B cell-regulated immune responses in tumor models and cancer patients (original) (raw)
Abstract
The essential role played by T cells in anticancer immunity is widely accepted. The immunosuppressive functions of regulatory T cells are central for tumor progression and have been endowed with a robust predictive value. Increasing evidence indicates that also B cells have a crucial part in the regulation of T-cell responses against tumors. Although experiments reporting the production of natural antitumor antibodies and the induction of cytotoxic immune responses have revealed a tumor-protective function for B cells, other findings suggest that B cells may also exert tumor-promoting functions, resulting in a controversial picture. Here, we review recent evidence on the interactions between B and T cells in murine models and cancer patients and their implications for cancer immunology.
Keywords: antitumor immunity, B cells, regulatory B cells, T cells, Tregs, tumor-specific immune responses
Introduction
The important role of T cells in antitumor immune responses is widely accepted and has been extensively studied. However, tumor-specific immune responses appear to be much more complex than other mechanisms of defense against pathogen, as demonstrated by the clinical inefficacy of T cell-based anticancer vaccines. As early as in 1956, Thomas and Burnet proposed the theory of immunosurveillance in humans, suggesting that lymphocytes act as sentinels that continuously eliminate neo-transformed cells to prevent the manifestation of overt neoplasms. Although this theory has been challenged several times, data accumulating in the late 1990s led to the widespread acceptance of its original formulation.1,2
B cells are mainly known for being in charge of the production of antibodies against a broad range of antigens. The discovery of B cells occurred in the mid-1960s, together with that of T cells. Cooper and Good demonstrated the functional distinction between cells in the chicken bursa of Fabricius (B cells), which were responsible for the secretion of antibodies, and cells that required an intact thymus (T cells), being associated with delayed-type hypersensitivity responses.3,4 Initially, B cells were defined as lymphocytes expressing clonally diverse cell-surface immunoglobulin receptors capable of recognizing specific antigens. In 1948, plasma cells were suggested to be the main source of antigen-specific antibodies.5
Besides their role in antibody generation, however, B cells mediate and regulate numerous other functions that are essential for immune homeostasis. Of crucial importance for T-cell immune responses, for instance, is the antigen-presenting capacity of B cells.6-12 In line with this notion, the congenital absence of B cells results in abnormalities within the immune system including a decrease in thymocyte number and diversity, defects in the splenic dendritic cell (DC) and T-cell compartments, the lack of Peyer’s patches, and an absence of macrophage subsets accompanied by decreased levels of specific chemokines.13
In addition to their role in the development of the immune system, B cells are indeed capable of modulating other immune cells by secreting cytokines and by expressing a specific set of receptors on their surface. These signals influence the function of T cells, DCs, and antigen-presenting cells (APCs), control the neogenesis and structural organization of lymphoid tissues, regulate wound healing, and play a role in transplant rejection. Considering clinical findings in septic and allergic conditions, B cell-initiated signaling cascades may have an impressive strength. Cytokines such as interleukin (IL)-4, IL-10, and transforming growth factor β (TGFβ) are among the most prominent immunosuppressive factors secreted by B cells in this setting.14-16 Further, in Hodgkin lymphoma, malignant Hodgkin and Reed-Sternberg cells can originate from cells of the B lineage at various stages of development.17 However, the role of B cells in antitumor immune responses as well as the impact of B-cell malfunctions in oncogenesis and tumor progression remain poorly understood.
Here, we discuss recent data elucidating the role of B cells in tumor progression with a special focus on the underlying immunological mechanisms, in particular the interaction between B and T cells.
B-Cell Immunology in Murine Tumor Models and Cancer Patients
Although during the last decade the field of oncoimmunology was largely focused on T cells, research has also been conducted to evaluate the potential involvement of B cells in carcinogenesis and tumor progression. To the knowledge of the authors, however, a systematic study of B cells in cancer patients has not been performed yet. Rather, most of the studies dissecting the regulatory functions of B cells relied on mouse models of autoimmune diseases or in vitro settings. Thus, it has been shown that T cell-mediated autoimmune responses can be prevented by a small subset of IL-10-producing B cells, which were characterized as CD1dhighCD5+ B cells.18 Along similar lines, mice can be protected from chronic colitis by B1b (CD5−CD1dhighB220lowCD11b+IgM+) regulatory cells, while CD19+CD24highCD38high B cells are associated with a protection from systemic lupus erythematosus in humans.19,20
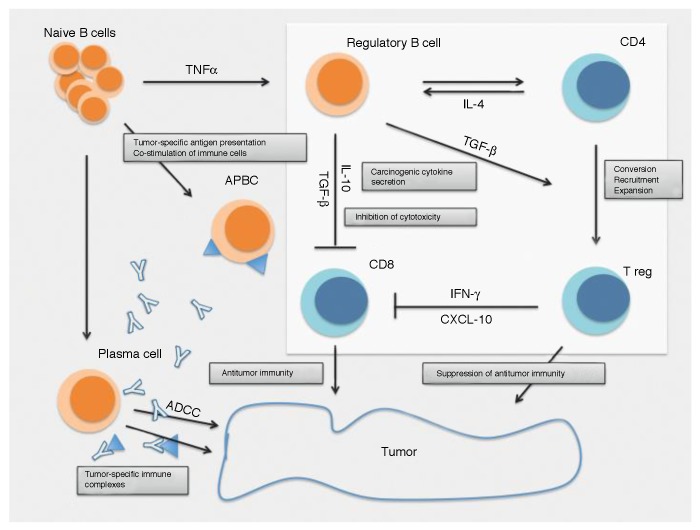
As early as in 1978, a tumor-promoting role was proposed for B cells in C57BL/6 mice injected with fibrosarcoma cells.21 Tumor growth and metastatic spread were indeed significantly reduced in mice depleted of B cells by an anti-IgM monoclonal antibody. These early findings have been supported by data from murine xenograft models collected throughout the 1990s, although the precise functions of B cells in antitumor immunity remained unclear.22 Seminal studies by Qin et al. based on wild-type and nude C57BL/6J mice subjected to the depletion of B cells by means of an anti-CD20 antibody revealed that the repertoire of CD4+ helper T cells is limited in the presence of B cells, resulting in reduced antitumor immune responses.23 More recently, Inoue et al. provided additional insights into the mechanisms mediating such an immunosuppressive function of B cells.24 To this aim, the authors screened tumors for their susceptibility to B cell-deficient immune networks by culturing splenic cells from wild-type or B cell-deficient C57BL/6 mice with irradiated tumor cells. Upon stimulation, the production of interferon γ (IFNγ) from CD8+ T cells and natural killer (NK) cells was found to be markedly increased in the B cell-deficient as compared with wild-type conditions. IFNγ production could be correlated directly with the expression of the CD40 ligand on the surface of cancer cells and inversely with IL-10 production by B cells. Confirming the findings described above, in a two-step model of skin carcinogenesis (as induced by the sequential administration of 7,12-dimethylbenz[α]anthracene and 12-O-tetradecanoylphorbol-13-acetate in the context of adoptive B-cell transfer), tumor necrosis factor α (TNFα) was identified as a key immunosuppressive mediator, owing to its ability to promote the accumulation of regulatory B cells (Bregs) (Fig. 1).26
Figure 1. Impact of B cells on carcinogenesis and tumor progression. B cells release cytokines, such as lymphotoxins, that mediate the androgen-independent activation of signal transducer and activator of transcription 3 (STAT3) and inhibitor of κB kinase α (IKKα), thus favoring tumor progression.29 Other cytokines such as tumor necrosis factor α (TNFα) stimulate the development of regulatory B cells (Bregs).26 While plasma cells are required for antibody-dependent cell cytotoxicity (ADCC), as they are the actual producer of tumor-specific antibodies,25 the resulting immune complexes may contribute to the establishment of a tumorigenic environment by stimulating Fcγ receptors (FcγRs).27 Furthermore, Bregs can promote oncogenesis by secreting interleukin-10 (IL-10) and transforming growth factor β (TGFβ), hence suppressing CD8+ T-cell cytotoxicity and converting/recruiting CD4+CD25+FOXP3+ regulatory T cells (Tregs).24,78 Such B cell activities normally shift the balance of tumor-specific immune response toward immunosuppression, hence sustaining tumor progression. APBC: antigen-presenting B cell.
Even deeper insights into the mechanisms accounting for B cell-mediated immunosuppression have been provided by de Visser et al. (Table 1).27 These authors used a model of human papillomavirus type 16 (HPV-16)-induced skin carcinogenesis and observed a reduced infiltration of immune cells in B cell-deficient mice as compared with wild-type animals. In line with this notion, the adoptive transfer of B lymphocytes into B cell-deficient mice restored the infiltration of innate immune cells into premalignant tissues. Although only low numbers of B cells infiltrate premalignant lesions, B cells may exert a distant effect on oncogenesis by secreting antibodies that are deposited at the tumor site in the form of immune complexes. Upon the binding of activating Fcγ receptors (FcγRs) to such immune complexes, myeloid cells including macrophages can be recruited to neoplastic lesions and become primed to exert tumor-supporting functions by secreting pro-angiogenic factors and immunosuppressive cytokines (Fig. 1). We have extended these findings to a clinical setting and demonstrated the presence of tumor-specific T-cell responses in the bone marrow of 40% breast carcinoma patients, correlating with an improved prognosis, as opposed to tumor-specific natural antibodies, which were detectable in approximately 50% of the patients and correlated with both the absence of bone marrow tumor-specific T cells and advanced tumor stage.25,31
Table 1. Impact of B cells on tumor growth in murine cancer models.
| Year | Mouse model | Cell line or carcinogen | Tumor type | Tumor growth on B-cell deficiency | Phenotypic markers | Ref. |
|---|---|---|---|---|---|---|
| 2005 | Rag1−/− Cd4−/− Cd8−/− | HPV16(transgenic) | Skin cancer | Reduced | - | 27 |
| 2005 | C57BL/6 | EL4 cellsMC38 cellsB16/F10 cells | LymphomaColon cancerMelanoma | ReducedReducedEnhanced | - | 28 |
| 2006 | C57BL/6 | EL-4 cellsD5 cellsMCA304 cells | LymphomaMelanomaSarcoma | ReducedReducedNo change | - | 24 |
| 2010 | C57BL/6 | TRAMP(transgenic) | Prostate cancer | Reduced | - | 29 |
| 2010 | C57BL/6 | B16/F10 cells | Melanoma | Enhanced | - | 30 |
| 2011 | C57BL/6 | DMBA/TPA | Skin cancer | Reduced | CD19+CD21+IL10+ | 26 |
| 2012 | BALB/cC57BL/6NOD/SCID | 4T1 cells | Breast cancer | Reduced | CD19+ CD25highB7-H1high CD81high CD86highCCR6high CD62LlowIgMint/low | 42 |
It is widely accepted that several distinct immunogenic cancers are driven by a chronic inflammatory milieu that, at least in part, may be maintained by B cells. Some cancers such as hepatocellular carcinoma and prostate cancer have indeed been epidemiologically linked to chronic inflammation.32-35 In line with this notion, chronic inflammation was identified as a key etiological mechanism, independent of carcinogen exposure, also in a mouse model of lung cancer.36
Although inflammation is generally linked to tumor progression, it may also stimulate antitumor response and can be harnessed for the immunotherapy of cancer patients.27,37,38 Since B cells have a major impact on the basic physiology of inflammatory responses to pathogens, numerous disorders are regulated by B cells. In the course of autoimmune diseases and antibody dependent cellular cytotoxicity (ADCC) reactions, B cells are indeed considered a command center interacting with other immune cells within a complex cytokine network (Fig. 1).13,39,40 Moreover, Ammirante et al. demonstrated the T cell- and complement-independent, lymphotoxin-mediated inhibition of prostate carcinoma by B cells.29 In this context, a link could be made with the activation of inhibitor of κB kinase α (IKKα), which stimulates metastasis by an NF-κB-independent, cell-autonomous mechanism. Ammirante et al. first demonstrated that immunoglobulins in the tumor stroma elicit at least 2 pro-inflammatory pathways, namely, by activating the complement system and by engaging FcγRs on the surface of immune cells.29 The stimulation of FcγRs in B cells was found to be a prerequisite to establish a tumor-promoting stroma. The authors reported a transient increase in the infiltration of tumors regressing upon castration by immune cells, including B cells. In this context, the production of lymphotoxin α/β by B cells was responsible for the androgen-independent activation of IKKα and signal transducer and activator of transcription 3 (STAT3) in tumor cells, which promoted relapse (Table 1). Thus, FcγRs are responsible for a functional link between adaptive and innate immune responses against developing tumors providing a platform for the interaction between circulating immunoglobulins and innate immune cells.41 Moreover, immunoglobulins can serve as a carrier for TGFβ and thereby exert suppressive effects on cellular immune responses.29 In a different study based on the mouse mammary adenocarcinoma 4T1 model, a population of CD19+B220+CD25+ B2 lymphocytes was also implicated in cancer progression.42 It should be noted that CD25 might simply identify activated cells, as it is upregulated on all activated T and B lymphocytes and expressed at high levels by thymic regulatory T cells (Tregs). Nonetheless, the abovementioned study demonstrated that the subcutaneous injection of 4T1 cells near the mammary gland of BALBc mice increased the proportion of Bregs in the peripheral blood and secondary lymphoid organs. Interestingly, this effect was dependent on the secretion of soluble factors by tumor cells. Indeed, the intraperitoneal injection of medium conditioned by these cells was sufficient to promote an accumulation of Bregs . The origin and then nature of such soluble factors warrant further investigation. The injection of an anti-B220 antibody to 4T1 carcinoma-bearing mice depleted approximately 20–50% of the B220 cell population in the lymph nodes and spleen, resulting in a significant decrease in the number of lung metastases. Surprisingly, however, although the authors showed that the majority of CD19+ B cells expressed B220, the depletion of B220+ cells was not accompanied by a decrease in the CD19+ B-cell population. Evaluating the direct effects of Bregs on oncogenesis and tumor progression in this model would be of particular interest.
The study mentioned above showed that CD19+B220+CD25+ B cells are able to inhibit T-cell proliferation and induce the conversion of CD4+ T cells into regulatory T cells (Tregs), both in vitro and in vivo, thus favoring metastasis (Fig. 1).42 The authors also demonstrated that conversion of CD4+ T cells into Tregs was dependent on physical contacts between T and B cells, as well as on the secretion of TGFβ by the latter. However, the molecular components involved in such a contact-dependent T-cell inhibition by B cells remain to be identified. The implication of TGFβ is in contrast with previous observations on Bregs, showing that the immunosuppressive functions of these cells is TGFβ-independent. Finally, the authors did not report a crucial role for IL-10, as recently confirmed by Zhang and colleagues in a similar experimental setting.42,43 Thus, the cytofluorometric characterization of Bregs based on standardized markers will be a major challenge for future research.
The question as to whether the use of different tumor cells in the same animal model may confirm the hypothesis that B cells exert major tumor-promoting functions was first addressed by Shah et al.28 These authors evaluated the immune responses to primary tumors of mice genetically engineered to lack B cells, finding the growth of EL4 thymomas and MC38 colon carcinomas to be significantly reduced. In contrast, the growth of B16 melanomas was significantly enhanced in B cell-deficient as compared with wild-type mice. These results have been subsequently confirmed by several other investigators using the same model.30 Also Sorrentino et al. were able to show that the absence of B cells promotes the growth of lung cancers in mice.44 By contrast, in B cell-deficient C57BL/6J mice implanted with metastatic B16-F10 melanoma cells, the adoptive transfer of B cells activated by CpG-oligodeoxynucleotides (ODNs), a Toll-like receptor 9 (TLR9) agonist, blocked the growth and induced the apoptotic demise of lung metastases. Along similar lines, the depletion of mature CD20+ B cells increased tumor growth in both CpG ODN-treated C57BL/6J and nude mice. In summary, these data demonstrate at least some degree of antitumor activity for CpG ODN-activated B cells. Many studies of this type, however, were performed using mice in which the immune system had developed in the complete absence of B cells (Table 1). These hosts harbor therefore a limited T-cell repertoire, lack follicular DCs as well as several macrophage subsets and show enhanced IL-12 production by DCs, which can skew TH1 responses.45-47 Besides the (partially controversial) findings described above, other potential mechanisms of B-cell malfunction or abnormalities in the B-cell compartment of cancer patients have not been characterized to date. For instance, a preferential loss of CD27+ and CD19+ B cells in patients bearing advanced melanomas and other solid tumors has been reported, but the mechanisms underlying this phenomenon still await elucidation.48,49
Crosstalk Between B and T Lymphocytes in the Course of Antitumor Immune Responses
The mutual interaction between immune cells through physical contacts, cell surface receptors, and soluble mediators represents the basic mechanism for the generation of immune responses. To this aim, the same evolutionarily perfected protocol of defense against a multitude of enemies, including malignant cells, is used. Thus, via a specific T-cell receptor (TCR), T cells recognize a peptidic fragment of the antigen in association with MHC molecules presented on the surface of APCs. Following this interaction, T cells massively proliferate and differentiate into effector T cells. The efficient priming of naïve CD8+ T cells depends on distinct signals by APCs (Fig. 1). First, APCs should present a peptidic fragment of the antigen in complex with MHC Class I molecules. Second, APCs must provide naïve CD8+ T cells with co-stimulatory signals conveyed by CD80 and CD86. Third, pro-inflammatory signals, such as those conveyed by IL-12 or type I IFNs, are necessary to attain an optimal CD8+ T-cell response. Several other molecules expressed or released by APCs have been shown to influence CD8+ T-cell responses at different stages, including several cytokines, co-stimulatory molecules of the TNF protein family, Notch ligands, and adhesion molecules. Lapointe and colleagues have shown that naïve resting B cells may induce a state of unresponsiveness in naïve CD4+ and CD8+ T cells.50 In contrast, CD40-activated B cells pulsed with melanoma cell lysates potently stimulated peripheral autologous T cells specific to melanoma-associated antigens. These studies suggest that B cells operate as efficient APCs for the expansion of tumor-associated antigen-specific CD8+ and CD4+ T cells.10 Moreover, exogenously pulsed B cells not only can present MHC Class II epitopes independent of their B-cell receptor (BCR) specificity but also can promote MHC Class I cross-presentation.
Further evidence on the involvement of B cells in the induction of optimal CD4+ and CD8+ T-cell response against tumors has been provided by DiLillo et al.30 Thus, in a murine B16 melanoma transfer model, the authors depleted mature B cells from wild-type adult mice by means of an anti-CD20 monoclonal antibody. In B cell-depleted hosts, tumor growth was markedly increased along with a significant impairment in CD4+ and CD8+ T-cell induction. Carpenter et al. demonstrated that B cells from patients with advanced stage solid tumors display a reduced ability to activate T cells to secrete IFNγ and IL2, while Blair et al., investigating the immunosuppressive functions of Bregs, could demonstrate that CD40-stimulated human B cells do suppress the differentiation of TH1 cells as partially mediated by IL-10 but not TGFβ.48,51 Such an immunosuppressive activity was reversed by means of anti-CD80 and anti-CD86 monoclonal antibodies. In a mouse model of lung cancer, Chapoval et al. demonstrated NK and T lymphocytes to be required for optimal responses to chemotherapy, while B cells appeared to suppress its antineoplastic effects.52 Accordingly, B cell-deficient mice were manifested a significant improvement in survival rates. In additional support of the immunosuppressive capacity of B cells, Perricone et al. reported an enhanced efficacy of melanoma-targeting vaccines in the absence of B lymphocytes.53 The vast majority of these studies has focused on how activated B cells can be used as effective APCs for T-cell priming.10,50,54-58 While lipopolysaccharide (LPS)-activated B cells have been reported to enhance the expansion of tumor-infiltrating lymphocytes in a culture system involving anti-CD3 monoclonal antibodies and IL-2, the adoptive transfer of LPS-activated B cells exposed to anti-CD3 antibodies to B16 melanoma-bearing mice induced the regression of lung metastases.59,60 Although the mechanisms whereby adoptively transferred B cells mediated antineoplastic effects require further clarification, an involvement of B cell-T cell interactions is likely.
Along similar lines, the presence of CD20+ tumor-infiltrating B cells (TIBCs) and Ig κ chains (IGKCs) within the tumor bed has been positively correlated with metastasis-free survival and response to chemotherapy in patients affected by a variety of malignancies including breast, ovarian, head and neck, non-small cell lung, and colorectal carcinoma as well as melanoma.61-66 The mechanisms underlying TIBC antitumor immunity may involve a role of B cells as APCs in that they can bind tumor-associated antigens via their BCR, process them, and then present the corresponding peptides to T cells in the context of MHC molecules. Moreover, by secreting lymphotoxin, TIBCs orchestrate the generation of local tertiary lymphoid structures, consisting of CD20+ B cells co-residing with CD8+ T cells in loose aggregates within and adjacent to tumor cell islets. Finally, effector TIBCs can produce cytokines IFNγ and IL-4 that may polarize T cells toward a TH1, TH2, or alternative functional phenotype.67-69 These observations might have strong implications for the development of prognostic tests based on TIBCs and inform the design of ever more effective anticancer immunotherapies.66
Recruitment and Conversion of Regulatory T Cells by B Cells
Sakaguchi et al. were the first to stimulate an interest in Tregs by identifying a population of CD4+ T cells specifically expressing the transcription factor FOXP3 and preventing autoimmunity in a murine model.70 Subsequently, numerous reports enlightened major aspects of the biology of Tregs, by characterizing different T-cell subpopulations with regulatory properties, including naturally occurring CD4+CD25high Tregs, induced Tregs (e.g., TR1 and TH3 cells) as well as CD4+CD25high Tregs developing in the periphery upon the conversion of CD4+CD25− T cells. All these T-cell populations with regulatory functions co-exist and contribute to immune suppression in the course of oncogenesis as well as in several other pathophysiological settings.70,71
Tregs suppress in a rather potent way TH1 immune responses as well as other CD8+ cytotoxic lymphocytes, hence favoring tumor growth. In line with this notion, CD4+CD25highFOXP3+ T regulatory cells are mainly characterized by an anergic state and by the capacity to actively inhibit CD4+CD25− T cells, CD8+ T cells, DCs, NK cells, natural killer T (NKT) cells, and B cells in a contact- and dose-dependent manner. Moreover, CD4+CD25highFOXP3+ Tregs are characterized by a phenotypic profile of antigen-experienced memory T cells. Recent evidence shows the Tregs can suppress the activity of plasma cells even without an intervention of helper T cells. In this context, it is important to note the distinct effects of Tregs on different B-cell subsets.72 Cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and glucocorticoid-induced TNFR-related protein (GITR) are among the most prominent cell-surface markers associated with the phenotype and function of Tregs. In addition, IL-10 and TGFβ, although rarely expressed in vitro, might be functionally relevant for Tregs in vivo, especially in settings of B cell-mediated immunosuppression (Fig. 1). Although Tregs have been intensively investigated during the last decade, it is still unclear whether these cells are prominently primed in the thymus or rather emerge in the periphery upon antigen-specific stimulation.73 In this respect, the work of Valzasina et al. supports the thymus-independent expansion of Tregs, as the inoculation of malignant cells to mice resulted in the expansion of CD4+CD25+ T cells even in thymectomized animals and independently of pre-existing CD25+ T cells (which had been removed by specific monoclonal antibodies).74
The first line of evidence demonstrating the regulation of Tregs by B cells in a murine model was provided by Wei et al., describing the B cell-mediated protection from colitis resulting from the conversion of naïve T cells into Tregs.75 The protective potential of Tregs in autoimmune diseases has been unveiled long time ago, but the underlying mechanisms, including the conversion of T cells and the involvement of specific and rather small subsets of B cells, have only recently begun to emerge.51,76,77
Kessel et al. have studied the effects of Bregs on T cells in human samples, with particular attention to the role of IL-10 and TGFβ, finding that the co-culture of Bregs (defined as CD25highCD27highCD86highCD1dhighIL-10highTGFβhigh) with stimulated autologous CD4+ T cells significantly decreased the proliferative properties of the latter.78 Furthermore, FOXP3 and CTLA-4 expression by Tregs were enhanced by non-stimulated (and even more by CD40L-stimulated) Bregs. The Treg-regulatory activity of Bregs was essentially dependent on direct cell-to-cell contacts as well as on TGFβ, but not IL-10. The recruitment of Tregs by B cells mainly relies on the secretion of chemokine (C-C motif) ligand 4 (CCL4), operating as a potent chemoattractant.42 Primary B lymphocytes are also capable of actively converting naïve T cells into FOXP3+ Tregs.79,80 Interestingly, in a murine 4T1 breast carcinoma model, Olkhanud et al. could demonstrate that so-called “tumor-evoked Bregs” promote the metastatic spread to the lung of breast cancer cells by converting resting CD4+ T cells into Tregs.42 Upon further analysis, such a metastatic spread turned out to require a chemokine (C-C motif) receptor 4 (CCR4)-mediated chemotactic response on both tumor cells and Tregs, which directly inactivated pulmonary NK cells.81 Olkhanud et al. speculate that Tregs and tumor-evoked Bregs may also functionally interact with myeloid-derived suppressor cells (MDSCs), which can further potentiate the Treg response, hence exerting immunosuppressive effects and promoting disease progression.42 Finally, Tadmor et al. compared tumor growth as well as the number and function of Tregs in wild-type immunocompetent and B cell-deficient mice.82 Mice were either naïve or had received EMT-6 mammary adenocarcinoma cells. Tumor growth was substantially inhibited in B cell-deficient mice as compared with wild-type animals. Tregs expanded in wild-type mice upon EMT-6 cell inoculation, a phenomenon that was much less pronounced in B cell-deficient animals. The percentage and absolute number of Tregs found in the spleen, tumor-draining lymph nodes, and within neoplastic lesions were significantly reduced in tumor-bearing B cell-deficient mice as compared with their wild-type counterparts, and so were Treg functions.82
Conclusions and Clinical Perspectives
Although increasing evidence indicates that B cells play a significant role in antitumor immune responses, the identification of distinct B-cell subsets and associated markers has been challenging, for several reasons. First, B cells are extraordinarily sensitive to isolation methods commonly used to purify other immune cells, e.g., T cells. Second, tumor progression seems to be a critical factor for the ever more pronounced malfunctioning of B cells. Therefore, the precise identification and clear distinction of pro-tumor vs. anti-tumor B-cell subsets will be a central goal for the forthcoming research. Going one step further, only a few translational approaches have been made in order to explore B cell-modulatory drugs in murine tumor models and cancer patients. In this regard, rituximab—a chimeric monoclonal antibody targeting CD20—has been shown to exert promising therapeutic activity in patients affected by some hematologic malignancies, including leukemia and lymphoma. Chronic lymphocytic leukemia patients, for example, significantly benefited in terms of overall and progression-free survival when receiving chemotherapy plus rituximab as compared with chemotherapy alone.83 The same held true in elderly patients with diffuse large B-cell lymphomas, as the addition of rituximab to the standard therapeutic regimen (based on the combination of cyclophosphamide, doxorubicin, vincristine and prednisone, CHOP) substantially enhanced the rate of complete responses and prolonged event-free and overall survival in the absence of a clinically significant increase in toxicity.84 Second-generation antibodies targeting CD20 (e.g., ofatumumab, veltuzumab, and ocrelizumab) are currently under clinical evaluation85 Anti-CD20 antibodies also attracted interest for the treatment of solid tumors. Aklilu et al. reported that the depletion of circulating B cells by rituximab in combination with IL-2-based immunotherapy did not increase the response rate of patients with metastatic renal cell carcinoma or melanoma.86 On the other hand, in a pilot clinical trial including patients with metastatic unresectable colon cancer, a reduction of tumor burden was observed in 5 out of 8 patients upon partial B-cell depletion with rituximab plus conventional chemotherapy.49 This said, some Breg subsets may escape anti-CD20 immunotherapies.87,88 Thus, the meticulous characterization of distinct Breg subsets might become crucially important in the future to finely tune targeted anticancer immunotherapies.
Another field of interest has been opened up by TIBCs strongly expressing IGKCs, in that the levels of IGKCs proved to constitute a single, robust immune biomarker predicting an improved metastasis-free survival and favorable responses to chemotherapy in patients affected by various solid tumors (see above).64,89 Due to such a reliable association with clinical endpoints, IGKC levels might therefore serve as an immunological biomarker of disease outcome in future clinical trials.90 Furthermore, a recently developed method for cloning and immortalizing TIBCs may prove instrumental for unraveling the actual relevance of these cells in tumor progression and response to therapy.91 Finally, even B cell-based antitumor therapies might be envisioned now that isolation and expansion procedures for tumor-infiltrating and antigen-specific B cells are available.66
Recently, increasing attention has been attracted by signal transducers operating downstream of the BCR, which regulate multiple processes including B-cell development and survival. Bruton tyrosine kinase (BTK) is positioned in a rather apical position within the BCR signaling cascade, thus presenting an attractive target for the selective inhibition of B cells.92 Accordingly, in a phase I clinical trial, ibrutinib—a selective and irreversible small-molecule inhibitor of BTK—has been shown to exert substantial therapeutic activity in patients affected by a variety of B-cell malignancies.92 Another small-molecule inhibitor of BCR signaling is dasatinib, which interferes with the enzymatic activity of SRC family tyrosine kinases. Interestingly, in a phase I/II trial involving patients with castration-resistant prostate cancer, dasatinib administered in combination with docetaxel, an established anti-mitotic chemotherapeutic agent, has been associated with a favorable toxicity and some partial responses, although the question of clinical efficacy remains to be clarified in randomized studies.93
In spite of several open questions, accumulating evidence suggests that B cells not only excel in antibody production but also play a critical role as part of the complex cast regulating cellular immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
References
- 1.Thomas L. Discussion. In: Lawrence HS, ed. Cellular and Humoral Aspects of the Hypersensitive States. New York, NY: Hoeber-Harper, 1959:529-33. [Google Scholar]
- 2.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 3.Cooper MD, Raymond DA, Peterson RD, South MA, Good RA. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966;123:75–102. doi: 10.1084/jem.123.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanyi J, Lydyard PM. Delineation of chicken lymphocyte populations by specific anti-thymus and anti-bursa sera. Cell Immunol. 1972;5:180–9. doi: 10.1016/0008-8749(72)90094-9. [DOI] [PubMed] [Google Scholar]
- 5.Fagraeus A. The plasma cellular reaction and its relation to the formation of antibodies in vitro. J Immunol. 1948;58:1–13. [PubMed] [Google Scholar]
- 6.Ron Y, De Baetselier P, Gordon J, Feldman M, Segal S. Defective induction of antigen-reactive proliferating T cells in B cell-deprived mice. Eur J Immunol. 1981;11:964–8. doi: 10.1002/eji.1830111203. [DOI] [PubMed] [Google Scholar]
- 7.Ron Y, Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987;138:2848–56. [PubMed] [Google Scholar]
- 8.Janeway CA, Jr., Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138:1051–5. [PubMed] [Google Scholar]
- 9.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 10.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidard L, Kovacsovics-Bankowski M, Kraeft SK, Chen LB, Benacerraf B, Rock KL. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J Immunol. 1996;156:2809–18. [PubMed] [Google Scholar]
- 12.Lanzavecchia A, Bove S. Specific B lymphocytes efficiently pick up, process and present antigen to T cells. Behring Inst Mitt. 1985:82–7. [PubMed] [Google Scholar]
- 13.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–80. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 15.Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J Immunol. 2005;175:7103–7. doi: 10.4049/jimmunol.175.11.7103. [DOI] [PubMed] [Google Scholar]
- 16.Schultze JL, Michalak S, Lowne J, Wong A, Gilleece MH, Gribben JG, et al. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1–12. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Küppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci U S A. 1994;91:10962–6. doi: 10.1073/pnas.91.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Shimomura Y, Mizoguchi E, Sugimoto K, Kibe R, Benno Y, Mizoguchi A, et al. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008;20:729–37. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- 20.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 21.Brodt P, Gordon J. Natural resistance mechanisms may play a role in protection against chemical carcinogenesis. Cancer Immunol Immunother. 1982;13:125–7. doi: 10.1007/BF00205312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monach PA, Schreiber H, Rowley DA. CD4+ and B lymphocytes in transplantation immunity. II. Augmented rejection of tumor allografts by mice lacking B cells. Transplantation. 1993;55:1356–61. doi: 10.1097/00007890-199306000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–30. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 24.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 25.Domschke C, Schuetz F, Ge Y, Seibel T, Falk C, Brors B, et al. Intratumoral cytokines and tumor cell biology determine spontaneous breast cancer-specific immune responses and their correlation to prognosis. Cancer Res. 2009;69:8420–8. doi: 10.1158/0008-5472.CAN-09-1627. [DOI] [PubMed] [Google Scholar]
- 26.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–7. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117:574–86. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 29.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–5. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–16. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domschke C, Schuetz F, Sommerfeldt N, Rom J, Scharf A, Sohn C, et al. Effects of distant metastasis and peripheral CA 15-3 on the induction of spontaneous T cell responses in breast cancer patients. Cancer Immunol Immunother. 2010;59:479–86. doi: 10.1007/s00262-009-0801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A. B cells and macrophages in cancer: yin and yang. Nat Med. 2011;17:285–6. doi: 10.1038/nm0311-285. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 38.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 39.Moshkani S, Kuzin II, Adewale F, Jansson J, Sanz I, Schwarz EM, et al. CD23+ CD21(high) CD1d(high) B cells in inflamed lymph nodes are a locally differentiated population with increased antigen capture and activation potential. J Immunol. 2012;188:5944–53. doi: 10.4049/jimmunol.1103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiLillo DJ, Horikawa M, Tedder TF. B-lymphocyte effector functions in health and disease. Immunol Res. 2011;49:281–92. doi: 10.1007/s12026-010-8189-3. [DOI] [PubMed] [Google Scholar]
- 41.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 42.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4⁺ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–15. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Eliav Y, Shin SU, Schreiber TH, Podack ER, Tadmor T, et al. B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer Immunol Immunother. 2013;62:87–99. doi: 10.1007/s00262-012-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorrentino R, Morello S, Forte G, Montinaro A, De Vita G, Luciano A, et al. B cells contribute to the antitumor activity of CpG-oligodeoxynucleotide in a mouse model of metastatic lung carcinoma. Am J Respir Crit Care Med. 2011;183:1369–79. doi: 10.1164/rccm.201010-1738OC. [DOI] [PubMed] [Google Scholar]
- 45.João C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. B cell-dependent TCR diversification. J Immunol. 2004;172:4709–16. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 46.Crowley MT, Reilly CR, Lo D. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J Immunol. 1999;163:4894–900. [PubMed] [Google Scholar]
- 47.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–82. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carpenter EL, Mick R, Rech AJ, Beatty GL, Colligon TA, Rosenfeld MR, et al. Collapse of the CD27+ B-cell compartment associated with systemic plasmacytosis in patients with advanced melanoma and other cancers. Clin Cancer Res. 2009;15:4277–87. doi: 10.1158/1078-0432.CCR-09-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF, Jr., Feng L, et al. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48:541–9. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 51.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Chapoval AI, Fuller JA, Kremlev SG, Kamdar SJ, Evans R. Combination chemotherapy and IL-15 administration induce permanent tumor regression in a mouse lung tumor model: NK and T cell-mediated effects antagonized by B cells. J Immunol. 1998;161:6977–84. [PubMed] [Google Scholar]
- 53.Perricone MA, Smith KA, Claussen KA, Plog MS, Hempel DM, Roberts BL, et al. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother. 2004;27:273–81. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 54.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–25. doi: 10.1182/blood.V99.9.3319. [DOI] [PubMed] [Google Scholar]
- 55.Kondo E, Topp MS, Kiem HP, Obata Y, Morishima Y, Kuzushima K, et al. Efficient generation of antigen-specific cytotoxic T cells using retrovirally transduced CD40-activated B cells. J Immunol. 2002;169:2164–71. doi: 10.4049/jimmunol.169.4.2164. [DOI] [PubMed] [Google Scholar]
- 56.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 57.Van den Bosch GA, Ponsaerts P, Nijs G, Lenjou M, Vanham G, Van Bockstaele DR, et al. Ex vivo induction of viral antigen-specific CD8 T cell responses using mRNA-electroporated CD40-activated B cells. Clin Exp Immunol. 2005;139:458–67. doi: 10.1111/j.1365-2249.2005.02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung Y, Kim BS, Kim YJ, Ko HJ, Ko SY, Kim DH, et al. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res. 2006;66:6843–50. doi: 10.1158/0008-5472.CAN-06-0889. [DOI] [PubMed] [Google Scholar]
- 59.Tamada K, Harada M, Okamoto T, Takenoyama M, Ito O, Matsuzaki G, et al. Specific antitumor activity of tumor-infiltrating lymphocytes expanded first in a culture with both anti-CD3 monoclonal antibody and activated B cells and then in a culture with interleukin-2. Cancer Immunol Immunother. 1995;41:339–47. doi: 10.1007/BF01526553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harada M, Okamoto T, Kurosawa S, Shinomiya Y, Ito O, Takenoyama M, et al. The antitumor activity induced by the in vivo administration of activated B cells bound to anti-CD3 monoclonal antibody. Cell Immunol. 1995;161:132–7. doi: 10.1006/cimm.1995.1017. [DOI] [PubMed] [Google Scholar]
- 61.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladányi A, Kiss J, Mohos A, Somlai B, Liszkay G, Gilde K, et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011;60:1729–38. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–92. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt M, Hellwig B, Hammad S, Othman A, Lohr M, Chen Z, et al. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin κ C as a compatible prognostic marker in human solid tumors. Clin Cancer Res. 2012;18:2695–703. doi: 10.1158/1078-0432.CCR-11-2210. [DOI] [PubMed] [Google Scholar]
- 65.Zirakzadeh AA, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J Immunol. 2013;190:5847–55. doi: 10.4049/jimmunol.1203279. [DOI] [PubMed] [Google Scholar]
- 66.Linnebacher M. Tumor-infiltrating B cells come into vogue. World J Gastroenterol. 2013;19:8–11. doi: 10.3748/wjg.v19.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linnebacher M, Maletzki C. Tumor-infiltrating B cells: The ignored players in tumor immunology. Oncoimmunology. 2012;1:1186–8. doi: 10.4161/onci.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nielsen JS, Nelson BH. Tumor-infiltrating B cells and T cells: Working together to promote patient survival. Oncoimmunology. 2012;1:1623–5. doi: 10.4161/onci.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whiteside TL, Ferrone S. For breast cancer prognosis, immunoglobulin kappa chain surfaces to the top. Clin Cancer Res. 2012;18:2417–9. doi: 10.1158/1078-0432.CCR-12-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J Clin Invest. 2003;112:1310–2. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuniyasu Y, Takahashi T, Itoh M, Shimizu J, Toda G, Sakaguchi S. Naturally anergic and suppressive CD25(+)CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol. 2000;12:1145–55. doi: 10.1093/intimm/12.8.1145. [DOI] [PubMed] [Google Scholar]
- 72.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–3. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 73.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev. 2006;212:163–9. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 74.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25- lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–95. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 75.Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, et al. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci U S A. 2005;102:2010–5. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–8. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwata S, Saito K, Tokunaga M, Yamaoka K, Nawata M, Yukawa S, et al. Phenotypic changes of lymphocytes in patients with systemic lupus erythematosus who are in longterm remission after B cell depletion therapy with rituximab. J Rheumatol. 2011;38:633–41. doi: 10.3899/jrheum.100729. [DOI] [PubMed] [Google Scholar]
- 78.Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012;11:670–7. doi: 10.1016/j.autrev.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 79.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–29. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 80.Chen X, Jensen PE. Cutting edge: primary B lymphocytes preferentially expand allogeneic FoxP3+ CD4 T cells. J Immunol. 2007;179:2046–50. doi: 10.4049/jimmunol.179.4.2046. [DOI] [PubMed] [Google Scholar]
- 81.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tadmor T, Zhang Y, Cho HM, Podack ER, Rosenblatt JD. The absence of B lymphocytes reduces the number and function of T-regulatory cells and enhances the anti-tumor response in a murine tumor model. Cancer Immunol Immunother. 2011;60:609–19. doi: 10.1007/s00262-011-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bauer K, Rancea M, Roloff V, Elter T, Hallek M, Engert A, et al. Rituximab, ofatumumab and other monoclonal anti-CD20 antibodies for chronic lymphocytic leukaemia. Cochrane Database Syst Rev. 2012;11:CD008079. doi: 10.1002/14651858.CD008079.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 85.Cang S, Mukhi N, Wang K, Liu D. Novel CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol. 2012;5:64. doi: 10.1186/1756-8722-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, et al. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol. 2004;15:1109–14. doi: 10.1093/annonc/mdh280. [DOI] [PubMed] [Google Scholar]
- 87.Hamaguchi Y, Uchida J, Cain DW, Venturi GM, Poe JC, Haas KM, et al. The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol. 2005;174:4389–99. doi: 10.4049/jimmunol.174.7.4389. [DOI] [PubMed] [Google Scholar]
- 88.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt M, Micke P, Gehrmann M, Hengstler JG. Immunoglobulin kappa chain as an immunologic biomarker of prognosis and chemotherapy response in solid tumors. Oncoimmunology. 2012;1:1156–8. doi: 10.4161/onci.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Whiteside TL. Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol. 2013;3:107. doi: 10.3389/fonc.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maletzki C, Jahnke A, Ostwald C, Klar E, Prall F, Linnebacher M. Ex-vivo clonally expanded B lymphocytes infiltrating colorectal carcinoma are of mature immunophenotype and produce functional IgG. PLoS One. 2012;7:e32639. doi: 10.1371/journal.pone.0032639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Araujo JC, Mathew P, Armstrong AJ, Braud EL, Posadas E, Lonberg M, et al. Dasatinib combined with docetaxel for castration-resistant prostate cancer: results from a phase 1-2 study. Cancer. 2012;118:63–71. doi: 10.1002/cncr.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]