The Aging Brain and Cognition: Contribution of Vascular Injury and Aβ to Mild Cognitive Dysfunction (original) (raw)
. Author manuscript; available in PMC: 2014 Apr 1.
Abstract
Importance
β-Amyloid (Aβ) deposition and vascular brain injury (VBI) frequently co-occur and are both associated with cognitive decline in aging. Determining whether a direct relationship exists between them has been challenging. We sought to understand VBI’s influence on cognition and clinical impairment, separate from and in conjunction with pathologic changes associated with Alzheimer disease (AD).
Objective
To examine the relationship between neuroimaging measures of VBI and brain Aβ deposition and their associations with cognition.
Design and Setting
A cross-sectional study in a community- and clinic-based sample recruited for elevated vascular disease risk factors.
Participants
Clinically normal (mean age, 77.1 years [N=30]), cognitively impaired (mean age, 78.0 years [N=24]), and mildly demented (mean age, 79.8 years [N=7]) participants.
Interventions
Magnetic resonance imaging, Aβ (Pitts-burgh Compound B–positron emission tomographic [PiB-PET]) imaging, and cognitive testing.
Main Outcome Measures
Magnetic resonance images were rated for the presence and location of infarct (34 infarct-positive participants, 27 infarct-negative participants) and were used to quantify white matter lesion volume. The PiB-PET uptake ratios were used to create a PiB index by averaging uptake across regions vulnerable to early Aβ deposition; PiB positivity (29 PiB-positive participants, 32 PiB-negative participants) was determined from a data-derived threshold. Standardized composite cognitive measures included executive function and verbal and nonverbal memory.
Results
Vascular brain injury and Aβ were independent in both cognitively normal and impaired participants. Infarction, particularly in cortical and subcortical gray matter, was associated with lower cognitive performance in all domains (P<.05 for all comparisons). Pittsburgh Compound B positivity was neither a significant predictor of cognition nor interacted with VBI.
Conclusions and Relevance
In this elderly sample with normal cognition to mild dementia, enriched for vascular disease, VBI was more influential than Aβ in contemporaneous cognitive function and remained predictive after including the possible influence of Aβ. There was no evidence that VBI increases the likelihood of Aβ deposition. This finding highlights the importance of VBI in mild cognitive impairment and suggests that the impact of cerebrovascular disease should be considered with respect to defining the etiology of mild cognitive impairment.
Deposition of β-amyloid (Aβ) and vascular brain injury (VBI) are the 2 most common pathologic changes seen in the aging brain. They frequently co-occur1; however, determining whether a direct relationship exists between them has proven challenging. Although the presence of Aβ is a diagnostic criterion for Alzheimer disease (AD),2,3 a significant portion of clinically normal elderly individuals have Aβ (approximately 25%).1,4,5 Thus, the deleterious effects of Aβ may be dependent on a subsequent “cascade” of events that lead to dementia.6 However, both postmortem and in vivo neuroimaging measures of Aβ have been inconsistently associated with cognitive deficits.7–15
Most studies to date that have examined the relationship between Aβ and cognition exclude individuals with evidence of VBI. The few neuroimaging studies that have directly examined VBI and Aβ found no relationship between the 2 entities.16–18 Two of these studies16,18 considered only clinically normal individuals; because the presence of an infarct and Aβ in the same individual may increase the likelihood of clinical impairment,1 evidence of a relationship might have escaped their detection.
We sought to understand VBI’s influence on cognition and clinical impairment separate from and in conjunction with AD pathologic changes.19,20 The aim of this study was to define how neuroimaging measures of Aβ deposition and VBI separately and together affect cognition in a cognitively heterogeneous population. Specifically, we asked: (1) Is there a relationship between Aβ and VBI that is dependent on cognitive status or lesion type? (2) What are the distinct contributions of Aβ and VBI to specific cognitive domains in a group of individuals ranging from normal cognition to mild dementia?
METHODS
PARTICIPANTS
Participants were recruited for an ongoing multisite research program, the Aging Brain project, designed to recruit individuals with substantial vascular disease risk factors and VBI. Inclusion criteria have previously been described in detail.19
The 61 participants in the present study included 30 who were clinically normal (mean [SD] age, 77.1 [7.3] years), 24 who were cognitively impaired (mean age, 78.0 [7.4] years; 7 with amnestic mild cognitive impairment [MCI], 13 with nonamnestic MCI, and 4 with other impairment), and 7 individuals with a diagnosis of dementia (mean age, 79.8 [3.3] years). For research purposes, cognitive status was defined by dividing participants into 2 groups according to their Clinical Dementia Rating (CDR) status (CDR = 0 [N = 30]; CDR ≥0.5 [N=31]). All participants underwent cognitive testing, Pitts-burgh Compound B–positron emission tomography (PiB-PET), and magnetic resonance imaging (MRI). Participants were monitored longitudinally, and the MRI and cognitive session closest to the PiB imaging date were used. The mean time between all imaging and cognitive examinations was approximately 2 months.
The study was approved by the institutional review boards of all participating institutions. Written informed consent was obtained from all participants or their legal representatives following institutional review board–approved protocols.
COGNITIVE TESTING
The Mini-Mental State Examination (MMSE) provided a measure of global cognitive functioning; the Geriatric Depression Scale, a measure of depressive symptoms.
Each participant completed a broad battery of standardized neuropsychological tests. Previously developed composite measures of executive function, verbal memory, and nonverbal memory were used. The scale of these composite measures was transformed so that each had a mean of 100 and an SD of 15. These scales were created using item response theory methods to attain psychometrically matched scales with linear measurement properties and high reliability across a 4-SD range of ability. Specific methods have been previously described.19,21
MAGNETIC RESONANCE IMAGING
Acquisition
Fifty-one participants underwent MRI using a 3-T scanner (Magnetom Trio System; Siemens) with an 8-channel head coil. Acquired images included a T1-weighted, volumetric, magnetization-prepared rapid gradient-echo (MPRAGE) image (repetition time [TR], 2500 milliseconds; echo time [TE], 2.94 or 2.98 milliseconds; inversion time [T1], 1100 milliseconds) and a fluid-attenuated inversion recovery (FLAIR) image (TR, 5000 milliseconds; TE, 403 milliseconds; TI, 1700 milliseconds; 1.0×1.0 mm2 in-plane resolution with 1.00-mm thickness). Three participants underwent scanning using a 1.5-T system (Signa Genesis; GE Healthcare). Each session included a T1-weighted, 3-dimensional, spoiled gradient recalled–echo scan (TR, 9 milliseconds; TE, 1.9 milliseconds); data from FLAIR images were not used owing to technical difficulties. Seven participants underwent scanning with a 4-T system (MedSpec System; Siemens) with an 8-channel head coil. A T1-weighted volumetric MPRAGE image (TR, 2300 milliseconds; TE, 2.84 or 3.37 milliseconds; TI, 950 milliseconds) and a FLAIR image (TR, 6000 milliseconds; TE, 405 milliseconds; TI, 2050 milliseconds; 1.0×1.0 mm2 in-plane resolution with 2.00-mm thickness) were acquired.
Image Analysis
The T1-weighted MRIs were used to identify infarcts and to assist with PiB-PET processing. Image analysis software (FreeSurfer, version 5.1.0; http://surfer.nmr.mgh.harvard.edu/) was used to process the T1-weighted MRIs for each participant following procedures previously described.22 A cerebellar gray matter mask was also derived from each participant’s native brain space to serve as a reference region for PiB processing. Each mask was manually edited to remove noncerebellar tissue.
White Matter Hyperintensity Quantification
The skull was removed from FLAIR images and a manually edited binary mask was created using the remaining brain. FLAIR images were corrected for image intensity inhomogeneity,23 and then white matter hyperintensities (WMHs) were segmented using location (guided by the brain mask) and an image intensity histogram.24 The WMH lesion segmentations were visually inspected to ensure that they included at least 80% of observed WMH.
White matter hyperintensity volume was regressed onto total cranial volume (derived from the image analysis software) to minimize the effect of head size,25 and the resulting unstandardized residuals were retained. After adding a constant (12 000) to the residuals to eliminate negative values, the residuals were then log transformed to normalize the data. The log-transformed residuals were used in subsequent analyses. Nine individuals did not have WMH data because of technical problems.
Infarct Classification
Infarcts were identified by a vascular neurologist (N.S.) blind to any other participant data by using the T1-weighted and FLAIR MRIs. Infarcts were categorized according to the vascular territory affected, structures involved, size (small, 3–10 mm; large, >10 mm), and severity (cystic, not cystic). Infarcts were then labeled: cortical gray matter (affecting any cortical region), white matter (affecting any subcortical white matter region and/or internal capsule), subcortical gray matter (affecting the basal ganglia, thalamus, amygdala, and/or hippocampus), and other (affecting the midbrain, pons, medulla, and/or cerebellum). The number of infarcts was also noted (range, 0–4 infarcts) and was recoded into a categorical variable with 3 levels: 0 infarcts (n=27), 1 infarct (n=24), and more than 1 infarct (n=10).
PET IMAGING
Acquisition
The PiB radiotracer was synthesized at Lawrence Berkeley National Laboratory, Berkeley, California, using a previously published protocol.26 Ninety minutes of dynamic PiB-PET data were acquired on a high-resolution PET scanner (ECAT EXACT; Siemens).
Image Analysis
Pittsburgh Compound B data were preprocessed with procedures previously described16 using a gray matter cerebellar reference region to calculate distribution volume ratio images27,28 that were then warped to MNI (Montreal Neurological Institute) space using the SPM (Statistical Parametric Mapping) T1 template. The Global PiB Index was generated from the mean distribution volume ratios from regions of interest vulnerable to early Aβ deposition, which include the frontal cortex (anterior to the precentral gyrus), lateral parietal cortex, lateral temporal cortex, posterior cingulate, and precuneus.22,29 The occipital cortex was also examined because of its susceptibility to cerebral amyloid angiopathy. Areas that overlapped with the stroke and peristroke regions in the individuals with cortical infarct were manually excluded. In individuals with cerebellar infarct, the contralesional cerebellar hemisphere served as the reference region. This method was validated by demonstrating that the ratio of PiB uptake in the pons (a region generally spared from PiB binding) to the contralesional cerebellar hemisphere was similar to that ratio in subjects without infarct when comparing both a single cerebellar hemisphere and the entire gray matter cerebellum.
PiB Positivity
To define PiB as a dichotomous variable, 11 young adults (mean [SD] age, 24.5 [3.4] years) underwent PiB-PET imaging using the same acquisition and processing procedures already described. Global PiB Index values 2 SDs above the average (>1.114) were established as defining values of PiB positivity.
STATISTICAL ANALYSIS
All analyses were conducted using SPSS statistical software (version 20; SPSS Inc). Differences between infarct (positive vs negative) and PiB (positive vs negative) groups were tested using Pearson χ2 tests for dichotomous variables and independent-sample t tests for continuous variables. Spearmanρ, Pearson R, and linear regressions were used to assess relationships between continuous variables. Independent measures analyses of variance were used to explore the relationship between infarct and PiB uptake. The Kolmogorov-Smirnov test with Lilliefors correction was used to test normality of distribution of cognitive measures.
We used a multistage multiple regression approach to evaluate the contributions of vascular- and amyloid-related measures to neuropsychological test performance. The independent variables were the neuroimaging measures of vascular injury (infarct number [0, 1, or >1] and location and WMH) and Aβ deposition (Global PiB Index). The dependent variables were composite cognitive test performance (verbal and nonverbal memory and executive function). The baseline stage of the model included demographic variables: sex, age, and educational level. In the second stage, we evaluated whether each marker of pathologic change (infarcts, WMH, and PiB), considered separately, was associated with cognition. There were multiple indicators of infarct status (ie, cortical gray matter, subcortical gray matter, white matter, other, and number of infarcts) that were distributed in overlapping patterns; we evaluated each separately and, if more than 1 indicator was significant, in simple combinations to identify the best infarct predictor for each cognitive outcome. In the third stage, we evaluated the joint and independent effects of pathologic markers by adding all (PiB, WMH, and the best infarct predictor) to the baseline demographic model. This approach was adopted to describe the effects of each type of pathologic change when considered alone, then to investigate their independent contributions when considered together. A 2-tailed α level of .05 was used to determine statistical significance.
RESULTS
PARTICIPANT CHARACTERISTICS
The demographic data, apolipoprotein E (APOE) allele status, and clinical status of participants stratified by PiB status are summarized in Table 1. The same characteristics stratified by infarct status are summarized in Table 2. Overall, 34 of the 61 participants (56%) had an MRI-identified infarct, and 29 (48%) were classified as PiB-positive. Participants classified as PiB-negative (n=32) and PiB-positive (n=29) did not differ in age, educational level, CDR, MMSE score, or Geriatric Depression Scale score. Participants who were PiB-positive were more likely to be male (P =.046) and to carry the APOE ε4 allele (P =.02). There was a significant positive relationship between increasing age and PiB status (ρ=0.27; P =.04).
Table 1.
Participant Characteristics Stratified by PiB Statusa
| PiB− (n = 32) | PiB+ (n = 29) | t | χ2 | P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 76.6 (7.5) | 79 (6.3) | _t_59 = 1.4 | .17 |
| Male sex | 19 (59) | 24 (83) | χ12=4.00 | .046 |
| Educational level, mean (SD), y | 13.8 (2.9) | 14.1 (2.5) | _t_59 = 0.5 | .64 |
| APOE ε4 alleleb | 3 (12) | 10 (40) | χ12=5.09 | .02 |
| MMSE score, mean (SD) | 28 (2.2) | 27.4 (2.3) | _t_59 = 1.0 | .32 |
| GDS score, mean (SD) | 1.5 (1.7) | 2.4 (2.6) | _t_57 = 1.5 | .14 |
| Infarct+ | 20 (62) | 14 (48) | χ12=1.25 | .26 |
| CDR ≥ 0.5 | 15 (47) | 16 (55) | χ12=0.42 | .52 |
Table 2.
Participant Characteristics Stratified by Infarct Statusa
| Infarct− (n = 27) | Infarct+ (n = 34) | t | χ2 | P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 79.3 (7.5) | 76.6 (6.4) | _t_59 = 1.5 | .13 |
| Male sex | 20 (74) | 23 (68) | χ12=0.30 | .59 |
| Educational level, mean (SD), y | 13.9 (2.8) | 14 (2.7) | _t_59 = 0.1 | .92 |
| APOE ε4 alleleb | 4 (17) | 9 (33) | χ12=1.64 | .20 |
| MMSE score, mean (SD) | 28.2 (2.1) | 27.3 (2.4) | _t_59 = 1.6 | .12 |
| GDS score, mean (SD) | 1.6 (1.7) | 2.2 (2.6) | _t_57 = 1.0 | .33 |
| PiB+ | 15 (56) | 14 (41) | χ12=1.25 | .26 |
| CDR ≥ 0.5 | 10 (37) | 21 (62) | χ12=3.68 | .055 |
Infarct-negative (n = 27) and infarct-positive (n = 34) participants did not differ in age, sex, educational level, APOE allele status, MMSE score, or Geriatric Depression Scale score. There was a trend toward worse cognitive status in infarct-positive participants (P = .055), which was especially apparent in individuals with a cortical infarct (CDR = 0: 2 of 11 participants [18%]; CDR ≥ 0.5: 9 of 11 [82%]; P = .02). Of the 34 infarct-positive individuals, 11 had infarcts in cortical gray matter, 19 in subcortical gray matter, 23 in cortical white matter, and 14 in another location. These numbers do not sum to 34 because 10 individuals had more than 1 infarct (mean [range], 2.7 [2–4] infarcts), and most infarcts affected multiple regions. Cortical gray matter infarcts primarily affected the frontal lobe (n = 9). All subcortical gray matter infarcts occupied the basal ganglia, with most specifically affecting the striatum (n = 17). Three participants had a lesion in cortical white matter only and 4 in subcortical gray matter only. No participant had an isolated cortical gray matter lesion. Five participants had infarcts affecting both cortical gray and white matter, 9 had lesions affecting subcortical gray and cortical white matter, and 6 had lesions affecting all 3 regions (cortical gray, white, and subcortical gray matter). The mean WMH volume as a percentage of total cranial volume across the sample was 0.7%. Infarct-positive individuals had greater WMH volume than did infarct-negative individuals (P = .02). The WMH volume did not differ by MRI field strength (between 3-T and 4-T scans; P = .20).
VBI RELATIONSHIP WITH PiB
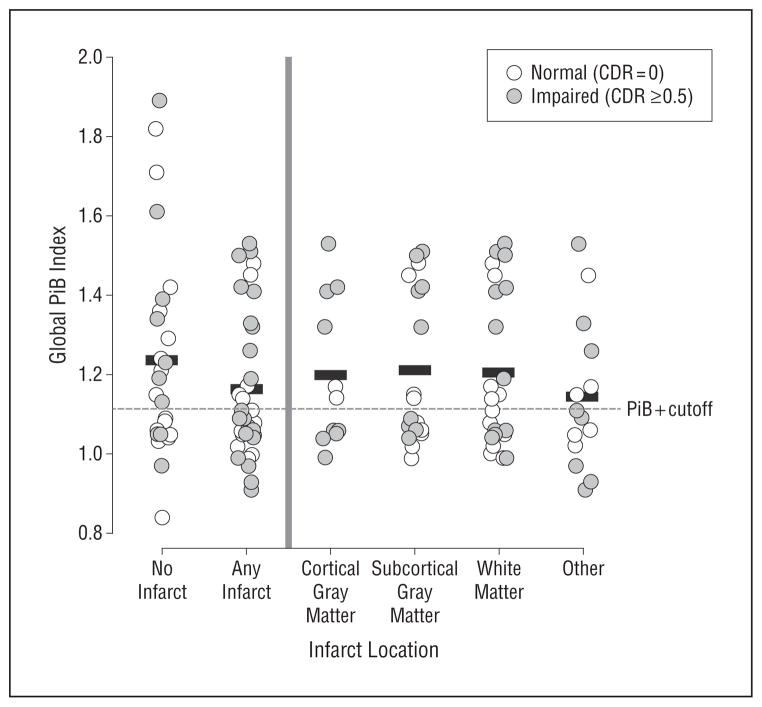
Presence of an infarct did not increase the likelihood that an individual was PiB-positive (P = .26 [Figure]). Dividing participants by cognitive status (CDR = 0 vs CDR ≥ 0.5) revealed no relationship between having an infarct and PiB uptake in normal (P = .64) or impaired (P = .16) individuals. There was no effect of infarct location (P >.30 for all comparisons) or cognitive status (P = .50) on PiB uptake and no interaction between presence of an infarct and cognitive status (P = .81 [Figure]). Individuals with a cortical gray matter infarct did not have greater Global PiB Index values (P = .96) and were not more likely to be PiB-positive compared with persons without a cortical gray matter infarct (P = .61). Infarct-positive and infarct-negative participants did not differ in any of the individual regions of interest constituting the Global PiB Index (P> .12 for all comparisons) or the occipital lobe (P = .44).
Figure.
Scatterplot of Global Pittsburgh Compound B (PiB) Index volume stratified by infarct presence and location. Black bars represent mean Global PiB Index for infarct groups. The dichotomous categorization of Global PiB Index (PiB+/−) is shown by the dashed line. Circles represent clinical status of individuals: white, normal (Clinical Dementia Rating [CDR] = 0); gray, impaired (CDR ≥ 0.5).
White matter hyperintensity burden showed no bivariate relationship with Global PiB Index (ρ= −0.07; P = .63). In addition, there was no relationship between the number of infarcts (0, 1, or >1) and the Global PiB Index (P = .42).
VBI AND PiB RELATIONSHIPS WITH COGNITION
All measures of cognition were normally distributed (P>.19 for all comparisons) and showed a similar range of performance (verbal memory: minimum score = 52.4, maximum score = 141.1; nonverbal memory: minimum = 46.8, maximum = 122.4; and executive function: minimum = 48.6, maximum = 123.6). To report the predictors of neuropsychological test performance, we provide standardized β values to allow for direct comparison of the strength of each variable’s independent contribution (Table 3).
Table 3.
Variance in Composite Cognitive Test Performance Explained by Demographics and Neuroimaging Markers of Pathologic Changea
| Stage | Demographic Characteristic | Neuroimaging Marker of Pathologic Change | |||||
|---|---|---|---|---|---|---|---|
| Infarct | WMH Volume | Aβ, Global PiB Index | |||||
| Age | Female Sex | Educational Level | Cortical Gray Matter | Subcortical Gray Matter | White Matter | Other | Number (0, 1, or >1) |
| Verbal Memory | |||||||
| 1 | −.11 | .06 | .24 | ||||
| 2 | −.06 | −.29b | −.06 | −.02 | −.19 | .02 | −.18 |
| 3 | −.24 | −.02 | .16 | −.34b | .19 | −.08 | |
| Nonverbal Memory | |||||||
| 1 | −.27b | .28b | .36c | ||||
| 2 | .06 | −.36c | .05 | −.05 | −.13 | −.08 | −.27b |
| 3 | −.31b | −.21 | .45d | −.23 | .02 | −.12 | |
| Executive Function | |||||||
| 1 | −.01 | .08 | .51d | ||||
| 2 | −.47d | −.33b | −.17 | −.20 | −.14 | −.21 | −.15 |
| 3 | −.08 | −.19 | .38c | −.54c | .05 | −.04 |
Verbal Memory
Demographic variables were not significant predictors of verbal memory performance in stage 1 (Table 3). In stage 2, presence of an infarct in subcortical gray matter was significantly related to memory performance (standardized β= −0.29; P = .02). Neither WMH volume nor PiB was a significant predictor of verbal memory. When all variables were included in the third stage (subcortical infarct from stage 2), the final equation explained 16.7% of the variance in verbal memory performance, and an infarct in the subcortical gray matter emerged as the only significant predictor after controlling for the effects of the other variables.
Nonverbal Memory
All demographic variables were significant predictors of nonverbal memory in stage 1; lower age, higher educational level, and female sex were associated with better performance (Table 3). In stage 2, an infarct in the subcortical gray matter again emerged as a significant predictor (standardized β= −0.36; P = .007). White matter hyperintensity volume was not a significant predictor; however, PiB emerged as a significant predictor of performance (standardized β = −0.27; P = .04). The stage 3 model included all variables (subcortical infarct from stage 2) and explained 48.0% of the variance in nonverbal memory performance. Age and educational level remained significant predictors, sex and an infarct in the subcortical gray matter were reduced to trends, and PiB was no longer a predictor, presumably because of its significant relationship with sex and age.
Executive Function
Educational level was a significant positive predictor of executive function in stage 1 (Table 3). In stage 2, when entered separately, infarcts in the cortical and subcortical gray matter were both significant predictors of executive function. When entered together, only cortical gray matter infarct emerged as a significant predictor (standardized β = −0.41; P = .002). Therefore, only having a cortical gray matter infarct was retained for the stage 3 model. Neither WMH volume nor Global PiB Index was a significant predictor of performance. The stage 3 model explained 43.3% of the variance in executive function performance, with cortical gray matter infarct and educational level remaining as significant predictors.
All the cognitive analyses were rerun excluding the 6 individuals with CDR >0.5 to confirm that the findings were not driven by individuals with dementia. In stage 2, the Global PiB Index showed a trend toward predicting verbal memory performance (standardized β = − 0.23; P = .09). The stage 3 models for all cognitive domains remained essentially unchanged.
COMMENT
The aim of this study was to determine the relative contributions of Aβ and VBI to specific areas of cognition in a sample of clinically normal, cognitively impaired, and mildly demented individuals enriched for vascular risk factors. Vascular brain injury had the greatest influence across all measured cognitive domains and was not related to Aβ. Dividing participants by cognitive status and regional location of the infarct (including the cortex) did not alter this finding. Approximately half the sample was classified as PiB-positive, and PiB was associated with both APOE genotype (with those having the e4 allele showing greater Aβ deposition) and increasing age, which demonstrates that the effects of well-established risk factors for Aβ were present and detectable in our sample. Thus, it may be that VBI and Aβ, both individually prevalent, frequently converge in dementia, each affecting function but neither bearing a causal relationship to the other.30–33
In this study, infarct affected all cognitive domains.34 Cortical gray matter infarcts influenced executive function, potentially because they predominantly encompassed the frontal lobe.35 Subcortical gray matter infarction, all of which involved the basal ganglia with the majority affecting the striatum, influenced verbal and non-verbal memory.36,37 There is suggestion that the striatum is involved in episodic memory learning,38 and subcortical infarct has a significant association with amnestic MCI.39 White matter hyperintensity burden was not related to any cognitive measure. The association between WMH and cognition (especially executive function) is generally small and most evident in cognitively normal samples.35,36 We may not have found a significant influence of WMH because of the cognitive heterogeneity of the sample and also potentially because the effects of infarct surpassed those due to WMH.
Through the use of amyloid imaging, we measured the separate and combined influence of Aβ and vascular disease on cognition. In this sample of participants enriched for increased vascular risk, infarct was associated with both clinical status and cognition, whereas PiB was not. Also, we did not find an additive negative effect of Aβ on function in that there was no greater number of infarct-positive/PiB-positive individuals in the clinically impaired group. Thus, we found no evidence of a negative effect of PiB on cognition. Why?
First, it may be an issue of statistical power because subjects in our study ranged from cognitively normal to mildly impaired. This sample, in contrast to others,15,37 may simply not be large enough to detect the modest effects of PiB within this somewhat restricted range of cognitive function. However, if this is the case, our results still say something about the relative strength of the effects of VBI and amyloid in normal to mildly impaired cognition.
A more substantive explanation is that, because amyloid deposition is an initiating event, the strength of association between PiB and cognition may depend on the cognitive syndrome studied. In cognitively normal persons, the downstream consequences (eg, neuronal death and synaptic loss) of amyloid have not yet occurred and the relationship of PiB to cognition is weak. In dementia, the downstream events have nearly always occurred to a degree, but Aβ may have already reached a plateau38; consequently, the relationship is only modest. In MCI, sometimes the downstream events have occurred but sometimes they have not, other causes of impairment (such as VBI) may be present, and the amyloid-cognition association is weak. Thus, across a wide range of disease severity, as Aβ levels increase, clinical function declines15,39; however, if the range of disease severity is limited, as it was here, amyloid effects may be difficult to detect.
In contrast to Aβ, VBI represents end-stage pathologic consequences of the vascular pathogenic process. Thus, in a sample such as this one that is restricted with regard to the range of cognitive impairment, it is perhaps not surprising that end-organ damage (VBI) has greater impact than do precipitating pathogenic events (Aβ). These results suggest that VBI, specifically infarction, warrants consideration when examining causes of MCI. Although our sampling does not permit generalizing the frequency of VBI to the aged population, other studies clearly show a high prevalence of this disorder.40
The literature suggests a link between vascular risk factors (eg, hypertension and diabetes mellitus) and Aβ aggregation,41–43 and indeed an association between elevated coronary risk and Aβ deposition has been reported recently41 (however, see Chui et al44). While it is tempting to conclude that the high rate of vascular risk in our sample accounts for the relatively high proportion of PiB-positive cases, more basic factors, such as the mean age of 78 years, might also account for this. In addition, half the participants were cognitively impaired or demented, groups in which the prevalence of cerebral Aβ exceeds 50%.45
If there is an association between vascular risk and Aβ, it does not appear to be mediated by VBI—at least as defined by MRI-measured infarcts and WMH. This does not exclude the possibility that more subtle types of vascular disease, such as microvascular damage or blood-brain barrier dysfunction, contribute to Aβ deposition.46
Cerebrovascular disease is highly prevalent in the elderly population. In this study of clinically normal to mildly demented individuals, we found that VBI negatively affected cognition. Although MCI is clearly a significant risk factor for AD, the present data suggest that the impact of VBI should be considered when defining the etiology of MCI. Reductions in cerebrovascular disease may be important in preventing MCI.
Acknowledgments
Funding/Support: This work was supported by grants AG012435 (Drs Reed and Chui), AG031563 (Dr Reed), AG00266 (Dr Marchant), and AG10129 (Dr DeCarli) from the National Institutes of Health.
Footnotes
Additional Contributions: Todd Bloom, MS, Shannon Buckley, BA, Adrian Herbert, MBA, and Ling Zheng, PhD, helped with data preparation, and Tadd Haight, PhD, Elizabeth Mormino, PhD, Gil Rabinovici, MD, Sylvia Villeneuve, PhD, and Miranka Wirth, PhD, provided helpful discussions throughout the project.
Author Contributions: Dr Marchant takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Marchant, Reed, Madison, Mack, Mungas, Chui, and Jagust. Acquisition of data: Reed, Sanossian, Kriger, Dhada, Mack, DeCarli, and Chui. Analysis and interpretation of data: Marchant, Reed, Madison, Kriger, DeCarli, Weiner, and Jagust. Drafting of the manuscript: Marchant. Critical revision of the manuscript for important intellectual content: Marchant, Reed, Sanossian, Madison, Kriger, Dhada, Mack, De-Carli, Weiner, Mungas, Chui, and Jagust. Statistical analysis: Marchant, Madison, Mack, and Mungas. Obtained funding: Reed, Weiner, Chui, and Jagust. Administrative, technical, and material support: Kriger, Dhada, DeCarli, Weiner, Chui, and Jagust. Study supervision: Jagust.
Conflict of Interest Disclosures: Dr Weiner reports (for 2010–2012) having served on scientific advisory boards for Eli Lilly & Company, Araclon and Institut Catala de Neurociencies Aplicades, the Research Advisory Committee on Gulf War Veterans’ Illnesses, Biogen Idec, and Pfizer Inc; having served as a consultant for AstraZeneca, Araclon, Medivation/Pfizer Inc, Ipsen, TauRx Therapeutics Ltd, Bayer Healthcare, Biogen Idec, Exonhit Therapeutics SA, Servier, Synarc, Janssen, Harvard University, and KLJ Associates; having received travel funds from NeuroVigil, Inc, CHRU-Hopital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Eli Lilly & Company, University of California, San Diego–Alzheimer’s Disease Neuroimaging Initiative (ADNI), Paris University, Institut Catala de Neurociencies Aplicades, University of New Mexico School of Medicine, Ipsen, Clinical Trials on Alzheimer’s Disease, Pfizer Inc, the AD/PD meeting (Conference on Alzheimer’s and Parkinson’s Diseases), Paul Sabatier University, Novartis, Tohoku University, Fundacio ACE, and Travel eDreams, Inc; having served on the editorial advisory boards of Alzheimer’s & Dementia and Magnetic Resonance Imaging; having received honoraria from NeuroVigil, Inc, Institute Catala de Neurociencies Aplicades, PMDA (Pharmaceuticals and Medical Devices Agency)/Japanese Ministry of Health, Labour, and Welfare, Tohoku University, and Alzheimer’s Drug Discovery Foundation; having received commercial entities research support from Merck and Avid; having received government entities research support from the Department of Defense and the Department of Veterans Affairs; and owning stock options in Synarc and Elan. Dr Weiner also reports that the following organizations contributed to the Foundation for the National Institutes of Health and thus to the National Institute on Aging–funded ADNI: Abbott, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Anonymous Foundation, AstraZeneca, Bayer Healthcare, BioClinica, Inc (ADNI 2), Bristol-Myers Squibb, Cure Alzheimer’s Fund, Eisai, Elan, Gene Network Sciences, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson & Johnson, Eli Lilly & Company, Medpace, Merck, Novartis, Pfizer Inc, Roche, Schering Plough, Synarc, and Wyeth.
References
- 1.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67 (3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 5.Rowe CC, Ng S, Ackermann U, et al. Imaging 3-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 8.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol. 2005;62(5):758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 9.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Medical Research Council Cognitive Function and Ageing Study. Age, neuropathology, and dementia. N Engl J Med. 2009;360(22):2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 10.Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, Haroutunian V. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66(1):49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigue KM, Kennedy KM, Devous MD, Sr, et al. 3-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-3 peptide deposition identified with Pitts-burgh compound B: cognitive decline associated with Aβ deposition. Arch Neurol. 2009;66(12):1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74(10):807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of A3 and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchant NL, Reed BR, Decarli CS, et al. Cerebrovascular disease, 3-amyloid, and cognition in aging. Neurobiol Aging. 2012;33(5):1006, e25–1006.e36. doi: 10.1016/j.neurobiolaging.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Kim SH, Kim GH, et al. Identification of pure subcortical vascular dementia using 11C-Pittsburgh compound B. Neurology. 2011;77(1):18–25. doi: 10.1212/WNL.0b013e318221acee. [DOI] [PubMed] [Google Scholar]
- 18.Hedden T, Van Dijk KR, Shire EH, Sperling RA, Johnson KA, Buckner RL. Failure to modulate attentional control in advanced aging linked to white matter pathology. Cereb Cortex. 2012;22(5):1038–1051. doi: 10.1093/cercor/bhr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed BR, Mungas DM, Kramer JH, et al. Profiles of neuropsychological impairment in autopsy-defined Alzheimer’s disease and cerebrovascular disease. Brain. 2007;130(pt 3):731–739. doi: 10.1093/brain/awl385. [DOI] [PubMed] [Google Scholar]
- 20.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore Longitudinal Study of Aging. Ann Neurol. 2008;64(2):168–176. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17(3):380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- 22.Mormino EC, Kluth JT, Madison CM, et al. Alzheimer’s Disease Neuroimaging Initiative. Episodic memory loss is related to hippocampal-mediated 3-amyloid deposition in elderly subjects. Brain. 2009;132(pt 5):1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadjidemetriou S, Studholme C, Mueller S, Weiner M, Schuff N. Restoration of MRI data for intensity non-uniformities using local high order intensity statistics. Med Image Anal. 2009;13(1):36–48. doi: 10.1016/j.media.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadjidemetriou S, Lorenzen P, Schuff N, Mueller S, Weiner M. Computational atlases of severity of white matter lesions in elderly subjects with MRI. Med Image Comput Comput Assist Interv. 2008;11(pt 1):450–458. doi: 10.1007/978-3-540-85988-8_54. [DOI] [PubMed] [Google Scholar]
- 25.Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Res. 1993;50(2):121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- 26.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46(13):2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 27.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 28.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain. 2010;133 (pt 2):512–528. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53(7):1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 31.Riekse RG, Leverenz JB, McCormick W, et al. Effect of vascular lesions on cognition in Alzheimer’s disease: a community-based study. J Am Geriatr Soc. 2004;52(9):1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA. 1997;277(10):813–817. [PubMed] [Google Scholar]
- 33.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 2010;119(4):421–433. doi: 10.1007/s00401-010-0654-5. [DOI] [PubMed] [Google Scholar]
- 34.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 35.Brickman AM, Siedlecki KL, Muraskin J, et al. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging. 2011;32(9):1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137(5):753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris JC, Roe CM, Grant EA, et al. Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic bio-markers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pike KE, Savage G, Villemagne VL, et al. 3-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 40.Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357(9251):169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 41.Reed BR, Marchant NL, Jagust WJ, Decarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging. 2012;33(9):1979–1987. doi: 10.1016/j.neurobiolaging.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pluta R, Ulamek M, Jabłoński M. Alzheimer’s mechanisms in ischemic brain degeneration. Anat Rec (Hoboken) 2009;292(12):1863–1881. doi: 10.1002/ar.21018. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Zhou K, Wang R, et al. Hypoxia-inducible factor 13 (HIF-13)–mediated hypoxia increases BACE1 expression and 3-amyloid generation. J Biol Chem. 2007;282(15):10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- 44.Chui HC, Zheng L, Reed BR, Vinters HV, Mack WJ. Vascular risk factors and Alzheimer’s disease: are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? an evidence-based review. Alzheimers Res Ther. 2012;3(6):36. doi: 10.1186/alzrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73(10):754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sagare AP, Bell RD, Zlokovic BV. Neurovascular defects and faulty amyloid-3 vascular clearance in Alzheimer’s disease [published online June 29, 2012] J Alzheimers Dis. doi: 10.3233/JAD-2012-129037. [DOI] [PMC free article] [PubMed] [Google Scholar]