Glycoepitopes of Staphylococcal Wall Teichoic Acid Govern Complement-mediated Opsonophagocytosis via Human Serum Antibody and Mannose-binding Lectin (original) (raw)
Background: The exact staphylococcal antigenic determinants of anti-staphylococcal IgG and mannose-binding lectin (MBL) have not been determined.
Results: The antigenic epitopes of the staphylococcal wall teichoic acid (WTA) for serum IgG and MBL are _O-N_-acetyl-d-glucosamine residues.
Conclusion: The sugar moiety of WTA is an important molecular determinant in host responses to S. aureus.
Significance: The results provide new insights into the host-pathogen interaction.
Keywords: Cell Wall, Complement System, Host Defense, Host-Pathogen Interactions, Innate Immunity, Gram-positive Bacteria, S. aureus
Abstract
Serum antibodies and mannose-binding lectin (MBL) are important host defense factors for host adaptive and innate immunity, respectively. Antibodies and MBL also initiate the classical and lectin complement pathways, respectively, leading to opsonophagocytosis. We have shown previously that Staphylococcus aureus wall teichoic acid (WTA), a cell wall glycopolymer consisting of ribitol phosphate substituted with α- or β-O-N_-acetyl-d-glucosamine (GlcNAc) and d-alanine, is recognized by MBL and serum anti-WTA IgG. However, the exact antigenic determinants to which anti-WTA antibodies or MBL bind have not been determined. To answer this question, several S. aureus mutants, such as α-GlcNAc glycosyltransferase-deficient S. aureus Δ_tarM, β-GlcNAc glycosyltransferase-deficient Δ_tarS_, and Δ_tarMS_ double mutant cells, were prepared from a laboratory and a community-associated methicillin-resistant S. aureus strain. Here, we describe the unexpected finding that β-GlcNAc WTA-deficient Δ_tarS_ mutant cells (which have intact α-GlcNAc) escape from anti-WTA antibody-mediated opsonophagocytosis, whereas α-GlcNAc WTA-deficient Δ_tarM_ mutant cells (which have intact β-GlcNAc) are efficiently engulfed by human leukocytes via anti-WTA IgG. Likewise, MBL binding in S. aureus cells was lost in the Δ_tarMS_ double mutant but not in either single mutant. When we determined the serum concentrations of the anti-α- or anti-β-GlcNAc-specific WTA IgGs, anti-β-GlcNAc WTA-IgG was dominant in pooled human IgG fractions and in the intact sera of healthy adults and infants. These data demonstrate the importance of the WTA sugar conformation for human innate and adaptive immunity against S. aureus infection.
Introduction
Staphylococcus aureus can cause serious infections of the skin, soft tissue, and bloodstream in the community and in hospitalized patients (1). The recent spread of methicillin-resistant S. aureus (MRSA)3 increases the difficulty of treating infections. S. aureus is a Gram-positive pathogen that is surrounded by glycopolymers, including wall teichoic acid (WTA), peptidoglycan, lipoteichoic acid, and capsular polysaccharide. S. aureus WTA is a glycopolymer that covalently links to peptidoglycan and is composed of an _N_-acetylmannosamine (ManNAc)-(β-1,3)-_N_-acetylglucosamine (GlcNAc) disaccharide with two glycerol phosphates followed by 10–40 ribitol phosphate repeating units (Fig. 1) (2, 3). The hydroxyls on the ribitol phosphate repeats are modified with cationic d-alanine esters and _O-_GlcNAc (4). Although WTA is dispensable for bacterial viability, it was reported to be involved in the adherence of S. aureus to nasal epithelial cells (5), resistance to lysozyme and antimicrobial peptides, and evasion of the innate immune response (6, 7). The zwitterionic S. aureus WTA has been reported to induce CD4+ T-cell proliferation in a major histocompatibility complex II-dependent manner, which in turn, modulates abscess formation in a mouse skin infection model (8).
FIGURE 1.
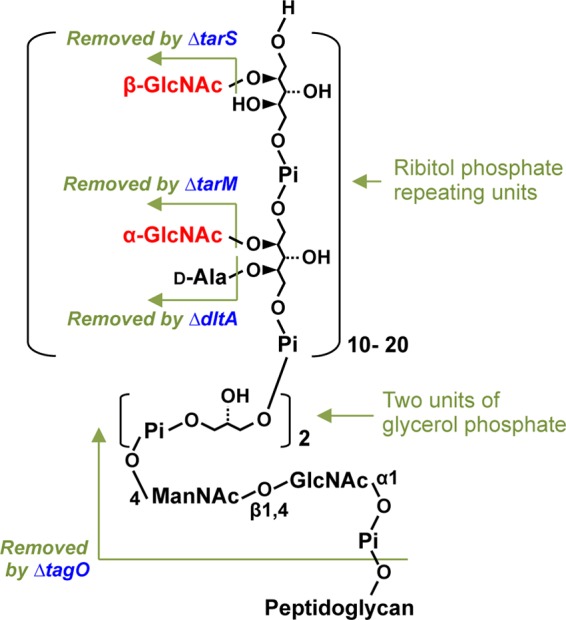
Schematic structure of S. aureus WTA. WTA of S. aureus is composed of a short linkage unit connected to peptidoglycan, consisting of a ManNAc-GlcNAc disaccharide with two glycerol phosphates, followed by a longer chain of ribitol phosphate repeating units substituted with α- or β-GlcNAc and d-alanine. Changes by Δ_tarS_, Δ_tarM_, Δ_dltA_, or Δ_tagO_ mutations on the WTA structure are indicated.
The complement system is the first line of host defense responses to invading pathogens (9). Pathogen-specific antibodies activate the classical complement pathway (10). Bacterial surface glycopolymers are also recognized by a variety of pattern recognition molecules, including mannose-binding lectin (MBL) (11, 12). MBL binds to mannose and the GlcNAc residues of sugar chains (13) and functions as an opsonin and an activator of the lectin complement pathway (14). The activation of the classical and lectin pathways mediates opsonization by complement fragments, such as C4b and C3b, proinflammatory signaling by anaphylatoxins for recruiting phagocytes, and cell lysis of Gram-negative bacteria by membrane attack complex formation. The immune complexes also induce phagocytosis via cell surface Fcγ receptors (FcγRs). Because S. aureus is a Gram-positive bacterium with a thick peptidoglycan layer, many reports have suggested that serum antibody-mediated opsonophagocytosis is necessary to combat pathogenic S. aureus infection (10, 15). Additionally, MBL deficiency in mice caused susceptibility to S. aureus infection (16). Therefore, complement-mediated opsonophagocytosis and FcγR-mediated phagocytosis are important components of protection from S. aureus infections, including those caused by MRSA strains, such as USA300 (17).
In the early 1960s, immunochemical studies were conducted by several research groups to determine the epitope structure of the S. aureus cell wall (18, 19). When a mixture of formaldehyde-treated S. aureus Copenhagen strain and Freund's adjuvant was injected into rabbits, the agglutination activity of rabbit antisera was inhibited by S. aureus WTA or by the purified α-GlcNAc-substituted ribitol phosphate. Based on these observations, the rabbit sera were believed to contain anti-WTA antibodies specific to α-GlcNAc epitopes on WTA (18, 19). On the other hand, when rabbit antisera were prepared by repeated intravenous injection of heat- or phenol-killed S. aureus NYH-6 or purified cell wall components, the antibody-binding epitope of the S. aureus NYH-6 was determined to be the β-GlcNAc residues of WTA based on a hemagglutination assay (20). The discrepancies between these results were explained by the specificity of the anti-WTA antibodies to various WTA types derived from the different strains used for immunization (18). Human immunization with S. aureus Copenhagen and NYH-6 strains resulted in the production of antibodies recognizing both α-GlcNAc and β-GlcNAc WTAs (21). Thus, until now, the exact epitope of S. aureus WTA and the specificity of antibodies after S. aureus infection were not clearly determined. One of the major reasons for this delay is the lack of genetic information regarding the GlcNAc transferases involved in the biosynthesis of S. aureus WTA and the difficulty of purifying WTA (specifically α-GlcNAcylated or β-GlcNAcylated WTAs) due to the absence of S. aureus mutants lacking β-GlcNAc or α-GlcNAc modifications of WTA.
Recently, we purified anti-WTA IgG from human intravenous IgG (IVIG) using an affinity column coupled with WTA isolated from S. aureus strain RN4220 (22). The purified anti-WTA IgG strongly induced activation of the classical complement pathway, leading to opsonophagocytosis of S. aureus (22). However, the exact epitope of WTA recognized by this anti-WTA IgG has not been determined. Additionally, we reported that S. aureus WTA functions as a ligand of MBL (23). Intriguingly, serum MBL from infants who had not yet fully developed adaptive immunity could bind to S. aureus WTA and induce complement C4 deposition. However, the exact motif used to bind WTA by human MBL is still not determined.
The molecular pathways of WTA glycosylation in S. aureus were recently elucidated (24, 25). Two WTA glycosyltransferases, TarM and TarS, are responsible for modifying WTA with α-GlcNAc and β-GlcNAc, respectively. Furthermore, methicillin-resistance in S. aureus requires a β-GlcNAc residue of WTA, identifying the TarS enzyme as a potential new drug target molecule whose inhibition would resensitize MRSA strains to β-lactam antibiotic agents (25). Because the tarS and tarM genes have been identified, we hypothesized that if we constructed S. aureus Δ_tarM_ or Δ_tarS_ single or Δ_tarMS_ double mutant cells from laboratory and highly pathogenic community-associated MRSA strains, it may be possible to determine which GlcNAc residue of WTA is recognized by serum anti-WTA IgG or MBL. Here, we report the novel findings that β-GlcNAc residues of WTA are required for the induction of the classical complement pathway-dependent opsonophagocytosis of S. aureus based on experiments with S. aureus mutant strains with altered WTA glycosylation. However, MBL recognized both α- and β-GlcNAc residues of WTA. We also found that antibodies specific to β-GlcNAc-modified WTA are major serum antibodies in humans, revealing an unexpected relationship between β-glycosylation of S. aureus WTA and host defense.
EXPERIMENTAL PROCEDURES
Ethics Statement
We obtained approval from the Institutional Review Board of Yangsan Pusan National University Hospital specifically for this study. The infant sera were collected with permission from parents/guardians who have provided written informed consent on behalf of all child participants. For the adult sera, we also obtained written informed content from all healthy participants.
Proteins, Sera, Bacteria, and Reagents
Native human MBL/MBL-associated serine protease (MASP) complex was purified from human sera as described previously (26). IgG-depleted sera were purchased from Sunnylab (Sittingbourne, UK), and MBL-deficient serum was purchased from Statens Serum Institute (Copenhagen, Denmark). S. aureus_-treated serum was prepared as described previously (22) using the S. aureus M0107 strain (Δ_spa), which is devoid of immunoglobulin-binding protein A. Monoclonal antibodies against human FcγRs included anti-human CD64 (clone 10.1; BioLegend), anti-human CD32 (AT10; Abcam), and anti-human CD16 (clone 3G8; BioLegend). S. aureus mutants were derivatives of strain RN4220 or USA300 are listed in Table 1. S. aureus and Escherichia coli strains were grown in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, and 1% NaCl) containing, where appropriate, 100 μg/ml ampicillin, 10 μg/ml erythromycin, 50 μg/ml kanamycin, 12.5 μg/ml chloramphenicol, or 20 μg/ml phleomycin at the appropriate temperature. In S. aureus T790 strain, the tarM gene, which encodes α-GlcNAc transferase, has been disrupted in RN4220 cells via integration of a pMutinT3 plasmid (27) harboring the central open reading frame region of the tarM gene at the HindIII and BamHI sites, which was amplified using primers of tarM_-F1 (ATGATGC GGACATACCTGCT) and tarM_-R2-BamHI (CACGGATCCTAAATGCACCCGTATCATCGAA). The tarM gene in S. aureus NCTC8325 genome, a parental strain of RN4220, was reported to split into two ORFs, but our sequencing result for the RN4220 tarM locus showed one nucleotide deletion that fuses the two ORFs into one TarM ORF as in other S. aureus strains. The tarS gene encoding β-GlcNAc transferase was disrupted via integration of a pSF151 plasmid (28) harboring the central open reading frame region of the tarS gene at the BamHI and EcoRI sites, which was amplified using primers of SA0248-F-BamHI (CAACTGGATCCAAATTCTGGTGGTCCAGGT) and SA0248-R-EcoRI (TTTCGAATTCGCGTAGTGCAACAATGGTCGT). Insertion into the chromosomal tarM or tarS gene, respectively, was confirmed by PCR. To construct a plasmid pS_tarM harboring the intact tarM gene, the tarM gene region was amplified with primers of pS_tarM_-F-BamHI (TGGAAAAGGGGATCCTCAATTACGATAATGC) and pS_tarM_-R-EcoRI (AGTGAATTCAGCTGGAAGAAATGGCTAC) and cloned into the BamHI and EcoRI sites of pKE515 plasmid (29). For a tarS_-harboring plasmid pS_tarS, TarS ORF was amplified using primers of pSA0248-F-KpnI (CAAAGTGGGAGAGGTACCATGATGAAA) and pSA0248-R2-EcoRI (AATGAATTCGAAAATGAAAATCAGGGAAATG) and cloned into the KpnI and EcoRI sites of pKE515pHU plasmid (30). The Δ_tarMS double mutant in strain USA300 was constructed using the pKOR1 shuttle vector (31). The Δ_tarM_Δ_tarS_ double mutant of USA300 strain was complemented with tarM or tarS by cloning the genes in the pLI50-PcadC shuttle expression vector under the control of a cadmium-inducible promoter (25). Mutations and plasmids were transduced using phage 80.
TABLE 1.
S. aureus strains used in this study
| Strains | Genotypes | Description | References |
|---|---|---|---|
| RN4220 | NCTC8324-5, restriction mutant | Parent strain | Ref. 53 |
| M0107 | RN4220 Δ_spa_::phleo | Protein A-depleted | Ref. 54 |
| T258 | M0107 Δ_tagO_::erm | WTA-deficient | Ref. 23 |
| T790 | M0107 Δ_tarM_::erm | WTA α-GlcNAc-deficient | This study |
| T803 | M0107 Δ_tarS_::km | WTA β-GlcNAc-deficient | This study |
| T807 | M0107 Δ_tarM_::erm Δ_tarS_::km | WTA α,β-GlcNAc-deficient | This study |
| T861 | M0107 Δ_dltA_::erm | WTA d-Ala-deficient | This study |
| T842 | T807 harboring pS_tarM_ | tarM gene-supplemented | This study |
| T844 | T807 harboring pS_tarS_ | tarS gene-supplemented | This study |
| T846 | T807 harboring pKE515 | Vector plasmid-supplemented | This study |
| USA300 | SCC_mec_ IVa, PVL+ | Community-associated MRSA | Ref. 25 |
| USA300 Δ_tarMS_ | USA300 Δ_tarM_ Δ_tarS_ | WTA α,β-GlcNAc-deficient | This study |
| USA300 Δtar_MS/M_ | USA300 Δ_tarMS_ harboring tarM plasmid | tarM plasmid-supplemented | This study |
| USA300 Δ_tarMS/S_ | USA300 Δ_tarMS_ harboring tarS plasmid | tarS plasmid-supplemented | This study |
| USA300 Δ_tagO_ | USA300 Δ_tagO_ | WTA-deficient | This study |
Preparation of WTA from S. aureus
WTA was prepared from S. aureus strains according to our published method (32) with some modifications. Briefly, WTA-bound insoluble peptidoglycan was prepared and incubated with 5% trichloroacetic acid for 18 h at room temperature to release the WTA. The digested materials were centrifuged, and the WTA released in the supernatant was precipitated with acetone. The WTA fraction (∼8 mg from 20 mg of WTA-attached insoluble peptidoglycan) was further purified with HitrapQ column chromatography. Fractions containing WTA were monitored by _A_220 and PAGE with silver staining, and pooled fractions were evaluated for inorganic phosphate (33) and GlcNAc (34) after acid hydrolysis with 6 n HCl at 100 °C for 3 h in vacuo to avoid the degradation of GlcNAc residues in the oxygen.
Purification of Anti-WTA IgG
Anti-WTA IgG was affinity-purified with some modifications from commercially available human IVIG (Green Cross, Korea) using a WTA-coated nitrocellulose membrane as described recently with some modifications (23). In brief, 100 μg of monomeric peptidoglycan-linked WTA in 200 μl of PBS was prepared from S. aureus strain T384 (RN4220 Δ_lgt_::phleo Δ_oatA_::erm) as described elsewhere (23), spotted onto a nitrocellulose membrane (10 × 90 mm, Whatman, 0.45-μm pore), and baked at 100 °C for 1 h. The membranes were washed with buffer A (20 mm Tris-HCl, pH 7.4, 150 mm NaCl) and blocked with buffer B (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, and 1% BSA) for 2 h at 4 °C. Three sheets of the membrane were incubated with 50 mg of IVIG in 40 ml of buffer C (10 mm Tris-HCl, pH 7.4, 140 mm NaCl, and 1% BSA) for 2 h at 4 °C. After washing with buffer A, bound IgGs were eluted with 1 ml of 0.1 m glycine (pH 2.8) and immediately neutralized with 1 m KOH to pH 7.5. The glycine in the eluted IgG fraction was removed by passing the fraction through a Vivaspin 20 (Sartorius) three times in the presence of buffer A. To remove the anti-peptidoglycan IgGs, the obtained IgG fraction was incubated at 4 °C with S. aureus Δ_spa_, Δ_tagO_ double mutant cells that were prefixed with formaldehyde. Then the S. aureus double mutant cells were pelleted by centrifugation, and the supernatant was collected, concentrated by Vivaspin 20, and used as a purified anti-WTA IgG fraction.
Flow Cytometry Analysis of S. aureus Cells
S. aureus Δ_spa_ mutant (M0107) that is deficient for IgG-binding protein A and its derivatives were used. To detect bound MBL, ethanol-fixed S. aureus cells (4 μl of a suspension at _A_600 = 3) were incubated with MBL/MASP (2–10 ng) or human serum in 20 μl of incubation buffer (10 mm Tris-HCl, pH 7.4, 140 mm NaCl, 10 mm CaCl2, and 1% BSA) for 2 h at 4 °C, washed, and incubated with mouse anti-human MBL mAb (Dobeel, Korea; diluted 1:200), followed by goat F(ab′)2 anti-mouse IgG antibodies conjugated with FITC (Beckman Coulter; diluted 1:200). Cells were appropriately washed with washing buffer (10 mm Tris-HCl, pH 7.4, 140 mm NaCl, 10 mm CaCl2, and 0.05% Tween 20). To detect bound human IgG, S. aureus cells were incubated under conditions identical to MBL binding conditions described above, except that mouse anti-human IgG mAb (Sigma; diluted 1:200) was used as the primary antibody. To detect C4 or C3 deposition on S. aureus cells, ethanol-fixed S. aureus cells were incubated with _S. aureus_-treated serum (10%) with or without MBL/MASP (5–30 ng) or intact infant sera (5%) in 20 μl of incubation buffer for 20 or 60 min at 37 °C. In some experiments, S. aureus cells were incubated with IgG-depleted serum (10%) or MBL-deficient serum (10%) for 20 min at 37 °C. To detect bound C4b, mouse anti-human C4 mAb (Bioporto; diluted 1:500) was used. To detect bound C3b, mouse anti-human C3 mAb conjugated with FITC (Beckman Coulter; diluted 1:200) was used. Washed S. aureus cells were sonicated for 15 s to disperse clumped cells before flow cytometry analyses (Accuri C6, Beckman Coulter).
ELISA for Anti-WTA IgG
The amounts of anti-WTA IgGs in human serum were determined by ELISA (22) with some modifications. Each WTA (5 nmol of phosphate) in 60 μl of PBS (pH 7.5) was applied to F96 Cert. maxisorp immunoplates (Nunc) in triplicate, and the plates were incubated overnight at room temperature. The WTA-coated microplates were incubated with an adequate volume of human serum in 50 μl of a buffer (10 mm Tris-HCl, pH 7.4, 140 mm NaCl, and 1% BSA) for 2 h at 4 °C, and bound IgGs were detected with mouse monoclonal anti-human-IgG antibodies (Sigma-Aldrich) and goat F(ab′)2 anti-mouse IgG (H+L) antibodies conjugated with horseradish peroxidase (HRP) (Beckman Coulter; 1:10000 dilution). The plates were developed with the substrate, 3,3′,5,5′-tetramethylbenzidine (Zymed Laboratories Inc.) in dark conditions, and the development reaction was stopped by the addition of 2 n H2SO4. Absorbance at 450 nm was recorded using a microplate reader (Thermo Scientific USA). Data were representative of at least three independent experiments. Each anti-WTA IgG concentration was estimated compared with that of the control, which was purified anti-WTA-IgGs using the parental S. aureus M0107 WTA.
ELISA for MBL
MBL binding to purified WTA and the resulting C4 deposition were evaluated by ELISA (23) with some modifications. Briefly, each WTA (3 μg in 60 μl of PBS, pH 7.5)-coated microplate was washed with washing buffer and blocked with 200 μl of buffer B for 1 h at room temperature. After washing, the wells were incubated with 0–60 ng of MBL/MASP in 50 μl of incubation buffer for 2 h at 4 °C. When C4 deposition was determined, wells were washed and incubated with C4 (100 ng) in 50 μl of the incubation buffer for 1 h at 37 °C. Alternatively, immobilized ligands were incubated with 5% human intact infant serum in 50 μl of the incubation buffer. Primary antibodies for MBL and C4b were the same as used for the flow cytometry experiments and were used at dilutions of 1:1,000 and 1:3,000, respectively. The secondary antibodies were goat anti-mouse IgG (H+L) conjugated with HRP (Beckman Coulter; 1:10,000 dilution).
Isolation of Human Polymorphonuclear Leukocytes (PMNs) and Opsonophagocytosis Assay
The peripheral blood mononuclear cells were isolated from healthy donors using Polymorphprep solution (Nycomed Pharm As, Torshov, Norway) as described (22). The PMNs were 99% viable, as shown in a trypan blue dye exclusion test. An opsonophagocytosis assay was performed with minor modifications as described (22). S. aureus strains grown from a postexponential growth phase culture in LB medium were washed, killed with 70% ethanol, labeled with 0.1 mm FITC (Sigma) in 0.1 m Na2CO3 buffer (pH 8.5) for 30 min at room temperature, and resuspended in Hanks' balanced salt solution. FITC-labeled bacteria (equivalent to 1.5 × 107 cfu) were opsonized with 10% _S. aureus_-treated sera with purified human MBL or anti-WTA IgG in 20 μl of Hanks' balanced salt solution containing 2 mm CaCl2, 1 mm MgCl2, 150 mm NaCl, and 0.4% BSA for 30 min at 37 °C with shaking. PMN suspension (1.5 × 105 cells, 35 μl) was added to 5 μl of the opsonized bacteria (corresponding to 3.7 × 106 cfu; multiplicity of infection ∼25) and incubated at 37 °C for 60 min with shaking. The phagocytosed FITC-labeled S. aureus cells in the PMNs were counted in fluorescent phase-contrast microscopy. More than 100 PMNs were counted. Extracellular FITC-labeled S. aureus were quenched by 0.2% trypan blue.
Opsonophagocytic Killing Assay
An opsonophagocytic killing assay was performed with modifications as described previously (35, 36). S. aureus strains were grown to the logarithmic phase (_A_578 = 0.5) in LB medium and diluted in RPMI medium containing 0.05% human serum albumin. Human PMNs were isolated from the freshly obtained blood of healthy volunteers (37). One hundred μl of IgG depleted serum (30%) or MBL-deficient serum (30%) were combined with 100 μl of PMNs (2.5 × 106 cells) and 2.5 × 106 cfu of S. aureus cells and incubated with end over end rotation at 37 °C for 90 min. The negative controls included tubes from which PMNs or sera were omitted. The viable cells were enumerated after overnight culture on tryptic soy agar plates. The percentage of killing was calculated with the equation, (number of cfu surviving in the tubes with bacteria, PMNs, and sera/cfu of control without PMNs) × 100.
Data Processing and Statistical Analysis
The results from the quantitative analyses were expressed as the mean ± S.D. of the data from at least three independent experiments, unless otherwise stated. Other data were representative of at least three independent experiments that yielded similar results. The statistical analyses were performed using Student's t test. p values less than 0.05 were considered significant and are indicated in the figures.
RESULTS
The tarS Mutation Abrogates Anti-WTA IgG Binding to S. aureus Cells and Subsequent Complement Activation and Opsonophagocytosis
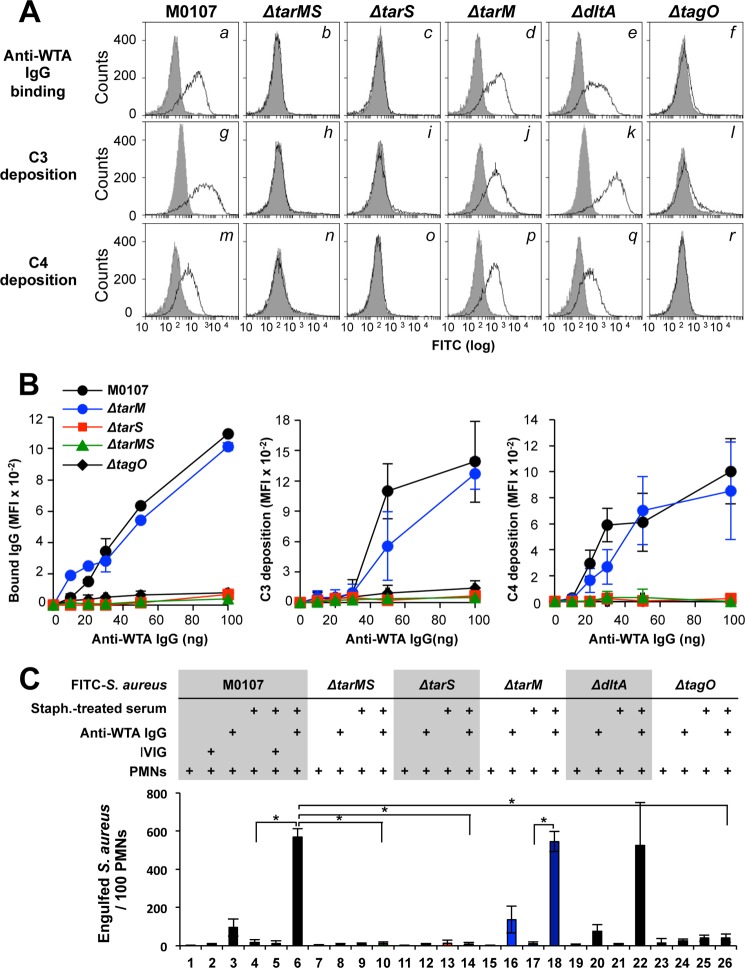
To examine which of the three WTA modifications, namely the α-GlcNAc, β-GlcNAc, and d-alanine substitutions of WTA, is recognized by anti-WTA IgG, binding ability of anti-WTA IgG to S. aureus mutant cells was examined via flow cytometry. As shown in Fig. 1 and Table 1, the Δ_tarS_ and Δ_tarM_ single mutants still have α-GlcNAc or β-GlcNAc WTA modifications, respectively, whereas the double mutant Δ_tarMS_ has no GlcNAc substitutions on its WTA. The Δ_dltA_ mutant cells lose the d-alanine substitution on WTA, and the Δ_tagO_ mutant cells lose WTA in the cell wall entirely. The anti-WTA IgG was affinity-purified from IVIG. As shown in Fig. 2A, anti-WTA IgG bound to the S. aureus M0107 cells (a) but not to isogenic Δ_tagO_ mutant cells (f), which is consistent with our previous report (22). In the same conditions, anti-WTA IgG bound to the Δ_tarM_ cells (d) and Δ_dltA_ cells (e). However, the anti-WTA IgG did not bind to the Δ_tarMS_ double mutant cells (b) or the Δ_tarS_ mutant cells (c). The dose response of anti-WTA-IgG between 0.5 and 5 μg/ml confirmed that the binding ability of anti-WTA IgG was completely lost in the Δ_tarS_, Δ_tarMS_, or Δ_tagO_ mutants (Fig. 2B, left). Consistent with these results, the Δ_tarMS_ double mutant harboring a plasmid containing intact tarS, but not the tarM gene, restored the anti-WTA IgG binding (supplemental Fig. S1_A_). These results suggest that the β-GlcNAc group of S. aureus WTA may function as an essential antigenic determinant for anti-WTA IgG binding, and the α-GlcNAc or d-alanine substitutions do not function in this way.
FIGURE 2.
Anti-WTA IgG-mediated complement activation and opsonophagocytosis of S. aureus cells are dependent on β-GlcNAc WTA. A, in the top panels, ethanol-killed S. aureus mutant cells were incubated without (gray area) or with anti-WTA IgG (50 ng; black line) in 20 μl of buffer, and bound IgG was detected by flow cytometric analysis. The middle and bottom panels show the measurement of C3 and C4 depositions in 10% S. aureus_-treated adult serum without (gray area) or with anti-WTA IgG (50 ng; black line) in 20 μl of buffer. C3 and C4 were detected by specific antibodies with flow cytometric analysis. B, anti-WTA IgG binding (left), C3 deposition (middle), and C4 deposition (right) on each S. aureus strain was examined as in A with respect to affinity-purified anti-WTA IgG concentrations. C, ethanol-killed Δ_spa (parent; columns 1–6) and the indicated isogenic mutant S. aureus cells were labeled with FITC (0.1 mm) and opsonized without or with _S. aureus_-treated serum (10%) in 20 μl of buffer. For descriptions of columns 1–26, see “Results.” Purified anti-WTA IgG (50 ng) or IVIG (50 ng) was simultaneously added, as indicated. Opsonized FITC-labeled S. aureus cells were incubated with human PMNs (1 × 105 PMNs) at a multiplicity of infection of 25 in 40 μl of RPMI 1640 medium at 37 °C for 1 h. Phagocytosed S. aureus cells per 100 PMNs were counted under fluorescent phase-contrast microscopy. Data are represented as the means ± S.D. (error bars) of the results of three independent experiments. *, p < 0.05.
We investigated anti-WTA IgG-mediated complement C3 and C4 deposition on S. aureus cells (Fig. 2A, g–r). For this experiment, we prepared S. aureus_-treated serum that was depleted of serum MBL, endogenous serum anti-WTA IgG, and other anti-S. aureus IgGs by incubating human sera with S. aureus Δ_spa mutant cells as described previously (22). Consistent with anti-WTA IgG binding to the S. aureus cells, C3b and C4b deposition was observed on S. aureus cells with the intact tarS gene, such as the parental cells (g and m), Δ_tarM_ (j and p), and Δ_dltA_ mutant cells (k and q), but not on the Δ_tarS_ (i and o) and Δ_tarMS_ mutant cells (h and n). The dose-response curve of anti-WTA IgG showed that C3 and C4 deposition on M0107 cells or Δ_tarM_ cells increased in a dose-dependent manner (Fig. 2B, middle and right). In the same anti-WTA IgG dose range, the C3 and C4 depositions on the Δ_tarS_ and Δ_tarMS_ mutants, which are deficient in the WTA β-GlcNAc modification, remained unchanged. The Δ_tarMS_ double mutant containing a plasmid harboring the tarS gene (ΔMS/pS) but not the tarM (ΔMS/pM) gene restored the anti-WTA IgG-mediated C3 and C4 depositions (supplemental Fig. S1_A_, d–i). Taken together, these results indicate that the β-GlcNAc residue of WTA generated by TarS is required for anti-WTA IgG-mediated complement activation by S. aureus.
Because anti-WTA IgG specifically induced C3 deposition on β-GlcNAc WTA-synthesizing S. aureus cells, we hypothesized that the Δ_tarS_ mutant might evade anti-WTA IgG-mediated opsonophagocytosis. To quantify the S. aureus cells engulfed by the human PMNs, we counted the number of FITC-labeled bacteria engulfed by 100 PMNs under a fluorescent microscope (Fig. 2C). In the absence of S. aureus_-treated serum, anti-WTA IgG alone increased PMN-mediated engulfment from 1 ± 1 (Fig. 2C, column 1) to 96 ± 44 (column 3), indicating that engulfment was induced in PMNs via FcγRs (38). The dependence of engulfment on FcγRs was confirmed by specific inhibition by commercially available anti-FcγR monoclonal antibodies (supplemental Fig. S1_C_, columns 3–6). In identical conditions, comparable levels of FcγR-dependent phagocytosis were observed for the Δ_tarM and Δ_dltA_ mutant cells but not the Δ_tarS_ or Δ_tarMS_ cells (Fig. 2C, columns 8, 12, 16, and 20). When the S. aureus parental M0107 cells were opsonized with S. aureus_-treated serum, the number of bacteria phagocytosed by 100 PMNs increased to 570 ± 41 compared with that of the absence of S. aureus_-treated serum (Fig. 2C, columns 3 and 6), suggesting the induction of complement-mediated opsonophagocytosis. This anti-WTA IgG-dependent engulfment was not inhibited by any of the anti-FcγR antibodies (supplemental Fig. S1_C_, column 9). Under the same conditions, the Δ_tarM (Fig. 2C, column 18) and Δ_dltA mutant cells (column 22), but not Δ_tarMS_ (column 10), Δ_tarS_ (column 14), or Δ_tagO_ mutant cells (column 26), were engulfed by the PMNs. These results clearly indicate that the TarS-mediated β-GlcNAc WTA modification is essential for anti-WTA IgG-mediated opsonophagocytosis of S. aureus.
We used the S. aureus MRSA strain USA300 and the isogenic Δ_tarMS_ mutant along with USA300 Δ_tarMS_ complemented with plasmids encoding tarM or tarS because the WTA structures may vary between different S. aureus lineages, and the laboratory strain M0107 may differ in its serum antibody-binding capacities from a primary, virulent S. aureus strain (Table 1). Using these mutant cells, when we compared C3 deposition by MBL-deficient human serum, the C3 deposition on the USA300 wild-type cells was lost due to the tagO mutation, suggesting that the C3 deposition in this MBL-deficient human serum was dependent on WTA. The USA300 Δ_tarMS_ double mutant cells (supplemental Fig. S2_A_, b) exhibited strongly abrogated C3 deposition compared with the parental USA300 wild-type cells, and complementation with the tarM or tarS genes led to partial or complete restoration (c and d), respectively (supplemental Fig. S2_A_). To further evaluate the effects of the tarMS mutation on the opsonization-dependent PMN-mediated killing of the S. aureus USA300 strain, USA300 cells were opsonized with the MBL-deficient human serum, incubated with or without PMNs, and the number of viable bacteria was determined. The killing of the USA300 wild-type cells by PMNs was reduced 4.5-fold by the tarMS mutation and complementation of the tarS gene, but the tarM gene restored susceptibility to PMNs-mediated bacterial killing (supplemental Fig. S2_B_). These results are the first evidence that WTA β-GlcNAc epitopes dictate the anti-WTA IgG-mediated complement activation that leads to opsonophagocytosis.
Purified β-GlcNAcylated WTAs Are Specifically Bound by Anti-WTA IgGs
To further characterize the requirement of β-GlcNAc WTA for anti-WTA IgG-mediated opsonization, WTAs were purified from the M0107, Δ_tarS_, Δ_tarM_, and Δ_tarMS_ mutant cells and analyzed by PAGE with silver staining (Fig. 3A). As expected, the WTA from the Δ_tarMS_ double mutant cells (lane 5) migrated faster than that of the M0107, Δ_tarS_, and Δ_tarM_ single mutant cells (lanes 1, 3, and 4), and the mock WTA faction from the Δ_tagO_ mutant did not have any signal (lane 2). These results further confirm that the WTA from the Δ_tarMS_ double mutant lost the β- and α-GlcNAc residues of the ribitol phosphate units.
FIGURE 3.
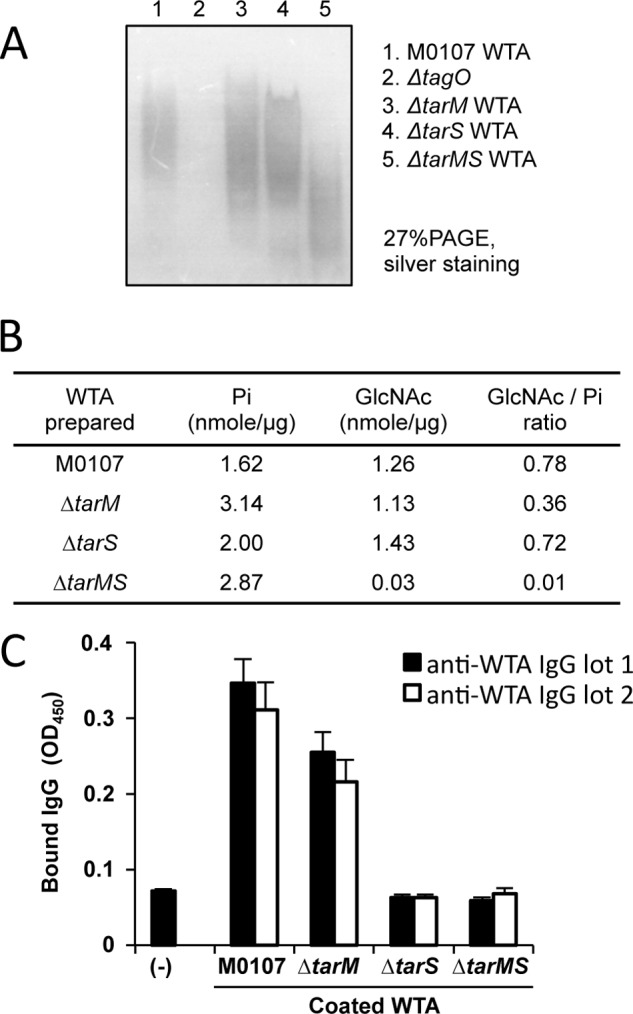
Biochemical characterization of purified WTAs and their affinities for purified anti-WTA IgG. A, purified WTAs (10 μg) from indicated strains were separated by PAGE (27%) and visualized by silver staining. B, amounts of phosphate, GlcNAc, and GlcNAc/inorganic phosphate (GlcNAc/Pi) ratio of purified WTA are shown. C, each purified WTA (5 nmol of phosphate) was used to coat a 96-well ELISA plate, and affinity-purified anti-WTA IgG (100 ng) was assayed for its binding ability to each coated WTA by ELISA. Two independently purified anti-WTA IgGs were used. Data are represented as the means ± S.D. (error bars) of the results of three independent experiments.
To exclude the possibility of insufficient GlcNAc modification of WTA in the Δ_tarS_ mutant, the GlcNAc and phosphate contents of each purified WTA were measured by the Morgan-Elson method (34) and the Chen method (33), respectively. The GlcNAc/inorganic phosphate (GlcNAc/Pi) ratio of the WTAs isolated from the M0107 cells and the Δ_tarMS_ mutant cells was determined to be 0.78 and 0.01, respectively, confirming that the Δ_tarMS_ mutant does not contain GlcNAc residues on its WTA. The GlcNAc/Pi ratio of the WTA was determined to be 0.72 for the Δ_tarS_ mutant, showing that the Δ_tarS_ mutant cells have amounts of GlcNAc residues comparable with those of the M0107 cells, although the Δ_tarS_ cells did not show anti-WTA IgG-mediated opsonophagocytosis (Fig. 2C, column 14). Although the value of the Δ_tarM_ mutant cells was 0.36, opsonophagocytosis was induced at a level similar to that of the M0107 cells (Fig. 2C, column 18). These results clearly indicate that the impaired anti-WTA IgG-mediated opsonophagocytosis of the Δ_tarS_ mutant cells is not due to insufficient amounts of α-GlcNAc residues in WTA.
We estimated the amounts of anti-WTA IgG bound to the purified WTAs (Fig. 3C). For this experiment, purified WTA amounts corresponding to 5 nmol of phosphate from M0107, Δ_tarM_, Δ_tarS_, or Δ_tarMS_ cells were coated onto 96-well microtiter plates, and the binding to two independently purified anti-WTA IgGs (100 ng) was determined by ELISA. The β-GlcNAc-depleted WTAs from the Δ_tarS_ and Δ_tarMS_ double mutant cells did not show binding to the purified serum anti-WTA IgGs. In contrast, the β-GlcNAc-sufficient WTAs obtained from the M0107 and Δ_tarM_ mutant cells showed similar levels of binding ability as the M0107 cells. These results further confirm the binding specificity of serum anti-WTA antibodies to β-GlcNAc WTA.
Adult Sera Predominantly Contain Anti-β-GlcNAc WTA Antibodies
The findings that serum anti-WTA IgGs are directed against glycosylated WTA and are specific for one of the two possible GlcNAc conformations raised two questions. The first question concerns an explanation of the finding that only β-GlcNAc WTA-specific antibodies were obtained from the IVIG when IVIG were used as a purification source for anti-WTA IgG. The second question asks whether anti-WTA antibodies specific for β-GlcNAcylated WTA are naturally generated in healthy humans. The latter has been an open question since 1960, although many groups have tried to answer it (18, 19). Because we obtained biologically functional β-GlcNAc- and α-GlcNAc-modified WTAs from the S. aureus mutant cells, we quantified the amount of IgGs specific to β-GlcNAcylated WTA or α-GlcNAcylated WTA in human sera and IVIG (Table 2). For this experiment, we collected six different adult sera. Adult sera 1–3 (A-1 to A-3) contained MBL and serum anti-WTA IgG. In contrast, A-4 to A-6 sera were deficient in MBL but contained anti-WTA IgG. When the amounts of anti-β-GlcNAc or anti-α-GlcNAc WTA-specific IgGs were estimated by ELISA using purified WTAs, an average of 76% of the total anti-WTA IgGs, which were specific to the WTA from the S. aureus parental M0107 cells, bound to the β-GlcNAcylated WTA. The average amounts of the serum IgGs specific to the α,β-GlcNAcylated WTA and the β-GlcNAcylated WTA of six adults were calculated as 540 ng/μl versus 420 ng/μl, respectively. Of these, an average of 4% of the IgGs showed specific binding to the α-GlcNAcylated WTA and undetectable binding to the non-glycosylated WTA, which was purified from the Δ_tarMS_ double mutant cells. Two commercially available IVIG contained 79% anti-WTA IgG specific to β-GlcNAc WTA (average 26 ng/μl) among the anti-WTA α,β-GlcNAc-specific IgGs (33 ng/μl). These results strongly suggest that human sera contain β-GlcNAc WTA-specific IgGs as the major S. aureus anti-WTA antibodies. The sera of infants are described below.
TABLE 2.
WTA glycoepitope-specific IgGs in human sera or IVIG
Purified WTAs from M0107, Δ_tarM_, Δ_tarS_, and Δ_tarMS_ cells as described in Fig. 3, A and B, were used to determine anti-α,β-GlcNAc WTA IgG, anti-β-GlcNAc WTA IgG, anti-α-GlcNAc WTA IgG, and anti-non-glycosylated-WTA IgG, respectively, of each human serum or IVIG by ELISA. <5 or <4 indicates the not-detected value in these experimental conditions. Serum numbers A-1 to A-6 represent six adult sera, IVIG-1 and -2 are IVIG from two different companies, and I-5 to I-10 are four infant sera. Average concentrations of each IgG in each serum category are determined, and the percentages relative to anti-α,β-GlcNAc WTA IgG concentrations are shown in parentheses. NG, non-glycosylated. NA, not available.
| Serum no. | Sex | Age | MBL | Anti-α,β-WTA IgG | Anti-β-WTA IgG | Anti-α-WTA IgG | Anti-NG-WTA IgG |
|---|---|---|---|---|---|---|---|
| ng/μ_l_ | ng/μ_l_ | ng/μ_l_ | ng/μ_l_ | ng/μ_l_ | |||
| A-1 | Male | 28 years | 4.6 | 610 | 400 | <5 | <5 |
| A-2 | Female | 26 years | 4.5 | 460 | 450 | 24 | <5 |
| A-3 | Female | 24 years | 5.6 | 540 | 350 | 22 | <5 |
| A-4 | Male | 28 years | 0.0 | 420 | 340 | <5 | <5 |
| A-5 | Female | 26 years | 0.0 | 540 | 480 | <5 | <5 |
| A-6 | Male | 30 years | 0.0 | 700 | 510 | 19 | <5 |
| Average | 550 (100%) | 420 (76%) | 22 (4%) | <5 (<1%) | |||
| IVIG-1 | NA | NA | NA | 33 | 27 | 8.6 | <4 |
| IVIG-2 | NA | NA | NA | 32 | 25 | 8.9 | <4 |
| Average | 33 (100%) | 26 (79%) | 8.8 (27%) | <4 (<12%) | |||
| I-5 | Male | 8 months | 4.9 | <5 | <5 | <5 | <5 |
| I-9 | Male | 10 months | 6.6 | <5 | <5 | <5 | <5 |
| I-8 | Male | 3 months | 4.1 | 123 | 92 | <5 | <5 |
| I-10 | Female | 3 months | 3.0 | 120 | 80 | <5 | <5 |
| Average | 122 (100%) | 86 (70%) | <5 (<4%) | <5 (<4%) |
GlcNAc Modification of WTA Is Essential for MBL Binding to S. aureus Cells
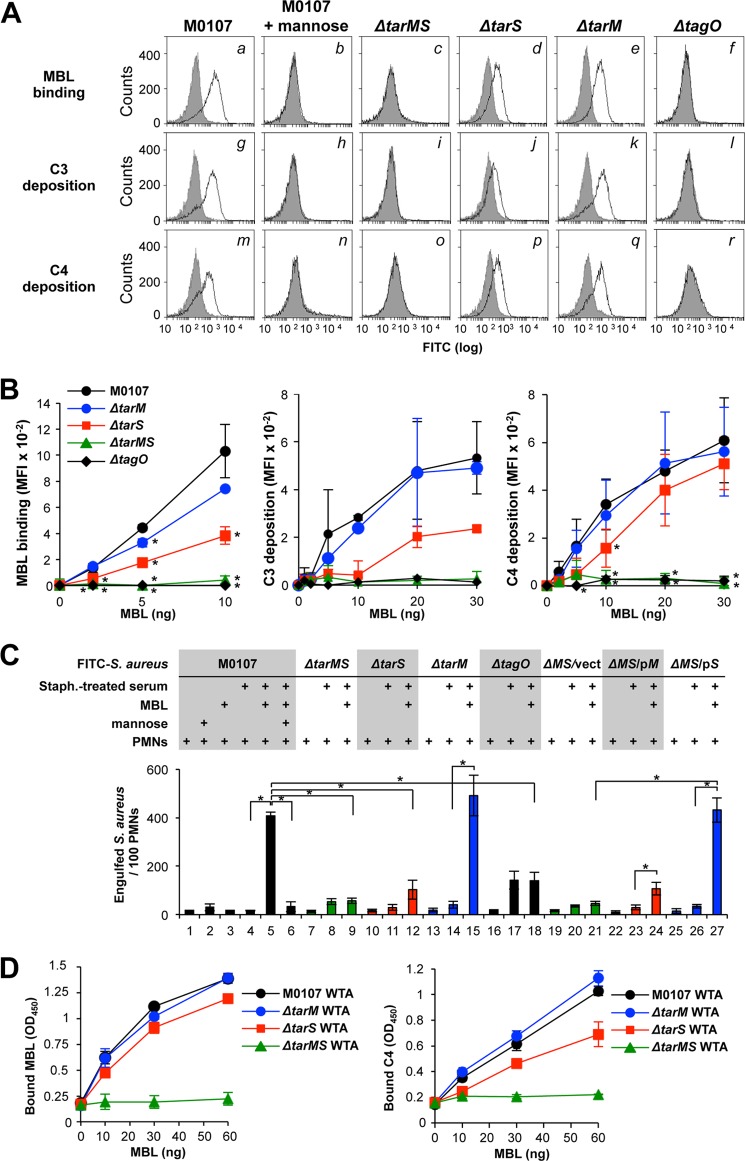
Recently, we reported that purified human MBL and the MBL-MASP complex recognize S. aureus WTA in vitro (23). The serum MBL-MASP complex in infants who have not fully developed adaptive immunity can bind to S. aureus WTA and induce complement activation, whereas MBL-MASP in adults does not bind to S. aureus WTA because the serum anti-WTA IgGs have a higher affinity for WTA than MBL, resulting in anti-WTA IgGs preferentially binding to WTA (23). These findings raised the question of which of the GlcNAc residues of the S. aureus WTA are recognized by serum MBL. This question is reasonable because MBL has been shown to bind mannose or GlcNAc monosaccharides and polysaccharides and to recognize the hydroxyl groups at the C3 and C4 positions of the mannose residue (13). S. aureus WTA contains ManNAc and GlcNAc groups in the linker region in addition to the GlcNAc residues of the ribitol phosphate repeat. It is uncertain which sugar residues of WTA are recognized by MBL. To address this question, we examined the ability of purified human MBL to bind to five different S. aureus cells via flow cytometry (Fig. 4A, a–f). MBL at 0.5 μg/ml bound to the S. aureus M0107 cells (a), which was abolished by the addition of mannose (b) and by the tagO mutation (f), indicating the binding specificity of MBL to WTA. The Δ_tarMS_ double mutant cells did not show any MBL binding (c). The Δ_tarS_ mutant cells did not show MBL binding (d), and the Δ_tarM_ mutant showed a greater MBL binding ability than the Δ_tarS_ mutant cells (e). The complement C3b (g–l) and C4b depositions (m–r) via the MBL-mediated complement lectin pathway in the S. aureus_-treated serum showed a similar tendency in terms of MBL binding ability. To further confirm these observations, the dose response to MBL was examined. As expected, Δ_tarMS double mutant cells did not show MBL binding or C3 and C4 depositions (Fig. 4B). In contrast to the anti-WTA IgGs, human MBL showed weak binding to the Δ_tarS_ mutant cells in a dose-dependent manner (left). C3 and C4 deposition showed similar tendencies (middle and right). Consistent with these results, the introduction of a plasmid harboring the tarM (ΔMS/pM) or tarS gene (ΔMS/pS) into Δ_tarMS_ double mutant cells restored all three abilities, including MBL binding and C3 and C4 deposition in the S. aureus_-treated serum (supplemental Fig. S1_B_). When the USA300 mutant strains were assessed for the level of bound C3 upon incubation with IgG-depleted serum, the results were similar to those of the M0107 cells (supplemental Fig. S2_C_). The complementation of the Δ_tarMS double mutant with tarM or tarS led to a weak or very pronounced increase of C3 deposition (c and d), respectively, supporting the idea that MBL may initiate C3 deposition more efficiently when MBL is bound to β-GlcNAc WTA compared with α-GlcNAc WTA. These results indicate that MBL recognizes both β- and α-GlcNAc WTA but has a higher affinity for β-GlcNAc WTA.
FIGURE 4.
WTA GlcNAc dependence of MBL-mediated complement activation and opsonophagocytosis of S. aureus cells by PMNs. A, in the top panels, ethanol-killed S. aureus M0107 (Δ_spa_) cells or their derivatives were incubated without (gray area) or with MBL (10 ng; black line) in 20 μl of buffer, and bound MBL was detected by flow cytometry analysis. In the middle and bottom panels, depositions of C3 and C4, respectively, onto S. aureus cells were carried out in 10% S. aureus_-treated adult serum without (gray area) or with MBL (10 ng, black line) in 20 μl of buffer. C3 and C4 were detected by specific antibodies with FCM analysis. B, MBL binding (left), C3 deposition (middle), and C4 deposition (right) were examined as in A with respect to MBL concentrations. Mean fluorescent intensities on flow cytometry analyses were expressed on the vertical axis. C, ethanol-killed M0107 (Δ_spa, parent; columns 1–6) and mutant S. aureus cells were labeled with FITC (0.1 mm) and opsonized without or with _S. aureus_-treated serum (10%) in 20 μl of buffer. Purified MBL (10 ng) and mannose (100 mm) were simultaneously added as indicated. Opsonized FITC-labeled S. aureus cells were incubated with human PMNs (1 × 105 PMNs) at a multiplicity of infection of 25 in 40 μl of RPMI 1640 medium at 37 °C for 1 h. Phagocytosed S. aureus cells per 100 PMNs were counted under fluorescent phase-contrast microscopy. Data are represented as the means ± S.D. (error bars) of the results of three independent experiments. *, p < 0.05. D, purified WTA was assayed for MBL binding and C4 deposition by ELISA. Left, each purified WTA (3 μg) was coated onto a 96-well ELISA plate, and purified MBL/MASP at the indicated amount was incubated at 4 °C for 2 h. Amounts of MBL bound to each coated WTA were determined by ELISA. Right, each purified WTA (3 μg) was coated onto a 96-well ELISA plate, and purified MBL/MASP at the indicated amount and C4 (100 ng) were incubated at 37 °C for 1 h, and C4 bound to each coated WTA was determined by ELISA. Data are represented as the means ± S.D. of results of three independent experiments.
Next, we examined MBL-mediated opsonophagocytosis by PMNs (Fig. 4C). The control experiment with purified MBL resulted in an increase in the total number of engulfed S. aureus M0107 cells (Fig. 4C, column 5) from 30 ± 15 (column 4) in an S. aureus_-treated serum-dependent manner, which was inhibited by the addition of mannose (column 6), indicating that MBL induced opsonophagocytosis of the S. aureus cells. In contrast, purified MBL did not enhance opsonophagocytosis of the Δ_tarMS mutant cells (columns 8 and 9) or the tagO mutant cells (columns 17 and 18). The weak opsonophagocytosis of Δ_tarS_ mutant cells was induced by MBL (columns 11 and 12), although marked enhancement of engulfment was observed in the Δ_tarM_ mutant cells in the presence of MBL (columns 14 and 15). A plasmid harboring the tarM or tarS gene restored MBL-mediated opsonophagocytosis of the Δ_tarMS_ mutant cells in a weak or strong manner, respectively (columns 23 and 24 and columns 26 and 27). This MBL-dependent opsonophagocytosis was not inhibited by anti-FcγR antibodies (supplemental Fig. S1_C_, column 11), providing evidence that this process is mediated by PMN complement receptors. When S. aureus USA300 and its mutant strains were opsonized with IgG-depleted serum and tested for PMN-mediated killing, the USA300 Δ_tarMS_ mutant cells escaped from the PMN-mediated killing, and complementation of the Δ_tarMS_ mutant with a tarS but not a _tarM_-expressing plasmid resensitized the double mutant cells to PMN-mediated killing (supplemental Fig. S2_D_). These results clearly demonstrate that the GlcNAc residues of WTA are an essential factor for MBL binding and subsequent lectin complement pathway activation leading to opsonophagocytosis of S. aureus cells. Collectively, serum MBL preferentially recognizes β-GlcNAc WTA over α-GlcNAc WTA during the MBL-mediated opsonophagocytosis response.
In addition, we examined the specificity of MBL binding to a series of purified WTAs by ELISA (Fig. 4D, left). As expected, MBL did not bind to WTAs purified from Δ_tarMS_ and Δ_tagO_ mutant cells. In contrast, MBL bound to the Δ_tarS_ WTA with similar characteristics to M0107 and Δ_tarM_ WTAs (Fig. 4D, left). C4 deposition was also not induced in the Δ_tarMS_ WTA, consistent with the MBL binding profiles, except that the Δ_tarS_ WTA showed slightly weaker binding than the M0107 and Δ_tarM_ WTAs (right). Therefore, MBL also requires GlcNAc residues on WTA for binding under the reconstituted conditions. The slight differences in MBL binding between the whole cells and the purified WTAs might be due to additional factors present on the bacterial cell surface.
Functional Relevance of MBL in Intact Infant Sera
Based on these results, we investigated the roles of MBL using intact infant sera. Eleven infant sera were collected, and we determined the MBL concentrations by ELISA. Four MBL-sufficient sera, I-5, I-9, I-8, and I-10, were selected for further experiments (Table 2). We first estimated the MBL binding abilities of these four sera to the S. aureus M0107 and mutant cells (supplemental Fig. S3). As expected, the endogenous MBL of these four infant intact sera bound to the S. aureus M0107 cells (a, g, m, and s), but this interaction was abolished by the addition of mannose (b, h, n, and t), demonstrating the binding specificity of MBL. MBLs in these four infant sera did not bind to the Δ_tarMS_ (c, i, o, and u) and Δ_tagO_ mutant cells (f, l, r, and x). In this condition, low levels of MBL binding were observed for the Δ_tarS_ (d, j, p, and v) and Δ_tarM_ mutant cells (e, k, g, and w). These results suggest that MBL in infant sera binds β-GlcNAc and α-GlcNAc on WTA.
Next, C3 deposition was examined (Fig. 5). All four infant sera induced C3 deposition (Fig. 5, a, g, m, and s). C3 depositions in two infant sera (I-5 and I-9) were clearly abolished by the addition of mannose (b and h), indicating that the C3 depositions in these sera were induced by MBL-mediated lectin complement activation. Consistent with the loss or reduction of MBL binding to the mutant cells as described above (supplemental Fig. S3), the MBL-mediated C3 deposition in the I-5 and I-9 sera was lost in the Δ_tarMS_ mutant cells (Fig. 5, c and i) and partially weakened in the Δ_tarS_ mutant cells (d and j). Reduction of C3 deposition in the Δ_tarM_ mutant cells was not evident (e and k). These results suggest that the MBL-mediated lectin complement pathway is the predominant route for C3 opsonization in the infant I-5 and I-9 sera. In contrast, C3 depositions in the I-8 and I-10 sera were not abolished by the addition of mannose (n and t), suggesting that another route is active in C3 deposition in the I-8 and I-10 sera. The C3 deposition in I-8 and I-10 sera was completely lost for the Δ_tarMS_ mutant cells (o and u). Therefore, C3 depositions in I-8 and I-10 sera require GlcNAc residues of WTA, indicating that the C3 depositions in I-8 and I-10 sera occur through the anti-WTA IgG-mediated classical pathway. Because I-8 and I-10 sera induced C3 deposition on the Δ_tarS_ mutant cells (p and v), whereas purified anti-WTA IgG targeted only β-GlcNAc WTA, as described above, the C3 depositions on the Δ_tarS_ mutant cells in the I-8 and I-10 sera might be induced by MBL. These results suggest that the infant I-8 and I-10 sera induced C3 deposition via a combination of the MBL-mediated lectin pathway and the anti-WTA IgG-mediated classical pathway.
FIGURE 5.
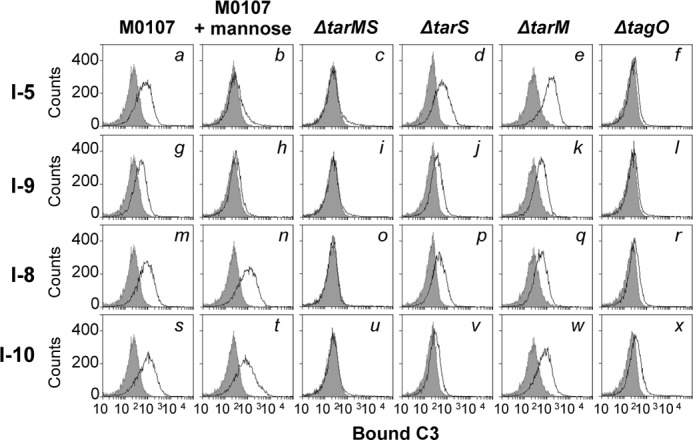
The MBL-mediated lectin pathway and anti-WTA IgG-mediated classical complement pathway are activated GlcNAc-dependently in intact infant sera. Ethanol-killed S. aureus M0107 (Δ_spa_) cells or its derivatives were incubated without (gray area) or with 5% of each intact infant serum (black line) in 20 μl of buffer at 37 °C for 1 h, and bound C3 was detected by flow cytometric analysis. Data are representative of at least three independent experiments.
Finally, we analyzed the amounts of serum anti-WTA antibodies specific for β-GlcNAc- or α-GlcNAc-modified WTA in infant sera by ELISA (Table 2). As expected, the I-5 and I-9 sera did not contain anti-WTA antibodies because the adaptive immune system of these infants was most likely not yet developed. The I-8 and I-10 sera contained lower levels of anti-WTA IgG than the adult sera, which is consistent with their ability to induce WTA GlcNAc-dependent and MBL-independent complement C3 activation. Among the anti-α,β-GlcNAc WTA-recognizing IgG in the I-8 and I-10 sera (average 120 ng/μl), 70% were specific for β-GlcNAc WTA (average 86 ng/μl). These antibodies are presumably maternally transferred. Alternatively, these results may provide evidence that infant sera predominantly produce anti-β-GlcNAc WTA antibodies when their adaptive immunity begins to develop.
DISCUSSION
Since 1960, many studies have indicated that injection of S. aureus teichoic acids into humans or rabbits results in the induction of circulating antibodies against β-GlcNAc or α-GlcNAc WTA (19, 21, 39, 40). Until recently, the unavailability of homogenous GlcNAc WTAs from S. aureus mutant cells hampered efforts to determine the epitope of anti-WTA antibodies. In this study, the availability of S. aureus Δ_tarM_, Δ_tarS_, and Δ_tarMS_ mutant cells and WTAs purified from these mutant strains enabled us to determine the exact antigenic determinant of anti-WTA antibodies and MBL.
In the current study, we describe two new findings. We first demonstrate that anti-WTA IgG and MBL require GlcNAc-modified S. aureus WTAs for binding. Between the α- and β-anomers of the GlcNAc substituent, the β-GlcNAc residue of WTA is critical for serum anti-WTA antibody-mediated complement activation and opsonophagocytosis, whereas both anomers bind to MBL. Second, we show that the anti-WTA β-GlcNAc IgG concentration in adult sera is a minimum of 3-fold higher than that of the anti-WTA α-GlcNAc IgG. Regarding possible explanations for the latter observations, we assumed that the β-GlcNAcylated WTA might be more antigenic than the α-GlcNAcylated WTA toward the host immune system or that the β-form is more stable than the α-form in vivo, in which case the α-GlcNAcylated WTA may be easily cleaved by the host's α-glycosidase in the bloodstream, tissues, or antigen-presenting cells. In agreement with this assumption, all known S. aureus genomes contain tarS, whereas tarM is absent in several lineages (24). It should be noted that S. aureus WTA has recently been shown to be presented to T-cells by antigen-presenting cells (8). Further studies might be necessary to discover how WTA is presented to T-cells. The fact that MBL and anti-WTA IgG target the same GlcNAc residues of ribitol phosphate repeats of S. aureus WTA intuitively explains the molecular mechanisms of anti-WTA IgG inhibition of MBL binding to WTA in our previous studies (22, 23). Additionally, we show that most of the human serum anti-S. aureus antibodies are directed against WTA. The anti-WTA antibodies and MBL preferentially recognize the β-configuration of WTA GlcNAc, suggesting that the α-configuration of WTA GlcNAc is minimally present in vivo or that S. aureus TarM has a minor role in S. aureus pathogenesis.
Recently, we reported that intradermal immunization with purified S. aureus WTA in mice induces production of anti-WTA IgG and that this anti-WTA IgG response protects against infection with both MW2, a community-associated MRSA, and COL, a health care-associated MRSA, even in the absence of MBL (41). These protective mechanisms of WTA immunization are assumed to be mediated at least in part by anti-WTA IgG-mediated opsonophagocytosis in the blood. In a previous study, WTA was purified from an S. aureus strain containing wild-type WTA. Therefore, new WTA derivatives purified from the Δ_tarS_, Δ_tarM_, or Δ_tarMS_ mutant strains would be valuable resources to address detailed WTA-mediated protection mechanisms in vivo.
The deficiency in serum MBL is associated with a common opsonization defect in children who have had recurrent infections (42, 43). Subsequent studies have shown that MBL deficiency increases susceptibility (44) to acute respiratory tract infections in early childhood (45). Our current data and a previous report (23) strongly support the clinical importance of MBL in children. First, we show that MBL in infant sera recognizes β-GlcNAc and α-GlcNAc on WTAs. As shown in Fig. 5, infant sera containing both MBL and anti-WTA IgG induced C3 deposition via a combination of the MBL-mediated lectin pathway and anti-WTA IgG-mediated classical pathway, whereas infant sera containing only MBL predominantly induce C3 deposition via the lectin complement pathway. These data suggest the molecular basis behind the susceptibility of MBL-insufficient infants with immature adaptive immune systems to S. aureus infections, such as acute respiratory tract infection and recurrent infection.
Among S. aureus strains, the USA300 strain is particularly notorious for evading human PMNs, which are crucial to the host immune response to S. aureus infection (46, 47). Recent studies showed that USA300 strains can produce up to five bicomponent pore-forming leukotoxins that have been implicated in the capabilities of these strains to kill PMNs (48, 49). The common feature of all these toxins is their ability to target and kill human PMNs in vitro. In this study, the in vitro anti-WTA IgG-mediated specific engulfment and killing effects of several S. aureus strains by human PMNs was shown for β-GlcNAc WTA-containing S. aureus cells. Although growth of S. aureus cells engulfed by PMNs is temporarily inhibited, escape is possible in vivo via the release of leukotoxins. To address the complicated host-S. aureus cross-talk, further delicate in vivo experiments will be necessary to define whether anti-WTA IgG or MBL can function as in vivo bona fide inducer molecules for host defense responses during S. aureus infection.
Finally, one aspect of our results relates to a recent report (25) showing that the S. aureus TarS enzyme is required to maintain β-lactam resistance in MRSA strains. The authors proposed a scaffolding role for the β-GlcNAc WTAs in methicillin resistance, as shown in previous studies (50–52), and they suggested that TarS is a unique target for novel antibiotics to treat MRSA infection in conjunction with β-lactams. In this study, we demonstrate that β-GlcNAc WTA is a critical target of serum anti-WTA antibodies. Therefore, induction of anti-β-GlcNAc WTA antibodies by appropriate vaccination or treatment with anti-β-GlcNAc WTA antibodies could be an effective strategies to combat infection with S. aureus, including MRSA, because β-GlcNAc WTA is an essential cell wall constituent of S. aureus, including MRSA strains.
*
This work was supported by Korean National Research Foundation Programs 2012-0139-0002 and 2011-002-7773 (to B. L. L.) and by German Research Council Grants TRR34 (to A. P.) and SFB766 (to G. X.).
3
The abbreviations used are:
MRSA
methicillin-resistant S. aureus
MBL
mannose-binding lectin
WTA
wall teichoic acid
MASP
MBL-associated serine protease
PMNs
human polymorphonuclear leukocytes
IVIG
intravenous IgG
ManNAc
_N_-acetylmannosamine
FcγR
Fcγ receptor.
REFERENCES
- 1.Lowy F. D. (1998) Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 [DOI] [PubMed] [Google Scholar]
- 2.Weidenmaier C., Peschel A. (2008) Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 6, 276–287 [DOI] [PubMed] [Google Scholar]
- 3.Swoboda J. G., Campbell J., Meredith T. C., Walker S. (2010) Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 11, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia G., Kohler T., Peschel A. (2010) The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int. J. Med. Microbiol. 300, 148–154 [DOI] [PubMed] [Google Scholar]
- 5.Weidenmaier C., Kokai-Kun J. F., Kristian S. A., Chanturiya T., Kalbacher H., Gross M., Nicholson G., Neumeister B., Mond J. J., Peschel A. (2004) Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10, 243–245 [DOI] [PubMed] [Google Scholar]
- 6.Tabuchi Y., Shiratsuchi A., Kurokawa K., Gong J. H., Sekimizu K., Lee B. L., Nakanishi Y. (2010) Inhibitory role for d-alanylation of wall teichoic acid in activation of insect Toll pathway by peptidoglycan of Staphylococcus aureus. J. Immunol. 185, 2424–2431 [DOI] [PubMed] [Google Scholar]
- 7.Kurokawa K., Gong J. H., Ryu K. H., Zheng L., Chae J. H., Kim M. S., Lee B. L. (2011) Biochemical characterization of evasion from peptidoglycan recognition by _Staphylococcus aureus_d-alanylated wall teichoic acid in insect innate immunity. Dev. Comp. Immunol. 35, 835–839 [DOI] [PubMed] [Google Scholar]
- 8.Weidenmaier C., McLoughlin R. M., Lee J. C. (2010) The zwitterionic cell wall teichoic acid of Staphylococcus aureus provokes skin abscesses in mice by a novel CD4+ T-cell-dependent mechanism. PLoS One 5, e13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklin D., Hajishengallis G., Yang K., Lambris J. D. (2010) Complement. A key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daha N. A., Banda N. K., Roos A., Beurskens F. J., Bakker J. M., Daha M. R., Trouw L. A. (2011) Complement activation by (auto-)antibodies. Mol. Immunol. 48, 1656–1665 [DOI] [PubMed] [Google Scholar]
- 11.Fujita T. (2002) Evolution of the lectin-complement pathway and its role in innate immunity. Nat. Rev. Immunol. 2, 346–353 [DOI] [PubMed] [Google Scholar]
- 12.Ip W. K., Takahashi K., Ezekowitz R. A., Stuart L. M. (2009) Mannose-binding lectin and innate immunity. Immunol. Rev. 230, 9–21 [DOI] [PubMed] [Google Scholar]
- 13.Weis W. I., Drickamer K. (1996) Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 65, 441–473 [DOI] [PubMed] [Google Scholar]
- 14.Walport M. J. (2001) Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 15.Joiner K. A., Brown E. J., Frank M. M. (1984) Complement and bacteria. Chemistry and biology in host defense. Annu. Rev. Immunol. 2, 461–491 [DOI] [PubMed] [Google Scholar]
- 16.Shi L., Takahashi K., Dundee J., Shahroor-Karni S., Thiel S., Jensenius J. C., Gad F., Hamblin M. R., Sastry K. N., Ezekowitz R. A. (2004) Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 199, 1379–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pozzi C., Wilk K., Lee J. C., Gening M., Nifantiev N., Pier G. B. (2012) Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS One 7, e46648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathenson S. G., Strominger J. L. (1962) Enzymatic synthesis and immunochemistry of _N_-acetylglucosaminylribitol linkages in the teichoic acids of Staphylococcus aureus stains. J. Biol. Chem. 237, 3839–3841 [PubMed] [Google Scholar]
- 19.Juergens W. G., Sanderson A. R., Strominger J. L. (1963) Chemical basis for an immunological specificity of a strain of Staphylococcus aureus. J. Exp. Med. 117, 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morse S. I. (1962) Studies on the chemistry and immunochemistry of cell walls of Staphylococcus aureus. J. Exp. Med. 116, 229–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torii M., Kabat E. A., Bezer A. E. (1964) Separation of Teichoic Acid of Staphylococcus aureus into Two Immunologically Distinct Specific Polysaccharides with α- and β-_N_-Acetylglucosaminyl Linkages Respectively. Antigenicity of Theichoic Acids in Man. J. Exp. Med. 120, 13–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung D. J., An J. H., Kurokawa K., Jung Y. C., Kim M. J., Aoyagi Y., Matsushita M., Takahashi S., Lee H. S., Takahashi K., Lee B. L. (2012) Specific serum Ig recognizing staphylococcal wall teichoic acid induces complement-mediated opsonophagocytosis against Staphylococcus aureus. J. Immunol. 189, 4951–4959 [DOI] [PubMed] [Google Scholar]
- 23.Park K. H., Kurokawa K., Zheng L., Jung D. J., Tateishi K., Jin J. O., Ha N. C., Kang H. J., Matsushita M., Kwak J. Y., Takahashi K., Lee B. L. (2010) Human serum mannose-binding lectin senses wall teichoic acid glycopolymer of Staphylococcus aureus, which is restricted in infancy. J. Biol. Chem. 285, 27167–27175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia G., Maier L., Sanchez-Carballo P., Li M., Otto M., Holst O., Peschel A. (2010) Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J. Biol. Chem. 285, 13405–13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown S., Xia G., Luhachack L. G., Campbell J., Meredith T. C., Chen C., Winstel V., Gekeler C., Irazoqui J. E., Peschel A., Walker S. (2012) Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc. Natl. Acad. Sci. U.S.A. 109, 18909–18914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita M., Endo Y., Fujita T. (2000) Cutting edge. Complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J. Immunol. 164, 2281–2284 [DOI] [PubMed] [Google Scholar]
- 27.Vagner V., Dervyn E., Ehrlich S. D. (1998) A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144, 3097–3104 [DOI] [PubMed] [Google Scholar]
- 28.Tao L., LeBlanc D. J., Ferretti J. J. (1992) Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120, 105–110 [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Kurokawa K., Matsuo M., Fukuhara N., Murakami K., Sekimizu K. (2004) Identification of temperature-sensitive dnaD mutants of Staphylococcus aureus that are defective in chromosomal DNA replication. Mol. Genet. Genomics 271, 447–457 [DOI] [PubMed] [Google Scholar]
- 30.Kurokawa K., Lee H., Roh K. B., Asanuma M., Kim Y. S., Nakayama H., Shiratsuchi A., Choi Y., Takeuchi O., Kang H. J., Dohmae N., Nakanishi Y., Akira S., Sekimizu K., Lee B. L. (2009) The triacylated ATP binding cluster transporter substrate-binding lipoprotein of Staphylococcus aureus functions as a native ligand for Toll-like receptor 2. J. Biol. Chem. 284, 8406–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae T., Schneewind O. (2006) Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55, 58–63 [DOI] [PubMed] [Google Scholar]
- 32.Shiratsuchi A., Shimizu K., Watanabe I., Hashimoto Y., Kurokawa K., Razanajatovo I. M., Park K. H., Park H. K., Lee B. L., Sekimizu K., Nakanishi Y. (2010) Auxiliary role for d-alanylated wall teichoic acid in Toll-like receptor 2-mediated survival of Staphylococcus aureus in macrophages. Immunology 129, 268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P. S., Toribara T. Y., Warner H. (1956) Microdetermination of phosphorus. Anal. Chem. 28, 1756–1758 [Google Scholar]
- 34.Enghofer E., Kress H. (1979) An evaluation of the Morgan-Elson assay for 2-amino-2-deoxy sugars. Carbohydr. Res. 76, 233–238 [DOI] [PubMed] [Google Scholar]
- 35.Peschel A., Jack R. W., Otto M., Collins L. V., Staubitz P., Nicholson G., Kalbacher H., Nieuwenhuizen W. F., Jung G., Tarkowski A., van Kessel K. P., van Strijp J. A. (2001) Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 193, 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maira-Litrán T., Kropec A., Goldmann D. A., Pier G. B. (2005) Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-_N_-acetyl-β-(1–6)-glucosamine. Infect. Immun. 73, 6752–6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dürr M. C., Kristian S. A., Otto M., Matteoli G., Margolis P. S., Trias J., van Kessel K. P., van Strijp J. A., Bohn E., Landmann R., Peschel A. (2006) Neutrophil chemotaxis by pathogen-associated molecular patterns. Formylated peptides are crucial but not the sole neutrophil attractants produced by Staphylococcus aureus. Cell Microbiol. 8, 207–217 [DOI] [PubMed] [Google Scholar]
- 38.Nimmerjahn F., Ravetch J. V. (2008) Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 [DOI] [PubMed] [Google Scholar]
- 39.Dryla A., Prustomersky S., Gelbmann D., Hanner M., Bettinger E., Kocsis B., Kustos T., Henics T., Meinke A., Nagy E. (2005) Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin. Diagn. Lab. Immunol. 12, 387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colque-Navarro P., Jacobsson G., Andersson R., Flock J. I., Möllby R. (2010) Levels of antibody against 11 Staphylococcus aureus antigens in a healthy population. Clin. Vaccine Immunol. 17, 1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi K., Kurokawa K., Moyo P., Jung D.-J., An J.-H., Chigweshe L, Paul E., Lee B. L. (2013) Intradermal Immunization with wall teichoic acid (WTA) elicits and augments an anti-WTA IgG response that protects mice from methicillin-resistant Staphylococcus aureus infection independent of mannose-binding lectin status. PLoS One 8, e69739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Super M., Thiel S., Lu J., Levinsky R. J., Turner M. W. (1989) Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet 2, 1236–1239 [DOI] [PubMed] [Google Scholar]
- 43.Sumiya M., Super M., Tabona P., Levinsky R. J., Arai T., Turner M. W., Summerfield J. A. (1991) Molecular basis of opsonic defect in immunodeficient children. Lancet 337, 1569–1570 [DOI] [PubMed] [Google Scholar]
- 44.Summerfield J. A., Sumiya M., Levin M., Turner M. W. (1997) Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ 314, 1229–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koch A., Melbye M., Sørensen P., Homøe P., Madsen H. O., Mølbak K., Hansen C. H., Andersen L. H., Hahn G. W., Garred P. (2001) Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA 285, 1316–1321 [DOI] [PubMed] [Google Scholar]
- 46.Voyich J. M., Braughton K. R., Sturdevant D. E., Whitney A. R., Saïd-Salim B., Porcella S. F., Long R. D., Dorward D. W., Gardner D. J., Kreiswirth B. N., Musser J. M., DeLeo F. R. (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919 [DOI] [PubMed] [Google Scholar]
- 47.Otto M. (2010) Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 64, 143–162 [DOI] [PubMed] [Google Scholar]
- 48.Vandenesch F., Lina G., Henry T. (2012) Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides. A redundant arsenal of membrane-damaging virulence factors? Front. Cell Infect. Microbiol. 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alonzo F., 3rd, Torres V. J. (2013) Bacterial survival amidst an immune onslaught. The contribution of the Staphylococcus aureus leukotoxins. PLoS Pathogens 9, e1003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell J., Singh A. K., Santa Maria J. P., Jr., Kim Y., Brown S., Swoboda J. G., Mylonakis E., Wilkinson B. J., Walker S. (2011) Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 6, 106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atilano M. L., Pereira P. M., Yates J., Reed P., Veiga H., Pinho M. G., Filipe S. R. (2010) Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 107, 18991–18996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frankel M. B., Schneewind O. (2012) Determinants of murein hydrolase targeting to cross-wall of Staphylococcus aureus peptidoglycan. J. Biol. Chem. 287, 10460–10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Novick R. P., Ross H. F., Projan S. J., Kornblum J., Kreiswirth B., Moghazeh S. (1993) Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12, 3967–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oku Y., Kurokawa K., Matsuo M., Yamada S., Lee B. L., Sekimizu K. (2009) Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J. Bacteriol. 191, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]