Cohort Study of Insulin Glargine and Risk of Breast, Prostate, and Colorectal Cancer Among Patients With Diabetes (original) (raw)
Abstract
OBJECTIVE
To examine whether use of insulin glargine, compared with another long-acting insulin, is associated with risk of breast, prostate, colorectal cancer, or all cancers combined.
RESEARCH DESIGN AND METHODS
Computerized health records from Kaiser Permanente Northern and Southern California regions starting in 2001 and ending in 2009 were used to conduct a population-based cohort study among patients with diabetes aged ≥18 years. With use of Cox regression modeling, cancer risk in users of insulin glargine (n = 27,418) was compared with cancer risk in users of NPH (n = 100,757).
RESULTS
The cohort had a median follow-up of 3.3 years during which there was a median of 1.2 years of glargine use and 1.4 years of NPH use. Among users of NPH at baseline, there was no clear increase in risk of breast, prostate, colorectal, or all cancers combined associated with switching to glargine. Among those initiating insulin, ever use or ≥2 years of glargine was not associated with increased risk of prostate or colorectal cancer or all cancers combined. Among initiators, the hazard ratio (HR) for breast cancer associated with ever use of glargine was 1.3 (95% CI 1.0–1.8); the HR for breast cancer associated with use of glargine for ≥2 years was 1.6 or 1.7 depending on whether glargine users had also used NPH.
CONCLUSIONS
Results of this study should be viewed cautiously, given the relatively short duration of glargine use to date and the large number of potential associations examined.
Although findings were not consistent, results of four observational studies conducted in Europe and reported in June 2009 raised concerns that use of the long-acting insulin analog, glargine (Lantus), may increase the risk of one or more forms of cancer (1–4). Insulin analogs are structurally altered human insulins. Because altering human insulin molecules may also alter mitogenicity, there was concern about the carcinogenic potential of glargine. Each of the four studies was conducted among patients with diabetes and used data from electronic records. The number of end points, especially for cancer at specific sites, was small; the period of observation was short; and data were not consistently complete or available on several potentially important confounding variables.
Subsequently, Sanofi, the manufacturer of glargine, supported the current study among Kaiser Permanente members to examine the potential association between use of insulin glargine and risk of breast, prostate, colorectal cancer, or all cancers combined. The final version of the full protocol for this study was submitted to the European Medicines Agency Committee for Medicinal Products for Human Use in March 2010. It was approved by the Committee for Medicinal Products for Human Use in April 2010.
RESEARCH DESIGN AND METHODS
Setting and source population
The study was conducted among enrollees of Kaiser Permanente’s Northern and Southern California regions (KPNC and KPSC). Together, these regions currently serve ~4.8 million adult members. Kaiser Permanente is a nonprofit, prepaid health plan. KPNC and KPSC own and run their hospitals and clinics, employ their own physicians, manage their own pharmacies, and archive data generated from clinical encounters.
Study cohort
The cohort included members 18 years old or older diagnosed with type 1 or type 2 diabetes identified from pharmacy records (fills for diabetes drugs), laboratory results (HbA1c levels), and outpatient, emergency room, and hospitalization records listing a diagnosis of diabetes. The cohort was restricted to patients with no history of cancer.
Primary exposures of interest
The primary exposure of interest was insulin glargine. The comparator was human NPH (including NPH premixed with regular insulin), which is an intermediate-acting insulin with indications for use similar to those with glargine. Information on medication use came from computerized outpatient pharmacy records. Records include dispense date and drug name, amount, and days supply.
Eligible cohort members were categorized as “ever users” of glargine or NPH if they filled at least two prescriptions for the specific drug within a 6-month period. Estimating cumulative duration of insulin use from prescription data is difficult because of problems with adherence and wastage. Thus, duration was calculated in two ways. For our main analyses, we added the days between prescriptions; if days between two prescriptions was >6 months, then the days counted for the earlier prescription was limited to 183. In sensitivity analyses, we summed the number of days supply for each prescription.
Outcomes of interest
The three primary outcomes included female breast, prostate, and colorectal cancer. The secondary outcome was all cancers combined, excluding nonmelanoma skin cancers.
Incident cancers through 31 December 2009 were identified by linkage with the KPNC and KPSC cancer registries, both of which are contributing sites to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program of cancer registries. The registries follow SEER practices and have comparable accuracy and completeness insured by standardized medical record abstraction.
Potential confounding variables
Electronic health records provided information on other diabetes medications and on demographics, laboratory tests (e.g., HbA1c), BMI, and inpatient and outpatient diagnoses. For some variables (e.g., race/ethnicity, BMI), data from the electronic record were supplemented with information obtained from prior surveys conducted on subsets of the study cohort.
Cohort entry and follow-up (time at risk)
Entry into the cohort occurred on May 2001 (when glargine became available in the U.S.), or later, when the following inclusion criteria were met: at least 12 months of continuous health plan membership and pharmacy benefits, 18 years of age or older, and at least two prescriptions for insulin glargine or for NPH insulin within a 6-month period. For the main analyses, follow-up ended at diagnosis of any cancer, death, a gap of >4 months in either membership or prescription benefits, or 31 December 2009—whichever came first.
Statistical analyses
Analyses were conducted using multivariable Cox or Poisson regression modeling. Use of insulins and other diabetes medications was treated as time-varying covariates in regression models. Analyses were adjusted for potential confounders, including demographic and clinical variables. Sensitivity analyses examining various sets of additional potential confounders obtained via questionnaire were conducted in subsets of the study cohort.
RESULTS
The final eligible cohort included 115,514 adult men and women with diabetes (Supplementary Fig. 1). At the end of follow-up, there were 27,418 patients who had used insulin glargine and 100,757 who had used NPH (12,661 individuals had used both). Among new users of insulin (i.e., no use of any insulin in the prior 12 months), there were 6,548 whose first insulin was glargine—or ~25% of all glargine users. There were 39,708 new users of NPH—or ~40% of all NPH users.
As expected, there was a marked increase in use of glargine over time (Table 1). In the full cohort, age at first eligible glargine prescription was slightly younger than age at first eligible NPH fill. In contrast, the age distribution was similar in the new insulin users. In both the full cohort and new insulin users, users of glargine were more frequently male. There were only minimal racial/ethnic differences by insulin type. The proportion of NPH users without a documented BMI was substantially higher than for glargine users. Note, this is largely because we only included BMI measures within 24 months of first eligible prescription and a large proportion of first eligible NPH fills occurred prior to 2007, when the BMI began to be systematically calculated and recorded in the electronic medical records. When we restricted comparisons to persons with known BMI, the differences were reduced and the BMI distribution was similar among new insulin users. The proportion of adults with low income, based on median household income of their residence census track, was similar for glargine and NPH users. When we restricted to those with known smoking status, NPH users were more frequently current smokers.
Table 1.
Selected characteristics at first eligible glargine or NPH prescriptiona
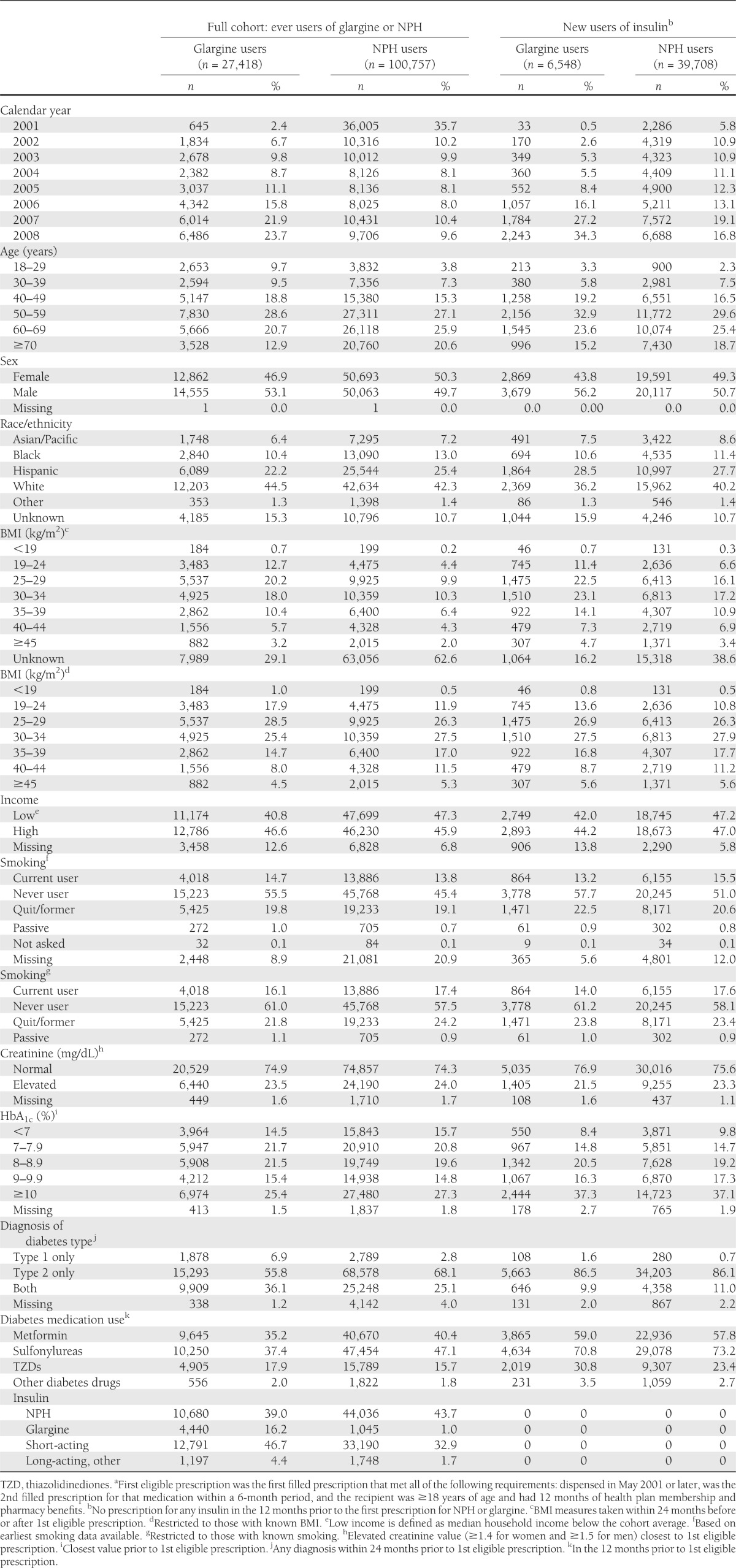
Among the full cohort, the proportion using NPH in the 12 months prior to their first eligible glargine prescription after entry into the cohort was similar to the proportion using NPH prior to first eligible NPH after entry (Table 1). Use of short-acting insulin was more common and metformin and sulfonylurea use were less common in the 12 months prior to first eligible glargine prescription than at the first eligible NPH prescription. Among new users of insulin, the use of other diabetes medications was generally similar in the 12 months prior to first eligible glargine or NPH prescription. Use of thiazolidinediones, however, was more common among the new glargine users.
In the full cohort, there was a median of 3.3 years of follow-up (maximum 8.6). From the first eligible prescription for glargine to end of follow-up was a median of 2.3 years vs. a median of 3.6 years for NPH (Supplementary Table 1). In general, insulin users received glargine or NPH for only a portion of their follow-up period. There was a median of 1.2 years of glargine use and 1.4 years of NPH use (Supplementary Table 2).
There were 5,851 cohort members with at least one cancer diagnosed during follow-up: 910 with breast cancer, 753 with prostate cancer, and 700 with colorectal cancer. Among new insulin users, there were 269 with breast cancer, 253 with prostate cancer, 205 with colorectal cancer, and a total of 1,856 with cancer at any site. Cancer incidence rates were consistent with those reported for California by SEER and expected rates based on the reported association between diabetes and cancer (5) (Supplementary Table 3).
Given that NPH was our primary comparator and it has been widely used for decades, we first explored whether use of NPH itself might be associated with cancer risk. These analyses, using Poisson regression, were conducted among a cohort of users of NPH with no prescription for insulin in the 12 months prior to their first NPH prescription (n = 46,390). (Note that these analyses included new users at KPSC from 2001–2008 and at KPNC from 1996–2008 and thus included more than the 39,708 new users of NPH for the period 2001–2008 that were included in our main analyses.) We saw no evidence of an association with duration of NPH use and risk of colorectal, prostate, or all cancers combined. Compared with risk with <2 years of NPH use, there was some suggestion of a very modest increase in risk of breast cancer associated with ≥5 years of NPH use (Supplementary Table 4). The risk ratio was 1.2 (95% CI 0.9–1.6) when we calculated duration based on time between prescriptions and 1.6 (1.0–2.4) when we calculated duration by summing days supply for each prescription. Given the lack of a strong association between duration of NPH use and cancer risk and that we found little evidence for large differences in prior NPH use at the time of first eligible glargine versus first eligible NPH prescription, we used NPH with all durations combined as the primary comparison for our analyses of glargine use.
We found little evidence of confounding by BMI, race/ethnicity, income, diabetes type, HbA1c levels, or ever use of other diabetes medications (Supplementary Tables 5 and 11). All analyses, therefore, were adjusted for KP region, age, sex (for colorectal cancer and all sites combined), year of cohort entry, use of metformin, and use of other insulins (short acting and other long acting). Metformin and other insulins were included because of interest by the European Medicines Agency in seeing models adjusted for these variables.
Among our full cohort of 115,514 glargine or NPH users, there was little support for an increased risk for prostate or colorectal cancer or all cancer sites combined associated with use of glargine (Tables 2–4, and Supplementary Table 12). However, there was a suggestion of a modestly increased risk of breast cancer among users of glargine for ≥2 years both among those who had and those who had not also used NPH (Table 2).
Table 2.
HRs for breast cancer associated with ever use and with duration of glargine use among all users of NPH or glargine, among NPH users at baseline, and among new users of insulin at baselinea
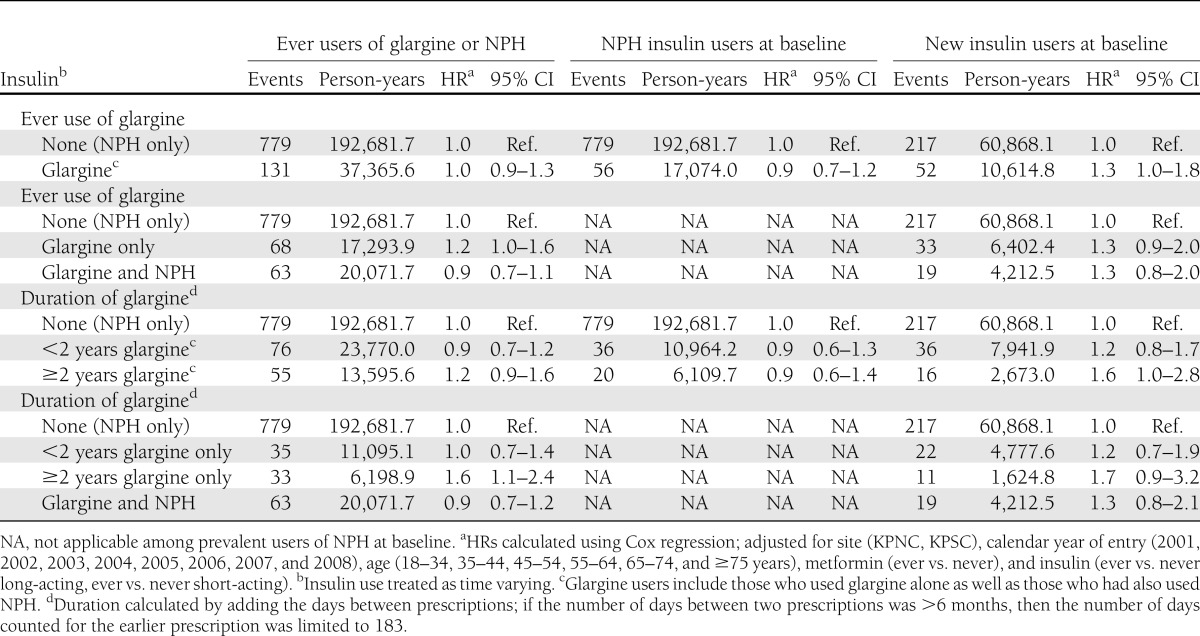
Table 4.
HRs for colorectal cancer associated with ever use and with duration of glargine use among all insulin users and among new users of insulin at baselinea
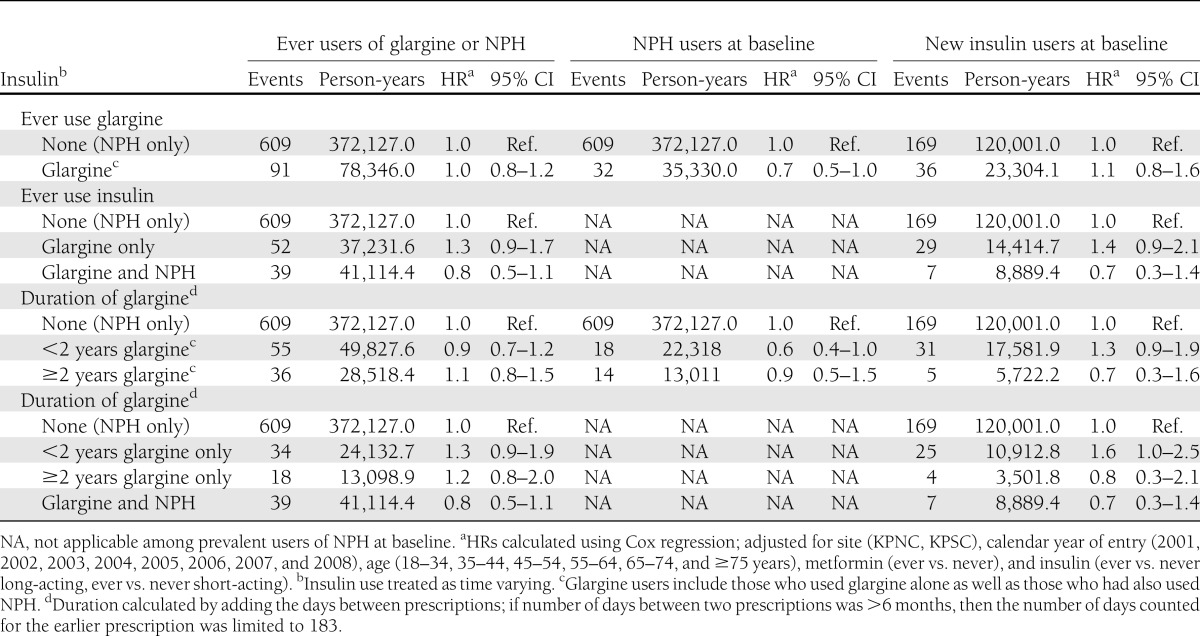
Table 3.
HRs for prostate cancer associated with ever use and with duration of glargine use among all insulin users and among new users of insulin at baselinea
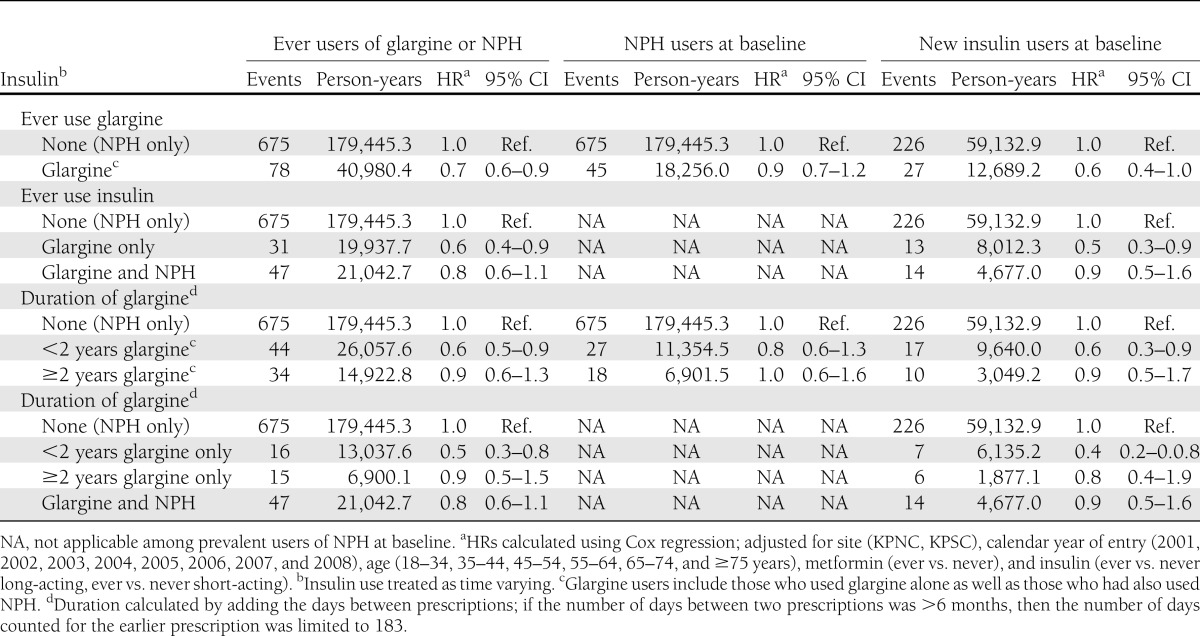
To examine whether switching to glargine after a history of using another long-acting insulin increased the risk of cancer, we looked at ever use of glargine and duration of glargine use among users of NPH at baseline (n = 99,506). We saw no evidence of an increase in risk for breast, prostate, or colorectal cancer or all cancer sites combined associated with ever use of glargine, and there was little evidence that risk increased with longer duration of glargine use (Tables 2–4 and Supplementary Table 12).
To examine whether, among new users of insulin, initiating glargine use versus another long-acting insulin increased the risk of cancer, we compared risk in 6,548 new glargine users with risk among 39,708 new NPH users (Tables 2–4 and Supplementary Table 12). Compared with risk in NPH users (all durations combined), there was no increase in risk observed for prostate, colorectal, or all cancers combined associated with ever use of glargine. However, there was a suggestion of a modest increase in risk of breast cancer (hazard ratio [HR] 1.3 [95% CI 1.0–1.8]) (Tables 2–4 and Supplementary Table 12). There also was a suggestion that risk of breast cancer increased with increasing duration of glargine use. The HR for breast cancer was 1.2 (95% CI 0.8–1.7) for < 2 years of glargine use and 1.6 (95% CI 1.0–2.8) for ≥2 years of glargine use. Duration results were similar when looking at glargine use only among those who had not used NPH. There was little evidence for an increase in risk of prostate, colorectal, or all cancer sites combined associated with longer duration of glargine use.
Other subgroup and sensitivity analyses
In analyses restricted to individuals with 48 months of membership and pharmacy benefits prior to baseline, the HRs for breast, prostate, and colorectal cancer and all cancer sites combined were similar to those reported above (Supplementary Table 13).
In sensitivity analyses, we examined cancer risk associated with duration of glargine use when duration was calculated by summing days supply for each prescription. Results were generally similar to those in our main analyses with duration calculated as time between prescriptions, although among new insulin users the HR for breast cancer associated with ≥2 years’ duration of glargine was slightly more elevated (HR 2.1 or 2.2, depending on whether patients had also used NPH) (Supplementary Table 14).
In additional sensitivity analyses among new insulin users, follow-up was censored as in the main analyses and additionally when a patient stopped using glargine or NPH or when they switched to another long-acting insulin. As in our main analyses, these sensitivity analyses were adjusted for Kaiser Permanente region, calendar year, sex, age, metformin use, and use of short-acting insulin, although use of metformin and short-acting insulin was fixed at baseline and not treated as time varying. We generated HRs for the period <2 years and for the period ≥2 years after insulin initiation (Supplementary Table 15). In these analyses, there was no evidence that ≥2 years of glargine use vs. ≥2 years of NPH increased the risk of prostate or colorectal cancer. There was a suggestion of a modest increase in risk of breast cancer associated with ≥2 years of glargine vs. ≥2 years of NPH (HR 1.6 [95% CI 0.9–3.1]). The HR for all cancers combined associated with ≥2 years of glargine use vs. ≥2 years of NPH was 1.2 (95% CI 0.9–1.7).
CONCLUSIONS
In this population-based cohort study, we found limited support for an association between use of glargine and increased risk of cancer. Results among prevalent users of NPH, another long-acting insulin with similar indications for use, suggested that risk of cancer was not increased among those switching to glargine. Among new users of insulin, ever use or longer duration of use of glargine versus use of NPH was not associated with increased risk of prostate or colorectal cancer. However, there was an ~1.5- to 2.0-fold increase in risk of breast cancer associated with ≥2 years of glargine use. Given the small number of breast cancer cases with ≥2 years of glargine use among the new users, these estimates were imprecise. In addition, these results conflict with the findings in the full cohort and among prevalent users of NPH. We believe it is implausible that duration of glargine use would be associated with risk of breast cancer among new users but not prevalent users of insulin and so believe that chance resulting from multiple comparisons is the most plausible explanation for the positive association with breast cancer incidence among new glargine users.
Since the initial four European studies were published in June 2009 (1–4), several additional observational studies have reported results on the association between use of glargine and cancer risk (6–14). Among all studies, only a small number reported results for individual cancers. Breast cancer–specific results have been reported for seven study populations (2,4,6,9,10,12–14). In three studies (6,9,12), risk was weakly to modestly higher among glargine users or a subset of glargine users (e.g., users of glargine only or long-term users of insulin) than in users of other insulin, whereas in two studies (4,13) the risk was modestly lower and in one study (10) there was no difference in risk. The eighth was the Swedish study published in 2009 (2), which found an increased risk of breast cancer. However, in subsequent analyses with an expanded cohort and extended follow-up, the initial finding was attenuated or disappeared, depending on the time periods examined (7,14). Two studies (9,12) found an elevated risk of prostate cancer associated with glargine use, while one (10) found no association. Two studies reported a decrease in risk of colon or colorectal cancer (10,12).
An analysis combining results from 10,880 patients in 31 different clinical trials, mostly of very short duration of glargine use, found that glargine was not associated with an increased risk of breast, colon, or prostate cancer, although the number of cancer cases at these sites was small and risk estimates were imprecise (15). However, the strongest evidence to date bearing on the potential carcinogenicity of glargine comes from a recent analysis of the ORIGIN (Outcome Reduction With Initial Glargine Intervention) trial, which was conducted among patients with cardiovascular risk factors plus impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes and compared outcomes in 6,254 patients randomized to glargine with outcomes in 6,273 patients randomized to standard care (16). After a median of 6.2 years of follow-up, no increased risk of breast, prostate, or colon cancer was observed among patients in the glargine arm. For breast cancer specifically, there were exactly the same number of cases—28—diagnosed among those assigned to receive glargine and those assigned to receive standard care, although these results included both men and women.
The differences in results across studies may in part be explained by chance, different study designs or study populations, different comparison groups and adjustment for covariates, different practice patterns for diabetes management, or different periods of follow-up. Limitations of all studies include only recent and short-term use of glargine and a small number of cancers at specific sites. In addition, several observational studies had incomplete information on potentially important confounders.
Our study was subject to the above limitations as well. Glargine was available for use in the U.S. only as of May 2001, and ~60% of glargine users in our cohort initiated use in 2006 or later. We therefore were able only to examine the association between relatively recent and short-term use of glargine and cancer risk. The induction period for many carcinogens is often years to decades. Thus, this study of relatively recent and short-term use would miss effects that require longer exposure or follow-up to become evident. While the study was conducted in a large cohort of patients with diabetes, we had relatively few glargine-exposed cancer cases at the sites of interest, limiting our precision, especially for risk estimates associated with particular durations of use.
We lacked complete information on several potentially important confounders on the full cohort. However, in analyses restricted to individuals with information on race/ethnicity, type of diabetes, duration of diabetes, BMI, and smoking, we found little evidence of confounding by these factors.
Although guidelines at Kaiser Permanente for insulin initiation and management are generally similar to those recommended by the American Diabetes Association (16,17), practice patterns may differ from those in other medical care settings. For example, in a study of insulin users conducted in the U.S. Medicare population, ~40% used glargine and 60% used only a nonglargine insulin (10). However, practice patterns will only bias results if they are related to unmeasured risk factors for the cancers of interest.
There are several strengths of this cohort study. First, enrollees of KPNC and KPSC receive virtually all of their health care from the prepaid, integrated health plans. In addition, the memberships include >730,000 patients with diabetes. Computerized clinical records allow for the identification of patients with diabetes based on diagnoses, laboratory tests, and pharmacy data, and the plans have high-quality cancer registries. This study is also strengthened by the availability of electronic pharmacy records for data on medication use. By requiring patients to fill two prescriptions within a 6-month period, we increased the likelihood that those classified as users actually took the medications of interest.
In conclusion, our results do not support an association between relatively short-term use of glargine and increased risk of colorectal or prostate cancer or all cancer sites combined. These results are consistent with and complementary to the results of ORIGIN, the only randomized clinical trial with a large number of participants and follow-up extending more than a few years. Our finding of a modest increase in risk of breast cancer associated with ≥2 years of glargine among new insulin users should be viewed cautiously. Given study limitations, particularly the ability to examine only very recent and short-term glargine use, additional follow-up of this cohort and others will be needed to determine whether glargine is associated with an increase in breast or other forms of cancer.
Acknowledgments
This study was funded by a grant from Sanofi to University of North Carolina-Chapel Hill (UNC-CH), with a subcontract from UNC-CH to Kaiser Foundation Research Institute. The sponsor was given the opportunity to review and comment on the manuscript before submission. L.A.H. has received other research funding from Takeda, Merck, and Genentech (Roche). A.F., S.K.V.D.E., and C.P.Q. have received additional research funding from Takeda. S.K.V.D.E. has also received research funding from GlaxoSmithKline. No other potential conflicts of interest relevant to this article were reported.
The opinions expressed are those of the authors.
L.A.H. designed the study, interpreted the results, and wrote, reviewed, and edited the manuscript. K.N.D. and C.P.Q. designed the study, interpreted results, and reviewed and edited the manuscript. A.C. collected, analyzed, and interpreted data and reviewed and edited the manuscript. S.K.V.D.E. and N.S.W. designed the study, interpreted results, and reviewed and edited the manuscript. A.F. designed the study, interpreted the results, and wrote, reviewed, and edited the manuscript. L.A.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank patients with diabetes at Kaiser Permanente Northern and Southern California regions. The authors also thank John Buse, MD, UNC-CH, for his assistance with obtaining funding and with initial drafts of the study protocol, and Maqdooda Merchant (Kaiser Permanente Northern California) and Richard Contreras (Kaiser Permanente Southern California) for their programming assistance.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
References
- 1.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009;52:1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia 2009;52:1745–1754 [DOI] [PubMed] [Google Scholar]
- 3.Colhoun HM, SDRN Epidemiology Group Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 2009;52:1755–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 [DOI] [PubMed] [Google Scholar]
- 5.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009;16:1103–1123 [DOI] [PubMed] [Google Scholar]
- 6.Suissa S, Azoulay L, Dell’Aniello S, Evans M, Vora J, Pollak M. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia 2011;54:2254–2262 [DOI] [PubMed] [Google Scholar]
- 7.Ljung R, Talbäck M, Haglund B, Jonasson JM, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of breast cancer - a four-year population-based observation. Acta Oncol 2012;51:400–402 [DOI] [PubMed] [Google Scholar]
- 8.Blin P, Lassalle R, Dureau-Pournin C, et al. Insulin glargine and risk of cancer: a cohort study in the French National Healthcare Insurance Database. Diabetologia 2012;55:644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lind M, Fahlén M, Eliasson B, Odén A. The relationship between the exposure time of insulin glargine and risk of breast and prostate cancer: an observational study of the time-dependent effects of antidiabetic treatments in patients with diabetes. Prim Care Diabetes 2012;6:53–59 [DOI] [PubMed] [Google Scholar]
- 10.Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care 2011;34:1965–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannucci E, Monami M, Balzi D, et al. Doses of insulin and its analogues and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 2010;33:1997–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Risk of cancer in patients on insulin glargine and other insulin analogues in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia 2012;55:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang CH, Toh S, Lin JW, et al. Cancer risk associated with insulin glargine among adult type 2 diabetes patients—a nationwide cohort study. PLoS ONE 2011;6:e21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljung R, Talbäck M, Haglund B, Jonasson JM, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies - a three-year population-based observation. Acta Oncol 2011;50:685–693 [DOI] [PubMed] [Google Scholar]
- 15.Home PD, Lagarenne P. Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia 2009;52:2499–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerstein HC, Bosch J, Dagenais GR, et al. ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 17.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]