Notch-dependent differentiation of adult airway basal stem cells (original) (raw)
. Author manuscript; available in PMC: 2013 Sep 20.
Published in final edited form as: Cell Stem Cell. 2011 Jun 3;8(6):639–648. doi: 10.1016/j.stem.2011.04.003
Summary
The epithelium lining the airways of the adult human lung is composed of ciliated and secretory cells together with undifferentiated basal cells (BCs). The composition and organization of this epithelium is severely disrupted in many respiratory diseases. However, little is known about the mechanisms controlling airway homeostasis and repair after epithelial damage. Here, we exploit the mouse tracheobronchial epithelium, in which BCs function as resident stem cells, as a genetically tractable model of human small airways. Using a reporter allele we show that the low level of Notch signaling at steady state is greatly enhanced during repair and the generation of luminal progenitors. Loss-of-function experiments show that Notch signaling is required for the differentiation, but not self-renewal, of BCs. Moreover, sustained Notch activation in BCs promotes their luminal differentiation, primarily towards secretory lineages. We also provide evidence that this function of Notch signaling is conserved in BCs from human airways.
- Notch signaling is active in steady state airways and increased during repair
- Notch is required for differentiation, but not self-renewal, of airway basal cells
- Notch promotes luminal differentiation of mouse basal stem cells
- Our data suggest this mechanism is conserved in human basal cells
Introduction
The epithelium lining the conducting airways of the lungs is the first line of defense against inhaled particles and pathogens. This vital function depends on the proper proportions, distribution, and physiological activity of differentiated cell types, including ciliated and secretory cells. The loss of these cells due to wear and tear, inflammation, or injury must be balanced with replacement from local stem and progenitor cells. In debilitating lung diseases, including cystic fibrosis, asthma, chronic obstructive pulmonary disease (COPD) and cancer, the airway epithelium undergoes pathological remodeling, with profound changes in the number and proportion of cell types (Randell, 2006; Rock et al., 2010; Shi et al., 2009). To develop new therapies there is a pressing need to understand the mechanisms regulating epithelial maintenance and repair by resident stem cells in the mature airway.
The tracheobronchial airways of adult mice histologically resemble the conducting airways of the human lung, providing a genetically tractable model for studying cell lineages and molecular mechanisms relevant to human airway disease (Rock et al., 2010). In both species the pseudostratified epithelium contains ciliated, secretory, and basal cells in roughly equal proportions (Boers et al., 1998; Nakajima et al., 1998; Rock et al., 2009). Work from our lab and others suggests that in these epithelia the basal cells (BCs), as a population, are the adult tissue stem cells that self-renew over the long term and give rise to specialized luminal cells (Hackett et al., 2008; Hong et al., 2004; Randell et al., 1991; Rock et al., 2009). However, little is known about the mechanisms that regulate their proliferation, self-renewal, and selection of daughter cell fate.
In many adult and embryonic tissues, the Notch signaling pathway plays critical roles in stem cell maintenance and differentiation (Chiba, 2006; Liu et al., 2010). In some adult organs, including the mammary gland and interfollicular epidermis, canonical Notch signaling is associated with differentiation of stem cells (Blanpain et al., 2006; Bouras et al., 2008). In other tissues including the hematopoetic and nervous systems, Notch signaling maintains the stem cell pool (Duncan et al., 2005; Imayoshi et al., 2010). The functions of the Notch signaling pathway are therefore context dependent and notoriously complex, necessitating stage and cell type specific analysis for each organ system examined. Studies on the role of Notch signaling in the lung have so far been confined to embryonic development. During branching morphogenesis, pharmacological inhibition of the pathway perturbs the balance of proximal and distal progenitors (Tsao et al., 2008). Later, several binary cell fate decisions in the epithelium are regulated by Notch, including the selection of neuroendocrine versus non-neuroendocrine (Ito et al., 2000) and secretory versus ciliated lineages (Guseh et al., 2009; Morimoto et al., 2010; Tsao et al., 2009). During the final stages of lung development, Notch signaling promotes the maturation of mesenchymal myofibroblasts that is critical for the formation of alveoli (Xu et al., 2010). These studies are extremely important to our understanding of lung development. However, they were either performed before the maturation of basal stem cells or in distal airways of the mouse lung that do not contain BCs. The functions of Notch signaling in BCs of adult airways, relevant to human respiratory disease, are currently unclear.
Here, we demonstrate that Notch signaling regulates behaviors of basal stem cells in adult mouse and human airways. We use a transgenic reporter to demonstrate that Notch signaling is active at steady state and increases during epithelial repair when basal stem cells generate undifferentiated, luminal daughters. Genetic and pharmacological loss-of-function studies show that the differentiation of airway BCs is Notch-dependent. Conversely, sustained genetic activation of the pathway promotes the luminal differentiation of BCs, primarily towards secretory lineages. We provide evidence that these functions of Notch signaling are conserved in human airway BCs and so may contribute to pathological airway remodeling in respiratory diseases.
Results
Expression of Notch signaling components in the pseudostratified airway epithelium
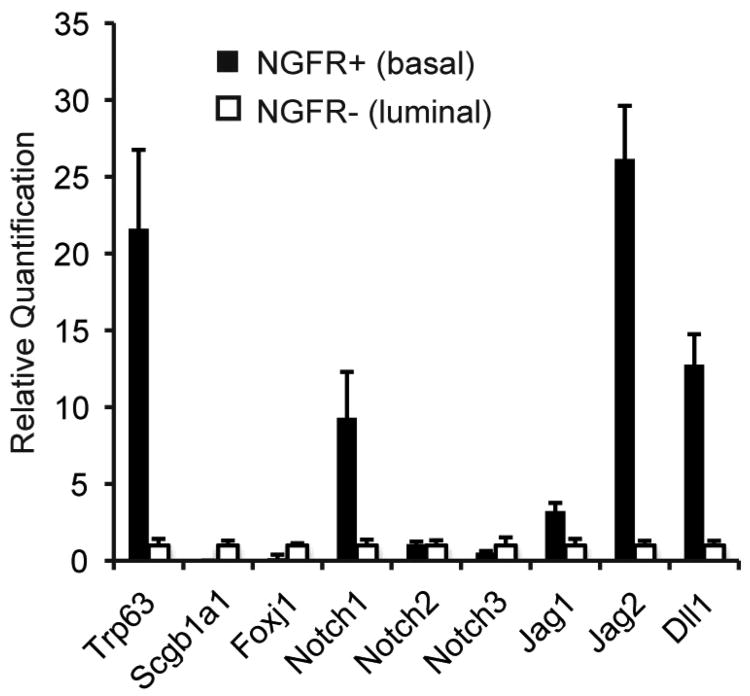
To characterize Notch signaling in adult airways, we first analyzed the expression of pathway genes in basal and luminal cells isolated from steady state mouse tracheobronchial epithelium. We purified NGFR+ BCs by FACS and confirmed by immunofluorescent staining of cytospin preparations that ∼90% of the population was positive for TRP63, a transcription factor expressed in BCs of stratified and pseudostratified epithelia throughout the body (Rock et al., 2009). Quantitative RT-PCR (qPCR) confirmed that the NGFR+ population was enriched for transcripts of Trp63 (Figure 1). The NGFR- population, mostly comprised of luminal secretory and ciliated cells, had higher levels of RNA encoding Scgb1a1, a secretory product of Clara cells, and FoxJ1, a transcription factor specific to ciliated cells (Figure 1). As a population, BCs were significantly enriched for transcripts encoding Notch1, Dll1 and Jag2. Jag1 was also expressed at slightly higher levels in BCs than luminal cells. There was no differential expression of Notch2 and Notch3, and transcripts for Notch4, Dll3, or Dll4 could not be detected in either population.
Figure 1. Expression of Notch pathway genes in adult airway epithelium.
Expression of Notch pathway components in BCs (black bars) and luminal cells (open bars) assessed by quantitative RT-PCR. y-axis is relative quantification (RQ) normalized to Gapdh and expression of each gene in luminal cells is set to 1 for comparison. Error bars show 95% confidence interval based on triplicate samples. Data shown are representative of three independent experiments.
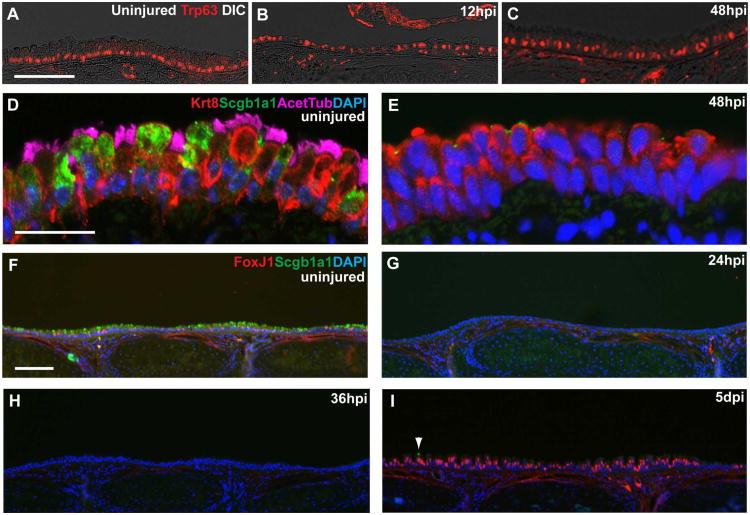
Compared to some other organs, cells of the airway epithelium are long-lived (Kauffman, 1980) and there is relatively little proliferation, self-renewal and differentiation of airway stem cells under steady state conditions. Therefore, we used an injury/repair model of the mouse tracheobronchial epithelium to study the role of Notch signaling in vivo during conditions of enhanced cell turnover. In this model, adult mice are exposed to SO2 for three hours and returned to room air. This treatment results in the death and sloughing of most luminal cells, but leaves behind BCs throughout the tracheobronchial region (Borthwick et al., 2001; Evans et al., 2001; Rawlins et al., 2007; Rock et al., 2009). BCs fuel an orderly and efficient repair process during which they remain in close proximity to the basal lamina and continue to express BC markers including Trp63, Krt5 and Pdpn (Figure 2A-C and data not shown). Cell proliferation peaks 24-hours post-injury (hpi) and the epithelium transiently stratifies, beginning around 36hpi (Figure 2C,E and Rawlins et al., 2007). At this time, abundant suprabasal cells are negative for markers of either BCs or differentiated ciliated and secretory cells. These undifferentiated suprabasal cells express Krt8, a cytokeratin expressed in luminal cells, including secretory and ciliated cells in the uninjured trachea (Figure 2D,E). We call these cells “early progenitors” (EPs) since they are derived from basal stem cells and generate ciliated and secretory lineages. Fully differentiated ciliated and secretory cells are first observed five days post-injury (Figure 2F-I).
Figure 2. SO2 inhalation injury model to study mechanisms regulating basal cell behaviors.
(A) Control uninjured trachea stained with anti-Trp63 (red, BCs). (B,C) Trp63+ BCs survive SO2 inhalation injury and remain close to the basal lamina 12 hours post-injury (hpi) and during transient stratification 48hpi. Low levels of Trp63 are detected in some suprabasal cells at this time. (D) In the uninjured trachea, ciliated (pink, acetylated tubulin) and Clara (green, Scgb1a1) cells are Krt8+ (red). (E) At 48hpi Krt8+ cells are abundant but negative for markers of ciliated and Clara cells. (F-I) Immunohistochemistry for FoxJ1 (red, ciliated cells) and Scgb1a1 (green, Clara cells) shows these cell types are present in roughly equal proportions in the uninjured trachea. However, neither luminal cell type is observed at (G) 24 or (H) 36hpi. (I) At 5dpi there are abundant FoxJ1+ cells but very few Scgb1a1+ Clara cells (arrowhead). Nuclei were stained with DAPI. Scale bars:A,D 25μm; F 100μm.
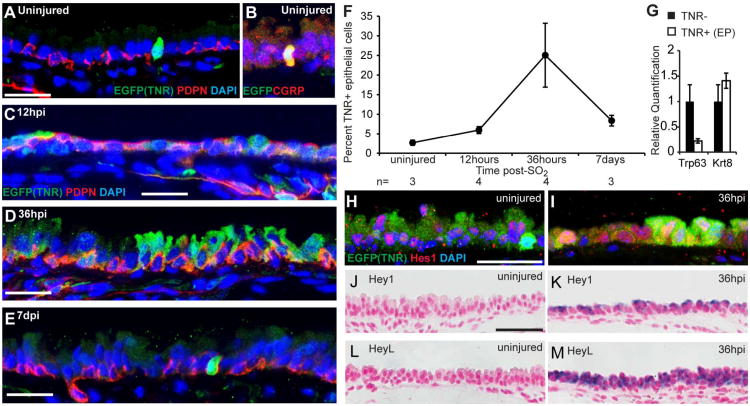
We used the Transgenic Notch Reporter (TNR) mouse line (Mizutani et al., 2007) to determine whether _Rbpj_-dependent canonical Notch signaling is active in airway epithelium in vivo. In this line, GFP is expressed in Notch-responsive cells under the control of four copies of the Rbpj binding site and a minimal promoter. We quantified Notch-responsive cells by manually counting TNR+ cells on confocal images of immunostained tissues sections. In the steady state adult trachea, only 2.7±0.7% of epithelial cells were TNR+ (Figure 3A,F). Of these, ∼75% were individual cells positive for CGRP and Prox1, markers of neuroendocrine cells (Figure 3B and data not shown). We hypothesized that the low level of Notch signaling reflected the low rate of cell turnover and paucity of putative EPs under steady state conditions. To test this, we used the SO2 injury model to increase the kinetics of proliferation, self-renewal, and the production of putative EPs and differentiated cell types in vivo. Twelve hours after injury, before stratification and the generation of abundant EPs, there was a modest increase in the proportion of TNR+ cells to 6.0±0.9% (Figure 3C,F). At 36hpi, when Trp63-;Krt8+ EPs are abundant, the proportion of TNR+ cells increased to 25.1±8.1% (Figure 3D,F). This value decreased to 8.4±1.4% at 7dpi (Figure 3E,F). To determine whether EPs proliferate during epithelial repair, we stained sections of TNR tracheas 36hpi with antibodies against GFP and phosphorylated histone H3 and found that the proliferative cells were almost exclusively GFP- (data from four mice not shown). However, this finding does not rule out the possibility that EPs (or their daughters) can proliferate after they have been specified towards a luminal fate and lost the expression of GFP or at other times during epithelial repair. In the future, as new markers of EPs are identified, it will be important to readdress this issue.
Figure 3. Notch signaling is active during epithelial repair.
(A-E) Sections of trachea from adult TNR mice stained with anti-GFP to show TNR+ Notch responsive cells (green) and anti-Pdpn (red, BCs) in (A,B) uninjured trachea or (C) 12hpi, (D) 36hpi, and (E) 7dpi. (B) In uninjured tracheas, ∼73% of TNR+ cells are CGRP+ (red, neuroendocrine cells). (F) Percent of epithelial cells scored as TNR+ during repair. Data are means ±SEM and n is the number of tracheas examined at each time. (G) qPCR on cDNA synthesized from TNR- cells (black bars) and TNR+ putative EPs (white bars) obtained by FACS at 36hpi. y-axis is relative quantification (RQ) compared to Gapdh and expression of each gene in TNR- cells (non-EPs) is set to 1 for comparison. Error bars show 95% confidence interval based on triplicate samples. Data are representative of two independent experiments. (H,I) Sections of tracheas from TNR mice stained with anti-Hes1 (red) and anti-GFP. Many luminal and basal cells express Hes1 in the uninjured trachea (H) and at 36hpi (I). In the repairing trachea, the expression of Hes1 is not restricted to GFP-expressing cells. (J-M) RNA in situ hybridization showing expression (purple) of (K) Hey1 and (M) HeyL 36hpi but not in uninjured tracheal epithelium (J,L). Nuclei were stained with DAPI or nuclear fast red. Fluorescent images are single confocal planes. Scale bars:A,C,D,E,H 25μm; J 50μm.
To begin characterizing the role of the Notch pathway at the molecular level, we purified GFP+ (EPs) and GFP- cells from TNR tracheas at 36hpi by FACS. qPCR on cDNA synthesized from these populations confirmed that GFP+ EPs have lower levels of Trp63 mRNA than the GFP- population that contains primarily BCs at this time (Figure 3G). This is consistent with our observation that the majority of TNR+ cells did not express Pdpn, a transmembrane glycoprotein expressed on BCs of the conducting airway epithelium, at any time examined. These data support the idea that Notch is active in EPs but not in Trp63+ BCs. Next, we performed immunohistochemistry and RNA in situ hybridization for Notch targets during the repair process. Many basal and luminal cells expressed the transcription factor Hes1 under steady state and reparative conditions (Figure 3H,I). Although Hes1 expression levels were qualitatively higher following SO2 injury, high levels of Hes1 were not restricted to TNR+ cells and not all TNR+ cells were Hes1+ (Figure 3H,I). The widespread distribution of Hes1 in the tracheobronchial epithelium is similar to that reported for the interfollicular epidermis (Blanpain et al., 2006), another tissue in which Notch signaling is associated with stem cell differentiation. In contrast to Hes1, transcripts for the bHLH transcription factors Hey1 and HeyL were strongly and specifically upregulated during epithelial repair, with highest levels at 36hpi (Figure 3J-M).
Notch signaling promotes luminal differentiation of basal cells
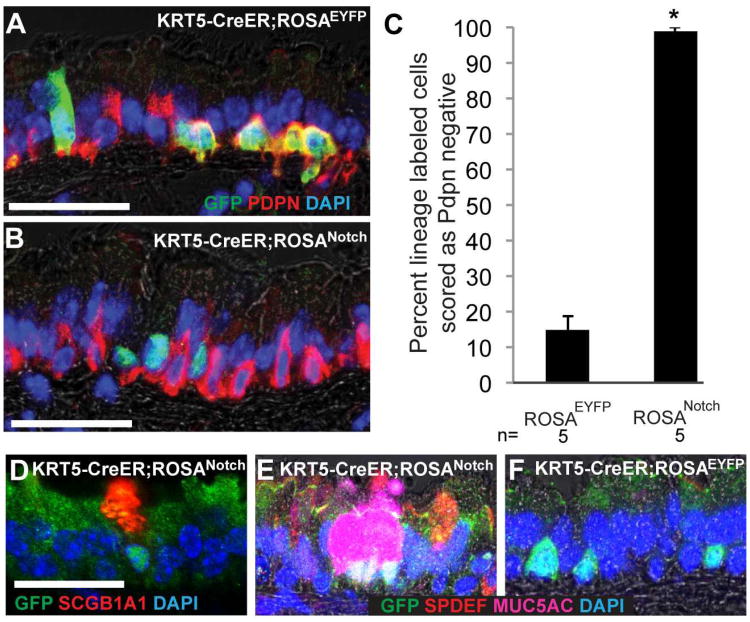
To test whether Notch signaling is sufficient to promote the luminal differentiation of basal stem cells in vivo, we activated the Notch pathway in BCs using the _ROSANotc_h mouse line (Murtaugh et al., 2003). Following Cre-mediated recombination, this allele results in the constitutive expression of nuclear GFP and the intracellular domain of Notch1 that is sufficient for ligand-independent, cell autonomous Notch activation. We injected adult KRT5-CreER;ROSANotch mice with tamoxifen to induce constitutive expression of the Notch1 intracellular domain and GFP specifically in BCs. KRT5-CreER;ROSAEYFP mice of similar ages were controls. After a two-week chase period, we assessed the expression of GFP/YFP and markers of differentiation on confocal images of tissues sections. In control tracheas, only 14.8±3.9% of EYFP+ lineage labeled cells had differentiated and lost expression of the BC marker Pdpn (Figure 4A,C). This demonstrates that the majority of BCs do not differentiate in this period under steady state conditions and is consistent with our previous findings (Rock et al., 2009). In contrast, 98.9±1.1% of cells expressing the Notch1 intracellular domain and nuclear localized GFP were scored as Pdpn- (Figure 4B,C). Therefore, Notch activation is sufficient to drive luminal differentiation of BCs in vivo. Importantly, the Notch+,Pdpn- cells were neither Cgrp+ nor Prox1+ (data not shown). This suggests that sustained Notch activation does not promote the differentiation of BCs into neuroendocrine cells, even though these cells are TNR+ at steady state (Figure 2B and data not shown). Rather, of the Notch+ cells, 81.2±10.5% stained positively for Scgb1a1, a secretory product of Clara cells (Figure 4D), and 56.9±12.6% were positive for markers of goblet secretory cells including the transcription factor Spdef (Park et al., 2007) and the mucin Muc5ac (Figure 4E). In control KRT5-CreER;ROSAEYFP tracheas, only 3.3±3.3% and 5.3±5.3% of EYFP+ cells were scored as Scgb1a1+ or Muc5ac+, respectively. Together, these data suggest that sustained activation of the Notch pathway induces luminal differentiation of BCs and, in particular, promotes the secretory lineage. The remaining Notch+;Pdpn- cells were not Foxj1+ ciliated cells. Therefore, they may be EPs or immature secretory cells. These findings are consistent with data suggesting that Notch promotes secretory cell differentiation from non-basal cell progenitors in the embryonic trachea and distal lung (Guseh et al., 2009; Tsao et al., 2009).
Figure 4. Notch gain-of-function promotes luminal differentiation of mouse airway basal cells in vivo.
(A) Adult KRT5-CreER;ROSAEYFP and (B) KRT5-CreER;ROSANotch mice were injected with tamoxifen and sections stained 2 weeks later with anti-GFP (green, lineage label is cytoplasmic for ROSAEYFP and nuclear for ROSANotch) and anti-Pdpn (red, BCs). (C) Percent of lineage labeled cells scored that had differentiated and lost expression of the BC marker Pdpn. Data shown are means ± SEM and n is the number of animals analyzed. *p<0.0001. (D) 81% of cells expressing the Notch1 intracellular domain and nuclear localized GFP stained positively for the Clara cell marker Scgb1a1 and (E) ∼57% were positive for markers of goblet cells including Spdef and Muc5ac. Note that these differentiated cells often appear in pairs or small clusters. (F) Only ∼5% of lineage labeled cells in controls were positive for markers of secretory cells including Scgb1a1, Muc5ac, or Spdef. Scale bars: A,B 40μm; D 30μm.
Notch gain-of-function analysis in human airway basal cells
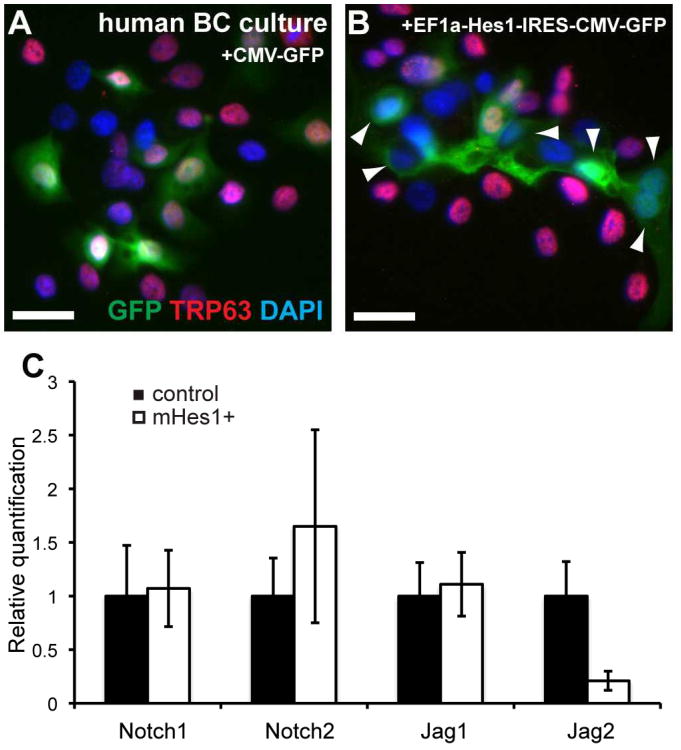
In humans, the pseudostratified epithelium containing BCs extends deep into the lung, right down to the alveolar duct (Boers et al., 1998; Nakajima et al., 1998; Rock et al., 2010). Our model predicts that activation of Notch signaling in human airway BCs will be associated with the downregulation of BC-associated genes and increased luminal differentiation. To test this hypothesis, we expressed constitutively high levels of the Notch target and bHLH transcription factor Hes1 in primary epithelial cells isolated from human lungs and grown in 2D culture (Fulcher et al., 2005). Under these conditions, the cells that survive and proliferate are initially mostly TRP63+ BCs. Cells were transduced with lentivirus encoding mouse Hes1 and GFP under the control of EF1a and CMV promoters, respectively (Yu et al., 2006). Cells transduced with lentivirus encoding GFP under control of the CMV promoter were used as controls. After 4 days, an average of 82.6% of GFP+ control cells were TRP63+ (n=2 independent, normal donors). By contrast, only 39.2% of cells expressing high levels of mHes1 were TRP63+ (Figure 5A,B). Similarly, 41.4% of BCs transduced with mHey1 retrovirus (Sakamoto et al., 2003) expressed TRP63 compared to 66.4% of BCs transduced with a GFP expressing retrovirus. Although these culture conditions do not support terminal mucociliary differentiation, our data suggest that Notch signaling plays an evolutionarily conserved role in the downregulation of BC-associated genes and luminal differentiation of BCs in the pseudostratified conducting airway epithelium. We performed qPCR using template cDNA synthesized from control GFP+ cells and _mHes1_-expressing GFP+ cells purified by FACS five days after transduction. Cells expressing mHes1 contained reduced levels of Jag2 transcripts compared to GFP+ control cells (Figure 5C). Importantly, Jag2 is the Notch ligand that is most differentially expressed between basal and luminal cells of the steady state mouse tracheobronchial epithelium (Figure 1). Together, these data support a model in which Notch promotes the luminal differentiation of human airway BCs. Notch-responsive putative EPs downregulate the expression of TRP63 and Notch ligand and show a trend toward upregulation of Notch receptor expression (Figure 5C) as they are generated by asymmetric division.
Figure 5. Notch gain-of-function analysis in human basal cells.
(A,B) Primary human BCs were transduced with lentivirus encoding (A) GFP or (B) mHes1 and GFP. After 4 days, 83% of control cells expressing GFP remained TRP63+ while only 39% of BCs expressing mHes1 and GFP were TRP63+. Arrowheads mark GFP+;TRP63- cells. Scale bars: 25μm. (C) qPCR for Notch pathway components was performed on cDNA synthesized from GFP+ cells that were obtained by FACS 5 days after transduction with lentivirus encoding either GFP alone (black bars) or mHes1 and GFP (white bars). y-axis is relative quantification (RQ) compared to Gapdh and expression in control cells is set to 1 for each gene. Error bars show 95% confidence interval based on triplicate samples.
Notch signaling is active in mouse luminal basal cell daughters in vitro
To confirm that Notch signaling is active in the luminal daughters of BCs at the single cell level, we used our recently described 3-dimensional organoid culture system. In this assay, primary NGFR+ tracheobronchial BCs purified by FACS are seeded into a mixture of Matrigel and growth medium (Rock et al., 2009). Individual basal stem cells proliferate and generate multicellular tracheospheres composed, after about two weeks, of basal, ciliated, and secretory cells surrounding a single central lumen. We seeded BCs from TNR mice in the assay and performed whole mount immunohistochemistry on spheres made up of 2 to 16 cells, before the differentiation of ciliated and secretory cells. At the 2-cell stage (∼2 days post seeding), no colonies contained two TNR+ cells. Moreover, all TNR+ cells were Trp63 negative at the 2, 4 and 16-cell stages (Figure 6A-D). Together, these data confirm our findings in vivo that Notch signaling is active in Trp63- daughter cells (putative EPs) at the time they are generated from BCs.
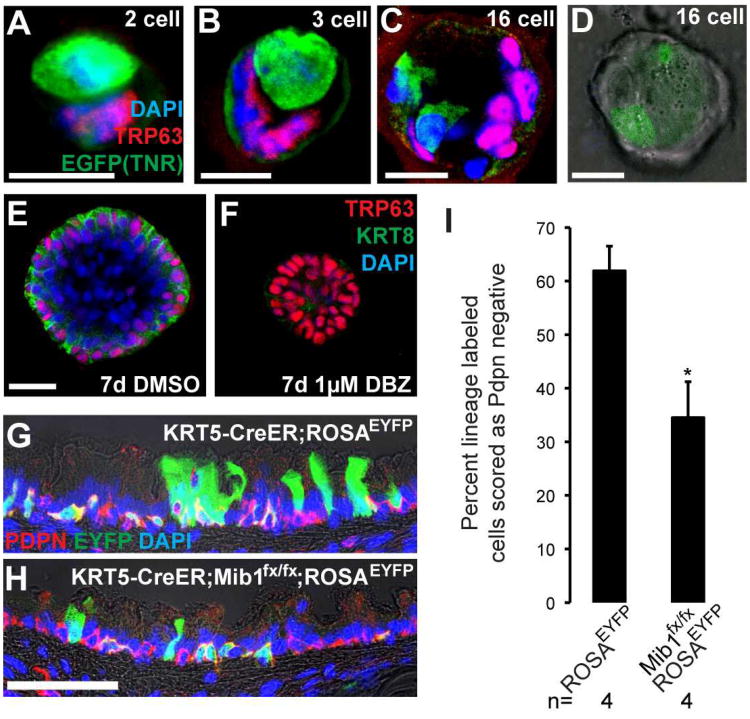
Figure 6. Loss-of-function analysis shows that Notch signaling is required for the luminal differentiation of airway basal cells.
(A-D) Tracheospheres grown from single BCs of TNR mice were stained in whole-mount with anti-GFP (green) to show TNR+ Notch responsive cells and anti-Trp63 (red, basal cells) from the 2-16 cell stages. Tracheospheres grown from wild type BCs were cultured with (E) DMSO or (F) gamma secretase inhibitor DBZ (1μM). After 7d, spheres were stained in whole-mount with anti-Trp63 (red, BCs) and anti-Krt8 (green, luminal cells). (G) Adult KRT5-CreER;ROSAEYFP and (H) KRT5-CreER;Mib1fx/fx;ROSAEYFP mice were injected with tamoxifen and exposed to SO2. Two weeks later, after epithelial repair, sections were stained with anti-GFP (green, lineage label), and anti-Pdpn (red, BCs). (I) Percent lineage labeled cells that had differentiated and lost expression of Pdpn. Data shown are means ± SEM and n is the number of anilmals examined. *p<0.05. Nuclei were stained with DAPI and images are single planes from confocal stacks. Scale bars: A-E 10μm; H 50μm.
Loss-of-function suggests that basal cell differentiation is Notch-dependent
Our gain-of-function experiments suggested that Notch promotes the luminal differentiation of airway basal stem cells. To test the hypothesis that Notch signaling is required for the differentiation of luminal daughters from basal stem cells, we pharmacologically inhibited Notch signaling by adding the gamma secretase inhibitor DBZ (1μM) or vehicle (DMSO) to wild type BCs at the time of seeding in the tracheosphere assay. After 7 days, control spheres (451/451 spheres examined in 4 independent experiments) developed a single lumen surrounded by a pseudostratified epithelium containing TRP63+ BCs and TRP63-;KRT8+ putative EPs (Figure 6E). In contrast, spheres generated from BCs cultured in the presence of DBZ contained only TRP63+ BCs and failed to form a lumen (446/459 spheres examined in 4 independent experiments) (Figure 6F). These data support a model in which Notch signaling is required for the differentiation of EPs from BCs, but is not required for BC proliferation or self-renewal in vitro.
To test this model in vivo, we used a conditional null allele of mindbomb homolog 1(Mib1) (Koo et al., 2007). Mib1 encodes an E3 ubiquitin ligase required in ligand-presenting cells to initiate Notch signal transduction (Itoh et al., 2003; Koo et al., 2007; Lai et al., 2005). Importantly, Mib1 null mice phenocopy null mutations of Notch signaling components (Koo et al., 2007). Adult KRT5-CreER;Mib1fx/fx;ROSAEYFP mice were injected with tamoxifen to delete Mib1 specifically from BCs and induce the heritable expression of EYFP for clonal lineage analysis. Age-matched KRT5-CreER;ROSAEYFP and KRT5-CreER;Mib1fx/+;ROSAEYFP mice were used as controls. We performed SO2 inhalation injury 4 days after the last tamoxifen injection to induce self-renewal and differentiation of the surviving BCs. We assessed the expression of YFP and markers of differentiation on confocal images of tissues sections two weeks post-injury. Consistent with our previous findings, 61.9±4.6% of lineage labeled cells had differentiated and lost expression of Pdpn (Figure 6G,I) (Rock et al., 2009). These data confirm that wild type BCs contribute to epithelial repair by generating luminal daughters. In contrast, the proportion of lineage labeled Mib1 null cells that were scored as luminal was significantly lower than wild type cells two weeks post injury. Only 34.5±6.7% of lineage labeled _Mib1_-null cells were scored as Pdpn- luminal cells (Figure 6H,I). Together, our pharmacological and genetic loss-of-function data support a model in which Notch signaling is required for the luminal differentiation of BC daughters. Moreover, since Mib1 is required specifically in ligand-expressing cells for Notch signaling, these data, combined with gene expression analysis, suggest that BCs are a significant source of Notch ligand during differentiative divisions to generate luminal EPs.
Discussion
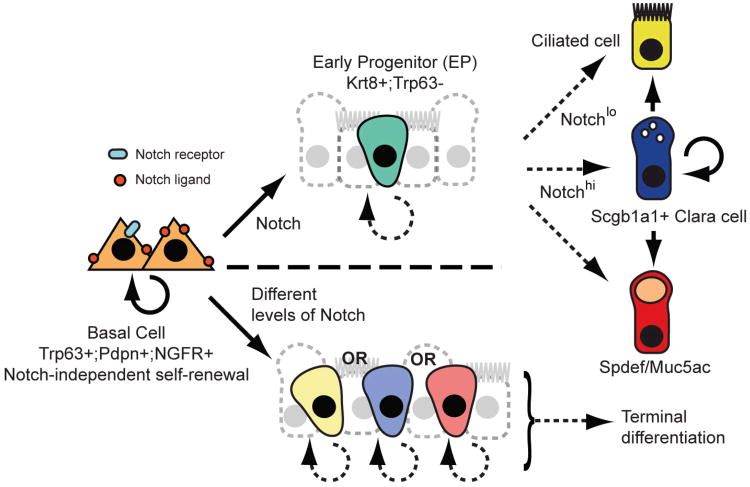
Here, we have used an in vivo reporter allele and genetic and pharmacological gain- and loss-of-function experiments to demonstrate that Notch is necessary and sufficient for the differentiation of adult airway basal stem cells. We have shown that the Notch pathway is active in relatively few cells under steady state conditions when there is little epithelial turnover. The number of Notch-responsive cells is enhanced when basal stem cells generate luminal daughters in vivo after epithelial injury and in vitro. Taken together, our data support the model depicted in Figure 7 (upper panel). According to this model, differentiating BCs divide asymmetrically to generate a multipotent Trp63-;Krt8+ luminal EP and this is a Notch-dependent process. We hypothesize that EPs have a limited capacity for proliferation and generate terminally differentiated ciliated or secretory cells in response to a second Notch signaling event. While TNR+ cells are not positive for phosphorylated histone H3 at 36hpi, there is indirect evidence that EPs constitute a transit amplifying population in repair. This comes from the observation that most of the Scgb1a1+, Spdef+ and Muc5ac+ secretory cells derived from the sustained activation of Notch in Krt5+ BCs were present in pairs or small clusters (Figure 4). EP behaviors, including self-renewal and differentiation, might be modulated by changes in their niche that includes neighboring epithelial cells, stroma, vasculature, smooth muscle, neurons, and inflammatory cells.
Figure 7. Models for Notch signaling in the adult pseudostratified airway epithelium.
Self-renewal of BCs is Notch-independent, but their luminal differentiation requires canonical Notch signaling. Upper panel: A multipotent early progenitor (EP) has limited capacity for proliferation and gives rise to mature ciliated and secretory cells in response to a second Notch signal. Lower panel: EPs are more lineage-restricted and their fates are specified by Notch input at the time of BC division. In either model, the abundance of EPs and the kinetics of their proliferation and differentiation may differ under steady state conditions versus repair. It is likely that the BC niche is dynamic and comprised of the extracellular matrix, secreted molecules and nearby epithelial, stromal, immune, neuronal, vascular and smooth muscle cells.
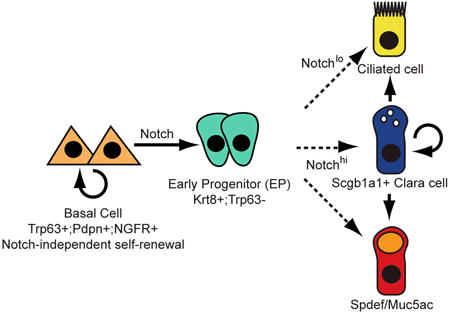
Currently, we cannot exclude alternative models for Notch regulation of BC differentiation in the adult airway epithelium. For example, as shown in Figure 7 (lower panel), there may only be one determinative Notch signaling event. In this scenario BCs express differing levels of Notch ligand, depending on signals from their local environment, including neighboring epithelial cells, and specify the lineage fate of their progeny at the time of the first asymmetric division. Accordingly, EPs would be more lineage-restricted than in the first model and would constitute a dynamically heterogeneous population, with some acquiring a high probability of generating ciliated cells and others secretory cells. This second scenario is similar to models proposed for stem cells in the Drosophila midgut intestinal epithelium (Jiang et al., 2009; Ohlstein and Spradling, 2007). In the future it should be possible to distinguish between alternative models by transiently inducing variable doses of Notch activation in individual BCs and following the fate of their daughters. It should also be possible to use the TNR allele to purify putative EPs and identify surface proteins and transcription factors that function as markers of this population. This will enable long term in vivo lineage tracing of individual EPs and definitive tests of the heterogeneity of this populations.
Fundamental to both models is the Notch-dependency of basal stem cell differentiation towards luminal lineages. Indeed, pharmacological inhibition of Notch signaling in the tracheosphere assay suggests that the differentiation of BCs depends on canonical Notch signaling. However, our in vivo genetic loss-of-function experiments, in which Mib1 was deleted from airway BCs using the KRT5-CreER driver, resulted in the reduction, but not absence, of lineage labeled luminal cells. To account for this, we propose that some daughters of _Mib1_-deficient BCs received a pro-differentiation Notch signal from neighboring wild-type BCs that had not undergone recombination due to the relatively low efficiency of the KRT5-CreER transgene. Consistent with our previous studies, we confirmed that only approximately 10% of BCs undergo recombination after a maximal regimen of four doses of tamoxifen (data not shown). Also, it is likely that some cells that recombined the ROSA-EYFP allele and expressed the lineage tag did not undergo recombination to delete both alleles of Mib1. Furthermore, the deletion of Mib1 may be compensated for by the expression of other E3 ubiquitin ligases. RT-PCR demonstrated that Mib2 is expressed in the tracheal epithelium under steady conditions (data not shown). Mice homozygous for a null allele of Mib2 are viable and fertile with no obvious respiratory defects (Koo et al., 2007). However, additional experiments are required to determine whether this protein partially compensates for the deletion of Mib1 in the adult tracheobronchial epithelium.
An important question is whether our proposed model for the role of Notch signaling in the regulation of airway BC differentiation is conserved in humans. Our data using primary human bronchial epithelial cells in culture certainly provide strong evidence that it is. BCs are found throughout the human lung, including small airways where pathological remodeling such as mucous hyperplasia and squamous metaplasia are particularly detrimental (Boers et al., 1998; Nakajima et al., 1998; Randell, 2006; Rock et al., 2010). Importantly, the KRT5+;KRT14+ BC population is expanded in areas of squamous metaplasia in patients with chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF) (Rock et al., 2010; Voynow et al., 2005). According to our models (Figure 7), this expansion could result from decreased levels of Notch signaling. Significantly, it has been reported recently that smokers with and without COPD express lower levels of Notch ligands and receptors in airway epithelial cells than non-smokers (Tilley et al., 2009). Furthermore, Notch gain-of-function has been linked to poor outcome in a subset of patients with non-small cell lung cancer (Westhoff et al., 2009). Together with our current findings, these studies suggest that the Notch signaling pathway represents a potential therapeutic target for airway remodeling and lung disease.
Experimental Procedures
Mice
KRT5-CreER (Rock et al., 2009), TNR (Mizutani et al., 2007), ROSAEYFP (Srinivas et al., 2001), ROSANotch (Murtaugh et al., 2003), and Mib1flox (Koo et al., 2007) mice have been described. Animal experiments were approved by the Duke IACUC. To induce recombination in BCs, 20mg/ml tamoxifen (Sigma) stock solution was prepared in Mazola corn oil. Adult mice were injected intraperitoneally with 0.25 mg per gram body weight every other day for a total of four injections as described (Rawlins et al., 2009). For SO2 experiments, the final tamoxifen injection was administered 2-4 days before injury. Adult mice were placed in individual compartments within a chamber and exposed to 500 ppm SO2 in air for 3 hours.
FACS
Tracheobronchial epithelial cells were isolated as described (Rock et al., 2009). Briefly, tracheas were incubated in 16U/ml Dispase (BD Biosciences), epithelial sheets were peeled from the underlying stroma with forceps, and single cells obtained by incubation with 0.1% trypsin at 37°C for 20 minutes. Cells were labeled with 9ug/ml rabbit anti-NGFR (Abcam 8875) or rabbit IgG for 45min in 2%FBS, 2%BSA in PBS on ice, washed and incubated with Alexa-Fluor 488 or PE-conjugated donkey anti-rabbit in 2%FBS, 2%BSA in PBS for 45 min on ice. Propidium iodide was added to exclude dead cells and FACS was performed in the Duke Cancer Center shared flow cytometry facility. To isolate EPs by FACS, the tracheal epithelium of TNR males was isolated and dissociated as above 36hpi, incubated with propidium iodide and sorted. Transduced human cells in 2D culture were trypsinized, incubated with propidium iodide and sorted.
RT-PCR
RNA was extracted with the RNeasy micro kit (QIAGEN) and cDNA synthesized with SuperScript III (Invitrogen). PCR was performed with SYBR green chemistry in a StepOnePlus (Applied Biosystems) and data were analyzed using the ΔΔCt method. Primer sequences are listed in Supp Table 1.
Immunohistochemistry and RNA in situ hybridization
Tracheas were dissected and fixed in 4% PFA for 3h at 4°C, washed with PBS, and embedded in OCT. Cryosections (10-14μm) were washed with 0.3% Triton X-100 in PBS and blocked in 10% donkey serum, 3% BSA, 0.1% Triton X-100 in PBS for at least 1 hour at room temperature. Sections were incubated with primary antibodies, diluted in blocking solution, overnight at 4°C. After washing, sections were incubated with secondary antibodies diluted in 5% donkey serum, 0.1% Triton X-100 in PBS for 3-4 hours at room temperature and then washed and counterstained with DAPI. For whole mount staining, tracheospheres were fixed with 4% PFA at room temperature for 10 minutes. After PBS washes, they were incubated in 5% BSA, 0.2% Triton X-100 for 15 minutes at room temperature and then incubated in primary antibody, diluted in 5% BSA, 0.02% Triton X-100, overnight at 4°C. After PBS washes, secondary antibody was added for 4 hours at room temperature diluted in 5% BSA, 0.02% Triton X-100, washed with PBS, and nuclei were stained with DAPI. Fluorescent images are single planes from confocal stacks. Antibodies used were: Abcam: Chicken anti-GFP (1:500); Developmental Studies Hybridoma Bank: hamster anti-podoplanin, 8.1.1 (1:1000), rat anti-Krt8, TROMA-I (1:200); Santa Cruz: mouse anti-p63, 4A4 (1:50-1:200); Peninsula labs: rabbit anti-Cgrp (1:200); LabVision: mouse anti-muc5ac (1:200); mouse anti-FoxJ1 (Steven Brody); rabbit anti-Scgb1a1 (Barry Stripp); guinea pig anti-Spdef (Jeffrey Whitsett); rabbit anti-Hes1 (Nadean Brown). All secondary antibodies were Alexa Fluor conjugates (488, 555, or 647) diluted 1:500 (Invitrogen).
For in situ hybridization, paraffin sections were dewaxed, rehydrated, fixed with 4% PFA before proteinase K treatment (20μg/ml for 10 minutes at room temperature), and fixed with 4% PFA again. Sections were treated with acetic anhydride in triethanolamine for 10 minutes, prehybridized at 65° for 3 hours, and hybridized at 65° overnight with DIG labeled probes. Slides were washed in decreasing concentrations of SSC and stained with AP-conjugated anti-DIG. Signal was visualized using BCIP/NBT and slides were counterstained with nuclear fast red.
Statistical Analysis
Unpaired Student's t-tests were used for all statistical analyses.
Cell culture and viral transduction
Plasmids encoding pGIPZ (Thermo Scientific) and EF.mHES1.CMV.GFP (Addgene) were transfected into 293T cells with lentiviral packaging vectors Δ8.9 and VSVG using Fugene 6. Viral particles were collected and concentrated by centrifugation and added to first passage primary human airway epithelial cells after 3d culture on uncoated plastic in BEGM medium as described (Fulcher et al., 2005). Pheonix cells were transfected with pClig and pClig-HesR1 plasmids (gift from Ryoichiro Kageyama) using Fugene 6 and grown at 32°C. Three days after transfection, retrovirus-containing supernatant was filtered. To transduce first passage human airway epithelial cells grown on plastic, cells were washed 2d after plating, incubated in retroviral supernatant +10% FBS +0.08 mg/ml Polybrene at 32°C for 15 minutes, and centrifuged for 30 minutes at 1100g before washing. Supernatant was removed and cells were grown in BEGM at 37°C for 96 h before immunofluorescence.
Tracheospheres were cultured as described (Rock et al., 2009) except that they were grown in 8-well chamber slides to facilitate whole-mount immunohistochemistry and confocal analysis. Five thousand viable basal cells obtained by FACS were resuspended in 500μl 2% growth factor-reduced Matrigel diluted in MTEC/Plus and seeded into each chamber. Prior to seeding, the chamber bottom was coated with 6μl 100% Matrigel that was allowed to gel. DBZ (EMD) was resuspended in DMSO and used at a final concentration of 1μm.
Supplementary Material
Supplemental Figure
Supplemental Table
Acknowledgments
This manuscript is dedicated to the memory of our friend and colleague Dr. Tim Oliver. The work was supported, in part, by NIH grants HL071303 (BLMH), F32-HL102920 (JRR) and R026-CR07 from the CFF (SHR). We thank Ryoichiro Kageyama, Nadean Brown, Jeffrey Whitsett, Chay Kuo, Terry Lechler, Emma Rawlins, Tannishtha Reya, Blanche Capel and their lab members for advice and reagents.
References
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27:401–415. doi: 10.1080/019021401300317125. [DOI] [PubMed] [Google Scholar]
- Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TL, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV, Jacoby DB, Kicic A, Stick SM, Knight DA. Characterization of side population cells from human airway epithelium. Stem Cells. 2008;26:2576–2585. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman SL. Cell proliferation in the mammalian lung. Int Rev Exp Pathol. 1980;22:131–191. [PubMed] [Google Scholar]
- Koo BK, Yoon MJ, Yoon KJ, Im SK, Kim YY, Kim CH, Suh PG, Jan YN, Kong YY. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS One. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC, Roegiers F, Qin X, Jan YN, Rubin GM. The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development. 2005;132:2319–2332. doi: 10.1242/dev.01825. [DOI] [PubMed] [Google Scholar]
- Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 2010;123:213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Kawanami O, Jin E, Ghazizadeh M, Honda M, Asano G, Horiba K, Ferrans VJ. Immunohistochemical and ultrastructural studies of basal cells, Clara cells and bronchiolar cuboidal cells in normal human airways. Pathol Int. 1998;48:944–953. doi: 10.1111/j.1440-1827.1998.tb03865.x. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Park KS, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana Hershey GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:718–725. doi: 10.1513/pats.200605-117SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell SH, Comment CE, Ramaekers FC, Nettesheim P. Properties of rat tracheal epithelial cells separated based on expression of cell surface alpha-galactosyl end groups. Am J Respir Cell Mol Biol. 1991;4:544–554. doi: 10.1165/ajrcmb/4.6.544. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci U S A. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Hirata H, Ohtsuka T, Bessho Y, Kageyama R. The basic helix-loop-helix genes Hesr1/Hey1 and Hesr2/Hey2 regulate maintenance of neural precursor cells in the brain. J Biol Chem. 2003;278:44808–44815. doi: 10.1074/jbc.M300448200. [DOI] [PubMed] [Google Scholar]
- Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:558–563. doi: 10.1513/pats.200905-031RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley AE, Harvey BG, Heguy A, Hackett NR, Wang R, O'Connor TP, Crystal RG. Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:457–466. doi: 10.1164/rccm.200705-795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J, Cardoso WV. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 2008;283:29532–29544. doi: 10.1074/jbc.M801565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med. 2005;172:1013–1018. doi: 10.1164/rccm.200410-1398OC. [DOI] [PubMed] [Google Scholar]
- Westhoff B, Colaluca IN, D'Ario G, Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G, Viale G, et al. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A. 2009;106:22293–22298. doi: 10.1073/pnas.0907781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Nieuwenhuis E, Cohen BL, Wang W, Canty AJ, Danska JS, Coultas L, Rossant J, Wu MY, Piscione TD, et al. Lunatic Fringe-mediated Notch signaling is required for lung alveogenesis. Am J Physiol Lung Cell Mol Physiol. 2010;298:L45–56. doi: 10.1152/ajplung.90550.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Alder JK, Chun JH, Friedman AD, Heimfeld S, Cheng L, Civin CI. HES1 inhibits cycling of hematopoietic progenitor cells via DNA binding. Stem Cells. 2006;24:876–888. doi: 10.1634/stemcells.2005-0598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure
Supplemental Table