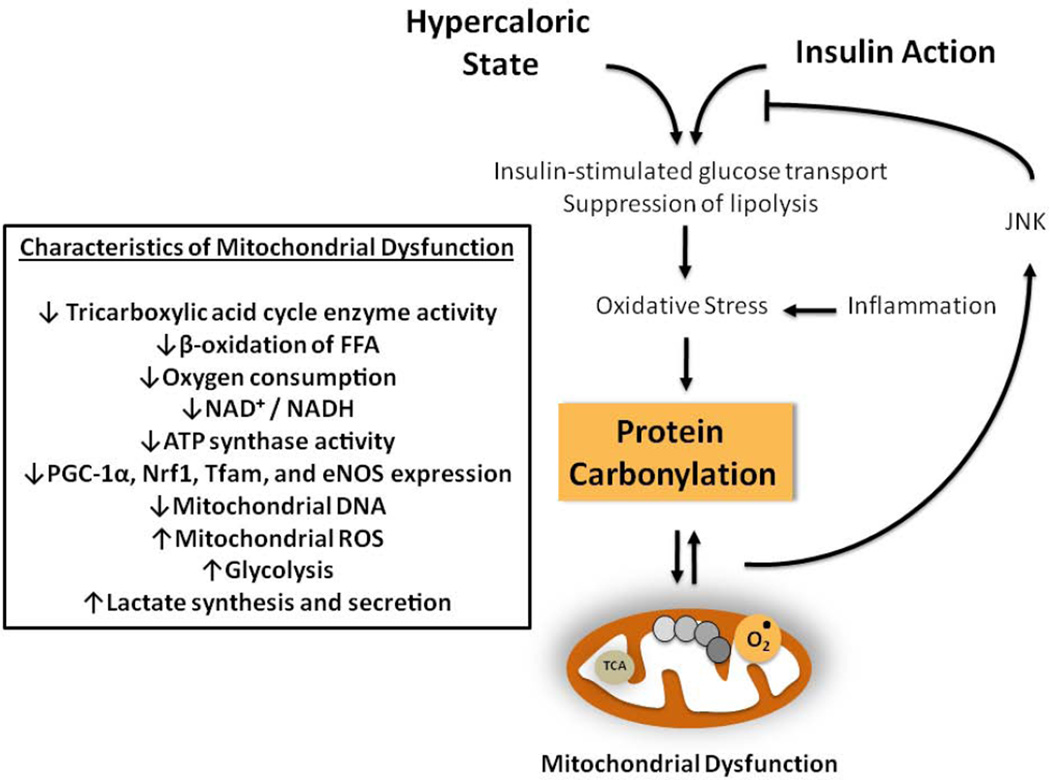
An important finding of the metabolic study of GSTA4 silenced 3T3-L1 adipocytes is a decrease in oxygen consumption of mitochondria isolated from the silenced cells. More specifically, these mitochondria display approximately 2-fold decrease in the state 2 respiration, as well as no increase in the state 3 respiration upon addition of ADP. This findings were accompanied by those of increased mitochondrial (but not whole cell) ROS, decreased NAD+/NADH ratio, reduced activity of ATP synthase for approximately 50%, and increased overall protein carbonylation, as well as carbonylation of some specific proteins.
Importantly, the increase in mitochondrial ROS is proposed as a possible mechanism linking the mitochondrial dysfunction to insulin resistance. In this model the increase in mitochondrial ROS is accompanied by an increase in apoptosis signal-regulating kinase 1 (ASK1), which is associated with an activation of the c-jun NH(2)-terminal kinases (JNK), increase in serine(307) phosphorylation of insulin receptor substrate 1 (IRS1), and decrease in its insulin-stimulated tyrosine phosphorylation, all of which leading to insulin resistance [85].
The expression of critical regulators of mitochondrial biogenesis: peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), nuclear respiratory factor 1 (Nrf1), mitochondrial transcription factor A (Tfam), and endothelial nitric oxide synthase (eNOS) was significantly decreased in the GSTA4 silenced 3T3-L1 adipocytes. At the same time, the expression of mitochondrial proteins: cyclooxygenase IV (COX IV) and cytochrome c, but not of manganese superoxide dismutase (Mn-SOD) and uncoupling protein 2 (UCP 2), was also decreased in the GSTA4 silenced 3T3-L1 adipocytes. The loss of mitochondrial DNA, manifested by reduced number of mitochondrial DNA copies of approximately 75%, was also found. However, further study revealed that all these changes did not lead to decrease in the number of mitochondria, but only to changes in their shape and internal organization (Figure 3).
Figure 3. Adipocyte protein carbonylation and mitochondrial dysfunction in obesity.
Characteristics of adipocyte mitochondrial dysfunction occurring as a consequence of hypercaloric overload or inflammation.
Targets of protein carbonylation in the white adipocyte
Development of advanced quantitative proteomic methods has enabled identification of specific carbonylated proteins and study of their influence in the cell metabolism. More specifically, using the method of incubation of adipose tissue soluble protein fraction with biotin hydrazide and subsequent detection of protein carbonyls with horseradish peroxidase-conjugated streptavidin, a significant increase in adipose tissue total protein carbonyls was detected in obese insulin resistant mice in comparison to their lean insulin sensitive counterparts. For identification of specific carbonylated proteins, biotin hydrazide coupled proteins were captured by avidin-Sepharose affinity chromatography, eluted, proteolytically digested, and analyzed by liquid chromatography – electrospray ionization tandem mass spectrometry (LC – ESI MS/MS). Among 37 carbonylated proteins, numerous enzymes involved in cellular stress response, signal transduction, and carbohydrate, lipid and protein metabolism were identified. The authors emphasize and focus on the carbonylation of adipocyte fatty-acid binding protein, a cytoplasmic fatty acid carrier which is implied in obesity induced insulin resistance through alteration of hormone-sensitive lipase activity [67].
Given the significant impairment of mitochondrial respiration in the GSTA4 silenced 3T3-L1 adipocytes, additional study was done to identify the specific mitochondrial targets of protein carbonylation, using both GSTA4 silenced and GSTA4 over-expressed 3T3-L1 adipocytes [86]. In a proteomic profiling approximately 20% of all mitochondrial proteins of the GSTA4 silenced adipocytes were identified as carbonylated, 33% of which are involved in the tricarboxylic acid cycle, 28% are involved in the branched chain amino acid catabolism, 19% are involved in oxidative phosphorylation, and with 10% or more of coverage the proteins of fatty acid, amino acid and ketone bodies metabolism were represented. However, only a small number of proteins which have an increased level of carbonylation in the GSTA4 silenced adipocytes as well as decreased level of carbonylation in the GSTA4 over-expressed cells were identified: NADH dehydrogenase (ubiquinone) 1 alpha subunit 2 (NDUFA2), NADH dehydrogenase (ubiquinone) 1 alpha subunit 3 (NDUFA3), mitochondrial inner membrane phosphate carrier (PiC), translocase of inner mitochondrial membrane 50, and valyl tRNA synthetase, the first three of them being further studied by a target protein silenced cell lines.
Previously it has been demonstrated that the inhibition of mitochondrial respiratory chain Complex I leads to an increase in superoxide anion production and decrease in mitochondrial respiration. Thus, the carbonylation of two of the subunits of Complex I: NDUFA2 and NDUFA3, along with the increased level of mitochondrial superoxide anion, which were found in GSTA4 silenced 3T3-L1 adipocytes, provide a link between adipocyte mitochondrial protein carbonylation and impaired respiration. Indeed, the oxygen consumption rate of the NDUFA2 and NDUFA3 silenced 3T3-L1 pre-adipocytes was considerably diminished in comparison to that of the GSTA4 silenced adipocytes, suggesting a significant impact of NDUFA2 and NDUFA3 carbonylation on the adipocyte respiration.
One of the functions of the mitochondrial inner membrane phosphate carrier is transport of inorganic phosphate through the inner mitochondrial membrane. In that way it cooperates in the ATP synthesis by the F0F1 ATPase (Complex V), and its carbonylation might be at least one of the reasons for decreased respiration of GSTA4 silenced 3T3-L1 adipocytes. Indeed, the PiC silenced 3T3-L1 pre-adipocytes exhibited markedly decreased level of oxygen consumption in comparison to the control cells.
Peroxiredoxin 3 and adipocyte protein carbonyls
Besides the GSTA4, in a recent study a peroxiredoxin 3 (Prx3) down-regulation in the white adipose tissue of obese mice and humans was described [87]. Peroxiredoxins are thiol-specific antioxidant enzymes that have ability to reduce peroxides and scavenge ROS. Notably, peroxiredoxins have higher affinity for H2O2 and scavenge it more efficiently than catalase. There are 6 isoforms of peroxiredoxins in mammalian cells: 1, 2 and 6 residing in the cytosol, 3 and 5 in mitochondria, and isoform 4 which is described in many tissues as a secretory protein [88]. The study of Prx3 knockout mice [87] revealed an increased mitochondrial protein carbonylation in their white adipocytes, along with impairment of adipocyte mitochondrial biogenesis, glucose and lipid metabolism, and oxidative phosphorylation.
In summary, these results are complementary to those from the study of GSTA4 silencing, suggesting involvement of common metabolic pathways, and probably protein – protein interactions, with adipocyte protein carbonyls having a significant role. However, further studies are needed to elucidate and explain the possible link between the down-regulation of these two antioxidant enzymes.
Animal models
Different animal models have been used in studies focused on oxidative stress and protein carbonylation in obese and insulin resistant white adipose tissue [65, 66, 87], mice being the most common.
The C57BL/6J mice have been proven as a very suitable animal model for study of obesity and insulin resistance which are induced by a high fat diet. As a consequence, there is approximately 2–3 fold increase in total protein carbonyls in their white adipose tissue, with concomitant 3–4 fold decrease in the abundance of the white adipose tissue GSTA4 protein [67]. Importantly, significant decrease of white adipose tissue GSTA4 expression is not limited to high fat feed C57BL/6J mice only, but is even more dramatic in the white adipose tissue from ob/ob mice [83]. Moreover, a 10-fold decrease in GSTA4 expression was found in genetically obese BTBR mice [97].In studies focused on function of GSTA4 as the key enzyme for 4-HNE and 4-ONE removal from the cells, GSTA4-null mice are also used. In an experiment both a)C57BL/6J GSTA4-null and b)C57BL/6J wild-type mice were divided in two groups: lean (on standard show) and obese (on high fat diet) [83]. Both lean and obese GSTA4-null mice exhibited no significant difference in body weight, fasting glucose and insulin levels in comparison to the corresponding wild-type. Not surprisingly, the level of superoxide in adipocyte mitochondrial matrix was 2–3 fold increased in obese wild-type mice in comparison to their lean littermates. Despite lack of evidence of obesity and insulin resistance, mitochondrial matrix superoxide was markedly increased in lean GSTA4-null mice compared to the controls (lean wild-type mice), which was even more evident in GSTA4-null obese mice. Moreover, increased adipocyte mitochondrial protein carbonylation, as well as impaired mitochondrial respiration (mitochondria from obese GSTA4-null mice displayed no increase in oxygen consumption in response to ADP, which was similar to mitochondria of GSTA4 silenced adipocytes) were found in obese GSTA4-null mice.
Subsequent experiments [98] confirmed that C57/BL6 GSTA4-null mice are not obese and that their adipose tissue 4-HNE content is only slightly, but not significantly higher in comparison to the wild-type. Further, the content of 4-HNE in skeletal muscle and liver of C57/BL6 GSTA4-null mice is almost equal to that measured in corresponding wild-type. In contrast, 129/sv GSTA4-null mice are obese, have hypertrophied adipocytes, and markedly elevated 4-HNE in adipose tissue, skeletal muscle and liver in comparison to the corresponding wild type. They exhibit a massive age-dependent infiltration of macrophages in the white adipose tissue. Although not glucose intolerant, they definitely tend to develop insulin resistance with age. The 129/sv GSTA4-null mice have increased levels of tissue malonyl-CoA which correlate with 4-HNE levels in skeletal muscle, adipose tissue and liver. Additionally, in 129/sv GSTA4-null mice were demonstrated: increased expression of mitochondrial acetyl-CoA carboxylase, decreased aconitase activity, and increased citrate level in skeletal muscle and liver. Authors propose the following mechanism by which 4-HNE induces fat deposition: a)Carbonylation of aconitase leads to its diminished function and citrate accumulation; b)Excess citrate is transported in cytosol, where supplies substrate for acetyl-CoA carboxylase and allosterically activates it; c)Depending of the tissue, the resulting malonyl-CoA is converted into fatty acids, or prevents β-oxidation, both resulting in fat deposition [99].
The results of experiments with GSTA4-null mice emphasize the importance of genetic background in development of obesity and insulin resistance, and suggest involvement of other 4-HNE detoxifying enzymes and/or compensatory mechanisms in C57/BL6 GSTA4-null mice. Their identification and targeting will be of utmost importance in intentions of development of novel, individualized therapeutic approaches in treatment of insulin resistance and T2DM.
Speaking about animal models, the 4-HNE has been also proven to promote fat accumulation even in Caenorhabditis elegans [100] and Saccharomyces cerevisiae [101], suggesting that this is a phylogenetically highly conserved biochemical process.
In sum, all these findings emphasize the role of 4-HNE (and probably 4-ONE) in development of obesity, as an important stakeholder of its self-sustaining nature. Namely, there is a vicious circle, or positive feedback loop between 4-HNE and excessive fat accumulation, regardless of its initial cause. More specifically, GSTA4 is down-regulated in obesity, leading to 4-HNE accumulation, which further leads to deposition of more fat. That was a favorable adaptation on limited and intermittent food access throughout the evolution, which results in epidemic of obesity in modern era [99].
Concluding remarks and perspectives
Many different carbonylated proteins are already identified in the white adipose tissue from obese insulin resistant mice [67], and GSTA4 silenced 3T3-L1 adipocytes [86]. The increased carbonylation is associated with complex metabolic and structural changes in the white adipocyte itself. However, in most cases, what is lacking is a definitive molecular analysis of protein carbonylation including determining the stoichiometry of carbonylation, the site(s) of modification and the role of such alkylation on enzyme/protein function. Advanced proteomic analysis will be required to address these issues. Although protein carbonylation typically leads to loss of function and degradation of affected molecules, there is also a significant role of protein carbonyls in cell signaling through activation (or inactivation) of specific metabolic pathways, and protein – protein interactions.
Some of the signaling functions of adipocyte protein carbonyls are already described. Thus, there is experimental evidence about reduced expression of the key transcription factors of mitochondrial biogenesis (PGC-1α, Nrf1, Tfam, and eNOS) in GSTA4 silenced 3T3-L1 adipocytes. These experimental findings suggest that increased protein carbonylation actually initiates a cascade of biochemical reactions linking to the mitochondrial biogenesis pathway [83]. For example, the carbonylation of peroxiredoxin 1 and glutathione peroxidase 1, which leads to their inactivation, might play a role in activation of NFκB stress response contributing to insulin resistance. The carbonylation of filamin A, the protein that interacts with the insulin receptor and inhibits insulin signal transduction, might increase this interaction and contribute to insulin resistance. Further, the carbonylation of calumenin, the protein involved in calcium efflux from the endoplasmic reticulum, might be involved in unfolded protein response and JNK activation, leading again to insulin resistance [67].
A large body of work suggests that obesity, insulin resistance, as well as the development of T2DM, are oxidative stress related pathologies with significant involvement of protein carbonyls. These findings have led to a widespread use of different kinds of antioxidant vitamins and supplements, which are taken by both general population and patients with different oxidative stress related pathologies, most often as uncontrolled self-prescription. However, recent analysis of available clinical data about the effect of antioxidant supplements: β-carotene, vitamin A, vitamin C, vitamin E, and selenium for prevention of mortality in adults shows no evidence to support their use in both primary and secondary prevention. Moreover, it seems that β-carotene and vitamin E supplementation, as well as high doses of vitamin A actually increase the mortality in adults [89]. However, although the indiscriminate supplementation with high doses of vitamin E cannot be recommended to the general public, specific groups of patients may benefit from this kind of treatment [90]. Further experiments are needed to identify novel molecular targets of redox status impairment in obesity induced insulin resistance that will further help in developing of targeted and possibly individualized antioxidant treatments.
HIGHLIGHTS.
- Adipose tissue oxidative stress as a cause in development of insulin resistance.
- Adipocyte protein carbonyls - critical factors regulating cellular (dys)function.
- Mitochondrial dysfunction in obese white adipocytes.
- Targets of protein carbonylation in the white adipocyte.
- Perspectives for further research - targeted and individualized treatment.
Acknowledgments
Financial support: The work of Tatjana Ruskovska is supported by COST CM1001 Action. DAB is supported by NIH DK 084669 and NIH DK050456.
Footnotes
Conflict of interest: Authors declare no conflict of interest.
REFERENCES
- 1.Fact sheet №311. 2012 May; http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Anderson AS. Nutrition interventions in women in low-income groups in the UK. Proc Nutr Soc. 2007;66:25–32. doi: 10.1017/S0029665107005265. [DOI] [PubMed] [Google Scholar]
- 3.Dammann KW, Smith C. Factors affecting low-income women's food choices and the perceived impact of dietary intake and socioeconomic status on their health and weight. J Nutr Educ Behav. 2009;41:242–253. doi: 10.1016/j.jneb.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, Farb M, Valentine RJ, Ruderman NB. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. 2012;53:792–801. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi K, Kim YB. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med. 2010;25:119–129. doi: 10.3904/kjim.2010.25.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 8.International Diabetes Federation. Annual Report. 2011 http://www.idf.org/publications/annual-report-2011.
- 10.Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Ismail-Beigi F. Pathogenesis and glycemic management of type 2 diabetes mellitus: a physiological approach. Arch Iran Med. 2012;15:239–246. [PubMed] [Google Scholar]
- 12.Gastaldelli A. Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;93(Suppl 1):S60–S65. doi: 10.1016/S0168-8227(11)70015-8. [DOI] [PubMed] [Google Scholar]
- 13.Vivekanadan-Giri A, Wang JH, Byun J, Pennathur S. Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage. Rev Endocr Metab Disord. 2008;9:275–287. doi: 10.1007/s11154-008-9093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamb RE, Goldstein BJ. Modulating an oxidative-inflammatory cascade: potential new treatment strategy for improving glucose metabolism, insulin resistance, and vascular function. Int J Clin Pract. 2008;62:1087–1095. doi: 10.1111/j.1742-1241.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Circu ML, Aw TJ. Reactive oxygen species, cellular redox systems and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsuyama M. NOX/NADPH Oxidase, the Superoxide-Generating Enzyme: Its transcriptional regulation and physiological roles. J Pharmacol Sci. 2010;114:134–146. doi: 10.1254/jphs.10r01cr. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP. Roles of the Lipid Peroxidation Product 4-Hydroxynonenal in Obesity, the Metabolic Syndrome, and Associated Vascular and Neurodegenerative Disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki YJ, Carini M, Butterfield DA. Protein Carbonylation. Antioxid Redox Sign. 2010;12:323–325. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxinonenal: generation and analysis of mGsta4null mice. Toxicol Appl Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Wonisch W, Falk A, Sundl I, Winklhofer-Roob BM, Lindschinger M. Oxidative stress increases continuously with BMI and age with unfavourable profiles in males. Aging Male. 2012;15:159–165. doi: 10.3109/13685538.2012.669436. [DOI] [PubMed] [Google Scholar]
- 22.Sankhla M, Sharma TK, Mathur K, Rathor JS, Butolia V, Gadhok AK, Vardey SK, Sinha M, Kaushik GG. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin Lab. 2012;58:385–392. [PubMed] [Google Scholar]
- 23.De Groote D, Van Belleghem K, Devière J, Van Brussel W, Mukaneza A, Amininejad L. Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann Nutr Metab. 2012;61:15–24. doi: 10.1159/000338634. [DOI] [PubMed] [Google Scholar]
- 24.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421–427. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez-Lopez L, Garcia-Sanchez JR, Rincon-Viquez Mde J, Lara-Padilla E, Sierra-Vargas MP, Olivares-Corichi IM. Hypocaloric diet and regular moderate aerobic exercise is an effective strategy to reduce anthropometric parameters and oxidative stress in obese patients. Obes Facts. 2012;5:12–22. doi: 10.1159/000336526. [DOI] [PubMed] [Google Scholar]
- 26.Codoñer-Franch P, Navarro-Ruiz A, Fernández-Ferri M, Arilla-Codoñer A, Ballester-Asensio E, Valls-Bellés V. A matter of fat: insulin resistance and oxidative stress. Pediatr Diabetes. 2012;13:392–399. doi: 10.1111/j.1399-5448.2011.00847.x. [DOI] [PubMed] [Google Scholar]
- 27.Karaouzene N, Merzouk H, Aribi M, Merzouk SA, Berrouiguet AY, Tessier C, Narce M. Effects of the association of aging and obesity on lipids, lipoproteins and oxidative stress biomarkers: a comparison of older with young men. Nutr Metab Cardiovasc Dis. 2011;21:792–799. doi: 10.1016/j.numecd.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Codoñer-Franch P, Boix-García L, Simó-Jordá R, Del Castillo-Villaescusa C, Maset-Maldonado J, Valls-Bellés V. Is obesity associated with oxidative stress in children? Int J Pediatr Obes. 2010;5:56–63. doi: 10.3109/17477160903055945. [DOI] [PubMed] [Google Scholar]
- 29.Uzun H, Konukoglu D, Gelisgen R, Zengin K, Taskin M. Plasma protein carbonyl and thiol stress before and after laparoscopic gastric banding in morbidly obese patients. Obes Surg. 2007;17:1367–1373. doi: 10.1007/s11695-007-9242-8. [DOI] [PubMed] [Google Scholar]
- 30.Codoñer-Franch P, López-Jaén AB, De La Mano-Hernández A, Sentandreu E, Simó-Jordá R, Valls-Bellés V. Oxidative markers in children with severe obesity following low-calorie diets supplemented with mandarin juice. Acta Paediatr. 2010;99:1841–1846. doi: 10.1111/j.1651-2227.2010.01903.x. [DOI] [PubMed] [Google Scholar]
- 31.Sledzinski T, Goyke E, Smolenski RT, Sledzinski Z, Swierczynski J. Decrease in serum protein carbonyl groups concentration and maintained hyperhomocysteinemia in patients undergoing bariatric surgery. Obes Surg. 2009;19:321–326. doi: 10.1007/s11695-008-9691-8. [DOI] [PubMed] [Google Scholar]
- 32.Brasil AR, Norton RC, Rossetti MB, Leão E, Mendes RP. C-reactive protein as an indicator of low intensity inflammation in children and adolescents with and without obesity. J Pediatr (Rio J) 2007;83:477–480. doi: 10.2223/JPED.1690. [DOI] [PubMed] [Google Scholar]
- 33.Reyes M, Gahagan S, Díaz E, Blanco E, Leiva L, Lera L, Burrows R. Relationship of adiposity and insulin resistance mediated by inflammation in a group of overweight and obese Chilean adolescents. Nutr J. 2011;10:4. doi: 10.1186/1475-2891-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illán-Gómez F, Gonzálvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragón-Alonso A, Pascual-Díaz M, Pérez-Paredes M, Lozano-Almela ML. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–955. doi: 10.1007/s11695-012-0643-y. [DOI] [PubMed] [Google Scholar]
- 35.Garanty-Bogacka B, Syrenicz M, Goral J, Krupa B, Syrenicz J, Walczak M, Syrenicz A. Changes in inflammatory biomarkers after successful lifestyle intervention in obese children. Endokrynol Pol. 2011;62:499–505. [PubMed] [Google Scholar]
- 36.Gauthier MS, Ruderman NB. Adipose tissue inflammation and insulin resistance: all obese humans are not created equal. Biochem J. 2010;430:e1–e4. doi: 10.1042/BJ20101062. [DOI] [PubMed] [Google Scholar]
- 37.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 38.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 39.Majka SM, Barak Y, Klemm DJ. Concise review: adipocyte origins: weighing the possibilities. Stem Cells. 2011;29:1034–1040. doi: 10.1002/stem.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 41.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 2010;88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 44.Itoh M, Suganami T, Hachiya R, Ogawa Y. Adipose tissue remodeling as homeostatic inflammation. Int J Inflam. 2011;2011:720926. doi: 10.4061/2011/720926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocr Metab Disord. 2011;12:163–172. doi: 10.1007/s11154-011-9168-2. [DOI] [PubMed] [Google Scholar]
- 47.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Snel M, Jonker JT, Schoones J, Lamb H, de Roos A, Pijl H, Smit JW, Meinders AE, Jazet IM. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol. 2012;2012:983814. doi: 10.1155/2012/983814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 50.Korsic M, Gotovac K, Nikolac M, Dusek T, Skegro M, Muck-Seler D, Borovecki F, Pivac N. Gene expression in visceral and subcutaneous adipose tissue in overweight women. Front Biosci (Elite Ed) 2012;4:2834–2844. doi: 10.2741/e587. [DOI] [PubMed] [Google Scholar]
- 51.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clément K. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 52.Barbarroja N, López-Pedrera R, Mayas MD, García-Fuentes E, Garrido-Sánchez L, Macías-González M, El Bekay R, Vidal-Puig A, Tinahones FJ. The obese healthy paradox: is inflammation the answer? Biochem J. 2010;430:141–149. doi: 10.1042/BJ20100285. [DOI] [PubMed] [Google Scholar]
- 53.Nickelson KJ, Stromsdorfer KL, Pickering RT, Liu TW, Ortinau LC, Keating AF, Perfield JW., 2nd A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice. Exp Diabetes Res. 2012;2012:859395. doi: 10.1155/2012/859395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakurai T, Izawa T, Kizaki T, Ogasawara JE, Shirato K, Imaizumi K, Takahashi K, Ishida H, Ohno H. Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue. Biochem Biophys Res Commun. 2009;379:605–609. doi: 10.1016/j.bbrc.2008.12.127. [DOI] [PubMed] [Google Scholar]
- 55.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galinier A, Carrière A, Fernandez Y, Carpéné C, André M, Caspar-Bauguil S, Thouvenot JP, Périquet B, Pénicaud L, Casteilla L. Adipose tissue proadipogenic redox changes in obesity. J Biol Chem. 2006;281:12682–12687. doi: 10.1074/jbc.M506949200. [DOI] [PubMed] [Google Scholar]
- 58.Sohet FM, Neyrinck AM, Dewulf EM, Bindels LB, Portois L, Malaisse WJ, Carpentier YA, Cani PD, Delzenne NM. Lipid peroxidation is not a prerequisite for the development of obesity and diabetes in high-fat-fed mice. Br J Nutr. 2009;102:462–469. doi: 10.1017/S0007114508191243. [DOI] [PubMed] [Google Scholar]
- 59.Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071–1077. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 60.Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, Masuzaki H, Park KS, Kim JB. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol. 2008;22:2176–2189. doi: 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi H, Matsuda M, Fukuhara A, Komuro R, Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Am J Physiol Endocrinol Metab. 2009;296:E1326–E1334. doi: 10.1152/ajpendo.90921.2008. [DOI] [PubMed] [Google Scholar]
- 62.Jobgen W, Fu WJ, Gao H, Li P, Meininger CJ, Smith SB, Spencer TE, Wu G. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids. 2009;37:187–198. doi: 10.1007/s00726-009-0246-7. [DOI] [PubMed] [Google Scholar]
- 63.Sugimoto M, Arai H, Tamura Y, Murayama T, Khaengkhan P, Nishio T, Ono K, Ariyasu H, Akamizu T, Ueda Y, Kita T, Harada S, Kamei K, Yokode M. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis. 2009;204:388–394. doi: 10.1016/j.atherosclerosis.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 64.DeFuria J, Bennett G, Strissel KJ, Perfield JW, 2nd, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr. 2009;139:1510–1516. doi: 10.3945/jn.109.105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sackmann-Sala L, Berryman DE, Lubbers ER, Vesel CB, Troike KM, List EO, Munn RD, Ikeno Y, Kopchick JJ. Decreased insulin sensitivity and increased oxidative damage in wasting adipose tissue depots of wild-type mice. Age (Dordr) 2012;34:1225–1237. doi: 10.1007/s11357-011-9304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krskova K, Eckertova M, Kukan M, Kuba D, Kebis A, Olszanecki R, Suski M, Gajdosechova L, Zorad S. Aerobic training lasting for 10 weeks elevates the adipose tissue FABP4, Giα, and adiponectin expression associated by a reduced protein oxidation. Endocr Regul. 2012;46:137–146. doi: 10.4149/endo_2012_03_137. [DOI] [PubMed] [Google Scholar]
- 67.Grimsrud PA, Picklo MJ, Sr, Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- 68.Moraes RC, Blondet A, Birkenkamp-Demtroeder K, Tirard J, Orntoft TF, Gertler A, Durand P, Naville D, Bégeot M. Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology. 2003;144:4773–4782. doi: 10.1210/en.2003-0456. [DOI] [PubMed] [Google Scholar]
- 69.Saito S, Fujiwara T, Matsunaga T, Minagawa K, Fukui K, Fukuda I, Osanai T, Okumura K. Increased adiponectin synthesis in the visceral adipose tissue in men with coronary artery disease treated with pravastatin: a role of the attenuation of oxidative stress. Atherosclerosis. 2008;199:378–383. doi: 10.1016/j.atherosclerosis.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Yokoyama H, Saito S, Daitoku K, Fukuda I, Higuma T, Hanada H, Osanai T, Okumura K. Effects of pravastatin and rosuvastatin on the generation of adiponectin in the visceral adipose tissue in patients with coronary artery disease. Fundam Clin Pharmacol. 2011;25:378–387. doi: 10.1111/j.1472-8206.2010.00847.x. [DOI] [PubMed] [Google Scholar]
- 71.Frohnert BI, Sinaiko AR, Serrot FJ, Foncea RE, Moran A, Ikramuddin S, Choudry U, Bernlohr DA. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring) 2011;19:1735–1741. doi: 10.1038/oby.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rudich A, Kozlovsky N, Potashnik R, Bashan N. Oxidant stress reduces insulin responsiveness in 3T3-L1 adipocytes. Am J Physiol. 1997;272:E935–E940. doi: 10.1152/ajpendo.1997.272.5.E935. [DOI] [PubMed] [Google Scholar]
- 73.Rudich A, Tirosh A, Potashnik R, Khamaisi M, Bashan N. Lipoic acid protects against oxidative stress induced impairment in insulin stimulation of protein kinase B and glucose transport in 3T3-L1 adipocytes. Diabetologia. 1999;42:949–957. doi: 10.1007/s001250051253. [DOI] [PubMed] [Google Scholar]
- 74.Shimoyama T, Yamaguchi S, Takahashi K, Katsuta H, Ito E, Seki H, Ushikawa K, Katahira H, Yoshimoto K, Ohno H, Nagamatsu S, Ishida H. Gliclazide protects 3T3L1 adipocytes against insulin resistance induced by hydrogen peroxide with restoration of GLUT4 translocation. Metabolism. 2006;55:722–730. doi: 10.1016/j.metabol.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 75.Ge X, Yu Q, Qi W, Shi X, Zhai Q. Chronic insulin treatment causes insulin resistance in 3T3-L1 adipocytes through oxidative stress. Free Radic Res. 2008;42:582–591. doi: 10.1080/10715760802158448. [DOI] [PubMed] [Google Scholar]
- 76.Fukuoka H, Iida K, Nishizawa H, Imanaka M, Takeno R, Iguchi G, Takahashi M, Okimura Y, Kaji H, Chihara K, Takahashi Y. IGF-I stimulates reactive oxygen species (ROS) production and inhibits insulin-dependent glucose uptake via ROS in 3T3-L1 adipocytes. Growth Horm IGF Res. 2010;20:212–219. doi: 10.1016/j.ghir.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Kameji H, Mochizuki K, Miyoshi N, Goda T. β-Carotene accumulation in 3T3-L1 adipocytes inhibits the elevation of reactive oxygen species and the suppression of genes related to insulin sensitivity induced by tumor necrosis factor-α. Nutrition. 2010;26:1151–1156. doi: 10.1016/j.nut.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Kim D, Han GD. Ameliorating effects of fermented rice bran extract on oxidative stress induced by high glucose and hydrogen peroxide in 3T3-L1 adipocytes. Plant Foods Hum Nutr. 2011;66:285–290. doi: 10.1007/s11130-011-0243-3. [DOI] [PubMed] [Google Scholar]
- 79.Yan J, Zhao Y, Suo S, Liu Y, Zhao B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic Biol Med. 2012;52:1648–1657. doi: 10.1016/j.freeradbiomed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 80.Chen B, Wei J, Wang W, Cui G, Zhao Y, Zhu X, Zhu M, Guo W, Yu J. Identification of signaling pathways involved in aberrant production of adipokines in adipocytes undergoing oxidative stress. Arch Med Res. 2009;40:241–248. doi: 10.1016/j.arcmed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Wang Z, Dou X, Gu D, Shen C, Yao T, Nguyen V, Braunschweig C, Song Z. 4-Hydroxynonenal differentially regulates adiponectin gene expression and secretion via activating PPARγ and accelerating ubiquitin-proteasome degradation. Mol Cell Endocrinol. 2012;349:222–231. doi: 10.1016/j.mce.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yen GC, Chen YC, Chang WT, Hsu CL. Effects of polyphenolic compounds on tumor necrosis factor-α (TNF-α)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J Agric Food Chem. 2011;59:546–551. doi: 10.1021/jf1036992. [DOI] [PubMed] [Google Scholar]
- 83.Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, Brestoff JR, Wiczer BM, Ilkayeva O, Cianflone K, Muoio DE, Arriaga EA, Bernlohr DA. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59:1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 85.Nishikawa T, Kukidome D, Sonoda K, Fujisawa K, Matsuhisa T, Motoshima H, Matsumura T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S161–S164. doi: 10.1016/j.diabres.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 86.Curtis JM, Hahn WS, Stone MD, Inda JJ, Droullard DJ, Kuzmicic JP, Donoghue MA, Long EK, Armien AG, Lavandero S, Arriaga E, Griffin TJ, Bernlohr DA. Protein carbonylation and adipocyte mitochondrial function. J Biol Chem. 2012;287:32967–32980. doi: 10.1074/jbc.M112.400663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huh JY, Kim Y, Jeong J, Park J, Kim I, Huh KH, Kim YS, Woo HA, Rhee SG, Lee KJ, Ha H. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxid Redox Signal. 2012;16:229–243. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madrigal-Matute J, Martinez-Pinna R, Fernandez-Garcia CE, Ramos-Mozo P, Burillo E, Egido J, Blanco-Colio LM, Martin-Ventura JL. Cell stress proteins in atherothrombosis. Oxid Med Cell Longev. 2012;2012:232464. doi: 10.1155/2012/232464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012 Mar 14;3:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dotan Y, Lichtenberg D, Pinchuk I. No evidence supports vitamin E indiscriminate supplementation. Biofactors. 2009;35:469–473. doi: 10.1002/biof.61. [DOI] [PubMed] [Google Scholar]
- 91.Fritz KS, Petersen DR. Exploring the biology of lipid-peroxidation derived protein carbonylation. Chem Res Toxicol. 2011;24:1411–1419. doi: 10.1021/tx200169n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimniak P. Relationship of electrophilic stress to aging. Free Radic Biol Med. 2011;51:1087–1105. doi: 10.1016/j.freeradbiomed.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Picklo MJ, Azenkeng A, Hoffmann MR. Trans-4-oxo-2-nonenal potently alters mitochondrial function. Free Radic Biol Med. 2011;50:400–407. doi: 10.1016/j.freeradbiomed.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 94.Demozay D, Mas JC, Rocchi S, Van Obberghen E. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenel on insulin signaling in 3T3-L1 adipocytes. Diabetes. 2008;57:1216–1226. doi: 10.2337/db07-0389. [DOI] [PubMed] [Google Scholar]
- 95.Demozay D, Rocchi S, Mas JC, Grillo S, Pirola L, Chavey C, Van Obberghen E. Fatty aldehyde dehydrogenase: potential role in oxidative stress protection and regulation of its gene expression by insulin. J Biol Chem. 2004;279:6261–6270. doi: 10.1074/jbc.M312062200. [DOI] [PubMed] [Google Scholar]
- 96.Cohen D. Rosiglitazone: what went wrong? BMJ. 2010;341:c4848. doi: 10.1136/bmj.c4848. [DOI] [PubMed] [Google Scholar]
- 97.Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res. 2008;18:706–716. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh SP, Niemczyk M, Saini D, Awasthi YC, Zimniak L, Zimniak P. Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry. 2008;47:3900–3911. doi: 10.1021/bi702124u. [DOI] [PubMed] [Google Scholar]
- 99.Zimniak P. 4-Hydroxynonenal and fat storage: A paradoxical pro-obesity mechanism? Cell Cycle. 2010;9:3393–3394. doi: 10.4161/cc.9.17.13123. [DOI] [PubMed] [Google Scholar]
- 100.Singh SP, Niemczyk M, Zimniak L, Zimniak P. Fat accumulation in Caenorhabditis elegans triggered by the electrophilic lipid peroxidation product 4-hydroxynonenal (4-HNE) Aging. 2008;1:68–80. doi: 10.18632/aging.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wonisch W, Zellnig G, Kohlwein SD, Schaur RJ, Bilinski T, Tatzber F, Esterbauer H. Ultrastructural analysis of HNE-treated Saccharomyces cerevisiae cells reveals fragmentation of the vacuole and an accumulation of lipids in the cytosol. Cell Biochem Funct. 2001;19:59–64. doi: 10.1002/cbf.888. [DOI] [PubMed] [Google Scholar]
- 102.Cohen G, Riahi Y, Shamni O, Guichardant M, Chatqilialoglu C, Ferreri C, Kaiser N, Sasson S. Role of lipid peroxidation and PPAR-δ in amplifying glucose-stimulated insulin secretion. Diabetes. 2011;60:2830–2842. doi: 10.2337/db11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]